Abstract
The present study examined alterations of spinal ubiquitin C-terminal hydrolase L1 (UCHL1), ubiquitin expression and glial activation in the cancer-induced bone pain rats. Furthermore, whether inhibition of spinal UCHL1 could alleviate cancer-induced bone pain was observed. The CIBP model was established by intrathecal Walker 256 mammary gland carcinoma cells in SD rats. The rats of CIBP developed significant pain facilitation in the Von Frey test. Double immunofluorescence analyses revealed that in the spines of CIBP rats, ubiquitin co-localized with NeuN, Iba-1 or GFAP; UCHL1 and NeuN were co-expressed and UCHL1 also co-localized with ubiquitin. The CIBP model induced up-regulation of ubiquitin and UCHL1 in the spines, as well as glial activation. Inhibition of spinal UCHL1 attenuated pain facilitation by down-regulation of ubiquitin expression and glial activation. in the CIBP rats. Our data suggests that UCHL1/ubiquitin distributed and increased in the spines of CIBP rats, that glial activation also increased in the CIBP model and that inhibition of spinal UCHL1 may be an effective method to alleviate cancer-induced bone pain.
Keywords: UCHL1, ubiquitin, cancer-induced bone pain, glial activation
Introduction
Cancer-induced bone pain (CIBP) is a common problem in the cancer patients and the treatments for CIBP are less effective with side effects [1]. Involvement of spinal glial activation in cancer-induced bone pain was proved and stimulations from tumor cells may induce the alteration of pain-related genes in spinal glial cells [1,2]. An abnormal ubiquitin-proteasome pathway (UPP) may be one of the transcription factor-mediated epigenetic mechanisms underlying the nervous system diseases in the colloid cells [3]. Ubiquitin C-terminal hydrolase L1 (UCHL1) is a de-ubiquitinating enzyme which maintains the level of mono-ubiquitin. In previous studies, UCHL1 was increased in neural and tumor tissues and plays important roles in the nervous system diseases. Spinal UCHL1 and its mRNA level were robustly increased in different rat models of chronic pain, supporting the hypothesis that UPP is involved in nociceptive processing [4]. Although the spinal UCHL1 involved in chronic pain modulation, the precise mechanisms remains unclear.
Previously, the UPP in the chronic pain diseases was mainly focused on neurons [3,4]. However pain modulation of UPP in colloid cells was still unknown. Glial cells secret different kinds of cytokines and chemokines after noxious stimulations in the chronic pain models. Therefore, colloid cells may interplay with disease-associated proteins and the UPP which may affect glial cells homeostasis and neuronal functions [3]. Therefore, the changes of UPP (involving ubiquitin-ligases or ubiquitin C-terminal hydrolase in neurons or colloid cells) in the nervous system diseases may be the new therapeutic targets for CIBP.
Presently, it is speculated that UCHL1 increased in the spines of CIBP rats, and inhibition of UCHL1 exhibits antinociceptive effects in the CIBP model. Thus, we asked the following questions: 1) Does UCHL1 or ubiquitin co-localize with spinal glial cells and neurons in CIBP rats? 2) Is the glial cells activation and expression of spinal UCHL1/ubiquitin affected by CIBP? 3) How does the inhibition of UCHL1 affect pain facilitation, ubiquitin expression and glial cells activation?
Material and methods
Preparation of cells and animals
The Walker 256 rat mammary gland carcinoma cells (each rat received approximately 0.5 ml, 2×107 cells/ml) were implanted into the right medullary cavity of the tibia in female Sprague Dawley rats (200-250 g) (tumor cell implantation, TCI). An equal volume of heat killed cells was injected into sham animals [5]. All experimental sequences were approved by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and Scientific Investigation Board of Shandong University, and the similar institutes of Xuzhou Medical University.
Intrathecal catheterization and drug
Lumbosacral intrathecal catheters were constructed and implanted by threading the catheter caudally from the cisterna magna [6]. Indwelling catheter was utilized to inject chemicals into the cerebrospinal fluid (CSF) encompassing the lumbosacral spinal segments. LDN-57444 (UCHL1 inhibitor, Sigma-Aldrich Company) was dissolved in DMSO. Dosages of drugs were selected according to the preliminary tests.
Mechanical allodynia
As previously described [6], SD rats were individually housed in the single cage (26×20×14 cm3) with 5×5 mm2 wires mesh grid floor. The hind paw withdrawal threshold (PWT) was recorded to observe mechanical allodynia after accommodating for 30 minutes. A series of filaments (0.40, 0.60, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0 and 15.0 g) was utilized to perform these tests. We placed every von Frey hairs for about 6-8 s. Each stimulus was used five times at 30-s intervals. Furthermore, 50% PWTs were calculated according to the up-down method.
Western blotting analysis
The right L4-5 spinal segments were collected and prepared for analysis. The bands were incubated with the primary antibodies (rabbit anti-UCHL1 (1:1000) and rabbit anti-ubiquitin (1:500)) at 37°C for 2 hours, and then incubated with the secondary antibodies (goat anti-rabbit IgG at 1:5000) at 37°C for 1 hour. The densities of the bands were quantified by Image J Software (version 1.45, NIH, USA). The average densities of three bands were calculated as protein expressions. All expressions of proteins were evaluated relative to GAPDH expression.
Immunohistochemistry
The frozen longitudinal slices (30 μm, L4-5 spinal segments after post-thawing and dehydration) were prepared. After blocked with 10% donkey serum, the slices were incubated with the primary antibodies for 40 hours, and then incubated with the secondary antibodies for 2 hours. The primary antibodies were anti-UCHL1 (1:1000), anti-ubiquitin (1:50), NeuN (1:500), GFAP (1:500), or Iba-1 (1:400). The secondary antibodies were FITC-conjugated IgG (1:200) or tetraethyl rhodamine isothiocyanate (1:200). The slices were viewed under a Leica fluorescence microscope after mounting. The optical density of the immunoreactive staining was measured with the Leica Qwin 500 image analysis system (Germany).
Statistical analysis
Data were presented as the mean ± SEM. All data were collected blindly. The Tukey test in ANOVA and repeated measures ANOVA were utilized to detect statistical differences. P < 0.05 was considered statistically significant.
Results
Double immunofluorescence of UCHL1 and ubiquitin
Immunofluorescence analysis indicates that ubiquitin-IR cells co-localized with NeuN, Iba-1 or GFAP; UCHL1-IR cells and NeuN were co-expressed, but not co-localized with Iba-1 or GFAP in the L4-5 spines of CIBP rats. Double immunofluorescence demonstrated that UCHL1 is co-expressing with ubiquitin in the L4-5 spines of CIBP rats (Figure 1). These results demonstrated that in the CIBP rats, UCHL1 was predominantly distributed in neurons, while ubiquitin existed in cells including neurons, astrocytes and microglia.
Figure 1.
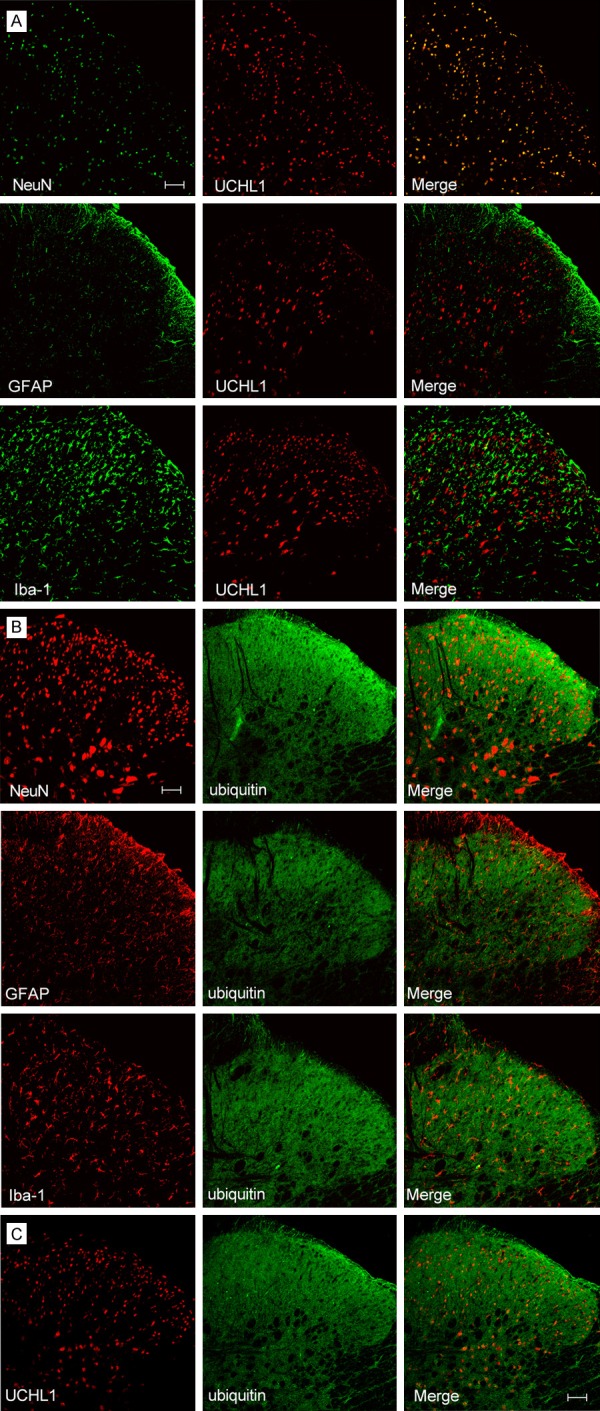
Spinal UCHL1 and ubiquitin expression in the ipsilateral L4-5 spines of CIBP rats (Scale bar = 100 μm). A. Dual labeling indicates that UCHL1-IR cells co-localized NeuN, but not co-localized with Iba-1 or GFAP; B. Ubiquitin-IR cells co-localized NeuN, Iba-1 and GFAP. C. Double immunofluorescence demonstrated that UCHL1 co-expressing with ubiquitin. CIBP: Cancer-induced bone pain group.
TCI resulted in pain facilitation on the ipsilateral side in cancer-induced bone pain rats
As illustrated in Figure 2, the rats developed lower PWTs in the Von Frey tests (P < 0.05), indicating the development of pain facilitation. The PWTs became more obvious on days 3-7 and lasted for 21 days following implantation (P < 0.05). Sham animals did not show any remarkable changes in PWTs (P > 0.05) (Figure 2).
Figure 2.
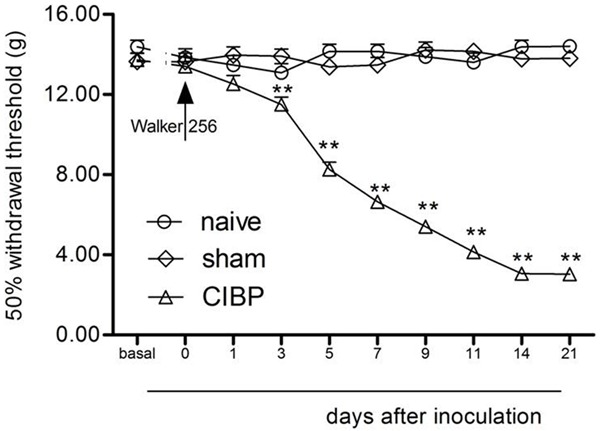
TCI-induced mechanical allodynia on the ipsilateral legs. Normal saline did not affect the paw withdrawal responses. **P < 0.01 vs. day 0 (mean ± SEM., n = 8). CIBP: Cancer-induced bone pain group.
Inhibition of UCHL1 attenuated the establishment of mechanical hypersensitivity induced by TCI
To observe whether inhibition of spinal UCHL1 can produce analgesia in the CIBP model, injection of LDN-57444 (UCHL1 inhibitor) or DMSO was intrathecal for three consecutive days after implantation (days 14-16). Repeated injection of LDN-57444 (2 μg or 4 μg, i.t., 14-16 days after implantation) significantly attenuated the establishment of TCI-induced low PWTs (P < 0.05). Repeated injection of LDN-57444 did not affect the PWTs in the sham animals (P > 0.05) (Figure 3). These data suggested that inhibition of spinal UCHL1 might produce analgesic effects during the period of CIBP.
Figure 3.
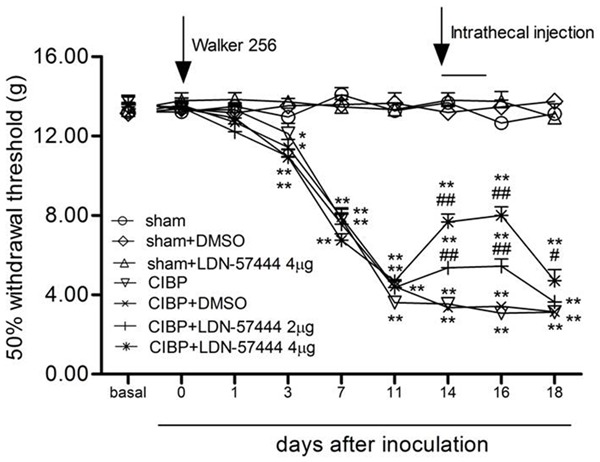
LDN-57444 inhibited the mechanical allodynia in the ipsilateral limbs following inoculation. LDN-57444 or DMSO was injected (i.t.) three consecutive days after inoculation (days 14-16) (mean ± SEM., n = 8). *P < 0.05, **P < 0.01, versus day 0. #P < 0.05 or ##P < 0.01, versus CIBP+DMSO (between the CIBP+DMSO rats and the CIBP+LDN-57444 rats). CIBP: Cancer-induced bone pain group.
UCHL1 and ubiquitin expression increased following TCI surgery
First, we demonstrated that the densities of UCHL1 and ubiquitin bands increased following tumor cell implantation (P < 0.05). The time-course of increased UCHL1 and ubiquitin expression closely corresponded with the development of pain facilitation after implantation (Figure 4).
Figure 4.
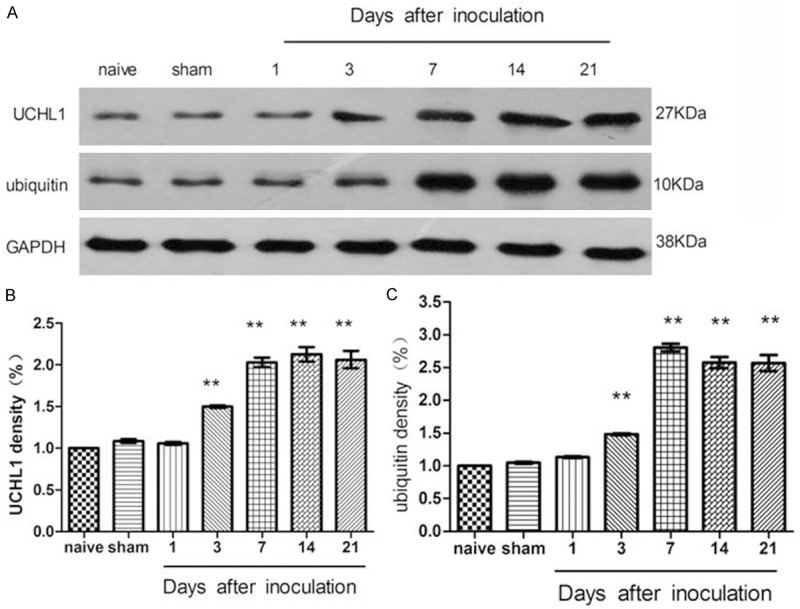
The level of spinal UCHL1 and ubiquitin expression increased following inoculation (L4-L5 segments). **P < 0.01, versus the naive rats (n = 3).
TCI-induced up-regulation of ubiquitin expression is antagonized by an intrathecal injection of LDN-57444
We found that repeated injection of UCHL1 inhibitor would decrease ubiquitin expression in contrast to CIBP/DMSO rats (P < 0.05). LDN-57444 did not affect spinal UCHL1 expression in CIBP rats (P > 0.05) (Figure 5).
Figure 5.
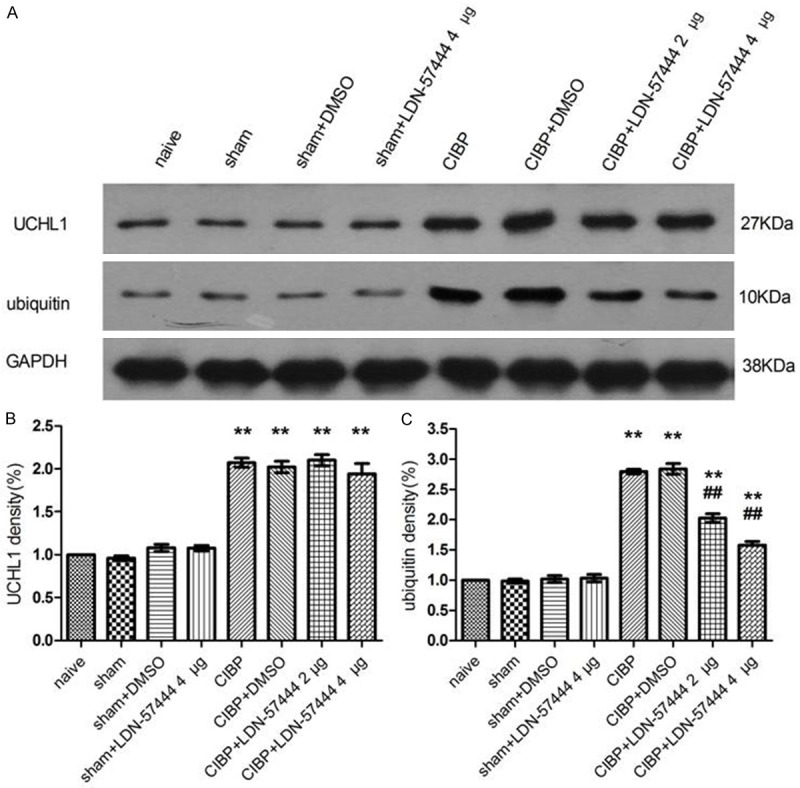
Up-regulation of ubiquitin expression were attenuated by an intrathecal injection of LDN-57444. UCHL1 expression was not affected by LDN-57444. Rats received injections of LDN-57444 or DMSO from 14 to 16 days following inoculation. Rats were sacrificed for western blotting analysis on day 16 after injection of LDN-57444. **P < 0.01, versus the naive rats; ##P < 0.01, versus the CIBP+DMSO rats (between the CIBP+DMSO rats and the CIBP+LDN-57444 rats) (n = 3). CIBP: Cancer-induced bone pain group.
The mean density of Iba-1 or GFAP was increased significantly in the CIBP animals in contrast to that of control animals (P < 0.05). Pre-injection of DMSO did not affect the increased density of Iba-1 or GFAP (P > 0.05). However, LDN-57444 pre-injection (4 µg/d) attenuated the up-regulation of Iba-1 or GFAP following the tumor cells inoculation, indicating inhibition of spinal glial cells (P < 0.05) (Figure 6).
Figure 6.
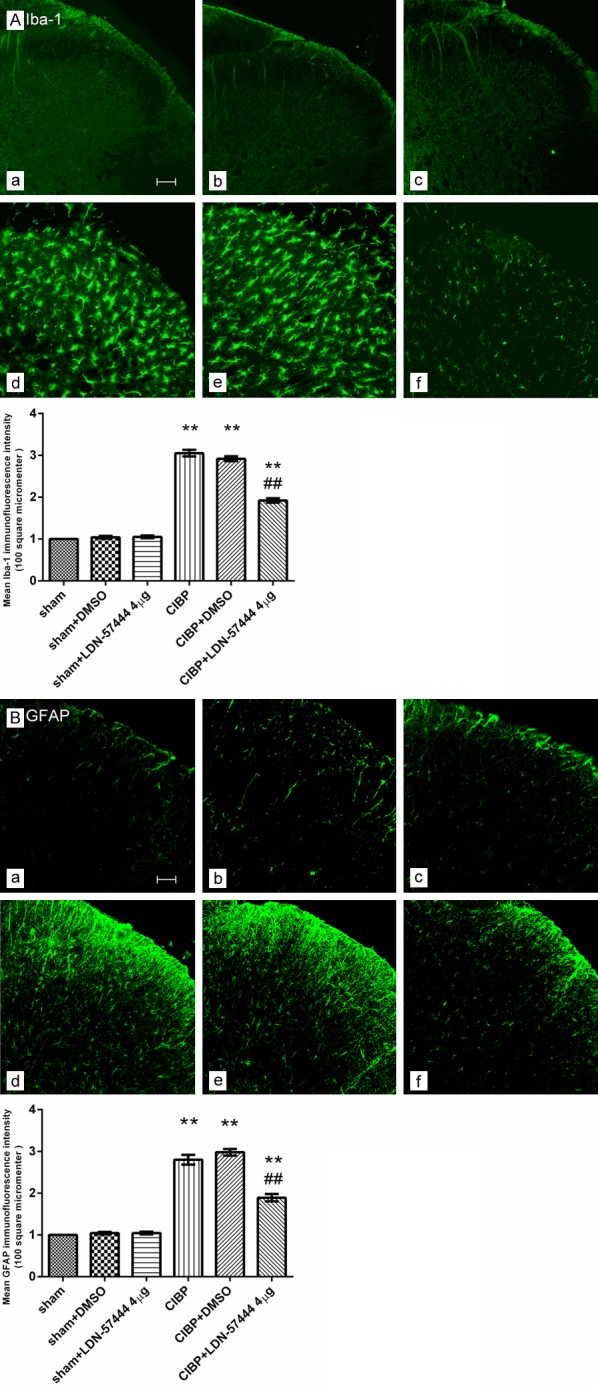
The UCHL1 inhibitor attenuated the microglia or astrocytes activation in the L4-5 segments after TCI (A: Iba-1, B: GFAP). (a) Sham group; (b) Sham+DMSO group; (c) Sham+LDN-57444 4 µg group; (d) CIBP group; (e) CIBP+DMSO group; (f) CIBP+LDN-57444 4 µg group. **P < 0.01 vs. the sham group; ##P < 0.01 vs. the CIBP+DMSO group. Scale bar = 100 μm. (mean ± SEM., n = 6). CIBP: Cancer-induced bone pain group.
Discussion
Our findings established as follows: (1) UCHL1/ubiquitin distributed in different kinds of cells in the spines of CIBP rats; (2) Spinal UCHL1/ubiquitin and glial activation increased after inoculation, which was corresponded with the development of pain behavior; (3) Through inhibiting ubiquitin expression and glial activation in the spines of CIBP rats, inhibition of spinal UCHL1 by LDN-57444 attenuated pain facilitation. Thus, demonstrating that spinal UCHL1 and ubiquitin expression were increased in the CIBP model and that the up-regulation was attenuated with intrathecal injection of UCHL1 inhibitor, our data extended the findings of previous experiments It is the first time to demonstrate that spinal UCHL1/ubiquitin and glial activation are involved in the antinociceptive effects of LDN-57444 in CIBP rats. Previous studies have shown that LDN-57444 inhibits UCHL1 hydrolase activity in vitro and in vivo [7]. We also found that the expression of UCHL1 did not change after intrathecal use of LDN-57444, while the expression of ubiquitin decreased in spinal cords.
However, how spinal UCHL1 and ubiquitin contribute to pain modulation is still not clear. Previous studies have demonstrated that UCH-L1 is involved in many pain-related pathways: (1) One recent study showed that UCH-L1 can be regulated by NF-κB. NF-κB increases the activity of UCH-L1 by binding to the 300 bp and 109 bp sites of the UCH-L1 promoter. Consequently, inflammatory stimulations may increase the activity of UCH-L1 via activating NF-κB in cultured murine podocytes [8]. (2) Up-regulation of UCHL1 activity and mono-ubiquitin expression following NMDARs activation may affect the transmission of neuronal information by maintaining the levels of mono-ubiquitin in hippocampus slices. LDN57444 (one UCHL1 inhibitor) could lead to de-phosphorylation of CREB (Ser133 site) and a subsequent increase in PSD-95/synapsin-1 [9]. (3) PKA is necessary in LTP. Corresponding to UCH or CREB, degradation of the PKA-R subunit may induce facilitation in Aplysia [10]. Exogenous UCHL1 may also attenuate Aβ-induced changes in LTP and PKA/CREB and may restore synaptic function [10,11]. LDN57444 inhibited 37% of UCHL1 activity and suppressed LTP in hippocampal slices [7]. (4) In many neurodegenerative diseases, protein degradation induced by UPP dysfunction is consistent with an alteration in microglia, astrocytes and neurons. Inhibition of proteasome by epoxomicin or MG-132 can decrease neuronal cell viability and glial cell activity. It appears that UPP can affect the activation of microglia and astrocytes by degrading aberrant proteins and maintaining protein homeostasis. [3,12].
Recent studies proved that extracellular ubiquitin is an endogenous CXCR4 agonist, CXCR4 activation with treatment of ubiquitin and SDF-1α may lead to similar Gαi-responses and a comparable magnitude of phosphorylation of ERK-1/2, p90 ribosomal S6 kinase-l and Akt, although phosphorylation induced by treatment of ubiquitin occur more transiently [13]. CXCR4 activation with treatment of ubiquitin and SDF-1α induces microglial migration and activation [14]. This activation, if occurred in the spinal cord, would in turn lead to pain facilitations [2].
It is demonstrated that activations of spinal glia could intensify the allodynia in cancer-induced bone pain. Spinal glia becomes activated after tumor cell inoculation, which was corresponding with the development of pain facilitation in the CIBP rats [1]. Previous studies showed that, UCHL1 was mainly expressed in neurons in the pain models [3,4]. Our data firstly prove that ubiquitin co-localize with NeuN, Iba-1 or GFAP in the spines of CIBP rats, while UCHL1 just co-localize with NeuN. Therefore, we speculate that neuron activation may increase the activity of UCHL1 which up-regulates ubiquitin expression in response to tumor cell inoculation. Ubiquitin and CXCR4 may lead to activation of glial cells. It has been reported that activated microglia or astrocytes can release varieties of neuro- and glial-excitatory substances (different kinds of cytokine and chemokine, et al.) [2], which may lead to the development of pain facilitation. This may explain why inhibition of spinal UCHL1 can attenuate the pain facilitation or glial activation after tumor cells inoculation.
In conclusion, in the spines of CIBP rats, UCHL1/ubiquitin distributed in different kinds cells. Spinal UCHL1/ubiquitin and glial activation increased in the CIBP models. Inhibition of spinal UCHL1 may be an effective approach to alleviate CIBP by inhibiting ubiquitin expression and glial activation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31100801, 81200858 and 81200861).
Disclosure of conflict of interest
None.
References
- 1.Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, McGinnis C, White FA. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007;21:581–591. doi: 10.1016/j.bbi.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cartier AE, Ubhi K, Spencer B, Vazquez-Roque RA, Kosberg KA, Fourgeaud L, Kanayson P, Patrick C, Rockenstein E, Patrick GN, Masliah E. Differential effects of UCHL1 modulation on alpha-synuclein in PD-like models of alpha-synucleinopathy. PLoS One. 2012;7:e34713. doi: 10.1371/journal.pone.0034713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chain DG, Casadio A, Schacher S, Hegde AN, Valbrun M, Yamamoto N, Goldberg AL, Bartsch D, Kandel ER, Schwartz JH. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron. 1999;22:147–156. doi: 10.1016/s0896-6273(00)80686-8. [DOI] [PubMed] [Google Scholar]
- 4.Jansen AH, Reits EA, Hol EM. The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front Mol Neurosci. 2014;7:73. doi: 10.3389/fnmol.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao-Ying QL, Zhao J, Dong ZQ, Wang J, Yu J, Yan MF, Zhang YQ, Wu GC, Wang YQ. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun. 2006;345:1292–1298. doi: 10.1016/j.bbrc.2006.04.186. [DOI] [PubMed] [Google Scholar]
- 6.Middeldorp J, Kamphuis W, Sluijs JA, Achoui D, Leenaars CH, Feenstra MG, van Tijn P, Fischer DF, Berkers C, Ovaa H, Quinlan RA, Hol EM. Intermediate filament transcription in astrocytes is repressed by proteasome inhibition. FASEB J. 2009;23:2710–2726. doi: 10.1096/fj.08-127696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss A, Blackburn-Munro G, Garry EM, Blakemore JA, Dickinson T, Rosie R, Mitchell R, Fleetwood-Walker SM. A role of the ubiquitin-proteasome system in neuropathic pain. J Neurosci. 2002;22:1363–1372. doi: 10.1523/JNEUROSCI.22-04-01363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poon WW, Carlos AJ, Aguilar BL, Berchtold NC, Kawano CK, Zograbyan V, Yaopruke T, Shelanski M, Cotman CW. beta-Amyloid (Abeta) oligomers impair brain-derived neurotrophic factor retrograde trafficking by down-regulating ubiquitin C-terminal hydrolase, UCH-L1. J Biol Chem. 2013;288:16937–16948. doi: 10.1074/jbc.M113.463711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saini V, Staren DM, Ziarek JJ, Nashaat ZN, Campbell EM, Volkman BF, Marchese A, Majetschak M. The CXC chemokine receptor 4 ligands ubiquitin and stromal cell-derived factor-1alpha function through distinct receptor interactions. J Biol Chem. 2011;286:33466–33477. doi: 10.1074/jbc.M111.233742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song H, Han Y, Pan C, Deng X, Dai W, Hu L, Jiang C, Yang Y, Cheng Z, Li F, Zhang G, Wu X, Liu W. Activation of Adenosine Monophosphate-activated Protein Kinase Suppresses Neuroinflammation and Ameliorates Bone Cancer Pain: Involvement of Inhibition on Mitogen-activated Protein Kinase. Anesthesiology. 2015;123:1170–1185. doi: 10.1097/ALN.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 11.Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, Zhang YQ. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain. 2007;129:64–75. doi: 10.1016/j.pain.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Xie M, Wang SH, Lu ZM, Pan Y, Chen QC, Liao XM. UCH-L1 inhibition involved in CREB dephosphorylation in hippocampal slices. J Mol Neurosci. 2014;53:59–68. doi: 10.1007/s12031-013-0197-z. [DOI] [PubMed] [Google Scholar]
- 13.Yin Q, Fan Q, Zhao Y, Cheng MY, Liu H, Li J, Lu FF, Jia JT, Cheng W, Yan CD. Spinal NF-kappaB and chemokine ligand 5 expression during spinal glial cell activation in a neuropathic pain model. PLoS One. 2015;10:e0115120. doi: 10.1371/journal.pone.0115120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Sun Y, Hu R, Luo W, Mao X, Zhao Z, Chen Q, Zhang Z. The regulation of the UCH-L1 gene by transcription factor NF-kappaB in podocytes. Cell Signal. 2013;25:1574–1585. doi: 10.1016/j.cellsig.2013.03.018. [DOI] [PubMed] [Google Scholar]


