Abstract
Erianin is a natural product extracted from Dendrobiumchrysotoxum. To investigate the antitumor activity of Erianin in estrogen receptor (ER) positive breast cancer, we treated T47D cells with Erianin and evaluated the effects of Erianin treatment on multiple cancer-associated pathways. Erianin inhibited the proliferation of T47D cells effectively. Erianin induced apoptosis in T47D cells through reducing Bcl-2 expression and activating caspase signaling. Furthermore, it also suppressed the expression of CDKs and caused cell cycle arrest. In addition, Erianin treatment suppressed the migration of T47D cells, most likely through regulating the homeostatic expression of MPP and TIMP. Meanwhile, Erianin did not affect the proliferation of normal breast epithelial cell line MCF10A. Together, these results demonstrated that Erianin might have the potential to be an effective drug to treat the ER positive breast cancer.
Keywords: Erianin, apoptosis, proliferation, breast cancer, estrogen receptor
Introduction
Erianin (2-methoxy-5-[2-(3,4,5-trimethoxy-phenyl)-ethyl]-phenol; Figure 1A) is a natural product extracted from Dendrobiumchrysotoxum and has been used as an antipyretic and an analgesic in traditional Chinese herbal medicine [1]. Erianin shows therapeutic potential to inhibit multiple cancers in vivo and in vitro. Itdemonstrates potent inhibitory activity on the proliferation of acute promyelocytic leukemia HL-60 cells, which is related to the apoptosis and the altered expression of bcl-2 and bax genes induced by erianin [2]. In addition, Erianin causes extensive tumor necrosis, growth delay and rapid vascular shutdown in xenografted human hepatoma Bel7402 and melanoma A375 tumors. It inhibits angiogenesis in vivo and in vitro and induces endothelial cytoskeletal disorganization [3]. Furthermore, Erianin inhibits metabolism in human umbilical vein endothelial cells in a JNK/SAPK-dependent manner, which is a potential mechanism involved in the anti-tumor and anti-angiogenic actions of Erianin [4].
Figure 1.
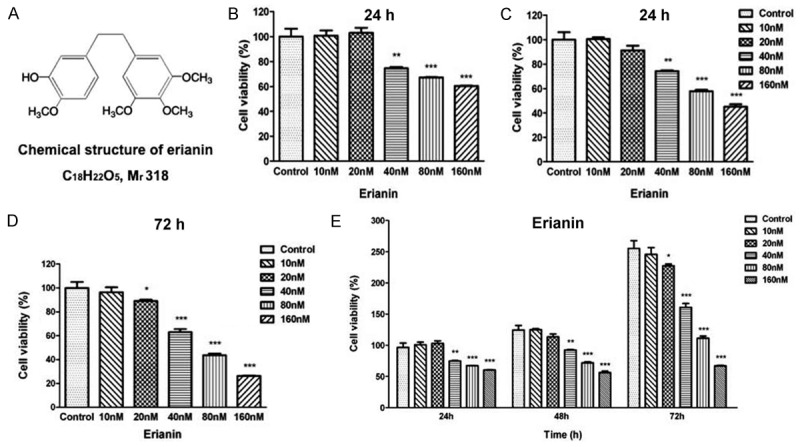
Erianin inhibits T47D cells proliferation in a does- and time-dependent. (A) Chemical structure of erianin. Inhibition effects of Erianin on T47D cells proliferation at 24 h (B), 48 h (C) and 72 h (D). Cells were treated with graded concentrations of Erianin (0, 10, 20, 40, 80, 160 nM) at different time points. Cell proliferation potential was evaluated by MTT assay. The viability of untreated control cells is represented as 100%. Data represent the means ± SD (n=5), *P<0.05, **P<0.01, ***P<0.001 versus control cells. (E) Erianin inhibits T47D cells proliferation in a dose- and time-dependent manner. Experimental data of MTT assay were integrated and means ± SD (n=5), *P<0.05, **P<0.01, ***P<0.001 compared to control cells at 24 h time points.
Breast cancer is the most prevalent cancer among women worldwide, with more than 1,300,000 new cases diagnosed and 450,000 deaths each year [5]. Breast cancer is a very heterogeneous disease, which is commonly categorized into three basic therapeutic groups, e.g. the estrogen receptor (ER) positive group, the HER 2 amplified group and triple-negative group [6]. The ER positive group is the most numerous and diverse, and approximately 80% of breast cancer cases is ER positive. The HER2 amplified group is characterized by a DNA copy number aberration of HER2. The triple-negative group lacks the expression of ER, progesterone receptor (PR) and HER2. The treatments for breast cancer include surgery, radiation therapy, chemotherapy, endocrine (hormone) therapy and targeted therapy [7]. For the ER positive breast cancer, endocrine therapy has represented the standard adjuvant treatment complementary to surgery, and has been an effective therapy to the majority of patients. However, a portion of patients relapse with incurable metastatic disease, likely due to the development of resistance to endocrine therapy, which underlines the clinical need to develop alternative treatments [8].
In the present study, we evaluated the anti-tumor effects of Erianin on an ER positive breast cancer cell line T47D, and investigated the key pathways crucial for the tumor growth, including cell cycling, apoptosis and migration, were investigated in the Erianin-treated T47D cells.
Materials and methods
Materials and chemicals
Erianin was purchased from Shanghai Yuanye Bio-Technology. All the cell culture reagents were from GIBCO. PVDF membranes were purchased from Milipore. The necessary apparatus for SDS-PAGE and Western blot were bought from Bio-Rad.
Cells culture
The human breast cancer cell lines T47D and MDA-MB-231 and the human breast epithelial cell line MCF10A were generously donated by Prof. Xin Hu (Jilin University, Jilin). T47D cells were cultured in Roswell Park Memorial Institute-1640 medium with 10% fetal bovine serum (FBS), 3.2 μg/ml insulin (Sigma) and 100 IU/ml penicillin/streptomycin. MCF10A cells were cultured in Ham’s F12 medium and Dulbecco’s modified Eagle’s medium with 2.5 mM l-glutamine (DMEM: F12, 5% horse serum, 20 ng/ml EGF (Peprotech, Germany), 100 ng/ml cholera toxin (Sigma, USA), 10 μg/ml insulin (Sigma), 0.5 mg/ml hydrocortisone (Sigma) and 100 IU/ml penicillin/streptomycin. MDA-MB-231 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and 100 IU/ml penicillin/streptomycin. Cells were maintained at 37°C and 5% CO2 in a humidified incubator.
MTT assay
Cells were seeded in a 96-well plate to a final concentration of 5000 cells/well and incubated in growth media with varying concentrations of Erianin for 24 h, 48 h and 72 h. Medium was removed and fresh medium was added to each well along with 10 ml of MTT solution (5 mg/ml). After 4 h incubation at 37°C, the medium was poured off and replaced by 150 ml of DMSO. The plates were read at wavelength of 490 nm using a micro-plate reader (BioTek, Winooski, VT, USA). Five reduplicate wells were used for each treatment, and experiments were repeated three times.
Western blot analysis
Cells were treated with Erianin (0 to 80 nM) for 48 h and 72 h. Western blot was performed as described previously [9]. The cells were collected in ice-cold PBS, and lysed in ice-cold whole cell extraction buffer (WCEB) containing 25 mM β-glycerophosphate (pH 7.3), 5 mM EDTA, 2 mM EGTA, 5 mM β-mercaptoethanol, 1% Triton X-100, 0.1 M NaCl, and a protease inhibitor mixture (Roche Applied Science). The protein concentrations the cell lysates were determined by use of Bradford methods and boiled in SDS sample buffer (50 mMTris [pH 6.8]; 100 mM DTT; 2% SDS; 0.1% bromophenol blue; 10% glycerol). The proteins were separated on 8~10% SDS polyacrylamide gel and electro-transferred to polyvinylidene fluoride (PVDF) membrane. After blocking with 3% BSA-TBST, primary antibodies were detected using HRP-conjugated anti-rabbit antibodies and visualized on Tanon-5200 Chemiluminescent Imaging System (Tanon Science & Technology). β-actin (ZSGB-BIO TA-09) was also tested to confirm equal loading.
Quantitative RT-PCR
Cells were treated with erianin (0 to 80 nM) for 48 h and 72 h. RNA isolation and qRT-PCR assays were performed as described previously [10,11]. Total RNA from cells was extracted with TRIzol reagent (Invitrogen) following the manufacturer’s instruction, and complementary DNA was synthesized using moloneymurine leukemia virus (M-MLV) reverse transcriptase with random primers. cDNA was generated with BioTeke super RT kit (Bioteke) according to the manufacture’s protocol. qRT-PCR was performed using SYBR Premix Ex TaqTM (TaKaRa). Primers are listed in Table 1.
Table 1.
RT-PCR primer sequences (all sequences from 5’ to 3’)
| RT-PCR Primers | Forward | Reverse |
|---|---|---|
| β-actin | GCCGCCAGCTCACCAT | TCGATGGGGTACTTCAGGGT |
| CDK1 | CTGGCTGATTTTGGCCTTGC | CCACTTCTGGCCACACTTCA |
| CDK2 | TTTGCTGAGATGGTGACTCG | CTTCATCCAGGGGAGGTACA |
| CDK4 | TGAGGGGGCCTCTCTAGCTT | CAAGGGAGACCCTCACGCC |
| CDK5 | CTCCGGGAGATCTGCCTACT | AGCTCCCCATTCTTTACAATCTCA |
| CDK7 | GGGACAGTTTGCCACCGTTT | ATGTCCAAAAGCATCAAGGAGAC |
| CDK8 | GGGATCTCTATGTCGGCATGTAG | AAATGACGTTTGGATGCTTAAGC |
| CDK9 | CAGTACGACTCGGTGGAGTG | TGTAATGGGGAACCCCTCCT |
| CDK10 | TGGACAAGGAGAAGGATG | CTGCTCACAGTAACCCATC |
| MMP2 | GAGTGCATGAACCAACCAGC | GTGTTCAGGTATTGCATGTGCT |
| MMP9 | CTTTGAGTCCGGTGGACGAT | TCGCCAGTACTTCCCATCCT |
| CDH2 | GGGAAATGGAAACTTGATGGCA | CAGTTGCTAAACTTCACTGAAAGGA |
| Fibronectin | GATAAATCAACAGTGGGAGCGG | GTCTCTTCAGCTTCAGGTTTACTC |
| TIMP1 | GCAATTCCGACCTCGTCATC | TAGACGAACCGGATGTCAGC |
| TIMP2 | CTGCGAGTGCAAGATCACG | TGGTGCCCGTTGATGTTCTT |
| CDH1 | TGAAAAGAGAGTGGAAGTGTCCGAG | GATTAGGGCTGTGTACGTGCTGTTC |
| Bax | CCCTTTTGCTTCAGGGTTTC | CCCTTTTGCTTCAGGGTTTC |
| Bcl-2 | GATAACGGAGGCTGGGATGC | TCACTTGTGGCCCAGATAGG |
| p12 | AAACATTCTCACCTCCTCTGGG | CCCAGTTCCCTGGGTGTAGC |
| p18 | TCCCAGCAGCGGAGGAC | TAGGGTCCCTTGTTCACGGT |
| p27 | GGCAAGTACGAGTGGCAAGA | AGAAGAATCGTCGGTTGCAGG |
| p53 | CCTCAGCATCTTATCCGAGTGG | TGGATGGTGGTACAGTCAGAGC |
| p21 | GTGAGCGATGGAACTTCGACTT | AGAGGTTTACAGTCTAGGTGGA |
Cell cycle analysis
Cells were treated with graded concentrations of erianin (0 to 80 nM) for 48 h and 72 h. Cell cycle distribution was evaluated using Cell cycle detection kit (BestBio, China) following the manufacturer’s instruction. Briefly, the cells were harvested, washed twice with PBS, and fixed at 4°C for 1 h with 70% ethanol, and then stained with a propidium iodide (PI) solution (containing RNase) at 4°C for 30 min. At least 20,000 cells were analyzed by Becton Dickinson FACScan cytoflurometer (Mansfield, MA, USA). Cell cycle distribution was calculated using ModFIT cell cycle analysis software (Version 2.01.2; Becton Dickinson).
Apoptosis staining
To visualize the development of apoptosis, cells were treated with graded concentrations of erianin (0 to 160 nM) for 48 h and 72 h and than stained with Annexin V-FITC Apoptosis Detection Kit (BestBio, China) according to the manufacture’s instruction. Briefly, cells were washed twice with PBS, resuspended in 400 μl of Annexin-V binding buffer, and then incubated with 5 μl of FITC conjugated Annexin-V for 15 min 4°C in the dark, followed by incubating with 10 μl of PI for 15 min 4°C in the dark. Microscopic analysis was performed using Olympus Fluorescence microscope. Viable cells do not take up either dye (FITC-/PI-), whereas cells undergoing early apoptosis reveal green fluorescence (FITC+/PI-), and cells in the late apoptotic phase take up both FITC and PI exhibiting both green and red fluorescence (FITC+/PI+).
Apoptosis assay
The apoptotic percentages status was determined by a flow cytometry analysis. Apoptosis assay was performed with Annexin V-FITC Apoptosis Detection Kit (BestBio, China) according to the manufacture’s instruction and than performed using Becton Dickinson FACScan cytoflurometer (Mansfield, MA, USA).
Migration determination
Migration was determined by wound healing assays. The cells were seeded at a density of 5×105 cells/well in a 12-well plate. After the cell monolayer was formed, a micropipette tip was used to scratch the attached cells to generate a wound space. The cells were then washed with PBS and replaced by serum-free RPMI-1640 medium containing various concentrations of erianin (0 to 20 nM). The cell migration progress into the wound was photographed using an inverted microscope at 0 h, 24 h, 48 h and 72 h. The average wound size represented the relative cell migration.
Statistical analysis
Data were presented as mean ± SD of at least three independent experiments. Statistical analysis of data was performed by Student’s t test and one-way ANOVA using Graph Pad Prism 5 software for two groups and multiple group comparison. P<0.05 represents the statistically significant difference.
Results
Erianin decreases the viability of T47D cells
To investigate the anti-proliferation effects of Erianin, MTT assays were used to evaluate the viability of the T47D cells treated with different dosages of Erianin. The MTT assay results showed that Erianin could inhibit the proliferation of T47D in a dosage-dependent manner. Erianin significantly decrease the viability of T47D cells at 40, 80 and 160 nM after 24 and 48 hours of treatment (Figure 1B, 1C). In addition, Erianin significantly decreased the viability of T47D cells at 20 nM after 72 hours of treatment, demonstrating that the treatment with low dosage but long time could also inhibit the viability of T47D cells (Figure 1D). Interestingly, integration of the dosage and time course of the MTT assays results demonstrated that only the treatment of Erianin at high dosage (80 and 160 nM) could inhibit the proliferation of the T47D cells completely (Figure 1E). Treatment with low dosages of Erianin could not inhibit the proliferation of the T47D cells completely (comparing 20 and 40 nM treatment at 72 h with control at 24 h).
Erianin suppresses the mobility of T47D cells
To investigate whether Erianin affects the mobility of T47D cells, wound healing assays were used to evaluate the migration of T47D cells that were treated with different dosages of Erianin. The results showed that the T47D cells treated with 10 and 20 nM Erianin showed significantly reduced migration compared with the control cells, demonstrating that even low dosages of Erianin could suppress migration of T47D cells effectively (Figure 2A, 2B). Importantly, the same low dosages of Erianin could not inhibit the viability of T47D cells significantly (Figure 1B, 1C). Therefore, the reduced migration of Erianin-treated T47D cells was not caused by the decreased cell proliferation. In addition, the T47D cells treated with Erianin for 48 and 72 hours showed greater reduced migration thanthose cells treated for 24 hours, indicating that longer treatment had greater inhibitory effects on cell migration than shorter treatment. Furthermore, we analyzed the effects of Erianin treatment on the expression of genes involved in the cell migration. The expressions of genes that promote migration, e.g. matrix metalloproteinase 2 (MPP2), MPP9, CDH2 and Fibronectin, were decreased in the Erianin-treated T47D cells. On the contrary, the expressions of genes that suppress migration, e.g. tissue inhibitor of MMPs 1 (TIMP1), TIMP2 and CDH1, were increased in the Erianin-treated T47D cells (Figure 2C).
Figure 2.

Erianin suppresses T47D cells migration. A. Cells were treated with various doses of erianin (0 to 20 nM) for 24, 48 or 72 h, and the migratory behavior was analyzed using wound healing assays. After the indicated treatment, migrating cells in the denuded zone were photographed. B. Effect of erianin on T47D migration at 24 h, 48 h and 72 h. C. qPCR analysis for MMP2, MMP9, CDH1, CDH2, TIMP1, TIMP2 and Fibronectin in erianin-treated cells. Data represent the mean ± SD (n=3), *P<0.05, **P<0.01, ***P<0.001 compared to control cells.
Erianin causes cell cycle arrests of T47D cells at G2/M phase
Since Erianin treatment caused decreased proliferation of T47D cells, we further investigated whether it inhibited the cell proliferation through inducing cell cycle arrests. Indeed, the Erianin-treated T47D cells showed significantly increased amounts of cells at G2/M phase, especially at 40 and 80 nM (Figure 3A). In addition, the percentages of cells at G2/M phase is also significantly increased in the Erianin-treated T47D cells (Figure 3B). Meanwhile, the Erianin-treated T47D cells showed greatly reduced percentage of cells at G0/G1 phase (Figure 3B). Furthermore, the transcription of cell cycle-related CDK genes were examined by quantitative PCR, and the transcription of CDK1, 2, 4, 7, 8, 9 and 10 were significantly reduced in Erianin-treated T47D cells, indicating that the cell cycle arrests in Erianin-treated T47D cells could be caused by reduced expression of these CDKs (Figure 3C).
Figure 3.
Erianin induces T47D cells cycle arrest at G2/M phase. A. Erianin induces T47D cells cycle arrest. Cells were treated with graded concentrations of erianin (0 to 160 nM) for 48 and 72 hours. Propidium iodide staining was done to determine the DNA content. B. The percentage of cells in different phases of cell cycle was analyzed by flow cytometry. C. qPCR analysis for CDK family expression in erianin-treated cells. Data represent the mean ± SD (n=3), *P<0.05, **P<0.01, ***P<0.001 compared to control cells.
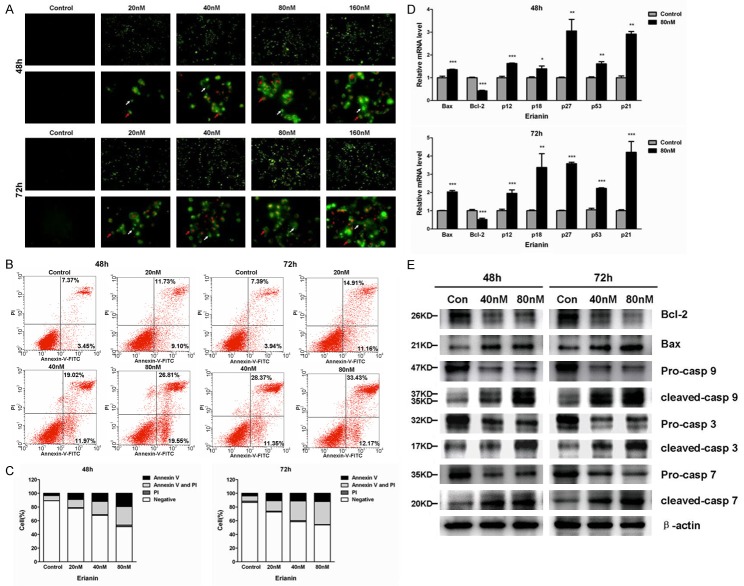
Erianin induces apoptosis of T47D cells
We also investigated whether Erianin treatment could induce apoptosis of T47D cells. The Annexin V/PI staining assays showed many Erianin-treated T47D cells were in the process of early apoptosis and late apoptosis (Figure 4A). Furthermore, the flow cytometry analysis demonstrated that the Erianin-treated T47D cells had significantly increased percentages of cells that were in the process of early and late apoptosis (Figure 4B, 4C). The potential mechanism underlying the Erianin-induced apoptosis was investigated by quantitative PCR. The expression of Bcl-2, a gene involved in the suppression of apoptosis, was decreased in the Erianin-treated T47D cells, while the expression of genes that were involved in the promotion of apoptosis, e.g., Bax, p12, p18, p27, p53 and p21 were increased in the Erianin-treated T47D cells (Figure 4D). The decreased expression of Bcl-2 and increased expression of Bax were confirmed at protein levels (Figure 4E). In addition, multiple caspases were cleaved in the Erianin-treated cells, demonstrating the activation of the caspase signaling pathway (Figure 4E).
Figure 4.
Erianin induced T47D cells apoptosis. A. Visualization of apoptotic cells by Annexin V/PI staining assay on T47D cells. White arrows indicate early apoptotic cells and red arrows indicate late apoptotic cells. B. The apoptotic status was determined by flow cytometry analysis. C. Percentages of negative (viable) cells, annexin V-positive (early apoptotic) cells, PI-positive (necrotic) cells, or annexin V and PI double-positive (late apoptotic) cells were shown. Data represent the mean ± SD (n=3), *P<0.05, **P<0.01, ***P<0.001 compared to control cells. D. qPCR analysis for Bax, Bcl-2, p12, p18, p27, p53 and p21 in erianin-treated cells. Data represent the mean ± SD (n=3), *P<0.05, **P<0.01, ***P<0.001 compared to control cells. E. Effects of erianin on protein levels of Bcl-2, Bax and caspases in T47D cells.
Erianin does not affect survival of normal breast epithelial cell line MCF10A
To investigate whether Erianin treatment affects the viability of normal breast epithelial cells, we treated MCF10A cells with multiple dosages of Erianin. Erianin treatment did not have significant effects on growth of MCF10A cells at all at 24 hours (Figure 5A). At 48 hours of treatment, while high dosage of Erianin did not have significant effects on growth of MCF10A cells, low dosages of Erianin promoted the growth of MCF10A (Figure 5B). At 72 hours of treatment, while low dosage of Erianin still promoted the growth of MCF10A cells, extremely high dosage of Erianin, e.g. 160 nM, inhibited the growth of MCF10A (Figure 5C). Overall, in contrast to the inhibitory effects of Erianin on the viability of T47D cells, Erianin treatment did not show great inhibitory effects on the viability of MCF10A cells (Figure 5D). These results demonstrated that the inhibitory effects on proliferation of Erianin could be specific to tumor cells, which indicate that Erianin might be an ideal drug to treat ER-positive breast cancers.
Figure 5.
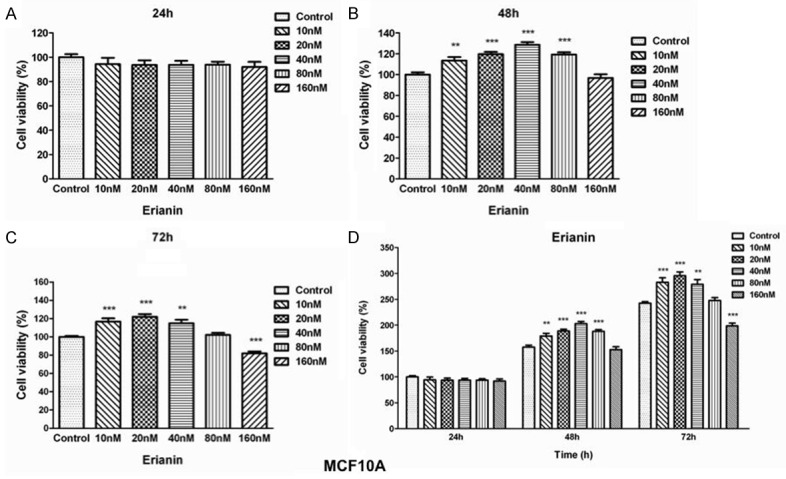
Erianin promotes MCF10A cell growth. (A) Effects of erianin on MCF10A cells proliferation at 24 h (A), 48 h (B) and 72 h (C). Cells were treated with graded concentrations of Erianin (0, 10, 20, 40, 80, 160 nM) at different time points. Cell proliferation potential was evaluated by MTT assay. The viability of untreated control cells is represented as 100%. Data represent the means ± SD (n=5), *P<0.05, **P<0.01, ***P<0.001 versus control cells. (D) Erianin promotes MCF10A cell growth in a dose- and time-dependent manner. Experimental data of MTT assay were integrated and means ± SD (n=5), *P<0.05, **P<0.01, ***P<0.001 compared to control cells at 24 h time points.
Discussion
Our previous study reported that Dendrobium. candidum could inhibit the proliferation of MCF-7 cells proliferation [9]. However, the Dendrobium. candidum is a mixture which contains multiple chemical constituents that might have potential anti-cancer effects [1]. In the present study, we evaluated the anti-cancer effects of one important component of the Dendrobium. Candidum, e.g. Erianin, on ER positive breast cancer cell line T47D.
The results demonstrated that the treatment of Erianin could significantly decrease the cell viability at different concentrations compared to the control group at 24 h, 48 h, and 72 h, respectively. More importantly, the treatment of Erianin induced apoptosis effectively in the T47D cells, and the expression of Bcl-2, an important anti-apoptotic protein, was significantly reduced in the Erianin-treated cells. Bcl-2 can bind to the pro-apoptotic proteins, such as Bax, and neutralize their pro-apoptotic functions [12]. Therefore, reduced expression of Bcl-2 could potentially make cells more susceptible to the stimulation of apoptosis signaling. Bcl-2 has been a target for therapeutic intervention to enhance the vulnerability to therapy in ER positive breast cancer [13,14]. Further investigation is required to elucidate how Erianin treatment could suppress the expression of Bcl-2.
In this study, we demonstrated that Erianininhibits the viability of T47D cells by enhancing the cell cycle arrest in the G2/M phase. CDKs play a key role in the controlling of cell proliferation through maintaining of cell cycling, and CDK inhibition has been an attractive therapeutic intervention to treat cancers, including ER positive breast cancer [15]. Our results demonstrated that the treatment of Erianin significantly suppresses the expression of multiple CDKs, indicating that Erianin could target multiple CDKs, which might be an effective way to suppress cell cycling completely.
We found that the migration of T47D cells was greatly inhibited by the treatment of Erianin. The migration ability is essential for metastasis, which is the main factor responsible for death in breast patients. The homeostatic expression of MPP and their inhibitors TIMP is essential for the migration, and involved in the process of metastasis of breast cancer cells [16]. Our results demonstrated that Erianin could suppress the expression of genes that promote migration and promote the expression of genes that suppress migration, which revealed an interesting mechanism on how Erianin could inhibit cell migration.
In conclusion, Erianin could inhibit several cancer-associated pathways in the ER positive breast cancer cells simultaneously, which indicates that Erianin has the potential to be an effective drug to treat ER positive the breast cancer. In addition, Erianin treatment does not affect the growth of normal breast epithelial cells (MCF10A), suggesting it might not have strong side effects in vivo.
Disclosure of conflict of interest
None.
References
- 1.Lam Y, Ng TB, Yao RM, Shi J, Xu K, Sze SC, Zhang KY. Evaluation of chemical constituents and important mechanism of pharmacological biology in dendrobium plants. Evid Based Complement Alternat Med. 2015;2015:841752. doi: 10.1155/2015/841752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li YM, Wang HY, Liu GQ. Erianin induces apoptosis in human leukemia HL-60 cells. Acta Pharmacol Sin. 2001;22:1018–1022. [PubMed] [Google Scholar]
- 3.Gong YQ, Fan Y, Wu DZ, Yang H, Hu ZB, Wang ZT. In vivo and in vitro evaluation of erianin, a novel anti-angiogenic agent. Eur J Cancer. 2004;40:1554–1565. doi: 10.1016/j.ejca.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Gong Y, Fan Y, Liu L, Wu D, Chang Z, Wang Z. Erianin induces a JNK/SAPK-dependent metabolic inhibition in human umbilical vein endothelial cells. In Vivo. 2004;18:223–228. [PubMed] [Google Scholar]
- 5.Qin L, Li R, Zhang J, Li A, Luo R. Special suppressive role of miR-29b in HER2-positive breast cancer cells by targeting Stat3. Am J Transl Res. 2015;7:878–890. [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nounou M, EIAmrawy F, Ahmed N, Abdelraouf K, Goda S, Syed-Sha-Qhattal H. Breast Cancer: Conventional Diagnosis and Treatment Modalities and Recent Patents and Technologies. Breast Cancer (Auckl) 2015;9:17–34. doi: 10.4137/BCBCR.S29420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumachi F, Santeufemia D, Basso S. Current medical treatment of estrogen receptor-positive breast cancer. World J Biol Chem. 2015;6:231–239. doi: 10.4331/wjbc.v6.i3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, Guo Y, Fu X, Wang Y, Liu Y, Huo B, Sheng J, Hu X. Dendrobiumcandidum inhibits MCF-7 cells proliferation by inducing cell cycle arrest at G2/M phase and regulating key biomarkers. Onco Targets Ther. 2016;9:21–30. doi: 10.2147/OTT.S93305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Bragt MP, Hu X, Xie Y, Li Z. RUNX1, a transcription factor mutated in breast cancer, controls the fate of ER-positive mammary luminal cells. Elife. 2013;3:e03881. doi: 10.7554/eLife.03881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin T, Ponn A, Hu X, Law BK, Lu J. Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene. 2010;29:4896–4904. doi: 10.1038/onc.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy S, Nicholson DW. Cross-talk in cell death signaling. J Exp Med. 2000;192:F21–F25. [PMC free article] [PubMed] [Google Scholar]
- 13.Vaillant F, Merino D, Lee L, Breslin K, Pal B, Ritchie ME, Smyth GK, Christie M, Phillipson LJ, Burns CJ, Mann GB, Visvader JE, Lindeman GJ, Agrawal A, Robertson JF, Cheung KL, Gutteridge E, Ellis IO, Nicholson RI, Gee JM. Biological effects of fulvestrant on estrogen receptor positive human breast cancer: short, medium and long-term effects based on sequential biopsies. Int J Cancer. 2016;138:146–159. doi: 10.1002/ijc.29682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling X, He X, Apontes P, Cao F, Azrak RG, Li F. Enhancing effectiveness of the MDR-sensitive compound T138067 using advanced treatment with negative modulators of the drug-resistant protein survivin. Am J Transl Res. 2009;1:393–405. [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer EL. Targeting Breast Cancer with CDK inhibitors. Curr Oncol Rep. 2015;17:20–24. doi: 10.1007/s11912-015-0443-3. [DOI] [PubMed] [Google Scholar]
- 16.Gomes LR, Terra LF, Wailmemann RA, Labriola L, Sogayar MC. TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 2012;12:26. doi: 10.1186/1471-2407-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]