Abstract
To investigate the expression of hypoxia inducible factor (HIF-1α) and vascular endothelial growth factor (VEGF) in the synovium of collagen-induced arthritis (CIA) joint, and whether the PI3K pathway regulates angiogenesis in rheumatoid arthritis or not. A randomized controlled according to the principle of the rats were divided into normal control group (10 rats) and the experimental group (40 rats). The experimental group rats were established as type II collagen plus adjuvant Freund’s complete adjuvant-induced arthritis model. HIF-1α and VEGF proteins’ expression in serum of CIA rats group and normal control group were detected by ELISA. Microvessel density (MVD) in synovial tissue of CIA rats group and normal control group were detected by immunohistochemistry (IHC) staining. The protein expression of PTEN, PI3K, and AKT in synovial tissue were detected by Western Blot. Compared with normal control group, toes and ankle swelling and arthritis index (AI) of CIA rat increased, and the expression of VEGF and HIF-1α proteins in peripheral serum increased, IHC showed that MVD was significantly higher than that of the control group, and the difference was statistically significant (p<0.05). Western Blot results showed that PI3K and AKT proteins expression in CIA synovial tissue of rats increased, while the expression of PTEN protein decreased. Correlation analysis showed that VEGF and HIF-1 levels in the peripheral serum of CIA rats were positively correlated with arthritis index (AI); the contents of HIF-1α and VEGF in the peripheral serum of CIA rats were positively correlated with MVD in synovium tissue. The CIA rat model regulated the expression of HIF-1α and VEGF proteins in peripheral serum by PI3K signaling pathway, and then regulated neovascularization in RA.
Keywords: RA, HIF-1α, VEGF, PI3K, CIA
Background
RA is a common chronic autoimmune disease, the incidence of the world is about 0.6%, RA is also one of the main reasons of loss productivity in population, but its pathogenesis has not been fully elucidated, so at present, the treatment of RA is mainly to alleviate the symptoms, but not to be cured completely [1-3]. Study demonstrated that the microenvironment of the joint cavity in patients with RA is the hypoxia environment, HIF-1α is a nuclear transcription factor that regulates the response of cells to hypoxia, and it also promotes angiogenesis [4,5]; while VEGF can directly promote the formation of pannus, and then chronic inflammation of the synovial hyperplasia is aggravated [6,7]. PI3K is an important signal regulated protein in cells, which can regulate cell migration and proliferation, and participate in angiogenesis [8-10]. But whether the RA synovial cells regulate the expression of VEGF and HIF-1 through the PI3K signaling pathway, and then regulation of RA angiogenesis is still unknown. This study will use type II collagen formation model induced SPF SD rats and establish CIA. The expression of HIF-1 and VEGF protein in peripheral blood of CIA rat group and the control group were detected by ELISA, the expression of PTEN, PI3K, and AKT protein in synovial tissue of the knee joint of CIA rat group and the control group were detected by Western blot, to investigate whether the RA synovial cells regulate the expression of HIF-1 and VEGF through the PI3K signaling pathway, and then regulate the angiogenesis of RA.
Materials and methods
Materials
SPF grade SD rats weighted at 80 g~200 g were purchased from Shanghai silaike experimental animal limited liability company. Female and male accounted for 50%, respectivelyBovine collagen type II collagen (C-II) was purchased from the United States Chondrex company. Complete Freund’s adjuvant (CFA) and incomplete Freund’s adjuvant (IFA) were purchased from the Sigma company in the United States. HIF-1 alpha and ELISA VEGF kits were purchased from Shanghai enzyme linked Biotechnology Co., Ltd. CD31 polyclonal antibody was purchased from the United States Cruz Biotechnology Santa company; monoclonal antibody of anti-PTEN, anti-PI3 Kinase, anti-Akt and anti-actin were purchased from Cell Signaling Technology company in the U.S. Mouse anti rabbit HRP antibody and goat anti mouse HRP antibody were purchased from Abcam company in the U.S.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of The General Hospital of Jinan Military Command.
Methods
CIA rat model construction
The 50 SPF grade SD rats were randomly divided into experimental group including 40 rats and control group including 10 rats, which had been adopted in the SPF animal house for 5 d. After 1% pentobarbital anesthetization, the rats were induced by 300 μl Type II collagen plus CFA, and the secondary immunity was carried out with 300 μl IFA in the same location after 7 days. The control group was injected with 300 μl saline solution, and repeated the operation after 7 days. Weighing every two days after the first immunization since second days, the degree of joint swelling was observed. According to the red and swelling degree of the ankle joints, articulationes metatarsophalangeae, toe joints and the affected joint numbers the evaluation was carried out. 0 score represents the normal; 1 score represents 1 joint swelling; 2 scores represent 2 and more than 2 joints swelling; 3 scores represent severe swelling of the ankle under foot; 4 scores represent that all the paw swelling are unable to bear weight besides ankle joints. The four limbs are scored, respectively. The cumulative scores are arthritis index (AI). AI >5 scores is regarded as a successful model [11]. On the forty-third day, all rats were killed and the blood and joint synovial tissue of rats were collected.
HIF-1α detection in CIA rat
The peripheral blood was collected from 28 CIA rats and 10 normal control rats, and the serum was obtained after 3000RPM centrifugation for 20 minutes. According to the instruction of ELISA kit the HIF-1α was detected. 50 μL of serum was added to the plate and incubated 1 h at 37°C, after washing, enzyme labeled antibody was added and incubated 1 h at 37°C, after washing, background color liquid was added and incubated 0.5 h at 37°C, then the reaction was ended.
VEGF detection in CIA rat
The peripheral blood was collected from 28 CIA rats and 10 normal control rats, and the serum was obtained after 3000RPM centrifugation for 20 minutes. According to the instruction of ELISA kit the VEGF was detected. 50 μL of serum was added to the plate and incubated 1 h at 37°C, after washing, enzyme labeled antibody was added and incubated 1 h at 37°C, after washing, background color liquid was added and incubated 0.5 h at 37°C, then the reaction was ended.
Detection of MVD in synovial membrane
The MVD of the synovial membrane was detected by IHC staining. 28 CIA rats and 10 normal control group rat synovial tissue of double knee joint were fixed and decalcified. Paraffin sections were prepared and stained with IHC staining. In our study, CD31 polyclonal antibody labeled vascular endothelial cells with 1:500 dilution, secondary antibody of anti-mouse IgG was diluted with 1:2000 dilution, generally DAB stain, hematoxylin staining, dehydration, transparent, and mounting, neovascularization was observed under the microscope. The region of the most abundant blood vessels were selected under the 40 times optical field of view, then microvessel counts (MVC) of three high microvessel density area were counted under the 100 times optical field of view and take the average reading, MVD was expressed in the optical field of view with a number of vascular/100 times [12].
Protein expression of PTEN, PI3K and AKT in synovial tissue of CIA rat
After the CIA rats were killed, double knee synovium of rat were collected, total protein were extracted from synovial tissue and quantified. Protein expression was detected by western blot. 50 μg proteins were separated by 10% SDS-PAGE, concentration gel with 80 V and separation gel with 160 V were carried out for 30 min and 70 min, respectively, then the target proteins of PTEN, PI3K and AKT were transferred to PVDF membrane with 240 mA for 60 min, which were closed with 10% skim milk powder for 2 h at room temperature, anti PTEN monoclonal antibody (1:1000), anti PI3 Kinase monoclonal antibody (1:1000), anti Akt monoclonal antibody (1:1000) and anti actin monoclonal antibody (1:2000) were added and put on the table overnight at 4°C. On second day 3 times washing were carried out with 10 min/time. Secondary antibody of mouse anti rabbit HRP (1:2000) and goat anti mouse HRP (1:2000) were added and incubated for 60 min, 3 times washing with PBST, 10 min/time, finally scan was carried out.
Statistical analysis
SPSS17.0 software were used for statistical analysis, t test were used for paired data comparison. P<0.05 was considered as statistical significance.
Results
Construction of CIA rat model and AI
During the construction of CIA model of rats, normal control group of 10 rats were all survived, body weight gradually increased with the increasing of feeding time, the mental status were good and active, there were no obvious swelling and ulcer at the injection point. 40 rats in experimental group were no death, among them, 28 rats were induced to become CIA model, and the successful rate was 70%. Compared with control group, the body weight of the experimental group was decreased with the time of the onset of the disease. The state of mind was bad with hair off and the color imperfection, poor appetite and less activity. Injection site of 28 CIA rats showed obvious red and swollen, ulcer appeared and gradually aggravated, finally, the ulcer was broken to form a hard callus and healing. AI was gradually increased in 28 CIA rats, which reached the peak at more than the thirteenth days, then gradually decreased. The degree of joint swelling of CIA rats was also firstly increased gradually, which reached the peak at more than the fifth days, then gradually decreased. Compared with control group, seventh days after initial immunization, there were differences in the mean of AI in CIA rats, and the difference was statistically significant (p<0.05) (Figure 1).
Figure 1.
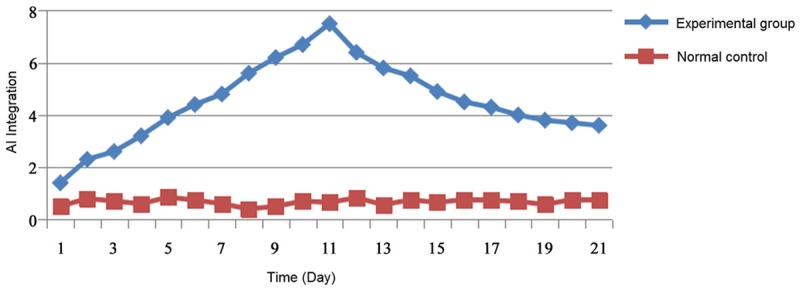
The average score of AI in rats.
The expression of HIF-1α in serum of CIA rats
The content of HIF-1α in peripheral serum of 28 CIA rats was (110.34 ± 24.82) ng/L, the content of HIF-1α in peripheral serum of 10 normal control SD rats was (37.55 ± 14.26) ng/L, the content of HIF-1α in peripheral serum of CIA rats was significantly higher than that in normal control group, the difference was statistically significant (p<0.05).
The expression of VEGF in serum of CIA rat
The content of VEGF in peripheral serum of 28 CIA rats was (82.34 ± 34.81) ng/L, the content of VEGF in peripheral serum of 10 normal control SD rats was (27.34 ± 12.52) ng/L, the content of VEGF in peripheral serum of CIA rats was significantly higher than that in normal control group, the difference was statistically significant (p<0.05).
MVD detection
As Figure 2 showed that the synovial tissue of CIA rats was rich in blood vessels, neovascularization quantities were intensive, MVD mean was (6.9 ± 1.1) vessels number/100 times optical sight, while the average value of MVD in the normal control group was (1.2 + 0.8) vessels number/100 times the optical field of view, the MVD of CIA rats was significantly higher than that of the control group, and the difference was statistically significant (p<0.05).
Figure 2.
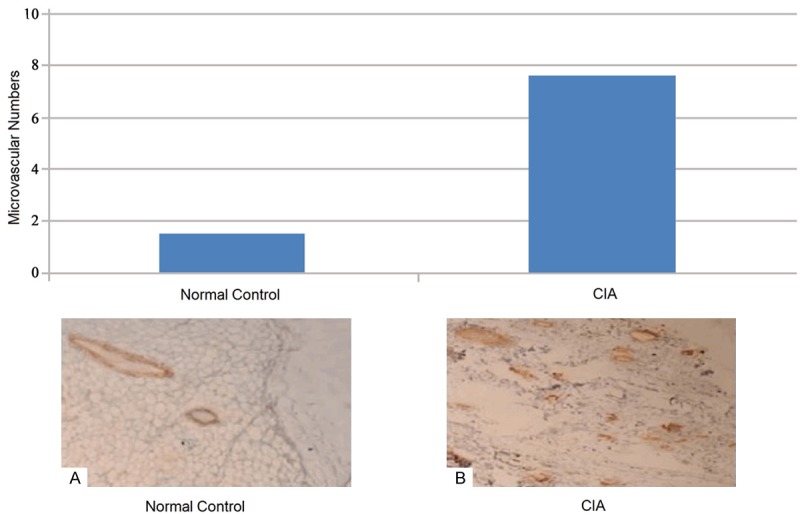
MVD comparison of synovial tissue in rats between normal control group and CIA group by IHC.
The protein expression of PTEN, PI3K and AK
As Figure 3 showed that the expression of total proteins PTEN in synovial tissue of CIA rats articular in experimental group decreased, while the PIK3 and AKT proteins increased in CIA rats experimental group.
Figure 3.
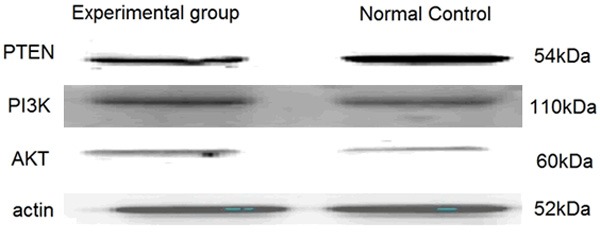
Protein expression of PTEN, PI3K and AKT of synovial tissue in rat by Western Blot.
Correlation analysis of HIF-1α and VEGF content with AI joint score in the peripheral serum of CIA rats
Correlation analysis of HIF-1α and VEGF content with AI joint score in the peripheral serum of CIA rats showed that the content of HIF-1α in the peripheral serum of CIA rats was positively correlated with the AI score, correlation coefficient was 0.895 (r2=0.895); correlation analysis of VEGF content and AI joint score in the peripheral serum of CIA rats showed that the content of VEGF in the peripheral serum of CIA rats was positively correlated with the AI score, correlation coefficient was 0.887 (r2=0.887) (Figure 4).
Figure 4.
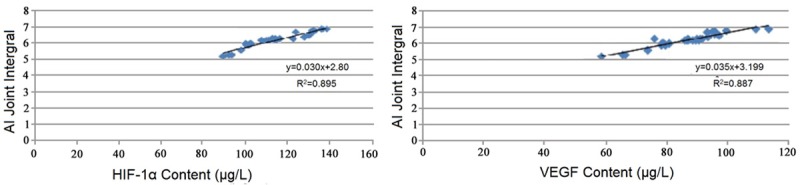
Correlation analysis of HIF-1α and VEGF content with AI joint score in the peripheral serum of CIA rats.
Correlation analysis of HIF-1α and VEGF levels in peripheral serum with MVD in CIA rats
Correlation analysis of HIF-1α level in peripheral serum with MVD in CIA rats showed that HIF-1α were positively correlated with the MVD, correlation coefficient was 0.815 (r2=0.815); correlation analysis of VEGF level in peripheral serum with MVD in CIA rats showed that VEGF were positively correlated with the MVD, correlation coefficient was 0.811 (r2=0.811) (Figure 5).
Figure 5.
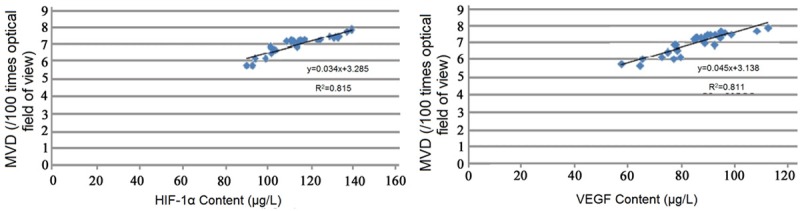
Correlation analysis of HIF-1α and VEGF levels in peripheral serum with MVD in CIA rats.
Discussion
The main pathological changes of RA is the chronic inflammation of the synovial membrane, synovial cell proliferation or apoptosis imbalance lead to synovial tumor like hyperplasia and accompanied by a large number of inflammatory cell infiltration, which induced a hypoxia status of RA microenvironment. Recently study found that the hypoxic microenvironment plays an important role in supporting the synovial angiogenesis [13,14]. The expression of HIF-1α in hypoxic microenvironment increased and promoted angiogenesis, made the body adapt to the hypoxic environment [15]. Our study showed that HIF-1α expression increased in the peripheral blood of CIA rats, and the increase of HIF-1α was significantly positively correlated with the AI and the MVD of CIA rats, which indicated that HIF-1α played an important role in the inflammatory and non-supportive hyperplasia and angiogenesis of RA synovial tissue in CIA rat. VEGF is one of the main cytokine to promote angiogenesis, when the tissue inflammation happened, the VEGF expression increased, while the hypoxia environment was one of the inducing factors of VEGF expression increase [16-18]. Our study showed that VEGF expression increased in the peripheral blood of CIA rats, and the increase of VEGF was significantly positively correlated with the AI and the MVD of CIA rats, which indicated that VEGF played an important role in the inflammatory hyperplasia and angiogenesis of RA synovial tissue in CIA rat.
PI3K and PTEN/AKT signal transduction pathways have a wide range of regulatory roles in cell migration, proliferation, apoptosis and angiogenesis [19]. The study found that the important proinflammatory factors IL-18 in pathogenesis can activate the PI3K pathway, and further promote the formation of new blood vessels [20,21].
Our results showed that the expression of HIF-1α and VEGF were regulated by CIA rat articular synovial cells through PI3K signaling pathway system, and then adjusted the RA angiogenesis. At the same time, our study also provided an experimental basis for further study of the mechanism of angiogenesis in RA.
Disclosure of conflict of interest
The authors declare no competing financial or commercial interests in this manuscript.
References
- 1.Goodman SM, Figgie MA. Arthroplasty in patients with established rheumatoid arthritis (RA): Mitigating risks and optimizing outcomes. Best Pract Res Clin Rheumatol. 2015;29:628–642. doi: 10.1016/j.berh.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Mullen MB, Saag KG. Evaluating and mitigating fracture risk in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2015;29:614–627. doi: 10.1016/j.berh.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Ince-Askan H, Dolhain RJ. Pregnancy and rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2015;29:580–596. doi: 10.1016/j.berh.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Lee YA, Choi HM, Lee SH, Hong SJ, Yang HI, Yoo MC, Kim KS. Hypoxia differentially affects IL-1beta-stimulated MMP-1 and MMP-13 expression of fibroblast-like synoviocytes in an HIF-1alpha-dependent manner. Rheumatology (Oxford) 2012;51:443–450. doi: 10.1093/rheumatology/ker327. [DOI] [PubMed] [Google Scholar]
- 5.Hu F, Shi L, Mu R, Zhu J, Li Y, Ma X, Li C, Jia R, Yang D, Li Y, Li Z. Hypoxia-inducible factor-1alpha and interleukin 33 form a regulatory circuit to perpetuate the inflammation in rheumatoid arthritis. PLoS One. 2013;8:e72650. doi: 10.1371/journal.pone.0072650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horai Y, Honda M, Nishino A, Nakashima Y, Suzuki T, Kawashiri SY, Ichinose K, Tamai M, Nakamura H, Motomura M, Origuchi T, Kawakami A. Anti-citrullinated protein antibody-positive rheumatoid arthritis associated with RS3PE syndrome-like symptoms and an elevated serum vascular endothelial growth factor level in a patient with myasthenia gravis. Intern Med. 2014;53:895–898. doi: 10.2169/internalmedicine.53.1897. [DOI] [PubMed] [Google Scholar]
- 7.Londono D, Cadavid D, Drouin EE, Strle K, McHugh G, Aversa JM, Steere AC. Antibodies to endothelial cell growth factor and obliterative microvascular lesions in the synovium of patients with antibiotic-refractory lyme arthritis. Arthritis Rheumatol. 2014;66:2124–2133. doi: 10.1002/art.38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartok B, Hammaker D, Firestein GS. Phosphoinositide 3-kinase delta regulates migration and invasion of synoviocytes in rheumatoid arthritis. J Immunol. 2014;192:2063–2070. doi: 10.4049/jimmunol.1300950. [DOI] [PubMed] [Google Scholar]
- 9.Tamura N. [Recent findings on phosphoinositide-3 kinase in rheumatic diseases] . Nihon Rinsho Meneki Gakkai Kaishi. 2012;35:8–13. doi: 10.2177/jsci.35.8. [DOI] [PubMed] [Google Scholar]
- 10.Haruta K, Mori S, Tamura N, Sasaki A, Nagamine M, Yaguchi S, Kamachi F, Enami J, Kobayashi S, Yamori T, Takasaki Y. Inhibitory effects of ZSTK474, a phosphatidylinositol 3-kinase inhibitor, on adjuvant-induced arthritis in rats. Inflamm Res. 2012;61:551–562. doi: 10.1007/s00011-012-0444-8. [DOI] [PubMed] [Google Scholar]
- 11.Pietrosimone KM, Jin M, Poston B, Liu P. Collagen-Induced Arthritis: A model for Murine Autoimmune Arthritis. Bio Protoc. 2015;5 doi: 10.21769/bioprotoc.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu JX, Zhang Y, Zhang XZ, Ma YY. [Anti-angiogenic effects of genistein on synovium in a rat model of type II collagen-induced arthritis] . Zhong Xi Yi Jie He Xue Bao. 2011;9:186–193. doi: 10.3736/jcim20110212. [DOI] [PubMed] [Google Scholar]
- 13.Ahn JK, Koh EM, Cha HS, Lee YS, Kim J, Bae EK, Ahn KS. Role of hypoxia-inducible factor-1alpha in hypoxia-induced expressions of IL-8, MMP-1 and MMP-3 in rheumatoid fibroblast-like synoviocytes. Rheumatology (Oxford) 2008;47:834–839. doi: 10.1093/rheumatology/ken086. [DOI] [PubMed] [Google Scholar]
- 14.Kang DW, Park MK, Oh HJ, Lee DG, Park SH, Choi KY, Cho ML, Min do S. Phospholipase D1 has a pivotal role in interleukin-1beta-driven chronic autoimmune arthritis through regulation of NF-kappaB, hypoxia-inducible factor 1alpha, and FoxO3a. Mol Cell Biol. 2013;33:2760–2772. doi: 10.1128/MCB.01519-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou LW, Wang J, Chang PL, Hsieh YL. Hyaluronan modulates accumulation of hypoxia-inducible factor-1 alpha, inducible nitric oxide synthase, and matrix metalloproteinase-3 in the synovium of rat adjuvant-induced arthritis model. Arthritis Res Ther. 2011;13:R90. doi: 10.1186/ar3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biscetti F, Flex A, Pecorini G, Angelini F, Arena V, Stigliano E, Gremese E, Tolusso B, Ferraccioli G. The role of high-mobility group box protein 1 in collagen antibody-induced arthritis is dependent on vascular endothelial growth factor. Clin Exp Immunol. 2016;184:62–72. doi: 10.1111/cei.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hah YS, Koh YJ, Lim HS, Kim HO, Cheon YH, Noh HS, Jang KY, Lee SY, Lee GM, Koh GY, Lee SI. Double-antiangiogenic protein DAAP targeting vascular endothelial growth factor A and angiopoietins attenuates collagen-induced arthritis. Arthritis Res Ther. 2013;15:R85. doi: 10.1186/ar4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KS, Lee YA, Ji HI, Song R, Kim JY, Lee SH, Hong SJ, Yoo MC, Yang HI. Increased expression of endocan in arthritic synovial tissues: effects of adiponectin on the expression of endocan in fibroblast-like synoviocytes. Mol Med Rep. 2015;11:2695–2702. doi: 10.3892/mmr.2014.3057. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang B. Trichostatin A, an Inhibitor of Histone Deacetylase, Inhibits the Viability and Invasiveness of Hypoxic Rheumatoid Arthritis Fibroblast-Like Synoviocytes via PI3K/Akt Signaling. J Biochem Mol Toxicol. 2016;30:163–169. doi: 10.1002/jbt.21774. [DOI] [PubMed] [Google Scholar]
- 20.Jia Q, Cheng W, Yue Y, Hu Y, Zhang J, Pan X, Xu Z, Zhang P. Cucurbitacin E inhibits TNF-alpha-induced inflammatory cytokine production in human synoviocyte MH7A cells via suppression of PI3K/Akt/NF-kappaB pathways. Int Immunopharmacol. 2015;29:884–890. doi: 10.1016/j.intimp.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Malemud CJ. The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med Chem. 2015;7:1137–1147. doi: 10.4155/fmc.15.55. [DOI] [PubMed] [Google Scholar]


