Abstract
Objective: This study aims to explore the treatment effects of chronic ulcer in diabetic rats with self assembling nanofiber gel encapsulated-polydeoxyribonucleotide. Methods: Diabetic skin ulcer mouse model was established in this study. They were divided into control group, common wound group and infectious wound group. Human embryonic fibroblast cells and vascular endothelial cells were treated with short poly-N-acetyl glucosamine nanofibers and polydeoxyribonucleotide. Their effects on cell proliferation, revascularization and inhibiting infection were detected by RT-PCR, western-blotting, HE staining and immunohistochemical methods respectively. Results: The expression levels of cytokines and angiogenic factors increased in the treatment groups especially in sNAG encapsulated-PDRN group. HE staining results indicated that PDRN, sNAG and sNAG encapsulated-PDRN could improve the wound healing, immunohistochemical results showed that PDRN, sNAG and sNAG encapsulated-PDRN promoted cell proliferation and new vessel formation especially sNAG encapsulated-PDRN. Conclusions: sNAG encapsulated-PDRN may have a potential application in the treatment of diabetic ulcers and chronic wound healing.
Keywords: Nanofibers, polydeoxyribonucleotide, poly-N-acetyl glucosamine, diabetes, cytokines, chronic ulcer, wound healing
Introduction
Chronic soft tissue ulcer, such as diabetic ulcer and bedsore, brought a heavy burden to the patients and even threatening their life, because its amputation rate and disability rate were high. The deficiency of growth factor and infection on wound surface of chronic soft tissue ulcer are important factors, they lead to the difficulty in healing of ulcer [1,2]. Local application of active growth factors can promote the healing of chronic ulcers, but half-life of foreign growth factor is short, it must be exploited repeatedly and costs much. Recent studies showed that the activation of adenosine A2A receptors can play an important role in the protection and healing of the wound [2-4].
Polydeoxyribonucleotide (PDRN) is a linear DNA sequence from human placenta, its length is between 50 and 2000 bp. It can effectively activate the adenosine A2A receptor and will not be degraded in vivo [5] It is widely used in the treatment of a series of ischemic and hypoxic diseases, such as myocardial infarction, cerebral infarction, peripheral arterial occlusive disease, etc [5-7]. PRDN could significantly improve the speed of wound healing, this therapeutic effect can be blocked by specific antagonists of adenosine A2A receptor [2]. PDRN has no immunogenicity in the human body and will not cause allergic reactions [5]. Therefore, we think that PDRN could be used in the treatment of chronic soft tissue ulcer.
How PDRN will be released into the chronic ulcer wound also directly affect the treatment. Self-assembling peptide nanofiber scaffold (SAPNS) is the latest findings in the field of biological material manufacturing. It is considered to be an ideal material for matrix repair and drug release system because of its good material cell interface compatibility and biological activity [8,9]. N-acetylglucosamine is one of the main components of the extracellular matrix and has a certain antibacterial anti-inflammatory and healing effects. Short poly-N-acetyl glucosamine (sNAG) nanofibers were prepared after the short chain poly N-acetyl glucosamine was modified. It was found that sNAG could promote cell proliferation and migration and accelerate the formation of new blood vessels, inhibit the growth of bacteria and control the infection [10-13].
In this study, we explored the treatment effects of chronic ulcer in diabetic rats with self assembling nanofiber gel encapsulated- PRDN, which will provide basis for the treatment of diabetic ulcers, chronic wound healing of bedsore etc.
Materials and methods
Cell culture
Human embryonic fibroblast (H2EL) cells and vascular endothelial (VE) cells were purchased from American type culture collection (ATCC). They were cultured with DMEM medium containing 2% fetal calf serum at 37°C with 5% CO2.
Experimental animals
Diabetic female C57BL/KsJ-m+/+Lept db mice (db+/db+) and their normal littermates (db+/+m) were used in this study. They were pre-feeding for one week with free access to food and water to adapt to the environment. Room temperature maintained at 18~25°C. Hair on the back was shaved and parallel 4-cm incision was produced on the back of all mice. They were divided into 16 groups according to different treatment methods (Table 1).
Table 1.
Experimental animals’ group
| Group1 | Group2 | Group3 | Group4 | |
|---|---|---|---|---|
| Diabetic mice with common wound | PDRN | sNAG | sNAG with PDRN | Control |
| Diabetic mice with infectious wound | PDRN | sNAG | sNAG with PDRN | Control |
| Normal mice with common wound | PDRN | sNAG | sNAG with PDRN | Control |
| Normal mice with infectious wound | PDRN | sNAG | sNAG with PDRN | Control |
The effects of PDRN, sNAG and sNAG encapsulated-PDRN on cell proliferation, revascularization and inhibiting infection
The cells were treated with PDRN, PDRN+DMPX (3, 7-dimethyl-propargilxanthine, specific antagonist of Adenosine A2A receptor), sNAG and sNAG encapsulated-PDRN respectively. The cell proliferation was determined with CCK8 detection kit according to the manual. The cytokines and angiogenic factors such as VEGF, Bax, Bcl-2, Ki67, inhibin b, cyclin D1, cyclin E etc were detected by RT-PCR and Western-bloting methods.
Total RNA was extracted using E.Z.N.A.® Total RNA Kit I (Omega Bio-Tek, Inc. Norcross, GA, USA) according to the manufacturer’s instructions. cDNAs were synthesized using PrimeScript RT Master Mix (Takara Bio, Inc. Shiga, Japan). The RT-PCR reaction was performed using ABI 7500 Fast (Applied Biosystems, Foster City, CA, USA) with SYBR Premix Ex Taq II (Takara). The cycle condition was: denaturation at 95°C for 30 sec, 40 amplification cycles at 95°C for 3 sec and 60°C for 30 sec. β-actin was used as the control. Primer sequences used in this study were shown in Table 2.
Table 2.
Real-time PCR primers
| Gene | Primer (5’-3’) | Length (bp) |
|---|---|---|
| VEGF | For: GGCAAAGTGAGTGACCTGCT | 112 |
| Rev: CGGTGTCTGTCTGTCTGTCC | ||
| Angiopoietin-1 | For: CACACTGGGACAGCAGGAA | 136 |
| Rev: CACAAGCATCAAACCACCAT | ||
| Transglutaminase 2 | For: CTCAGCCAAGACAAGGAGGT | 106 |
| Rev: CCCAGAGAGGAGAAGGCAGT | ||
| Bax | For: GGAAGAAGATGGGCTGAGG | 179 |
| Rev: TGTGTCCCGAAGGAGGTTTA | ||
| Ki67 | For: TAACACCATCAGCAGGCAAA | 105 |
| Rev: GCAGGTCCAGTTTCTCCACT | ||
| inhibin b | For: CAGAGCGAGAACCCTCAACT | 125 |
| Rev: ATTTGTCACCGCATCCATTT | ||
| cyclin D1 | For: CCCTCGGTGTCCTACTTCA | 108 |
| Rev: CTCCTCGCACTTCTGTTCCT | ||
| cyclin E | For: CTGGATGTTGACTGCCTTGA | 113 |
| Rev: ATGTCGCACCACTGATACCC | ||
| CDK2 | For: CAGGATGTGACCAAGCCAGT | 125 |
| Rev: TGAGTCCAAATAGCCCAAGG | ||
| CDK6 | For: GTGAACCAGCCCAAGATGAC | 132 |
| Rev: TGGAGGAAGATGGAGAGCAC | ||
| p15 | For: TGATTAGCACTTGGGTGACG | 130 |
| Rev: CCCTCCTCCACTTTGTCCTC | ||
| p27 | For: TATGACCCTCCCAAACCAAA | 144 |
| Rev: ACCATTTCCGTATCCACAGC | ||
| Integrin β1 | For: CCGCGCGGAAAAGATGAAT | 252 |
| Rev: ATGTCATCTGGAGGGCAACC | ||
| PECAM-1 | For: GTGCTGCAATGTGCTGTGAA | 133 |
| Rev: TGCTAGCCTTCTGCTTGGTC | ||
| Defensin | For: CAATTGCGTCAGCAGTGGAG | 116 |
| Rev: GGTCACTCCCAGCTCACTTG | ||
| β-actin | For: GTCGTACCACTGGCATTGTG | 291 |
| Rev: CTCTCAGCTGTGGTGGTGAA |
Cells were lysed in RIPA buffer with 10% phenylmethylsulfonyl fluoride. The cell extracts were loaded on 10% SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes. The membranes were blocked for 1 h at room temperature with 5% non-fat milk in TBST, and then incubated with primary antibodies at 4°C overnight. Following incubation with HRP-conjugated secondary antibody (diluted at 1:2,000, Abcam), immuno complexes were visualized by an enhanced chemiluminescence detection under FluorChem M Systerm (ProteinSimple, San Jose, CA, USA). Endogenous β-actin was used for normalization.
Therapeutic effects of skin ulcer in diabetic mice with PDRN, sNAG and sNAG encapsulated-PDRN
The wound was contaminated with staphyloccocus aureus in infectious diabetic mice groups. The cytokines and angiogenic factors in wound surface such as VEGF, Angiopoietin-1, Bcl-2, Ki67, cyclin D1, cyclin E etc were detected by RT-PCR and Western-blotting methods. They were performed as above described. The inflammatory cell infiltration in wound tissue was determined using HE stain. The samples were fixed in 10% formalin and embedded in paraffin routinely. The paraffin blocks of specimen were cut into continuous sections with 5 μm respectively. The sections were dewaxed with xylene and washed with ethanol and water. They were stained with Hematoxylin after that and then differentiated, washed and stained with eosin, then dehydrated, hyalinized and finally mounted on slides and observed under microscope, pictures were taken.
The expression levels of PECAM-1 and Ki67 were detected using immunohistochemical staining method to observe the situation of infection, newborn vessels, keratinocyte migration and wound healing. Briefly, samples fixed in 10% formalin were subsequently embedded in paraffin, and sections of 4-mm thickness were cut from the formalin-fixed samples. The sectioned tissue was deparaffinized in xylene and then rehydrated in a graded ethyl alcohol series. For increased specificity and sensitivity, tissues were microwaved for 10 min for antigen retrieval. Following cooling and rinsing in distilled water, endogenous peroxide activity was blocked with 3% H2O2 for 10 min, and the samples were then rinsed in 0.01 mol/l phosphate-buffered saline (PBS, pH 7.4) for 10 min. The sections were subsequently pre-incubated with a protein blocking solution for 10 min, prior to incubation with the primary antibodies at 4°C overnight in a humid chamber. The slides were then washed three times in PBS and incubated with secondary biotinylated antibody for 15 min at room temperature. The streptavidin-peroxidase method was used to detect the antigen-antibody complexes, and diaminobenzidine (DAB) was used as the chromogen substrate. The sections were stained and observed under microscope.
Statistical analysis
Statistical analysis was performed using one-way ANOVA or the Student’s t-test using SPSS 17.0 software. P<0.05 was considered to be statistically significant.
Results
PDRN could promote cell proliferation and the release of cytokines and angiogenic factors
The CCK8 detection results in H2EL cells and VE cells after treated by PDRN showed that the cell proliferation significantly increased after the treatment of PDRN, while they decreased with the treatment of PDRN+DMPX. The RT-PCR results of expression changes of cytokines and angiogenic factors in H2EL cells after treated by PDRN showed that the expression levels of VEGF, Bax, Bcl-2, Ki67, inhibin b, cyclin D1, cyclin E etc significantly increased after the treatment of PDRN, while they decreased with the treatment of PDRN+DMPX. The RT-PCR results of expression changes of cytokines and angiogenic factors in VE cells after treated by PDRN were similar with that of H2EL cells (data not shown).
The western blotting results of expression changes of cytokines and angiogenic factors in H2EL cells after treated by PDRN were shown in Figure 1. It also showed that the expression levels of VEGF, Bax, Bcl-2, Ki67, inhibin b, cyclin D1, cyclin E etc significantly increased after the treatment of PDRN, while they decreased with the treatment of PDRN+DMPX.
Figure 1.
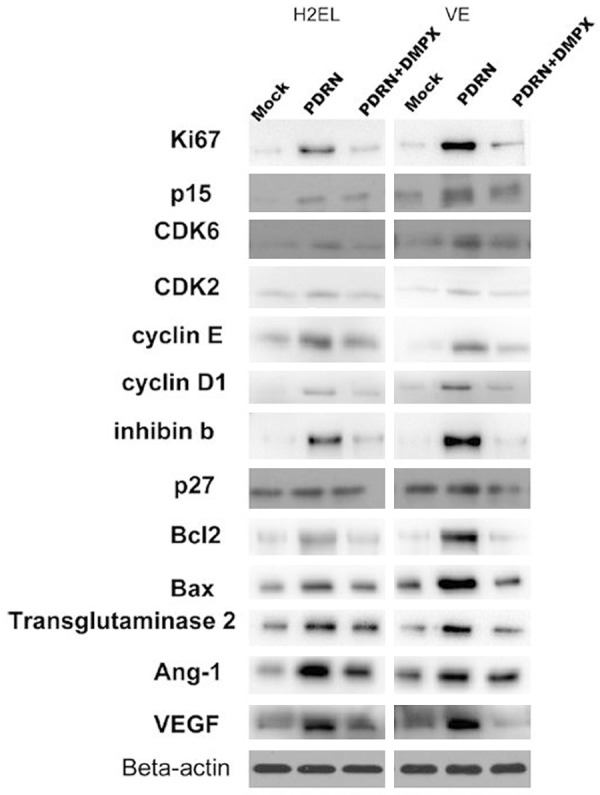
Western blotting results of expression changes of cytokines and angiogenic factors in H2EL cells after treated by PDRN. They significantly increased after the treatment of PDRN while they decreased after the treatment of PDRN+DMPX.
sNAG could promote cell proliferation, vessel formation and inhibit infection
The CCK8 detection results in H2EL cells and VE cells after treated by sNAG showed that the cell proliferation significantly increased after the treatment of sNAG. The RT-PCR results of expression changes of VEGF, Ki67, Integrin β, PECAM-1 and defensin in H2EL cells and VE cells after treated by sNAG showed that the expression levels of VEGF, Ki67, Integrin β, PECAM-1 and defensin in H2EL cells and VE cells significantly increased after the treatment of sNAG. The western blotting results of expression changes of VEGF, Ki67, Integrin β, PECAM-1 and defensin in H2EL cells and VE cells after treated by sNAG also showed that the expression levels of VEGF, Ki67, Integrin β, PECAM-1 and defensin significantly increased after the treatment of sNAG (data not shown).
sNAG encapsulated-PDRN could promote cell proliferation, revascularization and inhibit infection
The CCK8 detection results in H2EL cells and VE cells after treated by sNAG encapsulated-PDRN showed that the cell proliferation significantly increased after the treatment of sNAG encapsulated-PDRN. The RT-PCR results of expression changes of cytokines and angiogenic factors such as VEGF, Ki67, Bax, Bcl-2, Integrin β, PECAM-1, cyclin D1, cyclin E and defensin etc in H2EL cells and VE cells after treated by sNAG encapsulated-PDRN showed that the expression levels of these cytokines and angiogenic factors in H2EL cells and VE cells significantly increased after the treatment of sNAG encapsulated-PDRN (data not shown).
The western blotting results of expression changes of cytokines and angiogenic factors such as VEGF, Ki67, Bax, Bcl-2, Integrin β, PECAM-1, cyclin D1, cyclin E and defensin etc in H2EL cells and VE cells after treated by sNAG encapsulated-PDRN were shown in Figure 2. It also showed that the expression levels of these cytokines and angiogenic factors significantly increased after the treatment of sNAG encapsulated-PDRN.
Figure 2.
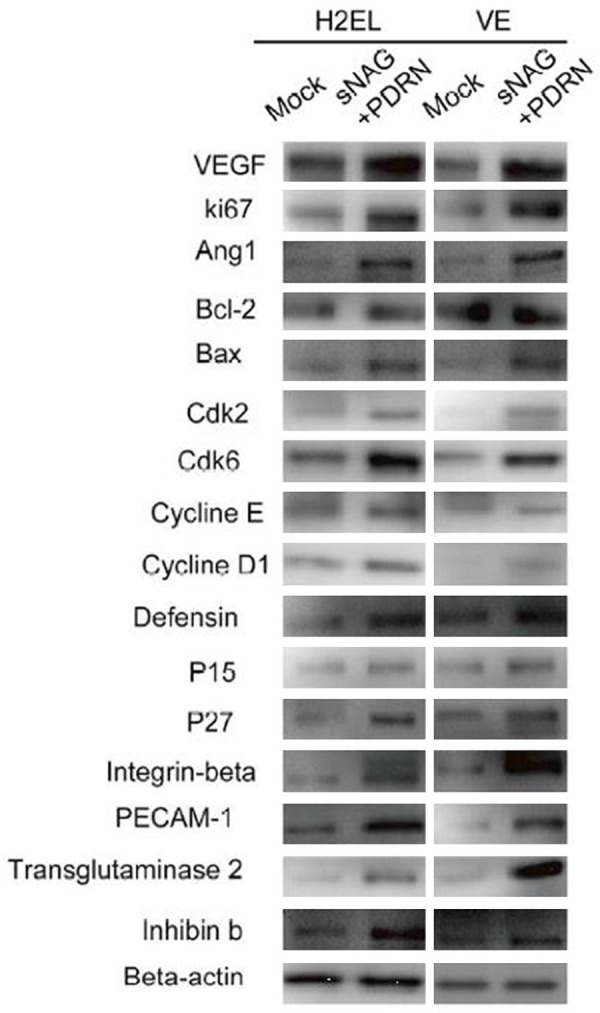
Western blotting results of expression changes of cytokines and angiogenic factors in H2EL cells and VE cells after treated by sNAG encapsulated-PDRN. The expression levels of these cytokines and angiogenic factors significantly increased after the treatment of sNAG encapsulated-PDRN.
Therapeutic effects of skin ulcer in diabetic mice with PDRN, sNAG and sNAG encapsulated-PDRN
The RT-PCR results of expression changes of cytokines and angiogenic factors such as VEGF, Ki67, Bax, Bcl-2, Integrin β, PECAM-1, cyclin D1, cyclin E and defensin etc in different groups after treated by PDRN, sNAG and sNAG encapsulated-PDRN showed that the expression levels of these cytokines and angiogenic factors significantly increased after the treatment of PDRN, sNAG and sNAG encapsulated-PDRN and they were the highest after the treatment of sNAG encapsulated-PDRN in different groups. The western blotting results of expression changes of cytokines and angiogenic factors such as VEGF, Ki67, Bax, Bcl-2, Integrin β, PECAM-1, cyclin D1, cyclin E and defensin etc in different groups after treated by PDRN, sNAG and sNAG encapsulated-PDRN also showed that the expression levels of these cytokines and angiogenic factors significantly increased after the treatment of PDRN, sNAG and sNAG encapsulated-PDRN and they were the highest after the treatment of sNAG encapsulated-PDRN in different groups (data not shown).
HE staining results showed that in diabetic mice group without treatment, there was poor re-epithelialization with partially organized granulation tissue, while there was moderate to complete re-epithelialization and well-formed granulation tissue in the treatment of PDRN, sNAG and sNAG encapsulated-PDRN groups especially in sNAG encapsulated-PDRN group without inflammatory infiltrates (Figure 3). In normal mice groups, dermis remodelling and wound closure processes were almost complete, and PDRN, sNAG and sNAG encapsulated-PDRN improved wound healing (data not shown).
Figure 3.
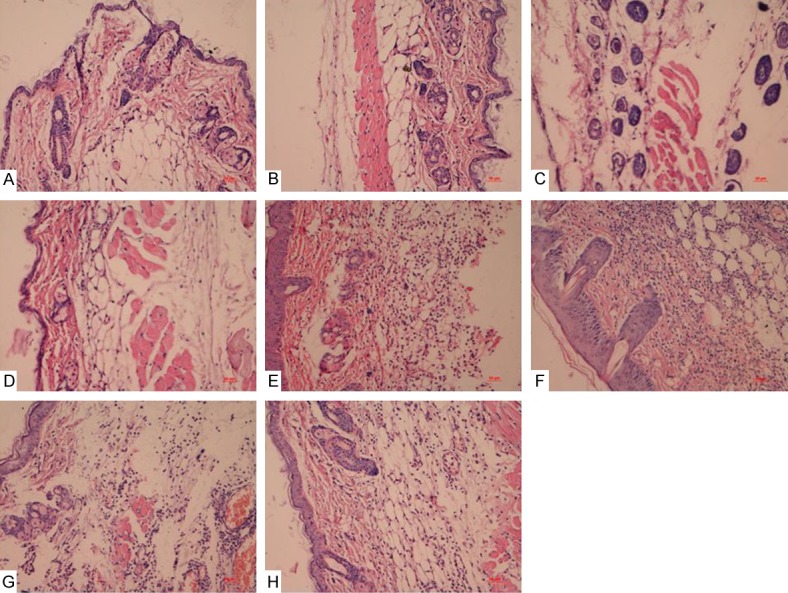
HE staining results of diabetic mice with common and infectious wound groups after treated by PDRN, sNAG and sNAG encapsulated-PDRN. There was poor re-epithelialization with partially organized granulation tissue in control group, while there was moderate to complete re-epithelialization and well-formed granulation tissue in the treatment of PDRN, sNAG and sNAG encapsulated-PDRN groups especially in sNAG encapsulated-PDRN group without inflammatory infiltrates. A: Diabetic mice with common wound control group; B: Diabetic mice with common wound treated by PDRN; C: Diabetic mice with common wound treated by SNAG; D: Diabetic mice with common wound treated by SNAG+PDRN; E: Diabetic mice with diabetic mice with common wound wound control group; F: Diabetic mice with infectious wound treated by PDRN; G: Diabetic mice with infectious wound treated by SNAG; H: Diabetic mice with infectious wound treated by SNAG+PDRN.
Ki67 immunostaining results of the different groups were shown in Figures 4 and 5. It showed that there were more positive cells in the treatment groups than that of control groups especially in sNAG encapsulated-PDRN group, which suggested that PDRN, sNAG and sNAG encapsulated-PDRN promoted cell proliferation.
Figure 4.
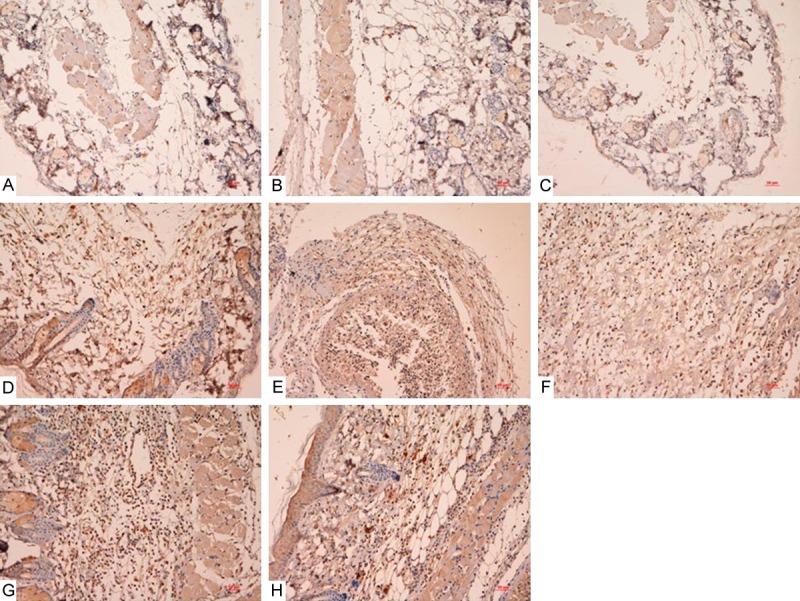
Ki67 immunostaining results of diabetic mice with common and infectious wound groups after treated by PDRN, sNAG and sNAG encapsulated-PDRN. There were more positive cells in the treatment groups than that of control groups especially in sNAG encapsulated-PDRN group. A: Diabetic mice with common wound control group; B: Diabetic mice with common wound treated by PDRN; C: Diabetic mice with common wound treated by SNAG; D: Diabetic mice with common wound treated by SNAG+PDRN; E: Diabetic mice with diabetic mice with common wound control group; F: Diabetic mice with infectious wound treated by PDRN; G: Diabetic mice with infectious wound treated by SNAG; H: Diabetic mice with infectious wound treated by SNAG+PDRN.
Figure 5.
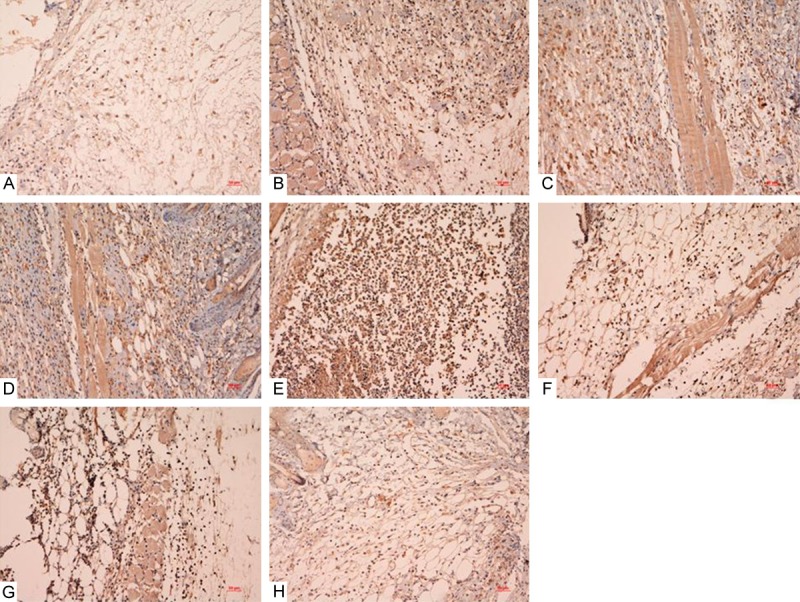
Ki67 immunostaining results of normal mice with common and infectious wound groups after treated by PDRN, sNAG and sNAG encapsulated-PDRN. There were more positive cells in the treatment groups than that of control groups especially in sNAG encapsulated-PDRN group. A: Normal mice with common wound control group; B: Normal mice with common wound treated by PDRN; C: Normal mice with common wound treated by SNAG; D: Normal mice with common wound treated by SNAG+PDRN; E: Normal mice with infectious wound control group; F: Normal mice with infectious wound treated by PDRN; G: Normal mice with infectious wound treated by SNAG; H: Normal mice with infectious wound treated by SNAG+PDRN.
PECAM-1 immunostaining results of the different groups also showed that PDRN, sNAG and sNAG encapsulated-PDRN increased PECAM-1 staining especially sNAG encapsulated-PDRN, which suggested that PDRN, sNAG and sNAG encapsulated-PDRN promoted neo-vessel formation (data not shown).
Discussion
Diabetic patients have disturbed wound-healing process which may enhance the overall morbidity and mortality of this population [14]. A complicated cellular and molecular processes containing inflammation, cell migration, angiogenesis, provisional matrix synthesis, collagen deposition and re-epithelization characterizes the normal skin repair [14,15]. There is a complex cascade of events in skin repair, angiogenesis and vasculogenesis played a key role in wound healing and is associated with the expression of several cytokines and angiogenic factors, such as VEGF, Ki67, PECAM-1 etc [16-21].
The wound healing potency of an aqueous extract of human placenta is clinically well established based on its numerous applications of chronic soft tissue ulcers in the stimulation of endogenous growth factors, anti inflammatory and anti-microbial properties [22-23]. However, it will be highly important for the therapeutic efficacy using appropriate vehicles to maintain the biological activity of human placenta extract (HPE) during the delivery process [24]. sNAG nanofibers gel was found to be the ideal vehicles [10-13]. So self-assembly sNAG nanofibers gel encapsulated aqueous extract of human placenta will be an ideal matrix material and tools for the treatment of chronic soft tissue ulcers.
Recent studies have shown that adenosine A2A receptor activation may play an important to protect and promote healing of wounds in ischemia hypoxia, inflammation, trauma, and many other pathological processes [25]. The A2A receptor activation can inhibit the generation of reactive oxygen species, and promote the release of cytokines, such as TNF alpha, accelerate proliferation of the osteoblast, fibroblasts, fat precursor cells, promote the release of VEGF, angiogenin and glutamine transferase II, and then promote the healing of the wound. To selectively activate A2A receptors will awaken the body’s self healing mechanisms [26].
In this study, we used sNAG encapsulated-PDRN to cure soft tissue ulcers in the back of diabetic mice. We found that it could activate adenosine A2A receptor and platelet integrin receptor, promote the release of many cytokines and angiogenic factors such as VEGF, Ki67, Bax, Bcl-2, Integrin β, PECAM-1, cyclin D1, cyclin E and so on, stimulate the cell proliferation and angiogenesis. It also could promote the release of defensin and inhibit infection to improve the wound healing. It not only realized the controllable release of PDRN but also provided the environment for soft tissue repair. It also had certain antibacterial ability, and could activate the body’s self healing mechanism, promote angiogenesis, accelerate wound healing.
In a word, the results of this study demonstrated that sNAG encapsulated-PDRN administration could ameliorate the diabetic wound healing through activate adenosine A2A receptor and platelet integrin receptor and promote the release of many cytokines and angiogenic factors. We believe that sNAG encapsulated-PDRN may have a potential application in the treatment of diabetic ulcers and chronic wound healing.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 81271968).
Disclosure of conflict of interest
None.
References
- 1.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23:594–608. doi: 10.1111/j.1464-5491.2006.01773.x. [DOI] [PubMed] [Google Scholar]
- 2.Altavilla D, Squadrito F, Polito F, Irrera N, Calo M, Lo Cascio P, Galeano M, La Cava L, Minutoli L, Marini H, Bitto A. Activation of adenosine A2A receptors restores the altered cell-cycle machinery during impaired wound healing in genetically diabetic mice. Surgery. 2011;149:253–261. doi: 10.1016/j.surg.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Palu A, Su C, Zhou BN, West B, Jensen J. Wound healing effects of noni (Morinda citrifolia L. ) leaves: a mechanism involving its PDGF/A2A receptor ligand binding and promotion of wound closure. Phytother Res. 2010;24:1437–1441. doi: 10.1002/ptr.3150. [DOI] [PubMed] [Google Scholar]
- 4.Clementina M, Giuseppe S. A2A receptor ligands: past, present and future trends. Curr Top Med Chem. 2010;10:902–922. doi: 10.2174/156802610791268765. [DOI] [PubMed] [Google Scholar]
- 5.Altavilla D, Bitto A, Polito F, Marini H, Minutoli L, Di Stefano V, Irrera N, Cattarini G, Squadrito F. Polydeoxyribonucleotide (PDRN): a safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc Hematol Agents Med Chem. 2009;7:313–321. doi: 10.2174/187152509789541909. [DOI] [PubMed] [Google Scholar]
- 6.Polito F, Bitto A, Galeano M, Irrera N, Marini H, Calo M, Squadrito F, Altavilla D. Polydeoxyribonucleotide restores blood flow in an experimental model of ischemic skin flaps. J Vasc Surg. 2012;55:479–488. doi: 10.1016/j.jvs.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 7.Galeano M, Bitto A, Altavilla D, Minutoli L, Polito F, Calo M, Lo Cascio P, Stagno d’Alcontres F, Squadrito F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008;16:208–217. doi: 10.1111/j.1524-475X.2008.00361.x. [DOI] [PubMed] [Google Scholar]
- 8.Bulut S, Erkal TS, Toksoz S, Tekinay AB, Tekinay T, Guler MO. Slow release and delivery of antisense oligonucleotide drug by self-assembled peptide amphiphile nanofibers. Biomacromolecules. 2011;12:3007–3014. doi: 10.1021/bm200641e. [DOI] [PubMed] [Google Scholar]
- 9.Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010;94:1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherer SS, Pietramaggiori G, Matthews J, Perry S, Assmann A, Carothers A, Demcheva M, Muise-Helmericks RC, Seth A, Vournakis JN, Valeri RC, Fischer TH, Hechtman HB, Orgill DP. Poly-N-acetyl glucosamine nanofibers: a new bioactive material to enhance diabetic wound healing by cell migration and angiogenesis. Ann Surg. 2009;250:322–330. doi: 10.1097/SLA.0b013e3181ae9d45. [DOI] [PubMed] [Google Scholar]
- 11.Muise-Helmericks RC, Demcheva M, Vournakis JN, Seth A. Poly-N-acetyl glucosamine fibers activate bone regeneration in a rabbit femur injury model. J Trauma. 2011;71:S194–196. doi: 10.1097/TA.0b013e3182258799. [DOI] [PubMed] [Google Scholar]
- 12.Scherer SS, Pietramaggiori G, Matthews JC, Gennaoui A, Demcheva M, Fischer TH, Valeri CR, Orgill DP. Poly-N-acetyl glucosamine fibers induce angiogenesis in ADP inhibitor-treated diabetic mice. J Trauma. 2011;71:S183–186. doi: 10.1097/TA.0b013e318225585b. [DOI] [PubMed] [Google Scholar]
- 13.Fischer TH, Thatte HS, Nichols TC, Bender-Neal DE, Bellinger AD, Vournakis JN. Synergistic platelet integrin signaling and factor XII activation in poly-N-acetyl glucosamine fiber-mediated hemostasis. Biomaterials. 2005;26:5433–5443. doi: 10.1016/j.biomaterials.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal SS, Gambhir RP, Agrawal A, Harish S. Efficacy of topical recombinant human platelet derived growth factor on wound healing in patients with chronic diabetic lower limb ulcers. Indian J Surg. 2010;72:27–31. doi: 10.1007/s12262-010-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 17.Salehi-Lalemarzi H, Shanehbandi D, Shafaghat F, Abbasi-Kenarsari H, Baradaran B, Movassaghpour AA, Kazemi T. Cloning and Stable Expression of cDNA Coding For Platelet Endothelial Cell Adhesion Molecule -1 (PECAM-1, CD31) in NIH-3T3 Cell Line. Adv Pharm Bull. 2015;5:247–253. doi: 10.15171/apb.2015.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hossain M, Qadri SM, Xu N, Su Y, Cayabyab FS, Heit B, Liu L. Endothelial LSP1 Modulates Extravascular Neutrophil Chemotaxis by Regulating Nonhematopoietic Vascular PECAM-1 Expression. J Immunol. 2015;195:2408–2416. doi: 10.4049/jimmunol.1402225. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Sorenson CM, Sheibani N. PECAM-1 isoforms, eNOS and endoglin axis in regulation of angiogenesis. Clin Sci (Lond) 2015;129:217–234. doi: 10.1042/CS20140714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006;14:558–565. doi: 10.1111/j.1743-6109.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 22.De D, Chakraborty PD, Bhattacharyya D. Regulation of trypsin activity by peptide fraction of an aqueous extract of human placenta used as wound healer. J Cell Physiol. 2011;226:2033–2040. doi: 10.1002/jcp.22535. [DOI] [PubMed] [Google Scholar]
- 23.De D, Chakraborty PD, Bhattacharyya D. Analysis of free and bound NADPH in aqueous extract of human placenta used as wound healer. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2435–2442. doi: 10.1016/j.jchromb.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Nath S, Bhattacharyya D. Cell adhesion by aqueous extract of human placenta used as wound healer. Indian J Exp Biol. 2007;45:732–738. [PubMed] [Google Scholar]
- 25.Keranen H, Gutierrez-de-Teran H, Aqvist J. Structural and energetic effects of A2A adenosine receptor mutations on agonist and antagonist binding. PLoS One. 2014;9:e108492. doi: 10.1371/journal.pone.0108492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beggiato S, Antonelli T, Tomasini MC, Borelli AC, Agnati LF, Tanganelli S, Fuxe K, Ferraro L. Adenosine A2A-D2 receptor-receptor interactions in putative heteromers in the regulation of the striato-pallidal gaba pathway: possible relevance for parkinson’s disease and its treatment. Curr Protein Pept Sci. 2014;15:673–680. doi: 10.2174/1389203715666140901103205. [DOI] [PubMed] [Google Scholar]
- 27.Phaechamud T, Yodkhum K, Charoenteeraboon J, Tabata Y. Chitosan-aluminum monostearate composite sponge dressing containing asiaticoside for wound healing and angiogenesis promotion in chronic wound. Mater Sci Eng C Mater Biol Appl. 2015;50:210–225. doi: 10.1016/j.msec.2015.02.003. [DOI] [PubMed] [Google Scholar]


