Abstract
The balance of osteogenesis and adipogenesis in bone marrow mesenchymal stem cells (BMSCs) is disrupted in osteoporosis. This study was designed to investigate the effects of extract of Ginkgo biloba (EGB) on proliferation, osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells in vitro. The effect of EGB on proliferation was evaluated by CCK-8 assay and flow cytometry. Osteogenic differentiation was evaluated by Alizarin Red S staining and Alkaline phosphatase assay. Adipogenic differentiation was evaluated by Oil Red O staining. Quantitative real-time polymerase chain reaction (Real-time PCR) was used to detect the expression of osteogenic specific genes (BMP-2, Runx2 and Colla1) and adipogenic specific genes (ap2, PPARγ). EGB did not significantly affect proliferation of BMSCs. However, it increased the calcium accumulation and significantly promoted the activity of alkaline phosphatase, especially when the concentration of EGB reached 150 µg/mL. EGB dose-dependently inhibited the adipogenic ability of BMSCs. The osteogenic-related genes (BMP-2, Runx2, Colla1) were overexpressed while the expression of genes involved in adipogenesis, such as PPAR-γ and ap2, was decreasing with the increase of EGB concentration. Our data proves that EGB inhibited adipocyte differentiation and enhanced osteogenic differentiation in BMSCs, but had no effect on the proliferation of BMSCs.
Keywords: Ginkgo biloba, bone marrow mesenchymal stem cells, proliferation, osteogenic differentiation, adipogenic differentiation
Introduction
Osteoporosis is a common bone disease worldwide and associated with bone loss and bone microstructure failure, which may lead to bone fragility and an increase in bone fracture [1,2]. Various risk factors are associated with osteoporosis, including menopause, amenorrhea or excessive alcohol consumption [3]. Thus, osteoporosis may develop as a result of nutritional dysfunction, lifestyle factors change or age. The long-term use and overuse of glucocorticoids also cause osteoporosis [4].
To prevent osteoporosis, various therapies have been developed. There is a range of anti-resorptive or anabolic options for the prevention of osteoporosis [5], such as alendronate, the most common drug for the treatment of postmenopausal osteoporosis. Hormone replacement therapy (HRT) is an alternative treatment for osteoporosis, but it may cause irregular bleeding and increase the risk of breast cancer [6]. Biphosphonates that are also used to treat osteoporosis can cause adverse effects such as osteonecrosis of the jaw, a rare but recognized side effect attributed to long-term administration of these drugs [7]. In fact, most of the existing treatments for osteoporosis are associated with side effects, thus prompting the search for alternatives.
Selective estrogen receptor modulators (SERMs) have estrogen-dependent biphasic effects on the target organs. Phytoestrogens (plant-derived compounds possessing estrogen-like activity) have similar characteristics as SERMs. Studies have shown that they are promising alternative drugs to treat osteoporosis [8]. Extract of Ginkgo biloba (EGB) has anti-estrogenic and estrogenic biphasic activities and might be a promising phytoestrogens [9,10].
Ginkgo biloba grows in China, Japan and Korea. Phytochemical studies have established a typical composition of the extract from this plant: 24% of phytoestrogens (kaempferol, kercetin and ishorhamnetin), 6% of terpenoids (ginkgolides and bilobilides) and less than 5 ppm of ginkgolic acid [11]. Ritu et al [12] proved that EGB have positive effects on the bone mineral density, bone microstructure, and osteoblast function in ovariectomized rats. Lucinda and coworkers [13] showed that EGB treatment significantly reverse the decrease of the percentage of the alveolar bone (PAB) in the mandible and the percentage of trabecular bone (PTB) in the femur.
As the pharmacological effects of EGB are associated with vascular and tissue protection and improvement in cognitive function, the aim of our study was to explore the effects of EGB on the balance of osteogenesis and adipogenesis in bone marrow mesenchymal stem cells (BMSCs) in vitro and to identify a novel therapeutic target of EGB for osteoporosis.
Materials and methods
Animals
This work was carried out in accordance with the guidelines issued by the Ethical Committee of Jilin University and approved by its Animal Care and Use Committee. One week-old Wistar mice were purchased from experimental animal center of Jilin university (protocol number SZXK 2013-0003 - Jilin University, China).
Reagents and chemicals
EGB was provided by Xuzhou Heng Kai Ginkgo Products Co. Ltd. (China). EGB contained 28.2% ginkgo flavonglycosides, 8.3% of terpenolactones, 15% of quercetin glycosides, 10.9% of kaempferol glycosides, 2.3% of ishorhamnetin glycosides and less than 5 ppm of ginkgolic acids. Cell culture medium and supplements were purchased from Hyclone (USA) and Gibco (USA).
Proliferation of bone marrow mesenchymal stem cells
Mesenchymal stem cells from the femur and tibia of 1-week old Wistar mice were isolated and cultured in the complete medium with 15% fetal bovine serum (FBS) according to the previously published protocol [14]. Firstly, the femur and tibia were excised from the mouse aseptically and the soft tissues were removed. The bones were washed three times and the epiphyses were cut off. L-DMEM was used to flush bone marrow cells and the cells were cultured in a humidified incubator containing 5% CO2 at 37°C. The proliferation of BMSCs was measured using the third passage of cells (1.0×104 cells/well) in a 96-well culture plate. After 24 h, the medium was supplemented with various doses of EGB (50, 100, 150, 200, 250 and 400 µg/mL). After 3, 5 or 7 days of culturing, cell proliferation assays were performed using CCK-8 assay kit (SAB, USA), in which the EGB-containing media were replaced with 100 µL of basal medium and 10 µL of CCK-8. One hour later, the plates absorption at 460 nm was measured using spectrophotometer (Bio-Tek ELX800, USA).
Cell cycle of BMSCs
The third passage of BMSCs was adjusted to 1.0×106 cells/well. When the cells reached 80% confluence, different concentrations of Ginkgo biloba extract were added. After 24 h, the cells were collected and rinsed twice with PBS, then pre-cooled 70% ethanol was used to fix cells at 4°C for over 12 h. Cells were re-suspended in 0.5 mL PBS and PI and RNase A were added to detect the stages of cell cycle using flow cytometry.
Mineralization of bone marrow mesenchymal stem cells
For osteogenic differentiation, BMSCs (1×106) were seeded in 24-well culture plate and cultured with FBS (10%), dexamethasone (100 nM), β-glycerophosphate (10 mM) and ascorbic acid (50 mM). Different concentrations of EGB (50, 100, 150, 200, 250 and 400 µg/mL) were added into BMSCs, and the cells were cultured for 7 and 14 days at 37°C with 5% CO2. Alizarin Red S and alkaline phosphatase (ALP) activity assay were used to test the mineralization of bone marrow mesenchymal stem cells.
Adipogenic differentiation in bone marrow mesenchymal stem cells
BMSCs (1×106) were added into 24-well plates and cultured in the adipogenic medium containing dexamethasone (1.0 μM), isobutylmethylxanthine (IBMX, 0.5 mM), indomethacin (200 μM), FBS (10%) and insulin (10 μg/mL), as well as different concentrations of EGB for 7 days. Staining with Oil Red O was used to test the adipogenic ability of EGB.
Real-time PCR
Real-time PCR was conducted to measure the expression of osteogenic and adipogenic specific genes in bone marrow mesenchymal stem cells. The primers for PCR are listed in Table 1. mRNA was isolated using TRIZOL (Life Technologies, USA) and quantified by the Nano-Drop spectrophotometer (Nano Drop 2000c, USA), cDNA was obtained using commercial kit (PrimeScriptTM RT reagent kit, Japan). cDNA was synthesized in a 40 μL reaction volume containing 20 μg mRNA. The PCR amplification protocol was as follows: 95°C, 30 s; 40 cycles (95°C for 5 s and 60°C for 20 s); 95°C, 1 min; 55°C, 30 s and 95°C, 30 s. Quantitative RT-PCR was carried out using the Light Cycler 480® (Roche, USA). The specificity of the expected PCR product was checked by melting curve analysis. The data were analyzed using the MxPro software, and observed changes in the gene expression were normalized to the GAPDH level.
Table 1.
Real-time PCR primers
| Gene | Primer sequence | Accession number |
|---|---|---|
| GAPDH | F-CTGAGGACCAGGTTGTCTCC | NM-017008 |
| R-TGTGAGGGAGATGCTCAGTG | ||
| PPARγ | F-ATTCTCAGTGGAGACCGCC | NM-013124.3 |
| R-GCAGAGGGTGAAGGCTCATA | ||
| ap2 | F-AATTCGATGAAATCACCCCA | NM-053365 |
| R-ATGCAAATTTCAGTCCAGGG | ||
| RUNX2 | F-CATGGCCGGGAATGATGAG | NM_053470 |
| R-TGTGAAGACCGTTATGGTCAAAGTG | ||
| Col1a1 | F-GACATGTTCAGCTTTGTGGACCTC | NM_053304 |
| R-AGGGACCCTTAGGCCATTGTGTA | ||
| Bmp-2 | F-ACCGTGCTCAGCTTCCATCAC | NM_017178 |
| R-CTATTTCCCAAAGCTTCCTGCATTT |
Data analysis
Each experiment was repeated at least three times. The data was analyzed by SPSS statistical analysis software. One-way analysis of variance (ANOVA) followed by Dunnett’s test was used to compare the data from different groups. P<0.05 was considered a significant difference.
Results
EGB did not affect the proliferation of BMSCs
To evaluate the effects of EGB on the proliferation of BMSCs, CCK-8 assay was chosen as it is convenient and highly sensitive. There was no statistically significant difference between different groups. EGB did not affect BMSCs proliferation when EGB was 50, 100, 150, 200, 400 μg/mL at days 1, 3 or 5 (Figure 1).
Figure 1.
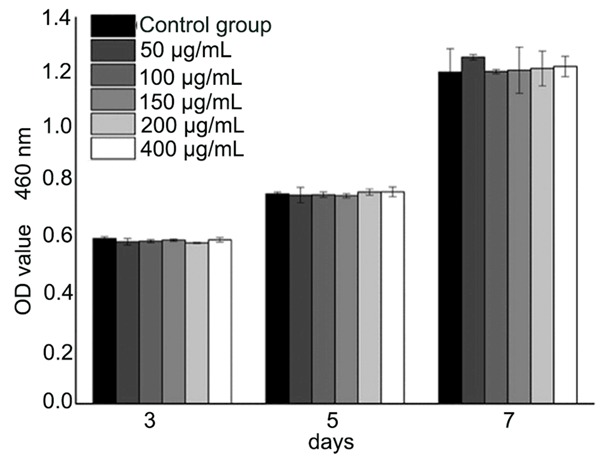
The effect of EGB on the proliferation of bone marrow mesenchymal stem cells. CCK-8 assay was used to evaluate the effect. The detection was undertaken after 3, 5 and 7 days of incubation by CCK-8 assay. There were no significant statistical differences between the groups (*P>0.05).
EGB arrested cells in S phase of cell cycle
Flow cytometry was performed to investigate the influence of EGB on the cell cycle. EGB in 0~150 µg/mL concentration range increased the proportion of cells in S phase, while EGB concentrations of 200~400 µg/mL decreased this proportion (Figure 2 and Table 2).
Figure 2.
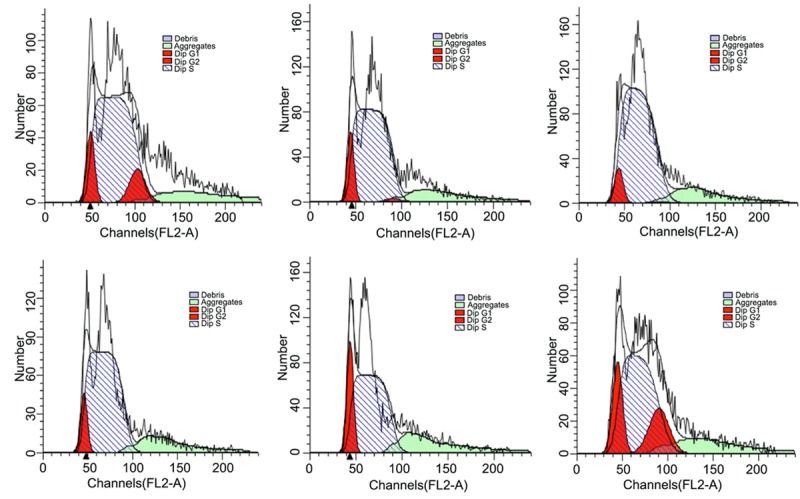
Cell cycle analysis of BMSCs cultured with different concentrations of EGB for 24 h.
Table 2.
The cell cycle changes of BMSCs after 24 h culturing with different concentrations of EGB
| Control | 50 μg/mL | 100 μg/mL | 150 μg/mL | 200 μg/mL | 400 μg/mL | |
|---|---|---|---|---|---|---|
| S phase | 78.31% | 85.39% | 89.08% | 91.3% | 77.31% | 64.42% |
| G1 phase | 11.23% | 13.28% | 10.92% | 8.05% | 22.69% | 17.83% |
| G2 phase | 10.46% | 1.33% | 0 | 0.64% | 0 | 17.75% |
EGB has a positive effect on the osteogenic differentiation of BMSCs
Alizarin Red S staining was used to assess the effects of EGB on the osteogenic differentiation of BMSCs. BMSCs were cultured with osteogenic supplements for 7 days and stained with Alizarin Red S to assess the calcium accumulation. Calcium accumulation in BMSCs is shown in Figure 3. Calcium deposition was significantly increasing by the EGB treatment when the concentration of extract was increased from 0 to 150 μg/mL. The greatest calcium deposition was observed at EGB concentration of 150 μg/mL, with further increases in extract concentration leading to fewer calcium deposits. The findings imply that EGB (in the range of 50 to 400 μg/mL) promoted mineralization in osteogenic groups compared to control group.
Figure 3.
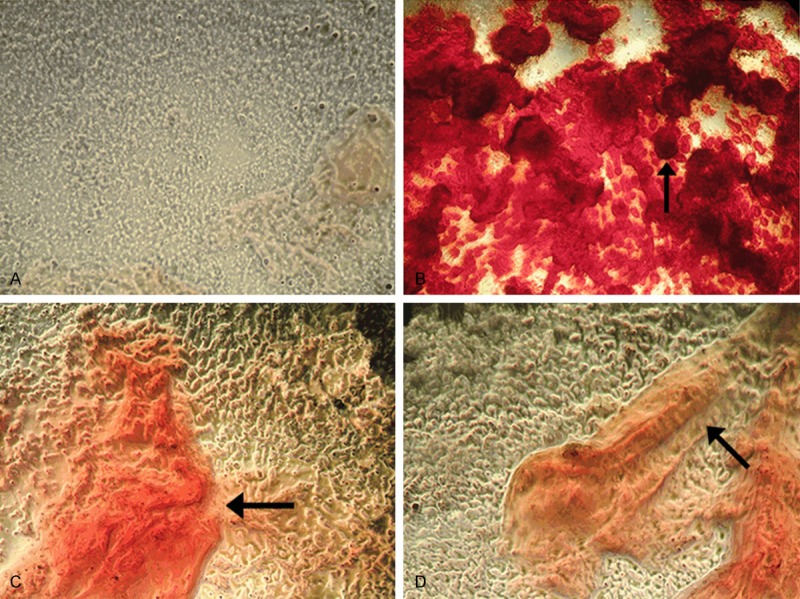
Alizarin Red S staining of calcium accumulation in BMSCs cultured with EGB under light microscope. (A) Control group with no EGB; (B) Cells incubated with 150 μg/mL EGB; (C) Cells incubated with 50 μg/mL EGB; (D) Cells incubated with 400 μg/mL EGB. The calcium deposits are indicated by black arrows (X40). The highest calcium accumulation was observed at 150 μg/mL EGB.
ALP is a representative marker for osteogenic differentiation. The examination of ALP was performed at day 7 and day 14 in osteogenic cultures. The results were consistently matched with those derived from the Alizarin Red-S staining. EGB (50-400 µg/mL) significantly increased ALP activity, and 150 µg/mL was the most effective concentration (Figure 4).
Figure 4.
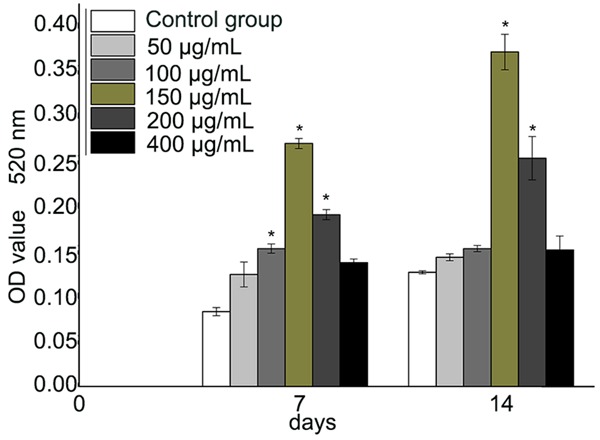
ALP activity in BMSCs at 7 and 14 days of incubation. The absorbance was measured at 520 nm. EGB treatment significantly increased the ALP activity compared to the controls at concentrations of 100, 150 and 200 μg/mL at 7 days (*P<0.05). EGB at 150 and 200 μg/mL also had statistically significant effect compared to control at 14 days (*P<0.05). The effect was the strongest at 150 μg/mL EGB (n=6).
EGB inhibited the adipogenic differentiation of BMSCs
BMSCs were cultured with adipogenic supplements for 7 days and stained with Oil Red O to assess accumulation of intracellular lipid droplets. Compared with control group, EGB treatment lowered the lipid accumulation and led to fewer numbers of lipid-filled cells. Oil Red O absorbance examination showed a significant inhibition of adipogenesis in BMSCs by EGB treatment (50, 200 and 400 μg/mL) (Figure 5).
Figure 5.
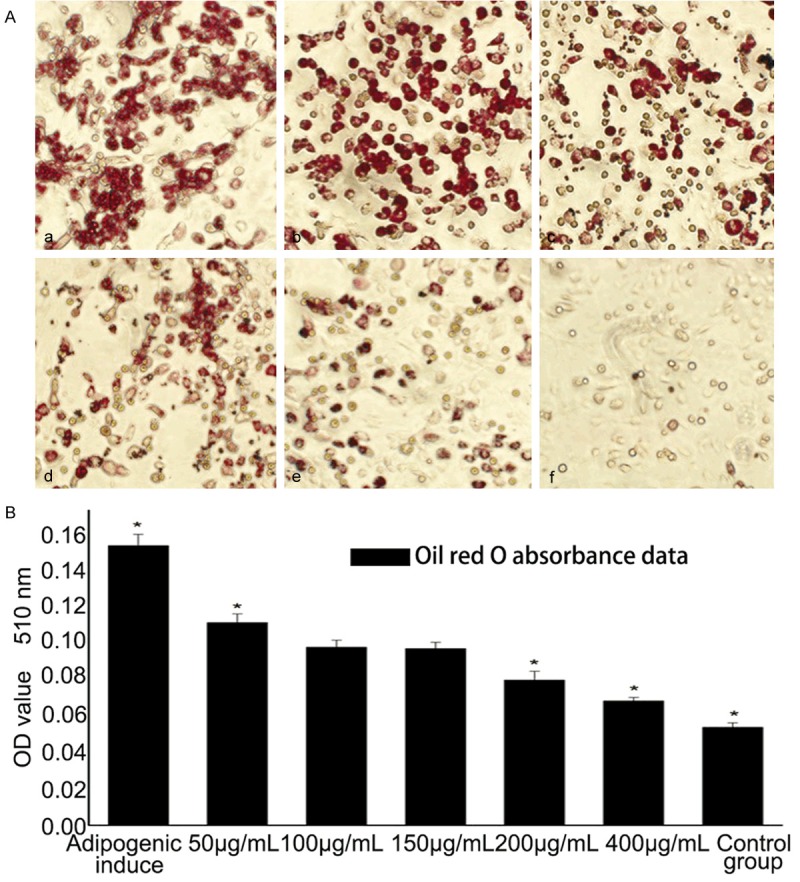
Effects of EGB on adipogenic differentiation of BMSCs. (A) Oil Red O staining for intracellular lipid droplets when cultured with different concentration of EGB in adipogenic medium. From (a-e), the concentrations of EGB were 50 μ0reEGB e μ0reEGB e μ0reE, 200eE, B e 400 μ00eE, respectively; (f) is a control group. The accumulation of intracellular lipid droplets decreased with the increase of EGB concentration. (B) Oil Red O absorbance was measured at 510 nm. EGB had significantly decreased the OD values compared to controls in a dose-dependent manner (*P<0.05) (n=7).
The osteogenic and adipogenic-related genes were diversely expressed in BMSCs upon EGB treatment
Real-time PCR was performed to explore whether EGB altered the expression of osteogenic and adipogenic specific genes involved in the differentiation of BMSCs. EGB (150 μg/mL) upregulated the expression of the osteogenic genes including BMP-2, RUNX2 and Col1a1 compared with the control group, and the difference was statistically significant (P<0.05, thus implying that EGB has potential to promote the osteogenic differentiation. We then tested the expression of adipogenic genes, PPARγ and ap2. The results showed that EGB decreased the mRNA expression of PPARγ and ap2 in a dose-dependent manner. The level of the PPARγ and ap2 mRNA reduced most significantly when EGB dose was 400 μg/ml (Figure 6).
Figure 6.
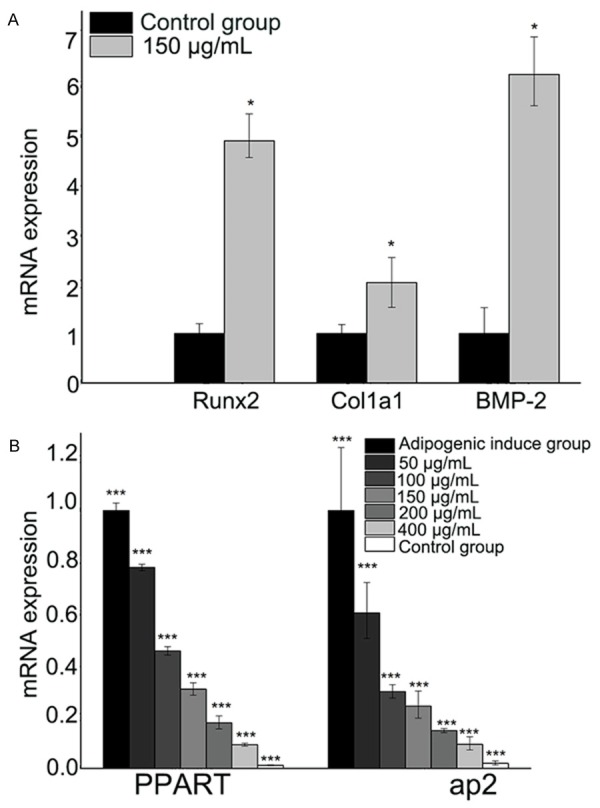
Expression of osteogenic (A) and adipogenic (B) specific genes upon EGB treatment. The level of mRNA was measured by Real-Time PCR.
Discussion
Osteoporosis is an increasingly common disease that correlates with age and overuse of hormones. Reciprocal relationship between increased adipocytes and decreased bone formation in conditions of bone loss like age-related osteoporosis has been reported [15]. Previous studies showed that bone fat increases with the bone losses in ovariectomized rats [16]. Bone marrow mesenchymal stem cells are pluripotent as they are able to differentiate into osteoblasts, adipocytes and other cells. When more BMSCs turn into adipocytes, less BMSCs are likely to turn into osteoblasts. These changes in differentiation may lead to osteoporosis. Our study proved that EGB has the active effects on the bone formation from BMSCs.
EGB is a phytoestrogen containing quercetin, kaempferol, and isorhamnetin, among which quercetin and kaempferol are the key factors [12] with potential estrogenic and anti-estrogenic activity mediated through ER pathway [9,10,17]. Our study investigated the osteogenic and adipogenic ability of EGB in BMSCs and proved that EGB promote BMSCs differentiation into osteoblasts, but inhibits their differentiation into adipocytes. ALP assay and Alizarin Red S staining are important tests for mineralization [18], both of which confirmed increased mineralization in BMSCs co-cultured with EGB for 7 and 14 days. This implies that EGB might be involved in the regulation of BMSCs mineralization. It was previously reported that EGB could protect the osteoblast-like MC3T3-E1 cells from cellular damages due to the presence of flavonoids that promote the bone formation [19]. In our study, EGB had no effects on the proliferation of BMSCs, thus suggesting that EGB promotes bone formation at least partly via the regulation of functions of the cells.
Our findings demonstrate that EGB could regulate BMSCs fate by increasing the number of osteoblasts and reducing the number of adipocytes. The findings point that EGB or its individual constituents might have a significant therapeutic potential in treating osteoporosis. The result of Real-Time PCR showed that EGB could increase the expression of osteogenic genes, but decrease the expression of adipogenic genes, suggesting the relationship between osteogenic and adipogenic specific genes in the BMSCs differentiation. Runx2, Col1a1 and BMP-2 are osteoblast transcription factors and were shown to be over-expressed in our study, whereas PPAR-γ and ap2, as adipogenic regulators, were down-expressed.
BMP-2 is one of the most important extracellular transcription factors to promote bone formation and induce the osteogenic differentiation. It plays an important role in the osteogenesis by activating the conduction of the signaling of Smads pathway and regulating the transcription of osteogenic related gene [20]. Runx2 is one of the target genes of BMP-2 signaling pathway and has a synergy with BMP-2. It is considered a key transcription factor for the BMSCs differentiation and bone formation [21]. Runx2 is a necessary factor for the expression of OSX, and OSX is involved in the osteogenic differentiation of BMSCs. Thus, Runx2 plays a central role in the osteogenesis differentiation as it participates in the cross-talk between many signaling pathways [22]. Col1a1 is also a significant factor in the bone-formation and considered to be the marker of the osteogenesis differentiation. Above all, our results further supported the hypothesis that, through transcription level regulation, BMSCs may differentiate to osteogenic lineage when it is co-cultured with appropriate drug.
Our data showed that there was a significant reduction in the expression of PPAR-γ and ap2 when cells were treated with EGB. PPAR-γ is principally regarded as the master regulator of adipogenesis, and all pro-adipogenic cell signaling pathways converge with PPARγ [23]. In addition, the expression of ap2, another adipogenic marker, also decreased.
Recently, some studies elucidate that PPARγ ligands have the potential to inhibit β-catenin signaling [24] and inhibit Runx2 mediated transcription of osteogenesis-related genes [25]. This may explain why EGB improves osteogenesis differentiation and inhibits adipogenesis differentiation in BMSCs. With EGB present, the expression of PPARγ and ap2 decreases but Runx2, Col1a1 and BMP2 expression does up. Several studies proved that EGB improves the bone mineral density and bone microstructure in osteoporosis rats [13,26]. Although there are limitations in our study, the obtained data clearly demonstrate that EGB can positive contribution to the prevention and treatment of osteoporosis.
Acknowledgements
This study was supported by Industrial Technology Research and Development Program of Jilin Province (No. 2013C023-5), and The Twelfth Five-Year Plan for Scientific and Technological Research of Educational Department of Jilin Province (No. 2015-530).
Disclosure of conflict of interest
None.
References
- 1.Hallworth RB. Prevention and treatment of postmenopausal osteoporosis. Pharm World Sci. 1998;20:198–205. doi: 10.1023/a:1008682921480. [DOI] [PubMed] [Google Scholar]
- 2.Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:349–356. doi: 10.1016/j.tripleo.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr. 2003;78:593S–609S. doi: 10.1093/ajcn/78.3.593S. [DOI] [PubMed] [Google Scholar]
- 4.Ilias I, Ghayee H. Glucocorticoid-Induced Osteoporosis. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. Endotext. South Dartmouth (MA): 2000. [Google Scholar]
- 5.Das S, Crockett JC. Osteoporosis - a current view of pharmacological prevention and treatment. Drug Des Devel Ther. 2013;7:435–448. doi: 10.2147/DDDT.S31504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedblad B, Merlo J, Manjer J, Engstrom G, Berglund G, Janzon L. Incidence of cardiovascular disease, cancer and death in postmenopausal women affirming use of hormone replacement therapy. Scand J Public Health. 2002;30:12–19. [PubMed] [Google Scholar]
- 7.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 8.Dang ZC, Lowik C. Dose-dependent effects of phytoestrogens on bone. Trends Endocrinol Metab. 2005;16:207–213. doi: 10.1016/j.tem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Oh SM, Chung KH. Estrogenic activities of Ginkgo biloba extracts. Life Sci. 2004;74:1325–1335. doi: 10.1016/j.lfs.2003.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Oh SM, Chung KH. Antiestrogenic activities of Ginkgo biloba extracts. J Steroid Biochem Mol Biol. 2006;100:167–176. doi: 10.1016/j.jsbmb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Ji YB, Alaerts G, Xu CJ, Hu YZ, Vander Heyden Y. Sequential uniform designs for fingerprints development of Ginkgo biloba extracts by capillary electrophoresis. J Chromatogr A. 2006;1128:273–281. doi: 10.1016/j.chroma.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 12.Trivedi R, Kumar A, Gupta V, Kumar S, Nagar GK, Romero JR, Dwivedi AK, Chattopadhyay N. Effects of Egb 761 on bone mineral density, bone microstructure, and osteoblast function: Possible roles of quercetin and kaempferol. Mol Cell Endocrinol. 2009;302:86–91. doi: 10.1016/j.mce.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Lucinda LM, Vieira BJ, Oliveira TT, Sa RC, Peters VM, Reis JE, Guerra MO. Evidences of osteoporosis improvement in Wistar rats treated with Ginkgo biloba extract: a histomorphometric study of mandible and femur. Fitoterapia. 2010;81:982–987. doi: 10.1016/j.fitote.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Heidari B, Shirazi A, Akhondi MM, Hassanpour H, Behzadi B, Naderi MM, Sarvari A, Borjian S. Comparison of proliferative and multilineage differentiation potential of sheep mesenchymal stem cells derived from bone marrow, liver, and adipose tissue. Avicenna J Med Biotechnol. 2013;5:104–117. [PMC free article] [PubMed] [Google Scholar]
- 15.Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002;55:693–698. doi: 10.1136/jcp.55.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin RB, Zissimos SL. Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone. 1991;12:123–131. doi: 10.1016/8756-3282(91)90011-7. [DOI] [PubMed] [Google Scholar]
- 17.Prouillet C, Maziere JC, Maziere C, Wattel A, Brazier M, Kamel S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem Pharmacol. 2004;67:1307–1313. doi: 10.1016/j.bcp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Bellows CG, Aubin JE, Heersche JN. Initiation and progression of mineralization of bone nodules formed in vitro: the role of alkaline phosphatase and organic phosphate. Bone Miner. 1991;14:27–40. doi: 10.1016/0169-6009(91)90100-e. [DOI] [PubMed] [Google Scholar]
- 19.Yirmibesoglu E, Karahacioglu E, Kilic D, Lortlar N, Akbulut G, Omeroglu S. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387–394. doi: 10.1111/j.1365-2230.2011.04253.x. [DOI] [PubMed] [Google Scholar]
- 20.Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21:637–646. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999;13:1025–1036. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 23.Tzameli I, Fang H, Ollero M, Shi H, Hamm JK, Kievit P, Hollenberg AN, Flier JS. Regulated production of a peroxisome proliferator-activated receptor-gamma ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J Biol Chem. 2004;279:36093–36102. doi: 10.1074/jbc.M405346200. [DOI] [PubMed] [Google Scholar]
- 24.Lu D, Carson DA. Repression of beta-catenin signaling by PPAR gamma ligands. Eur J Pharmacol. 2010;636:198–202. doi: 10.1016/j.ejphar.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS, Park SW, Kim SY, Shin CS. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem. 2003;278:23270–23277. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- 26.Lucinda LM, de Oliveira TT, Salvador PA, Peters VM, Reis JE, Guerra Mde O. Radiographic evidence of mandibular osteoporosis improvement in Wistar rats treated with Ginkgo biloba. Phytother Res. 2010;24:264–267. doi: 10.1002/ptr.2924. [DOI] [PubMed] [Google Scholar]


