Abstract
Progressive loss of cardiac myocytes through apoptosis contributes to heart failure (HF). In this study, we tested whether tanshinone IIA, one of the most abundant constituents of the root of Salvia miltiorrhiza, protects rat myocardium-derived H9C2 cells against apoptosis. Treatment of H9C2 cells with tanshinone IIA inhibited angiotensin II-induced apoptosis by downregulating the expression of PTEN (phosphatase and tensin homolog), a tumor suppressor that plays a critical role in apoptosis. Furthermore, tanshinone IIA was found to inhibit PTEN expression by upregulating the microRNA miR-152-3p, a potential PTEN regulator that is highly conserved in both rat and human. Notably, the antiapoptotic effect of tanshinone IIA was partially reversed when H9C2 cells were transfected with an inhibitor of miR-152-3p. Collectively, our findings reveal a previously unrecognized mechanism underlying the cardioprotective role of tanshinone IIA, and further suggest that tanshinone IIA could represent a promising drug candidate for HF therapy.
Keywords: Apoptosis, cardiac myocyte, heart failure, miR-152-3p, PTEN, tanshinone IIA
Introduction
Heart failure (HF), which is occasionally also referred to as chronic HF, is caused by various heart diseases and is considered the end-stage form of heart disease that is associated with high mortality [1]. The exact mechanism of HF remains unclear, but studies conducted to date have indicated that HF progression involves multiple contributing factors, such as coronary artery disease, blood pressure, alcohol consumption, smoking, infectious diseases, and other unknown causes [2-5]. In HF pathology, a major contributing factor is reported to be the progressive loss of functional cardiac myocytes due to apoptosis, and this notion is supported by evidence from studies on both humans and animal models [6]. Moreover, in heart transplantation, a high level of apoptotic cells has been observed in the recipients’ replaced failed hearts [7-9].
In healthy people, apoptosis in the myocardium is extremely rare: the rate was reported to be 0.01%-0.001% based on measurements of TUNEL-positive cardiac myocytes [10]. Although the rate of apoptosis in the human failing heart was reported to be substantially below 1% [11], low levels of apoptosis can still produce profound effects over a period of years due to the limited ability of cardiac myocytes to proliferate [10]. An apoptotic rate of 0.1% was hypothesized to result in a 37% reduction in the number of cardiac myocytes over one year, and this speculation was supported by results obtained with transgenic mice that expressed an inducible caspase 8 specifically in cardiac myocytes [12,13]. Moreover, p53, a widely recognized tumor suppressor, was also demonstrated to critically influence HF development due to its role in apoptosis [14-16]. Conversely, although HF has been commonly treated by modulating β-adrenergic receptor (β-AR)-mediated signaling by using β-blockers [2], studies have also reported β-AR-stimulation-induced apoptosis in the heart due to long-term application of β-AR-blocking therapy [17,18]. Therefore, prevention of the apoptosis of cardiac myocytes has emerged as a primary target in HF therapy.
Recently, for the treatment of HF, numerous researchers from China have started to investigate the application of traditional Chinese medicine (TCM) and TCM-based herbology. In TCM, Salvia miltiorrhiza has been used for managing cardiovascular diseases for centuries [19]. Thus, in this study, we tested whether tanshinone IIA-one of the most abundant constituents of the root of S. miltiorrhiza-protects rat myocardium-derived H9C2 cells against apoptosis. Our results indicated that tanshinone IIA inhibits angiotensin II (AngII)-induced apoptosis by downregulating the expression of phosphatase and tensin homolog (PTEN). Tanshinone IIA treatment of H9C2 cells upregulated microRNA-152-3p (hereafter miR-152-3p), a potential PTEN regulator that is highly conserved in both rat and human, and the antiapoptotic effect of tanshinone IIA was partially antagonized by an inhibitor of miR-152-3p. Collectively, our data further highlight the cardioprotective action of tanshinone IIA and suggest that tanshinone IIA could represent a promising drug candidate for HF therapy.
Materials and methods
Cells and chemicals
H9C2 cells (ATCC® CRL-1446™) were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (Life Technologies, Carlsbad, CA, USA); cultures were maintained in a Fisher Scientific™ Isotemp™ CO2 Incubator (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C under 5% CO2. Apoptosis was induced in H9C2 cells by adding 0.2 μM AngII (Sigma-Aldrich, St. Louis, MO, USA) to the cell-culture medium as previously described [20]. Tanshinone IIA was purchased from Sigma-Aldrich and dissolved in methanol at 5 mg/mL according to the manufacturer’s instruction. The rat and human miR-152-3p mimic and inhibitor and the miRNA-mimic negative control (miRNA-scramble control) were purchased from Applied Biological Materials Inc. (Richmond, BC, Canada). Cells were transfected with the microRNA-mimic or plasmids by using Lipofectamine 2000 (Thermo Fisher Scientific) according to manufacturer instructions.
miRNA microarray and real-time PCR
Total RNA was purified using TRIzol® Reagent (Life Technologies), as per manufacturer guidelines. RNase-free DNase (Promega, Madison, WI, USA) was used to eliminate genomic-DNA contamination during RNA isolation. RNA samples from both control and tanshinone IIA-treated H9C2 cells were hybridized with the Agilent Rat miRNA Microarray Release 16.0, 8x15K (Agilent Technologies, Santa Clara, CA, USA), by following the manufacturer’s protocol. Arrays were scanned using an Agilent Microarray Scanner and Feature Extractor (software version 9.5.1). Array results were confirmed for miRNA-152-3p by using qPCR.
H9C2-cell RNA for qPCR was also isolated using TRIzol® Reagent. The cDNAs indicating miRNA expression were analyzed by using a Hairpin-it™ miRNA RT-PCR Quantitation kit (GenePharma, Shanghai, China) with ABM mo-miR-152-3p Primers (Applied Biological Materials Inc.) according to the manufacturer’s instructions. To quantify cellular gene transcripts, RNA was reverse-transcribed by using AMV reverse transcriptase (Promega) with random hexamers as per manufacturer instructions, and then real-time PCR (qPCR) detection was performed with SYBR Green Mix (Life Technologies) for the targeted genes as previously described [21,22]. GAPDH and U6 transcripts were also amplified from the same samples and used as internal controls for normalization; we purchased RNU6 House Keeping Primers for rat U6 from Applied Biological Materials Inc. Relative gene expression was calculated using the 2-ΔΔCT method [23]. The sequence of primers for PTEN and GAPDH was listed as Table 1.
Table 1.
qPCR Primers and their sequence used in this study
| Primer name | Sequence |
|---|---|
| PTEN-F1 | ACACTCCAGCTGGGATCTGTAAGAGAA |
| PTEN-R1 | TGGTGTCGTGGAGTCG |
| GAPDH-F1 | CAGTGCCAGCCTCGTCTCAT |
| GAPDH-R1 | AGGGGCCATCCACAGTCTTC |
Cell-viability assay
Trypsinized H9C2 cells were stained with trypan blue (Sigma-Aldrich) for counting viable cells before further seeding, and then viable cells were seeded into 96-well plates at 2 × 104 cells/well. After various treatments, the cells were lysed with Passive Cell Lysis Buffer (Promega) and then cell viability was measured using the CellTiter-Glo® Luminescent Cell Viability Assay kit (Promega), according to the manufacturer’s instructions.
Western blotting
H9C2 cells were lysed using Laemmli Sample Buffer as previously described [22,24], and then the lysates were separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to PVDF membranes, and western blotted as described before [24]. Briefly, the PVDF membranes were first probed with a rabbit anti-PTEN antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and then the specific immunoreactive band was detected using a goat anti-rabbit IgG conjugated with horseradish peroxidase (Sigma) and visualized using a chemiluminescence substrate. Membranes were also blotted with an antibody against tubulin (Santa Cruz), which was used as a protein-loading control. The chemiluminescence signal was recorded digitally using a ChemiDoc MP system (Bio-Rad Laboratories, Hercules, CA, USA), and data were recorded and analyzed using ImageLab Program Version 6.1.
Flow cytometry (FCM)-based apoptosis assay
H9C2 cells were exposed to various treatments, and then the treated cells were trypsinized and 1 × 106 cells from each group were fixed with 4% paraformaldehyde (Sigma-Aldrich). Next, single-cell suspensions were stained with FITC-labeled Annexin V and propidium iodide, and then the stained cells were analyzed using a FACSCalibur machine (BD Biosciences, San Jose, CA, USA) to measure apoptosis levels.
Luciferase-based miRNA functional assay
Assays were performed using the human PTEN 3’UTR reporter plasmid psiCHECK2-PTEN-3’UTR (plasmid #50936, Addgene, Cambridge, MA, USA). HEK293T cells were cotransfected with psiCHECK2-PTEN-3’UTR and either miR-152-3p or miR-scramble control, and then luciferase activity was evaluated by using the Dual-Glo® Luciferase Assay System (Promega) according to manufacturer instructions. The luminescence signal was measured using a VICTOR-X5™ Multilabel Counter (Perkin-Elmer, Waltham, MA, USA). Percentages of luminescence intensity were normalized relative to that measured for Renilla luciferase and were calculated in comparison with cells transfected with miR-scramble control.
Statistical analysis
Student’s t tests were used to assess the significance of differences in apoptosis, PTEN mRNA levels, miR-152-3p expression, cell viability, and luciferase activity among the groups in the presence or absence of tanshinone IIA, AngII, or miR-152-3p mimic/inhibitor. Two-tailed P < 0.05 was considered statistically significant.
Results
Tanshinone IIA protects H9C2 cells against AngII-induced apoptosis
Tanshinone IIA has been demonstrated to protect against cardiac hypertrophy [25], but whether tanshinone IIA can inhibit apoptosis in myocardial cells is not known. Here, we tested the potential protective role of tanshinone IIA in the rat myocardium-derived cell line H9C2. When H9C2 cells were treated with 0.2 μM AngII to induce apoptosis, cells undergoing early and late apoptosis were increased to 22.8% and 11.799%, respectively, relative to control (Figure 1A). However, tanshinone IIA addition resulted in a reduction in AngII-induced early and late apoptosis in H9C2 cells, to 14.587% and 1.1312%, respectively, which suggested an antiapoptotic role of tanshinone IIA. We repeated the FCM-based apoptosis measurement for different groups at least 3 times each, and statistical analysis confirmed that tanshinone IIA suppressed AngII-induced apoptosis (Figure 1B).
Figure 1.

Tanshinone IIA inhibits angiotensin II (AngII)-induced apoptosis in H9C2 cells. A. Flow cytometry (FCM)-based analysis of apoptosis in H9C2 cells. H9C2 cells were treated with AngII or AngII plus tanshinone IIA or left untreated, and then stained with Annexin V and propidium iodide (PI) for FCM-based apoptosis analysis. B. Quantification of apoptosis. Each bar represents data from at least 3 repeated experiments. Significant differences between groups: *P < 0.05.
Tanshinone IIA inhibits PTEN expression
Because tanshinone IIA inhibited AngII-induced apoptosis in H9C2 cells, we sought to investigate the underlying mechanism. In screens performed to detect genes that are regulated by tanshinone IIA, we had noted that PTEN expression was markedly decreased in cells treated with tanshinone IIA, and we found that this inhibition of PTEN mRNA expression was dose-dependent (Figure 2A). The results of cell-viability analysis suggested that this PTEN downregulation was not due to the cytotoxicity of tanshinone IIA (Figure 2B). Moreover, examination of PTEN protein levels also confirmed that PTEN expression was inhibited following tanshinone IIA treatment (Figure 2C).
Figure 2.

Tanshinone IIA treatment inhibits PTEN expression in H9C2 cells. In all assays presented here, H9C2 cells were treated with the indicated doses of tanshinone IIA for 36 h. A. PTEN mRNA expression in H9C2 cells treated with tanshinone IIA, analyzed using qPCR. B. Viability of H9C2 cells treated with tanshinone IIA, measured using the CellTiter-Glo kit. For statistical analysis, experiments were repeated 3 times for each group. C. PTEN protein expression in H9C2 cells after tanshinone IIA treatment; cells were harvested using Laemmli Sample Buffer and samples were subjected to SDS-PAGE and western blotting.
Tanshinone IIA promotes microRNA-153-3p expression and thereby targets PTEN expression
PTEN is a critical negative regulator of the PI3K-Akt signaling pathway and is considered to be a tumor suppressor [26,27], and PTEN overexpression can lead to apoptosis and inhibit cell proliferation [28]. However, the mechanism by which tanshinone IIA downregulated PTEN expression was unclear. In other experiments that we conducted to screen for microRNA expression patterns in tanshinone IIA-treated H9C2 cells, we noted that miR-152-3p was significantly upregulated following exposure to tanshinone IIA (unpublished data). The results of sequence analyses indicated that the 3’UTR of the PTEN mRNA contains 2 putative binding sites for miR-152-3p (Figure 3A), and that human and rat miR-152-3p sequences and the putative binding sites in PTEN 3’UTR are highly conserved between the species (Figure 3A). Furthermore, qPCR results confirmed that tanshinone IIA treatment upregulated miR-152-3p in a dose-dependent manner (Figure 3B). Thus, our data suggested that tanshinone IIA-mediated upregulation of miR-152-3p protected rat myocardium-derived cells against AngII-induced apoptosis by targeting PTEN; this raised the intriguing question of whether human miR-152-3p functions similarly as its rat counterpart. In HEK293T cells transfected with human miR-152-3p and the PTEN 3’UTR reporter plasmid, luciferase activity was significantly lower than that in cells transfected with miR-scramble control, which implied that PTEN is also targeted by human miR-152-3p (Figure 3C). Because miR-152-3p and the gene PTEN are highly conserved in both human and rat, these data again suggested that tanshinone IIA potentially inhibits apoptosis in myocardial cells by inducing the expression of miR-152-3p, which is a regulator of PTEN.
Figure 3.
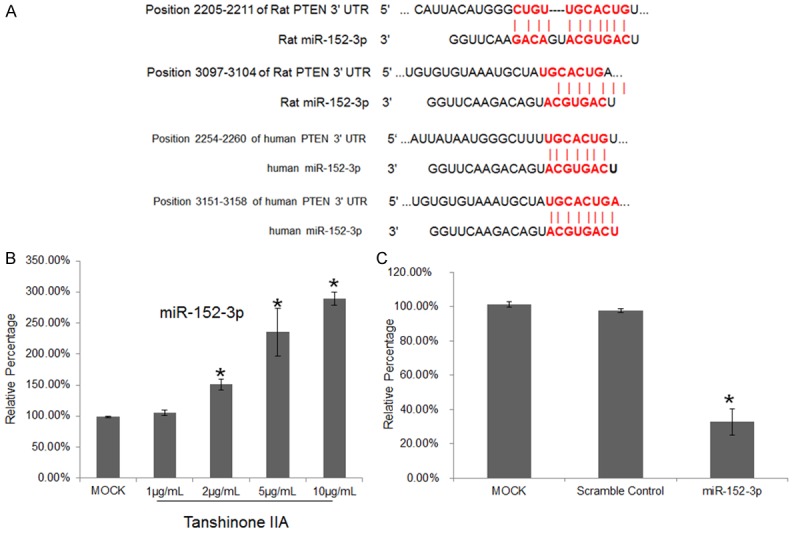
miR-152-3p is a potential PTEN regulator and it is upregulated following tanshinone IIA treatment. A. Alignment of 2 putative binding sites for miR-152-3p in human and rat PTEN 3’UTR. B. Tanshinone IIA treatment upregulates miR-152-3p expression. H9C2 cells were treated with different doses of tanshinone IIA for 36 h, and then the cells were harvested for qPCR analysis of PTEN mRNA expression. Each bar represents data from at least 3 repeated experiments. C. Luciferase-reporter assay to examine miR-152-3p-mimic targeting of PTEN 3’UTR. Each bar represents data from at least 3 repeated experiments. Significant differences between groups: *P < 0.05.
To further confirm PTEN targeting by miR-152-3p, H9C2 cells were transfected with a rat miR-152-3p mimic for 36 h and then subjected to qPCR analysis to measure PTEN transcripts. Transfection of the cells with the miR-152-3p mimic potently lowered the PTEN mRNA level (Figure 4A), and PTEN downregulation by the miR-152-3p mimic was also confirmed through western blotting (Figure 4B). Conversely, transfection of the cells with an inhibitor of miR-152-3p increased PTEN expression both at the mRNA level and protein level (Figure 4A and 4C).
Figure 4.

PTEN is targeted by miRNA-152-3p in H9C2 cells. (A) PTEN mRNA level is regulated by miRNA-152-3p. H9C2 cells were transfected with miRNA-152-3p mimic, miRNA-152-3p inhibitor, or miRNA-scramble control and then PTEN mRNA was analyzed using qPCR. Each bar represents data from at least 3 repeated experiments. Significant differences between groups: *P < 0.05. (B, C) Western blotting analysis of PTEN expression in H9C2 cells transfected with miR-152-3p mimic or scramble control (B) or miR-152-3p inhibitor or scramble control (C).
Antiapoptotic effect of tanshinone IIA is mediated by miR-152-3p
The aforementioned results demonstrated that tanshinone IIA inhibited PTEN expression by upregulating miR-152-3p, which is a potential regulator of PTEN mRNA expression; this implied that the protective role of tanshinone IIA in H9C2 cells might depend on the upregulation of miR-152-3p. To confirm this, we transfected H9C2 cells with the miR-152-3p mimic or inhibitor and then treated the cells with AngII. Following transfection with miR-scramble control, 27% of the cells underwent apoptosis, whereas transfection with the miR-152-3p mimic led to a significant inhibition of AngII-induced apoptosis (Figure 5A and 5B). However, when H9C2 cells were transfected with the miR-152-3p inhibitor, which antagonized tanshinone IIA-induced miR-152-3p upregulation, the cells undergoing early apoptosis were increased to a similar level as in AngII-treated cells (Figure 5A and 5B), although the cells undergoing late apoptosis were still at a lower level than in cells treated with AngII alone (Figure 5A and 5B). Collectively, these data suggest that tanshinone IIA-mediated protection partially depends on the ability of tanshinone IIA to upregulate miR-152-3p.
Figure 5.
Inhibition of apoptosis by tanshinone IIA partially depends on miR-152-3p upregulation. A. FCM-based analysis of apoptosis in H9C2 cells exposed to various treatments. H9C2 cells were transfected with miR-152-3p mimic, miR-152-3p inhibitor, or miRNA-scramble control, treated with AngII alone or AngII plus tanshinone IIA or left untreated, and then stained with Annexin V and PI for FCM-based apoptosis analysis. B. Quantification of apoptosis. Each bar represents data from at least 3 repeated experiments. Significant differences between groups: *P < 0.05.
Discussion
HF refers to a pathological condition that is characterized by insufficient cardiac output [5]. Several factors have been shown to contribute to HF development and progression, and the apoptosis of myocardial cells is considered to represent the key factor. Currently, other than cardiac transplantation, limited therapeutic options are available for managing HF. In China and its neighboring countries, S. miltiorrhiza has been long used for treating patients with myocardial infarction, angina pectoris, stroke, diabetes, sepsis, and other adverse health conditions [29]. Tanshinone IIA, the major lipophilic component and the most abundant constituent of the root of S. miltiorrhiza, has been shown to exert antioxidant and anti-inflammatory effects in various experimental disease models [30,31]. Moreover, tanshinone IIA has been widely demonstrated to produce cardioprotective effects such as reduced inflammatory responses during myocardial infarction, endothelium injury, atherosclerosis, and cardiovascular hypertrophy [29,32-34]. Therefore, this study was focused on examining the cytoprotection provided by tanshinone IIA.
We examined the protective effect of tanshinone IIA in the rat myocardial cell line H9C2. Our data indicated that treatment of H9C2 cells with tanshinone IIA inhibited AngII-induced apoptosis by downregulating PTEN expression. The results of further analysis demonstrated that PTEN downregulation was due to tanshinone IIA-induced miR-152-3p expression, and our findings suggested that miR-152-3p is potential PTEN regulator. Accordingly, inhibition of miR-152-3p function by using its inhibitor in H9C2 cells partially antagonized the antiapoptotic effect of tanshinone IIA. Because miR-152-3p and its putative target PTEN are highly conserved in both rat and human, we expect tanshinone IIA to similarly protect human myocardial cells against apoptosis.
Previously, miR-152 was considered to be the only functional microRNA generated from the miR-152 precursor [35]. However, the current database (Target Scan) lists 2 microRNAs, miR-152-3p and miR-152-5p, as the mature microRNAs generated by the miR-152 precursor. The previously defined miR-152 is now classified as the new miR-152-3p, but the function of miR-152-5p remains unknown.
Our current results only partially explain the mechanism underlying the cytoprotective effect mediated by tanshinone IIA: How tanshinone IIA treatment of H9C2 cells induces the upregulation of miR-152-3p is unclear. Moreover, when miR-152-3p function was blocked by transfecting cells with the miR-152-3p inhibitor, tanshinone IIA treatment still resulted in partial protection: the total number of cells undergoing apoptosis was markedly lower after treatment with AngII plus tanshinone IIA than after treatment with AngII alone. Combined with previous reports regarding the complex manner in which tanshinone IIA functions, our results imply that tanshinone IIA might modulate additional pathways involved in apoptosis and thereby protect myocardial cells. In conclusion, our data reveal a previously unknown function of tanshinone IIA and further suggest that tanshinone IIA might serve as a promising drug candidate for HF therapy.
Acknowledgements
This article was supported by a fund in our country. The fund number is 20160101025JC.
Disclosure of conflict of interest
None.
References
- 1.Cleland JG, Khand A, Clark A. The heart failure epidemic: exactly how big is it? Eur Heart J. 2001;22:623–626. doi: 10.1053/euhj.2000.2493. [DOI] [PubMed] [Google Scholar]
- 2.Fujita T, Ishikawa Y. Apoptosis in heart failure. -The role of the beta-adrenergic receptor-mediated signaling pathway and p53-mediated signaling pathway in the apoptosis of cardiomyocytes- Circ J. 2011;75:1811–1818. doi: 10.1253/circj.cj-11-0025. [DOI] [PubMed] [Google Scholar]
- 3.Grazette LP, Rosenzweig A. Role of apoptosis in heart failure. Heart Fail Clin. 2005;1:251–261. doi: 10.1016/j.hfc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care: Partial Update. London: 2010. [PubMed] [Google Scholar]
- 5.McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–1889. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- 6.Kang PM, Izumo S. Apoptosis and heart failure: A critical review of the literature. Circ Res. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 7.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 8.Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med. 1996;335:1190–1196. doi: 10.1056/NEJM199610173351604. [DOI] [PubMed] [Google Scholar]
- 9.Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997;95:320–323. doi: 10.1161/01.cir.95.2.320. [DOI] [PubMed] [Google Scholar]
- 10.Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA, Lavandero S. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2:e244. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Empel VP, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res. 2005;67:21–29. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Mani K. Programmed cell death in cardiac myocytes: strategies to maximize post-ischemic salvage. Heart Fail Rev. 2008;13:193–209. doi: 10.1007/s10741-007-9073-7. [DOI] [PubMed] [Google Scholar]
- 13.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsusaka H, Ide T, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, Kinugawa S, Tsutsui H. Targeted deletion of p53 prevents cardiac rupture after myocardial infarction in mice. Cardiovasc Res. 2006;70:457–465. doi: 10.1016/j.cardiores.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Naito AT, Okada S, Minamino T, Iwanaga K, Liu ML, Sumida T, Nomura S, Sahara N, Mizoroki T, Takashima A, Akazawa H, Nagai T, Shiojima I, Komuro I. Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circ Res. 2010;106:1692–1702. doi: 10.1161/CIRCRESAHA.109.214346. [DOI] [PubMed] [Google Scholar]
- 16.Das B, Young D, Vasanji A, Gupta S, Sarkar S, Sen S. Influence of p53 in the transition of myotrophin-induced cardiac hypertrophy to heart failure. Cardiovasc Res. 2010;87:524–534. doi: 10.1093/cvr/cvq068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Tomita H, Nazmy M, Kajimoto K, Yehia G, Molina CA, Sadoshima J. Inducible cAMP early repressor (ICER) is a negative-feedback regulator of cardiac hypertrophy and an important mediator of cardiac myocyte apoptosis in response to beta-adrenergic receptor stimulation. Circ Res. 2003;93:12–22. doi: 10.1161/01.RES.0000079794.57578.F1. [DOI] [PubMed] [Google Scholar]
- 18.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Wang Y, Liu H, Li N, Sun Y, Liu Z, Yang P. Ghrelin protects H9c2 cardiomyocytes from angiotensin II-induced apoptosis through the endoplasmic reticulum stress pathway. J Cardiovasc Pharmacol. 2012;59:465–471. doi: 10.1097/FJC.0b013e31824a7b60. [DOI] [PubMed] [Google Scholar]
- 21.Patel D, Opriessnig T, Stein DA, Halbur PG, Meng XJ, Iversen PL, Zhang YJ. Peptide-conjugated morpholino oligomers inhibit porcine reproductive and respiratory syndrome virus replication. Antiviral Res. 2008;77:95–107. doi: 10.1016/j.antiviral.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel D, Nan Y, Shen M, Ritthipichai K, Zhu X, Zhang YJ. Porcine reproductive and respiratory syndrome virus inhibits type I interferon signaling by blocking STAT1/STAT2 nuclear translocation. J Virol. 2010;84:11045–11055. doi: 10.1128/JVI.00655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Nan Y, Wang R, Shen M, Faaberg KS, Samal SK, Zhang YJ. Induction of type I interferons by a novel porcine reproductive and respiratory syndrome virus isolate. Virology. 2012;432:261–270. doi: 10.1016/j.virol.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan X, Li J, Wang X, Chen N, Cai B, Wang G, Shan H, Dong D, Liu Y, Li X, Yang F, Zhang P, Yang B, Lu Y. Tanshinone IIA protects against cardiac hypertrophy via inhibiting calcineurin/NFATc3 pathway. Int J Biol Sci. 2011;7:383–389. doi: 10.7150/ijbs.7.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu EC, Tarnawski AS. PTEN regulatory functions in tumor suppression and cell biology. Med Sci Monit. 2004;10:RA235–241. [PubMed] [Google Scholar]
- 27.Wang X, Jiang X. Post-translational regulation of PTEN. Oncogene. 2008;27:5454–5463. doi: 10.1038/onc.2008.242. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H, Dupont J, Yakar S, Karas M, LeRoith D. PTEN inhibits cell proliferation and induces apoptosis by downregulating cell surface IGF-IR expression in prostate cancer cells. Oncogene. 2004;23:786–794. doi: 10.1038/sj.onc.1207162. [DOI] [PubMed] [Google Scholar]
- 29.Shang Q, Xu H, Huang L. Tanshinone IIA: A Promising Natural Cardioprotective Agent. Evid Based Complement Alternat Med. 2012;2012:716459. doi: 10.1155/2012/716459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin HQ, Kim YS, Choi YJ, Kim YC, Sohn DH, Ryu SY, Lee BH. Effects of tanshinone IIA on the hepatotoxicity and gene expression involved in alcoholic liver disease. Arch Pharm Res. 2008;31:659–665. doi: 10.1007/s12272-001-1209-2. [DOI] [PubMed] [Google Scholar]
- 31.You Z, Xin Y, Liu Y, Han B, Zhang L, Chen Y, Gu L, Gao H, Xuan Y. Protective effect of Salvia miltiorrhizae injection on N(G)-nitro-D-arginine induced nitric oxide deficient and oxidative damage in rat kidney. Exp Toxicol Pathol. 2012;64:453–458. doi: 10.1016/j.etp.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Jin UH, Suh SJ, Chang HW, Son JK, Lee SH, Son KH, Chang YC, Kim CH. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J Cell Biochem. 2008;104:15–26. doi: 10.1002/jcb.21599. [DOI] [PubMed] [Google Scholar]
- 33.Li YS, Liang QS, Wang J. [Effect of tanshinone II A on angiotensin II induced nitric oxide production and endothelial nitric oxide synthase gene expression in cultured porcine aortic endothelial cells] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27:637–639. [PubMed] [Google Scholar]
- 34.Huang KJ, Wang H, Xie WZ, Zhang HS. Investigation of the effect of tanshinone IIA on nitric oxide production in human vascular endothelial cells by fluorescence imaging. Spectrochim Acta A Mol Biomol Spectrosc. 2007;68:1180–1186. doi: 10.1016/j.saa.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Yang JS, Li BJ, Lu HW, Chen Y, Lu C, Zhu RX, Liu SH, Yi QT, Li J, Song CH. Serum miR-152, miR-148a, miR-148b, and miR-21 as novel biomarkers in non-small cell lung cancer screening. Tumour Biol. 2015;36:3035–3042. doi: 10.1007/s13277-014-2938-1. [DOI] [PubMed] [Google Scholar]



