Abstract
Avascular necrosis of femoral head (AVFH) is a clinically recalcitrant disease of hip that leads to joint destruction. Osteoprotegerin (OPG), receptor activator of nuclear factor kappa-B (RANK) and RANK ligand (RANKL) regulates the balance, maturation and function of osteoclast and bone remodeling. This study aims to investigate molecular pathways which leads to AVN by studying expression profile of OPG, RANK and RANKL genes. Quantitative Real Time-PCR is used to evaluate mRNA expression of OPG, RANK and RANKL. mRNA and protein level in normal and necrotic tissue from 42 samples of ANFH specimens were analyzed. OPG and RANKL protein levels are estimated by western blotting. The results indicated that OPG mRNA levels are higher but not significantly different in necrotic tissue than that in normal tissue (P>0.05). Although expression of RANK and RANKL is significantly lower than that of OPG, RANK and RANKL mRNA levels are higher in necrotic tissue than normal tissue (P<0.05). Protein levels of OPG and RANKL show no significant difference. In conclusion, OPG, RANK and RANKL play important role in progress of bone remodeling in necrotic area and in disturbance of bone homeostasis, which might have an effect on bone destruction and subsequent collapse of hip joint.
Keywords: Avascular necrosis of the femoral head, osteopretegerin, RANK, RANKL, gene expression
Introduction
Avascular necrosis of the femoral head (ANFH) is one of the relatively difficult diseases in department of orthopedics. Patients usually suffer exquisite pain and the collapse of the femoral head and the destruction of the hip joint occur because of the limitation of the effect of intervention therapy [1]. With the destruction of the bone and the organic component of bone marrow, then ischemic damage occurs in subchondral bone. The illness will be aggravated by aking steroids, excessive drinking, systemic lupus erythematosus, crescent cell anemia, suppression, tumor diseases or wounds and so on [2,3]. In the early stage of necrotic tissue and adjacent tissues, with the transient activity of osteoblast, existing fibrovascular tissue hyperplasia and osteoclastic activity. But this plerosis function is quite limited, bone formation effect will be suppressed and osteoclast will continue to clean necrotic tissue. Finally, all of these will lead to the collapse of subchondral bone and damage of articular surface [4-6]. The balance of osteoblast and physiological effect of osteoclast are controlled by different molecular pathways which are all influencing the moulding of bone. If this balance is disturbed, then stability of local bone metabolism will be destroyed. Osteoprotegerin (OPG), receptor activator of nuclear factor kappa-B (RANK) and RANK ligand (RANKL) are all related to osteogenesis and bone resorption. OPG is molecule in RANK-RANKL-OPG axis which was the first to be found. By competition and combination of OPG and RANK, RANKL will inhibit the formation of osteoclast. If RANKL combines with homologous receptor RANK that will activate osteoclast by signal cascade reaction to promote bone absorption [7,8]. Considering the effect on bone remodeling and resorption of these three parts, many researches apply them to the therapy of osteoporosis, arthritis, vascular disease, tumor and cataclasis [9-11]. In this study, the expression level of these three genes in 42 cases of avascular necrosis of the femoral head of clinical specimens will be preliminary assessed. The relevant effect of these three genes in bone remodeling will be explored by comparative analysis of normal tissue and necrotic tissue.
Materials and methods
Patients
Samples are all chosen from 44 cases of patients who are with ischemic necrosis of femoral head and all the patients have received total hip arthroplasty (THA). While 2 cases are excluded because the amount of protein samples are too low, so finally 42 cases are included. According to the standard of bone circulation research organization classification (ARCO), the level of osteonecrosis of femoral head for all patients are regarded as level III-IV. This study is admitted by ethics committee of our hospital and all the relevant operations are informed consent by patients and their family members.
Samples
Cut the specimens of femoral head which are taken from the operation into two parts along the longitudinal axis. Extract same volume of bone tissue from necrotic tissue and normal tissue to do RNA and protein analysis. RNA and protein analysis of the same volume of bone tissue which are extracted from necrotic tissue and normal tissue. Take photos for cross section of bone during the operation, then the necrotic tissue and normal tissue boundaries (Figure 1) will be judged by 3 physicians who are participating in this study. Get necrotic tissue from necrotic area in subchondral bone (subchondral: 1-3 mm), normal tissue are taken from the femoral head and neck, more than 1 cm away from the boundary. The tissue will be cleaned by normal saline twice and clean the marrow and residual blood components every 5 minutes. Then extract and analysis of RNA and protein based on the methods which are used in Baelde and other researches [1]. Put the fresh tissue specimens that are taken from femoral head into reagent Trizol and cut it along the longitudinal axis after one hour. To extract total RNA (nvitrogen, Life Technologies, UK) with Trizol and after that, purify RNA according to the instruction of Extraction Kits (RNeasy Mini-kit, Qiagen, Germany). Then use 1% ethidium bromide stained agarose gel electrophoresis to estimate the quantity of separated RNA. By ultraviolet spectroscopy, centrifuge and purified RNA and test the spectral absorption rate of dilution of RNA in 260 nm and 280 nm, when the ratio between 1.8-1.8, it indicate higher purity of RNA. At the beginning of the study, we followed the methods Tuli used in the research, extracted and cultured specimens of 7 cases of femoral head necrosis in the tissue and normal tissue cells. Through the 85 l-2 kit (Sigma Aldrich, UK) staining it was found in the necrotic tissue also have part of osteoblast cells survived.
Figure 1.
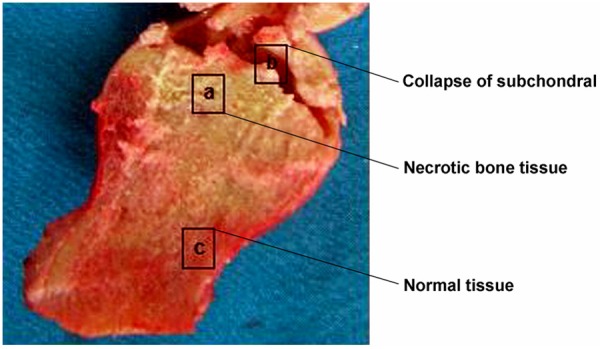
The images for the cross section of bone during the operation, the necrotic tissue and the normal tissue boundaries. (a) Necrotic bone tissue; (b) Collapse of subchondral; (c) Normal tissue.
Real-time PCR detects for OPG, RANKL and RANK
In order to quantify the 3 kinds of gene transcription, We use the real-time fluorescent PCR analysis (LC; Roche Diagnostics). Added 18 ul of water into reverse transcription reaction product, each reaction reagent samples contain the same amount 2 ul of water. In each of the PCR reaction, we use the same cDNA to evaluate the expression of human porphobilinogen deaminase (H-PBGD). Amplification mixture contains 2 μl 10 × reactants, using 3 mm/1 mm/1 mm magnesium chloride on OPG and RANKL and RANK respectively. The concentration of oligonucleotide primers is 0.5 μM, oligonucleotide probe concentration is 0.15 μM, 2 μl cDNA template, the reagent amount is 20 μl. Sample amplification of specific parameters is that initial degeneration stage under 95°C for 10 min, to activate the FastStart Taq DNA polymerase, 95°C 5 s degeneration 45 cycle. OPG, RANKL and RANK respectively at 55°C/54°C/54°C annealing 10 s, and at 72°C Extending in 6 s. Temperature change rate is 20°C/s.
With the reference to the same sample of internal stability in the expression of genes (such as housekeeping gene, h-PBGD) expression level of a quantitative analysis for each target gene transcription. On the basis of each gene and h-standard curve of PBGD gene cDNA of molecular targets of copy number cycle threshold (Ct), the latter for the fluorescent signal exceeds the threshold cycle number as needed. The absolute value of each target cDNA molecules of BMP and the ratio h-PBGD target cDNA molecules as a result.
Using TIB Molbiol (Berlin, Germany) synthetic oligonucleotide primers and DNA hybridization probes, hybridization probes of the adjacent side of fluorescent tags. The first 5’ end of probe is marked by the acceptor fluorophore LC Red 640 and fluorescein isothiocyanate marks the second 3’ end of probe. The probe which is marked 5’ prevent the polymerase extension during the process of PCR3’-phosphorylation. The nucleotide sequence of primer and hybridization probe are as follows: OPG [primer (coding strand: 5’-gaagctggaaccccagag-3’, antisense strand: 5’-gtgttgcatttcctttctgagtta-3’), probe: (5’-ccagatctaaccatgagccatccacc-FL/5’-LC640-tcg ctttctctgctctgatgtgctgtg-PH)], RANKL [primer (coding strand: 5’-gcaaaaggaattacaaca tatcgtt-3’, antisense strand: 5’-actttatgggaaccagatggg-3’), probe: (5’-ccagatctaaccatga gccatccacc-FL/5’-LC640-tcgctttctctgctctgatgtgctgtg-PH)], RANK [primer (coding strand: 5’-agggaaagcactcacagctaat-3’, antisense strand: 5’-acatgctccctgctgacc-3’), probe: (5’-agtggagataaggagtcctcaggtgacaFL/5’-LC640-ttgtgtcagtacacacacggcaaactt-PH)]. The genic PCR product of OPG, RANKL, RANK are 147 bp, 164 bp and 132 bp respectively.
Western blotting
Forty-two cases of protein in necrotic tissue and normal tissue in the sample for western blotting analysis, organization by saline and fresh PBS mechanical cleaning twice at low temperature, using. net-Triton cracking liquid (Tris-Cl 0.01 M, 0.1 M NaCl, 1 mm pH 7.4 slightly EDTA, Triton X-100, % 1% glycerol, 0.1% SDS, mixture of 0.5% sodium deoxycholic acid and protease inhibitors) for cracking. Electrophoresis cracking for the aliquot of lysate, and in NuPAGE TrisAcetate gel (Invitrogen, Carlsbad, CA) on separation, later will be transferred to the PVDF membrane protein (BioRad), 5% of skim milk powder are closed. Using specific antibodies against RANKL and OPG [rat antibody RANKL monoclonal antibody (1:100, Santa Cruz), people antibody OPG monoclonal antibody (1:250 dilution, Abcam, UK)] on membrane. Human F-actin monoclonal antibodies (Serotec, UK) as a marker protein quantitative protein band. Two fight for anti mouse IgG and fight people IgG horseradish peroxidase (HRP) (dilution 1:5000, Abcam, UK), after soaking the membrane in the ECL detection (Santa Cruz, USA), in the solution and exposed to XAR-5 film (Kodak, SIGMA, UK) radiation self developing, protein with the epson GT-8000 laser scanner. Calculate intensity ratio of the RANKL and OPG in relative to the strength of β-actin stripe.
Data analysis
By using SPSS 16.0 software (Systat Software Inc., San Jose, USA) to do data statistics and analysis and the data are all mean value, median, minimum and maximum. By using Wilcoxon rank tests to compare matched groups. If P<0.05, then the differences have statistical significance.
Results
Characteristics of patients
In the 42 cases of patients, 23 cases are female patients (13 cases are in non-menopausal period, 10 cases are postmenopausal), 19 cases are male patients, the average age is 43±2.3 years old (20 years old to 70 years old). 4 cases are in ARCO classification phase III, 38 cases (90%) are in phase IV. 12 patients are smokers. About the reason for osteonecrosis of the femoral head: 15 patients have taken steroids before; one patient had kidney transplant; 3 patients are with drepanocytic anaemia; 3 patients are diagnosed as systemic lupus erythematosus; 4 patients are addicted for alcohol; and other patients are found no correlated factors, so they are diagnosed as idiopathic avascular necrosis of the femoral head.
Alkaline phosphatase was enhanced in necrotic tissues
The alkaline phosphatase was detected in normal tissue and necrotic tissue in Femoral specimens. The cells were cultured for 3 weeks, and the alkaline phosphatase staining images were with 40 times magnification. The result indicated that the alkaline phosphatase staining in necrotic tissue (Figure 2A, 2B) were significantly increased compared to the normal tissue (P<0.05, Figure 2C, 2D).
Figure 2.
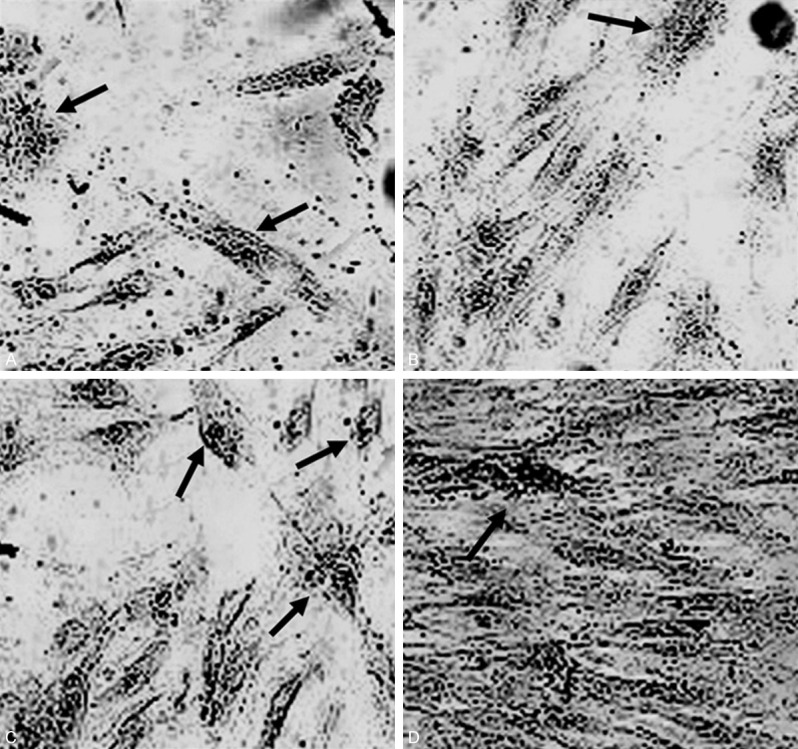
Cell culture in normal tissue (A, B) and necrotic tissue (C, D) in Femoral specimens. The cell culture were with 3 weeks, and the images were with 40 times magnification. Arrows indicate the alkaline phosphatase staining of osteoblasts.
Quantify the level detection for OPG, RANKL, RANK and mRNA
The test result of Q-RT-PCR shows that the ratio of OPG/h-PBGD in necrotic tissue (average value: 4.45, range: 0-41.9) is higher than which in normal tissue (average value: 3.53, range: 0.033-31.7). There is no statistical significance in the differences (P>0.05). The expression levels of RANKL and RANK are almost same which are all obviously lower than the expression of OPG gene. The ratio of RANKL/h-PBGD in necrotic tissue and normal tissue are 0.77 (range: 0.015-26.2), 0.27 (range: 0-13.98) respectively, (P<0.05). The ratio of RANK mRNA/h-PBGD mRNA in necrotic tissue and normal tissue are 0.97 (range: 0-20.7), 0.83 (range: 0.01-4.7) respectively (P<0.05) (Figure 3). The ratio of RANKL/OPG and RANK/OPG in normal tissue (average value: 0.659, range: 0-9.32; average value: 0.478, range: 0.015-3.33 respectively). The ratio of RANKL/OPG and RANK/OPG in necrotic tissue (average value: 1.210, range: 0.004-14.83; average value: 0.619, range: 0.025-5.248 respectively). So there is no statistical significance in the differences (P>0.05).
Figure 3.
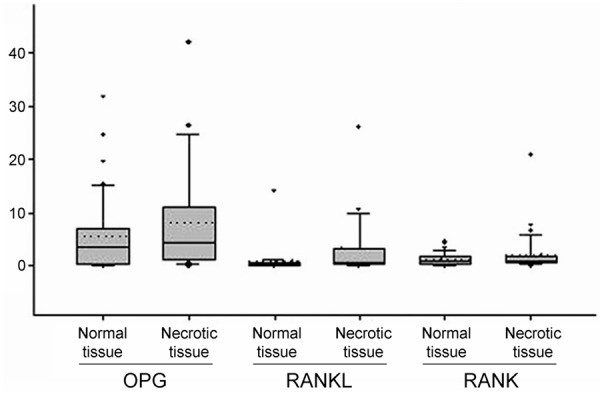
Quantitative RT-PCR detection of OPG, RANKL, RANK/h-PBGD mRNA levels. The box plot shows that the figure in the bottom is close to 0 which tips for twenty-fifth percentile. The line inside of the box plot is average value and its upper edge is seventy-fifth percentile. The error bars between top and bottom of the box plot are ninetieth percentile and tenth percentile relatively. The dotted lines show median value and the outliers are extreme values. The level differences between RANK and RANKL in normal tissue and necrotic tissue have statistical significance (P<0.05).
Analysis of western blot
The expression level of OPG and RANKL protein in 42 samples of necrotic tissue and normal tissue can be found in Figure 4. The expression of OPG protein that can be used to compare with other data can be found in normal tissue. The intensity ratio of OPG/F and the actin band is 0.57 (range: 0.37-4.91). The expression level of OPG protein in necrotic tissue is almost the same with which in normal tissue (average value: 0.62, range: 0.45-4.32) (P>0.05). RANKL protein can be detected in all the samples. The band intensity ratio of RANKL/F-actin in normal tissue and necrotic tissue are 0.48 (range: 0.45-0.49) and 0.5 (range: 0.45-0.58) respectively (Figure 5). RANK protein cannot be detected in all the samples.
Figure 4.
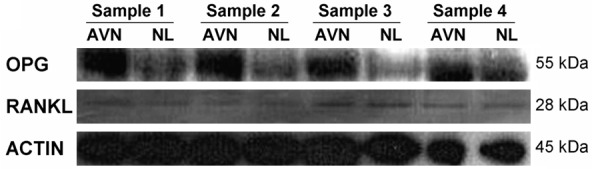
OPG (GGkDa) and RANKL (28 kDa) Protein western blot analysis of 4 samples of osteonecrosis of the femoral head; actin (45 kDa) is the control group; AVN and NL are protein lysate which are taken from necrotic tissue and normal tissue respectively.
Figure 5.
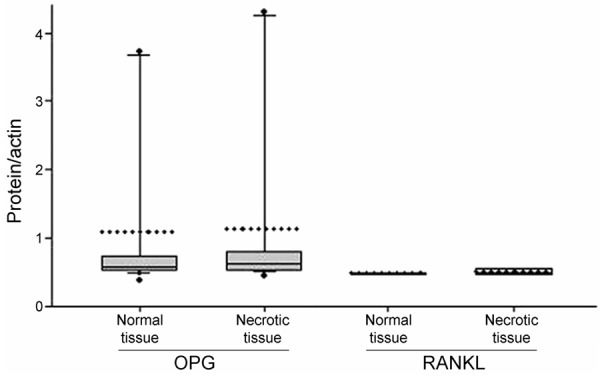
The expression level comparison of OPG and RANKL/F-actin in normal tissue and necrotic tissue, the box plot shows no obvious difference between them.
Expression of OPG, RANKL, RANK mRNA and analysis of related risk factors
Patients are divided into 3 groups based on age. Group 1: the patients are between 20 years old and 35 years old, group 2: the patients are the age between 36 years old and 50 years old, group 3: the patients are older than 50 years old. Compare each group with the expression of the corresponding sampling position, at the same time, we will compare the relative expression level of necrotic tissue and normal tissue for premenopausal and postmenopausal female patients because female patients are in different periods of menstruation. There is no significant correlation between patients’ age and gender (P>0.05). The expression level of RANK for necrotic tissue in female patients is obviously higher than premenopausal female patients (average value is 3.36 vs. 0.95, P<0.05). The expression level of RANK for normal tissues in non-smoking patients is higher than smoking patients (2.33 vs. 1.23 P<0.05).
Discussion
Many factors participate in the ischemic necrosis of the femoral head, and the patients are relatively young. It will seriously affect the quality of patients’ life since the destruction of subchondral bone, dysfunction of the hip caused by collapse which aroused by combining femoral head. The balance between osteoblasts and osteoclasts is critical on normal bone shape but balance is often destroyed when osteonecrosis happens [12]. Ischemia can cause the thenecrosis of bone and bone marrow, initially triggers the body’s self-healing function, but this is only for the small lesions (less than 15% of the femoral head). In most situations, the repair function almost is invalid [13,14]. Corresponding surgical treatment including core decompression, osteotomy, vascularized, or the non-vascularized bone graft and so on, the latter often needs to use the growth and differentiation factor to enhance its role, for the more serious hip necrosis, the best way is replacement surgery. Because the age of patient is relatively young,the late revision surgery and related complications will bring serious negative effect to patients [15].
Growth, development and maintenance of bone are the highly regulated process. Cell level reflects interaction between osteoblasts which prompt the growth of bone and osteoclasts which prompt the absorption of bone, in which, OPG and RANKL and RANK are the key factors to keep balance of two kinds of cells [16]. A lot of researches have proved the interaction of imbalance can lead to a variety of bone diseases [17-19]. In this study, we focus on the expression of OPG and RANKL and RANK genes that comes from specimens of patients with ischemic necrosis of femoral head, respectively select the necrotic tissue and normal tissue balance for further analysis three kinds of gene mRNA and protein expression level. OPG mRNA level in the necrotic tissue is generally higher than that of normal tissue, but the difference is not significant, in the two kinds of tissue, the protein expression mainly is consistent. The expression level of RANKL and RANK mRNA in the necrotic tissue was significantly higher than that in normal tissue, especially the expression level of the RANKL mRNA. Results of OPG are similar. There is no obvious difference that of RANKL protein expression level in two groups, RANK protein may be due to too little, which cannot be detected. In a word, RANKL mRNA and protein expression levels were significantly lower than that of OPG expression. mRNA expression levels in normal tissue is consistent with the protein expression level, but in the necrotic tissue mRNA expression of OPG and RANKL is increased, in which, the corresponding protein expression, the former is significantly increased, however, the latter is reduced. Therefore, the control of transcription is likely to be mainly happens on the area of the femoral head necrosis.
In this study, it is the first time for us to analysis the mRNA and protein level of OPG and RANKL and RANK gene which consist in clinical specimens organization of ischemic necrosis of femoral head, study results suggest the high level of OPG mRNA expression in necrotic tissue (although no statistical difference compared with normal tissue) may be associated with lower osteoclast activity, affect the balanced relationship between osteoclasts and osteoblasts, and then affect the remodeling of the bone. Studies have confirmed that the excess of OPG expression in transgenic mice or recombinant OPG treatment will appear in the increase of bone mineral density and bone sclerosis, in contrast to the former, if knocking out of OPG gene in mice, then can make its early bone loss appear.
A latest clinical study on the osteoporosis patients shows that OPG, RANKL, RANK, bone mineral density and fracture risk have obvious relevance, and OPG plays an important role in postnatal bone mass. Some researches focus on the relevance among RANK, OPG gene mutations and osteolysis. Paget’s disease of fractures and progressive bone malformations, further confirm that the three factors maintaining the body’s bone mass by interacting with each other [20-23]. Femoral head necrosis in our research group in the OPG mRNA expression increases significantly, which is consistent with the hardening signal in the necrosis area that lies in imaging examination, and foregoing result that in mice bone mineral density increase [24], but it is still not clear that elevated OPG is precisely the result of ischemic necrosis of femoral head or the initial factors.
By comparing the ratio of RANKL/OPG and RANK/OPG in the necrotic tissue and normal tissue, even though the necrotic tissue RANKL/OPG ratio nearly 2 times that of the normal tissue, there is no statistically significant difference (P>0.05). The two groups within necrotic tissue are higher than that in normal, side reflects the necrotic tissue in need of osteoblast in bone shaping. The genes that effect osteoblast maturation and function has been confirmed that is associated with bone mass and bone lesions, such as BMP, its transcription factor RUNX2 and signal molecules auxiliary to participate in the activities of osteogenesis [25,26]. Recent studies suggest that BMPs by RANKL and OPG pathways induces osteoclast formation reduced bone mass, this is a key regulatory factor of osteoclast [27,28].
In conclusion, the gene expression of OPG, RANK and RANKL play an important role in the development of avascular necrosis of the femoral head which influence the stability of bone regulation. They may also have certain effect on osteoclasia and the collapse of hip joint.
Disclosure of conflict of interest
None.
References
- 1.Bian Y, Qian W, Li H, Zhao RC, Shan WX, Weng X. Pathogenesis of glucocorticoid-induced avascular necrosis: a microarray analysis of gene expression in vitro. Int J Mol Med. 2015;36:678–684. doi: 10.3892/ijmm.2015.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li JG, Lin JJ, Wang ZL, Cai WK, Wang PN, Jia Q, Zhang AS, Wu GY, Zhu GX, Ni LX. Melatonin attenuates inflammation of acute pulpitis subjected to dental pulp inury. Am J Transl Res. 2015;7:66–78. [PMC free article] [PubMed] [Google Scholar]
- 3.Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86:631–637. doi: 10.1210/jcem.86.2.7192. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 5.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 6.Celik A, Tekis D, Saglam F, Tunali S, Kabakci N, Ozaksoy D, Manisal M, Ozcan MA, Meral M, Gulay H, Camsari T. Association of corticosteroids and factor V, prothrombin, and MTHFR gene mutations with avascular osteonecrosis in renal allograft recipients. Transplant Proc. 2006;38:512–516. doi: 10.1016/j.transproceed.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 7.Chong B, Hegde M, Fawkner M, Simonet S, Cassinelli H, Coker M, Kanis J, Seidel J, Tau C, Tuysuz B, Yuksel B, Love D. Idiopathic hyperphosphatasia and TNFRSF11B mutations: relationships between phenotype and genotype. J Bone Miner Res. 2003;18:2095–2104. doi: 10.1359/jbmr.2003.18.12.2095. [DOI] [PubMed] [Google Scholar]
- 8.Cundy T, Hegde M, Naot D, Chong B, King A, Wallace R, Mulley J, Love DR, Seidel J, Fakner M, Banovic T, Callon KE, Grey AB, Reid IR. A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum Mol Genet. 2002;11:2119–2127. doi: 10.1093/hmg/11.18.2119. [DOI] [PubMed] [Google Scholar]
- 9.Estrada K, Styrkarsdottir U, Evangelou E. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glueck CJ, Freiberg RA, Fontaine RN, Tracy T, Wang P. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res. 2001;386:19–33. doi: 10.1097/00003086-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hadjigeorgiou G, Dardiotis E, Dardioti M, Karantanaas A, Dimitroulias A, Malizos K. Genetic association studies in osteonecrosis of the femoral head: mini review of the literature. Skeletal Radiol. 2008;37:1–7. doi: 10.1007/s00256-007-0395-2. [DOI] [PubMed] [Google Scholar]
- 12.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97:105–114. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 13.Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, van Hul W, Whyte MP, Nakatsuka K, Hovy L, Anderson DM. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet. 2000;24:45–48. doi: 10.1038/71667. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen HL, Kusk P, Madsen B, Fenger M, Lauritzen JB. Serum osteoprotegerin (OPG) and the A163G polymorphism in the OPG promoter region are related to peripheral measures of bone mass and fracture odds ratios. J Bone Miner Metab. 2004;22:132–138. doi: 10.1007/s00774-003-0461-3. [DOI] [PubMed] [Google Scholar]
- 15.Samara S, Dailiana Z, Varitimidis S, Chassanidis C, Koromila T, Malizos KN, Kollia P. Bone morphogenetic proteins (BMPs) expression in the femoral heads of patients with avascular necrosis. Mol Biol Rep. 2013;40:4465–4472. doi: 10.1007/s11033-013-2538-y. [DOI] [PubMed] [Google Scholar]
- 16.Soucacos PN, Beris AE, Malizos K, Koropilias A, Zalavras H, Dailiana Z. Treatment of avascular necrosis of the femoral head with vascularized fibular transplant. Clin Orthop Relat Res. 2001;386:120–130. doi: 10.1097/00003086-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Tuli R, Seghatoleslami MR, Tuli S, Wang ML, Hozack WJ, Manner PA, Danielson KG, Tuan RS. A simple, high-yield method for obtaining multipotential mesenchymal progenitor cells from trabecular bone. Mol Biotechnol. 2003;23:37–49. doi: 10.1385/MB:23:1:37. [DOI] [PubMed] [Google Scholar]
- 18.Whyte MP, Mills BG, Reinus WR, Podgornik MN, Roodman GD, Gannon FH, Eddy MC, McAlister WH. Expansile skeletal hyperphosphatasia: a new familial metabolic bone disease. J Bone Miner Res. 2000;15:2330–2344. doi: 10.1359/jbmr.2000.15.12.2330. [DOI] [PubMed] [Google Scholar]
- 19.Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y Acad Sci. 2008;1143:123–150. doi: 10.1196/annals.1443.016. [DOI] [PubMed] [Google Scholar]
- 20.Kamiya N. The role of BMPs in bone anabolism and their potential targets SOST and DKK1. Curr Mol Pharmacol. 2012;5:153–163. doi: 10.2174/1874467211205020153. [DOI] [PubMed] [Google Scholar]
- 21.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–192. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5:618–625. doi: 10.1016/j.coph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Zalavras CG, Malizos KN, Dokou E, Vartholomatos G. The 677C-->T mutation of the methylene-tetrahydrofolate reductase gene in the pathogenesis of osteonecrosis of the femoral head. Haematologica. 2002;87:111–112. [PubMed] [Google Scholar]
- 24.Zupan J, Komadina R, Marc J. The relationship between osteoclastogenic and anti-osteoclastogenic pro-inflammatory cytokines differs in human osteoporotic and osteoarthritic bone tissues. J Biomed Sci. 2012;19:28. doi: 10.1186/1423-0127-19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuli R, Seghatoleslami MR, Tuli S, Wang ML, Hozack WJ, Manner PA, Danielson KG, Tuan RS. A simple, high-yield method for obtaining multipotential mesenchymal progenitor cells from trabecular bone. Mol Biotechnol. 2003;23:37–49. doi: 10.1385/MB:23:1:37. [DOI] [PubMed] [Google Scholar]
- 26.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Trouvin AP, Goeb V. Receptor activator of nuclear factor-kappaB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin Interv Aging. 2010;5:345–354. doi: 10.2147/CIA.S10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Yi W, Jin A, Duan Y, Min S. Effects of sequentially released BMP-2 and BMP-7 from PELA microcapsule-based scaffolds on the bone regeneration. Am J Transl Res. 2015;7:1417–1428. [PMC free article] [PubMed] [Google Scholar]


