Abstract
Objectives: The purpose of our study was aimed to investigate the effects of microRNA-21 (miR-21) and microRNA-24 (miR-24) inhibitors on ischemic stroke. Methods: MiR-21 inhibitor or miR-24 inhibitor was delivered to Sprague Dawley (SD) rats by continuous intracerebroventricular infusion. Two days later, middle cerebral artery occlusion (MCAO) was performed to induce ischemic stroke. Quantitative real-time PCR was performed to confirm transfection efficiency. The number of apoptotic neurons was detected using TUNEL method. Besides, primary hippocampal or cortical neuronal cultures were prepared from embryonic day 16-18 C57BL/6 mice. These cells were transfected with miR-21 inhibitor, miR-24 inhibitor, or negative scramble RNA. Then the cell viability was detected after transfection, as well as the protein levels of Caspase-3, B-cell lymphoma (Bcl)-xL, and heat shock protein (HSP) 70. Results: Both the levels of miR-21 and miR-24 were significantly reduced by transfection with inhibitors compared to control group or scramble RNA group (both P < 0.05). The apoptosis was significantly reduced in both hippocampal neuron and cortical neuron by miR-24 inhibitor rather than miR-21 inhibitor (P < 0.05), while the cell viability was significantly increased compared to the control group or the scramble group (P < 0.05). In addition, the levels of Bcl-xL and HSP70 were significantly increased, and the levels of Caspase-3 were statistically decreased by transfection with miR-24 inhibitor. Conclusion: MiRNA-24 but not miR-21 inhibitor prevents apoptosis in ischemic stroke by regulation of Bcl-xL, Caspase-3 and HSP70.
Keywords: Ischemic stroke, miRNA-24 inhibitor, miR-21 inhibitor, apoptosis
Introduction
Stroke is a major cause of functional impairment. It represents the third most common cause of death in developed countries, following coronary heart diseases and malignancy [1]. Approximately 85% of all strokes are ischemic in origin [2,3]. Multiple factors are associated with high risk of stroke, such as hypertension, atherosclerosis, type 2 diabetes, hyperlipidemia, hyperhomocysteinemia, and smoking and alcohol consumption [4]. Nevertheless, the prevalence of ischemic stroke is projected to increase among young adults [5]. It has been estimated that about 2.5 million people suffer from stroke and 1 million die from stroke-related disability every year in China, imposing a substantial financial burden on healthcare system [6]. Although tremendous progress and strides have been made in recent years in identifying the molecular basis of ischemic cerebral stroke, there is still a great deal of room for improvement.
Recently, microRNAs (miRNAs) have been described to play a possible role in the pathophysiology of various human diseases. MiRNAs are a novel family of small, endogenous, non-coding RNAs that regulate gene expression by binding to mRNAs [7]. It has been reported that more than 20% of the miRNAs alter in the ischemic brain, demonstrating that miRNAs are promising mediators in the pathogenesis of ischemic stroke [5,8-12]. Therefore, identification of these miRNAs may not only facilitate the illumination of the underlying molecular mechanisms of ischemic stroke, but also provide novel therapeutic targets for early diagnosis and treatment of ischemic stroke. Among the miRNAs identified in ischemic stroke, miR-21 has been reported to have an anti-apoptotic effect on N2A neuroblastoma cells that are exposed to oxygen-glucose deprivation (OGD) and reoxygenation, while miR-24 may have a pro-apoptotic effect by regulating anti-apoptotic and pro-apoptotic proteins [13]. However, little information is available regarding the effects of miR-21 and miR-24 inhibitors on middle cerebral artery occlusion (MCAO) model in vivo and on primary hippocampal or cortical neuronal cultures in vitro, as well as the underlying mechanism.
Therefore, we aimed to explore the effects of transfection of miR-21 and miR-24 inhibitors on neuron apoptosis, and whether these effects were involved in targeting anti-apoptotic (B-cell lymphoma (Bcl)-xL), pro-apoptotic protein (Caspase 3), and heat shock proteins (HSP) 70. Our results might provide new insights into the possible novel therapeutic targets for the treatment of ischemic stroke.
Material and methods
Animals
Eight-week-old male Sprague Dawley (SD) rats (weighing 250-300 g) and embryonic day 16-18 C57BL/6 mice were used in the study. All the animals were purchased from Slaccas Lab Animal Ltd (Shanghai, China). The animals were housed in polypropylene cages under a 12:12 light/dark cycle with free access to tap water and a standard diet prior to the test. All methods and procedures concerning use of the animals were reviewed and approved by the Institutional Animal Ethics Committee.
MCAO
The SD rats were subjected to undergo MCAO by electrocoagulation as previously described [14]. Briefly, the rats were anesthetized with 4% isoflurane during induction and 2% for maintenance in a 30/70 mixture of 30% O2/N2O. Body temperature was controlled at 37.0 ± 0.5°C with a Homeothermic Blanket System (Harvard apparatus, USA). The rats were placed in a supine position, and then a 2 cm skin incision was implemented. Right common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA) were exposed and carefully isolated. CCA and ECA were ligated with a 5-0 surgical sutures suture, and while ICA was temporarily clipped with an artery clip. A modified PE-50 catheter (BD, San Jose, CA, USA) that was filled with a fibrin rich clot was gently advanced from the right ECA into the ICA lumen, near the origin of the MCA. After injection, the catheter was withdrawn immediately and the right ECA was ligated. The blood flow was restored after occlusion. The changes of blood flow were recorded using a laser-Doppler flowmetry monitor (moorVMS-LDF, Moor Instruments, UK). One hour after MCAO, the rats were maintained in an air-ventilated incubator at 24.0 ± 0.5°C for 24 hours. Thereafter, the animals were sacrificed under anesthesia, and the brains were quickly harvested for biochemical assays and infarct determination.
In vivo miR inhibitors delivery to the rat brain
The procedure was carried out as previously described [15-17]. Briefly, the SD rats were anesthetized and fixed to a stereotaxic apparatus. The left lateral ventricle of the brain was stereotaxically implanted with a brain infusion cannula (Bregma: -0.22 mm; Dorsoventral: 3 mm; Lateral: 1 mm) connected with a micro-osmotic pump (Model 1004, Alzet, Cupertino, CA, USA). Continuous intracerebroventricular infusion of miR-21 inhibitor, negative control of scramble RNA for miR-21 inhibitor (Exiqon, Woburn, MA, USA), miR-24 inhibitor, and negative control of scramble RNA for miR-24 inhibitor (Dharmacon and Shanghai GenePharma Co, China) was delivered to the brain at a rate of 0.2 ml/minute. After two days of delivery, MCAO was performed to the animals.
RNA isolation and quantitative real-time PCR for miRNA
The cells were lysed and total RNA was isolated using the miRNeasy Mini kit (Qiagen, Valencia, CA, USA). For miRNA cDNA synthesis, miRNA was reverse transcribed with the miRNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) and amplified by the SYBR Green reporter (Applied Biosystems). Quantitative PCR reactions were performed by using an ABI PRISM® 7000 instrument (Applied Biosystems). U6 was used to normalize the expression of miRNAs for each sample. Data of gene expression was determined by using 2-ΔΔCT method.
Determination of apoptosis
The number of apoptotic neurons was detected using TUNEL method (Roche Diagnostics AG, Rotkreuz, Switzerland). Briefly, the brains were quickly harvested, embedded in paraffin, deparaffinized with dimethylbenzene, hydrated with in a gradient of ethanol, and washed with distilled water. Apoptosis assay was identified using an Apoptag Red in situ apoptosis detection kit (Chemicon International Inc.) according to manufacturer’s instruction. The samples were identified by fluorescence microscopy (Olympus, Tokyo, Japan). Ten fields were randomly selected to assess the percentage of apoptotic cells.
Primary hippocampal or cortical neuronal cultures
Hippocampal or cortical neuronal were prepared from embryonic day 16-18 C57BL/6 mice according to previously described methods [18]. Briefly, the cerebral cortices and hippocampus of fetus mice were dissected out and the meninges were carefully removed. Cells (1 × 106 cells/mL) were maintained in poly-D-lysine (Sigma, St. Louis, MO) coated plates in Dulbecco’s modified eagle medium (DMEM) medium (Life Technologies) with 10% fetal bovine serum (FBS) (Life Technologies). After 4-6 h of culture, the cultures were replenished with Neurobasal medium (Life Technologies) containing 100 U/mL penicillin, 100 μg/mL streptomycin, 2% B27, and 0.5 mM glutamine (Life Technologies) at 37°C with 5% CO2. The medium was changed every three days.
In vitro transfection of miR inhibitors
Cells were plated in 96-well plates (5 × 105 cells/well) in antibiotic-free medium for 24 h prior to transfection. The cells were transfected with miR-21 inhibitor, miR-24 inhibitor, or their corresponding negative scramble RNA at a final concentration of 50 nM using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocols. After 48 h of transfection, the cells were harvested for further analysis.
Determination of cell viability
After transient transfection with miR-21 inhibitor, miR-24 inhibitor, or negative scramble RNA, cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. Briefly, the neuron cells were placed in 96-well plates at a final concentration of 1 × 105 per mL in culture medium, and were incubated at 37°C with 5% CO2. At different time points (0 h, 24 h, and 72 h), 10 μl MTT was added to each well, and incubated at 37°C for another 2 h. The absorbance at 595 nm was read on a microplate reader (Bio Tek Instruments, Winooski, VT, USA).
Western blotting analysis
Proteins were extracted from hippocampus, and the protein concentrations were assessed using a BCA assay kit (TaKaRa BIO INC, Japan). The protein samples were resolved in a 10-12% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Proteins were then transferred to polyvinylidene fluoride (PVDF) membrane, and blocked with 5% nonfat milk in Tris-buffered saline-Tween (TBST) 20 for 2 h at room temperature. Membranes were then incubated with primary antibody overnight. The antibodies were shown as follows: anti-Caspase-3 (1:1000; Santa Cruz Biotechnology), anti-Bcl-xL (1:1000; Cell Signaling Technology, Beverly, MA), or anti-HSP70 (1:1000; Santa Cruz Biotechnology). An anti-GAPDH antibody was used as a loading control. Membranes were washed and incubated for 2 h in the presence of appropriate horseradish peroxidase (HRP)-conjugated secondary antibody. The positive reaction was visualized by using 3, 3’-diaminobenzitine (DAB) solution (Sigma, St. Louis, MO) with a chemiluminescent Immobilon Western blotting detection system (Millipore Corp., Billerica, MA).
Statistical analysis
Data are reported as mean ± standard deviation (SD). Measurement data were tested by using Student’s t-test or one-way analysis of variance (ANOVA) followed by post-hoc Tukey test. Statistical analyses were conducted using statistical package for the social sciences (SPSS, version 19.0, SPSS Inc., Chicago, IL). A statistical significance was defined when P < 0.05.
Results
Transfection efficiency
To confirm the effects of miR-21 and miR-24 inhibitors on stroke, miR-21 inhibitor, miR-24 inhibitor, or the negative control of scramble RNA was delivered to brain using stereotaxic injection. The levels of miR-21 and miR-24 in brain tissue were determined by real-time PCR. As shown in Figure 1A and 1B, the levels of miR-21 and miR-24 in the ischemic area were both significantly lower than those in the control group or scramble RNA group (both P < 0.05), indicating that the brain tissue had been successfully transfected with miR-21 and miR-24 inhibitors.
Figure 1.
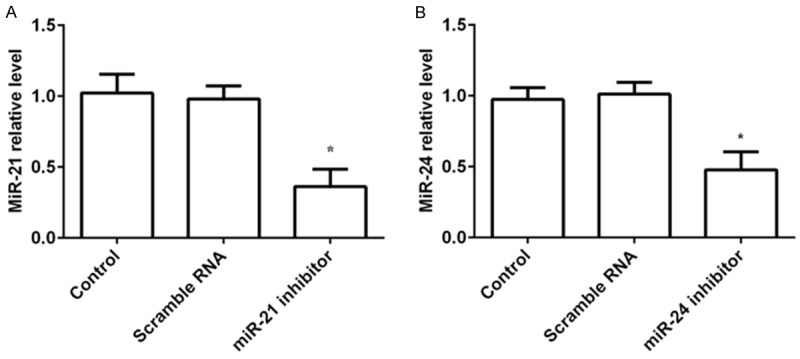
Transfection efficiency in the rat brain. MiR-21 inhibitor, miR-24 inhibitor, or scramble RNA was delivered to brain, and then the transfection efficiency was determined by real-time PCR. A. Relative miR-21 level was significantly lower in the ischemic area than those in the control group or scramble RNA group; B. Relative miR-24 level was markedly decreased in the ischemic area compared to those in the control group or scramble RNA group. *P < 0.05 compared to the control or scramble RNA group.
Apoptosis rate after transfection with miR inhibitors
To explore the effects of miR-21 and miR-24 inhibitors on neuronal apoptosis, TUNEL method was performed after transfection with miR inhibitors. The number of apoptotic cells was examined by ten high magnification of each sample. The results revealed that there were significantly less apoptotic cells (either in the hippocampal neuron or the cortical neuron) in the miR-24 inhibitor group compared to the control group or the scramble group (both P < 0.05). Although the apoptotic hippocampal neuron cells or cortical neuron cells in the miR-21 group were reduced compared to the control group or the scramble group, no significant differences were found (Figure 2A-D). These results demonstrated that inhibition of miR-24 but not miR-21 prevented MCAO-induced neuronal apoptosis and death.
Figure 2.
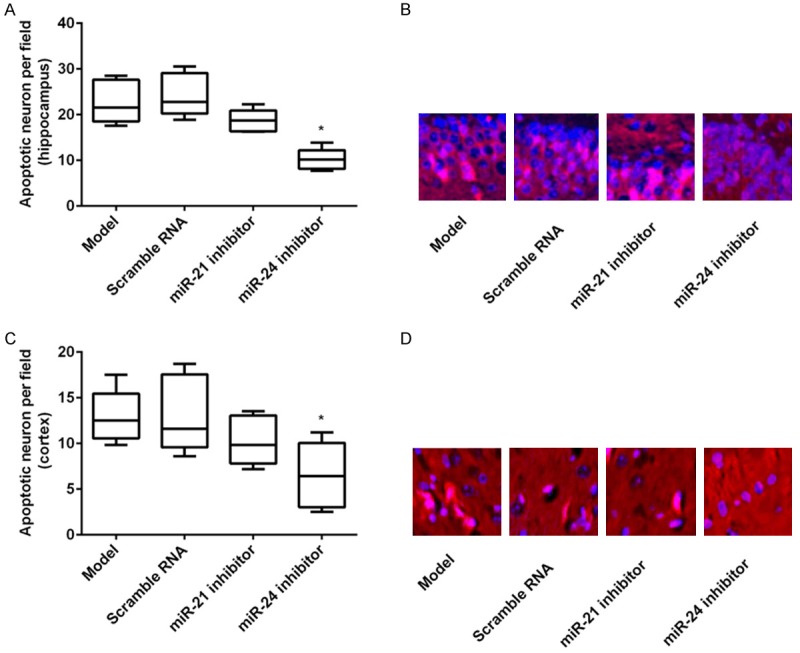
Apoptosis rate after transfection with miR inhibitors. MiR-21 inhibitor, miR-24 inhibitor, or scramble RNA was delivered to brain, and then the neuronal apoptosis was assessed by TUNEL method. The apoptotic cell numbers were counted by ten high magnification of each sample. A and B. MiR-24 inhibitor, but not miR-21 inhibitor, significantly reduced the apoptotic cells of the hippocampal neuron; C and D. MiR-24 inhibitor, but not miR-21 inhibitor, statistically decreased the apoptotic cells of the cortical neuron. *P < 0.05 compared to the control or scramble RNA group.
Cell viability after transfection with miR inhibitors
To explore the effects of miR-21 and miR-24 inhibitors on neuronal cell viability, MTT assay was carried out after transfection with miR inhibitors. As indicated in Figure 3A and 3B, the results showed that both the cell viability in hippocampal and cortical neuron was significantly increased at 24 h and 72 h by transfection with miR-24 inhibitor compared to the control group or the scramble group (P < 0.05); however, there were no significant differences among the control group, scramble group, and miR-21 inhibitor group. The results suggested that inhibition of miR-24 but not miR-21 could increase the cell viability.
Figure 3.
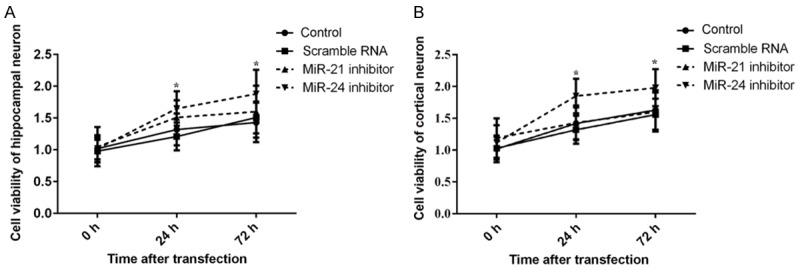
Cell viability after transfection with miR inhibitors. After transient transfection with miR-21 inhibitor, miR-24 inhibitor, or negative scramble RNA into hippocampal or cortical neuron, cell viability was measured by MTT assay. A. MiR-24 inhibitor, but not miR-21 inhibitor, significantly increased the cell viability of the hippocampal neuron at 24 h and 72 h after transfection; B. MiR-24 inhibitor, but not miR-21 inhibitor, obviously elevated the cell viability of the cortical neuron at 24 h and 72 h after transfection. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. *P < 0.05 compared to the control or scramble RNA group.
Expression of Bcl-xL, Caspase-3 and HSP70 after transfection with miR inhibitors
To further investigate the possible underlying mechanism of apoptosis, we determined the expression levels of anti-apoptosis protein (Bcl-xL) and pro-apoptosis protein (Caspase-3) after transfection with miR inhibitors. We found that the expression levels of Bcl-xL were significantly increased by transfection with miR-24 inhibitor, and while the expression levels of Caspase-3 were statistically decreased compared to the control group or the scramble group (both P < 0.05). However, there were no significant differences in the expression levels of Bcl-xL or Caspase-3 by transfection with miR-21 inhibitor (Figure 4A and 4B). Our results indicated that miR-24 inhibitor could prevent apoptosis by increasing the levels of anti-apoptosis proteins and decreasing the levels of pro-apoptosis proteins. Additionally, the expression levels of HSP70 were statistically increased by miR-24 inhibitor (P < 0.05) but not miR-21 inhibitor (Figure 4C and 4D), suggesting that miR-24 inhibitor could prevent apoptosis also by regulating the levels of HSP70.
Figure 4.
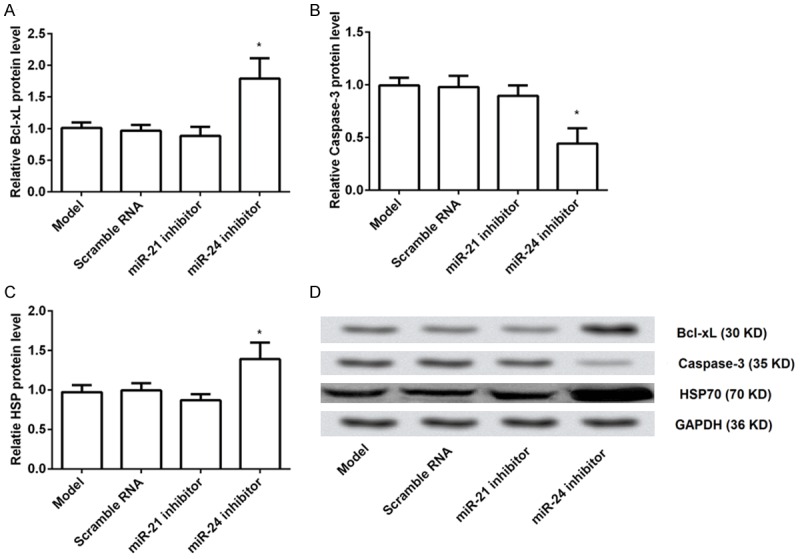
Expression of Bcl-xL, Caspase-3 and HSP70 after transfection with miR inhibitors. MiR-21 inhibitor, miR-24 inhibitor, or scramble RNA was delivered to brain, and then the expression levels of Bcl-xL, Caspase-3 and HSP70 were determined. A. MiR-24 inhibitor, but not miR-21 inhibitor, significantly increased the relative expression levels of Bcl-xL compared to the control group or the scramble group; B. MiR-24 inhibitor, but not miR-21 inhibitor, statistically decreased the relative expression levels of Caspase-3; C. MiR-24 inhibitor, but not miR-21 inhibitor, markedly elevated the relative expression levels of HSP70; D. Representative Western blotting picture of Bcl-xL, Caspase-3 and HSP70. Bcl, B-cell lymphoma; HSP, heat shock protein. *P < 0.05 compared to the control or scramble RNA group.
Discussion
In the present study, we investigate the functional role of miR-24 inhibitor and miR-21 inhibitor in ischemic stroke. Our study provides novel evidence in vivo and in vitro for a protective role of miR-24 inhibitor but not miR-21 inhibitor in ischemic stroke. Inhibition of miRNA-24 prevents hippocampus or cortex apoptosis and increases cell viability, leading to a protective effect on ischemic stroke. These effects might be mediated through regulation of the levels of Bcl-xL, Caspase-3 and HSP70.
The precise mechanisms of neuronal damage in cerebral ischemia are not completely understood, but recent studies have implicated that miRNAs function as important regulators in the pathological processes of ischemic injury [19-21]. MiRNAs have been suggested to play significant roles in preventing apoptosis in numerous diseases [22], including ischemic stroke [12,23]. A previous study suggested that both miR-21 and miR-24 could be identified as diagnostic biomarkers in cerebral ischemia and might have potential therapeutic targets for the treatment of post-ischemic injury [13]. However, the effects of miR-21 and miR-24 inhibitors on the animal models of ischemic stroke have not been investigated. To better understand the role of miR-21 and miR-24 inhibitors in the pathology of ischemic stroke, as well as the possible therapeutics against ischemic stroke, we used viral vectors to repress the expression of miRs in our study. In addition to inhibition of pathogenic miRs by modified antagomirs [24,25], miR sponges [26], or miRs decoys [27], improved viral vectors has also exhibited therapeutic efficacy [28]. Therefore, we speculated that miR-21 or miR-24 inhibitors might show the therapeutic efficacy in ischemic stroke.
To confirm the hypothesis, we first inhibited the expression of miR-21 and miR-24 by viral vectors, and then induced the models of ischemic stroke by MCAO method. The transfection efficiency were determined. The lower levels of miR-21 and miR-24 in the miRs inhibitors indicated successful transfection into the brain tissues. After transfection with miR inhibitors, neuronal apoptosis were examined. The results showed that hippocampal neuron or the cortical neuron caused less apoptosis rate by transfection with miR-24 inhibitor but not miR-21 inhibitor, suggesting that inhibition of miR-24 protects against MCAO-induced neuronal apoptosis and death. However, our results were somewhat different from other studies. MiRNA-21 has been identified as one of anti-apoptotic factors partly by targeting a host of pro-apoptotic genes [29], but in our study, miR-21 inhibitor showed no effect on apoptosis in neuron. MiR-24 has emerged as an important but controversial miR involved in apoptosis. Some researchers have suggested that miR-24 acts as an inhibitor in apoptosis, such as by partly repressing the BH3-only domain-containing protein Bim in mouse cardiomyocytes [30] and by inhibiting pro-apoptotic gene BCL2L11 in rat cardiomyocytes [31]. On the contrary, several studies revealed that miR-24 functions as a promotor in apoptosis and angiogenesis, such as by regulating Bcl-xL/Bcl-2-associated death promoter [24] and X-linked inhibitor of apoptosis protein (XIAP) [32]. In our study, we confirmed that miR-24 also showed its pro-apoptotic function.
We further determined the cell viability after transfection with miR inhibitors in vitro. As indicated in the results, the cell viability in hippocampal neuron and cortical neuron was significantly enhanced by transfection with miR-24 inhibitor but not miR-21 inhibitor, demonstrating that inhibition of miR-24 could increase the cell viability. Besides, the expression levels of anti-apoptotic protein (Bcl-xL) and pro-apoptotic protein (Caspase-3) after transfection with miR inhibitors were determined. Both Bcl-xL and Caspase-3 were important apoptosis-related proteins within the intrinsic apoptotic pathway [33]. Caspase-3 is well known to act downstream of Bcl-xl:Bax, and plays an important role in the execution of apoptosis [34]. The activity of Caspase-3 has been considered as an index of the process of apoptosis [35]. In line with previous studies, we also found that levels of Bcl-xL were significantly increased by transfection with miR-24 inhibitor, and whereas the expression levels of Caspase-3 were statistically decreased. Moreover, the expression levels of HSP70 were statistically increased by miR-24 inhibitor. It has been well acknowledged that HSP70 protects the brain from many insults including ischemic stroke by its chaperone functions and modulation of inflammatory responses [36,37]. In addition, HSP70 has shown its ability to modulate apoptosis by inhibiting various pro-apoptotic proteins such as Bax, cytochrome C, Caspase-9 and Apaf-1 [38,39]. In our study, the results showed that miR-24 inhibitor could increase the expression levels of HSP70 and subsequently exert its protective effect against ischemic stroke.
In conclusion, the present study identifies that miR-24 inhibitor but not miR-21 inhibitor protects against apoptosis in ischemic stroke. These effects might be through regulating the expression of Bcl-xL, Caspase-3 and HSP70. MiR-24 inhibitor might be a potential therapeutic target for the treatment of ischemic stroke.
Acknowledgements
This research was supported by Zhejiang Provincial Natural Science Foundation of China (No. Y2110807).
Disclosure of conflict of interest
None.
References
- 1.Read SJ, Hirano T, Davis SM, Donnan GA. Limiting neurological damage after stroke: a review of pharmacological treatment options. Drugs Aging. 1999;14:11–39. doi: 10.2165/00002512-199914010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Beal CC. Gender and stroke symptoms: a review of the current literature. J Neurosci Nurs. 2010;42:80–87. [PubMed] [Google Scholar]
- 3.Chen PH, Gao S, Wang YJ, Xu AD, Li YS, Wang D. Classifying Ischemic Stroke, from TOAST to CISS. CNS Neurosci Ther. 2012;18:452–456. doi: 10.1111/j.1755-5949.2011.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein LB, Adams R, Becker K, Furberg CD, Gorelick PB, Hademenos G, Hill M, Howard G, Howard VJ, Jacobs B, Levine SR, Mosca L, Sacco RL, Sherman DG, Wolf PA, del Zoppo GJ. Primary prevention of ischemic stroke: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2001;32:280–299. doi: 10.1161/01.str.32.1.280. [DOI] [PubMed] [Google Scholar]
- 5.Tan JR, Tan KS, Koo YX, Yong FL, Wang CW, Armugam A, Jeyaseelan K. Blood microRNAs in low or no risk ischemic stroke patients. Int J Mol Sci. 2013;14:2072–2084. doi: 10.3390/ijms14012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42:3651–3654. doi: 10.1161/STROKEAHA.111.635755. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Saito Y, Saito H. MicroRNAs in cancers and neurodegenerative disorders. Front Genet. 2012;3:194. doi: 10.3389/fgene.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011;43:521–528. doi: 10.1152/physiolgenomics.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jickling GC, Ander BP, Zhan X, Noblett D, Stamova B, Liu D. microRNA expression in peripheral blood cells following acute ischemic stroke and their predicted gene targets. PLoS One. 2014;9:e99283. doi: 10.1371/journal.pone.0099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol. 2013;9:328–339. doi: 10.1038/nrneurol.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan JL, Liu X. MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci Ther. 2013;19:813–819. doi: 10.1111/cns.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Zhang J. Identification of miRNA-21 and miRNA-24 in plasma as potential early stage markers of acute cerebral infarction. Mol Med Rep. 2014;10:971–976. doi: 10.3892/mmr.2014.2245. [DOI] [PubMed] [Google Scholar]
- 14.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 15.Barbash S, Hanin G, Soreq H. Stereotactic injection of microRNA-expressing lentiviruses to the mouse hippocampus ca1 region and assessment of the behavioral outcome. J Vis Exp. 2013:e50170. doi: 10.3791/50170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab. 2009;29:675–687. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu AJ, Zang P, Guo JM, Wang W, Dong WZ, Guo W, Xiong ZG, Wang WZ, Su DF. Involvement of acetylcholine-alpha7nAChR in the protective effects of arterial baroreflex against ischemic stroke. CNS Neurosci Ther. 2012;18:918–926. doi: 10.1111/cns.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab. 2010;30:1564–1576. doi: 10.1038/jcbfm.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziu M, Fletcher L, Rana S, Jimenez DF, Digicaylioglu M. Temporal differences in microRNA expression patterns in astrocytes and neurons after ischemic injury. PLoS One. 2011;6:e14724. doi: 10.1371/journal.pone.0014724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima RT, Busacca S, Almeida GM, Gaudino G, Fennell DA, Vasconcelos MH. MicroRNA regulation of core apoptosis pathways in cancer. Eur J Cancer. 2011;47:163–174. doi: 10.1016/j.ejca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One. 2012;7:e32662. doi: 10.1371/journal.pone.0032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, Galuppo P, Kneitz S, Pena JT, Sohn-Lee C, Loyer X, Soutschek J, Brand T, Tuschl T, Heineke J, Martin U, Schulte-Merker S, Ertl G, Engelhardt S, Bauersachs J, Thum T. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 25.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caporali A, Meloni M, Vollenkle C, Bonci D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH, Agami R, le Sage C, Condorelli G, Madeddu P, Martelli F, Emanueli C. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 28.Meloni M, Marchetti M, Garner K, Littlejohns B, Sala-Newby G, Xenophontos N, Floris I, Suleiman MS, Madeddu P, Caporali A, Emanueli C. Local inhibition of microRNA-24 improves reparative angiogenesis and left ventricle remodeling and function in mice with myocardial infarction. Mol Ther. 2013;21:1390–1402. doi: 10.1038/mt.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YJ, Hwang SJ, Bae YC, Jung JS. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells. 2009;27:3093–3102. doi: 10.1002/stem.235. [DOI] [PubMed] [Google Scholar]
- 30.Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med. 2011;208:549–560. doi: 10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li DF, Tian J, Guo X, Huang LM, Xu Y, Wang CC, Wang JF, Ren AJ, Yuan WJ, Lin L. Induction of microRNA-24 by HIF-1 protects against ischemic injury in rat cardiomyocytes. Physiol Res. 2012;61:555–565. doi: 10.33549/physiolres.932270. [DOI] [PubMed] [Google Scholar]
- 32.Xie Y, Tobin LA, Camps J, Wangsa D, Yang J, Rao M, Witasp E, Awad KS, Yoo N, Ried T, Kwong KF. MicroRNA-24 regulates XIAP to reduce the apoptosis threshold in cancer cells. Oncogene. 2013;32:2442–2451. doi: 10.1038/onc.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujita N, Nagahashi A, Nagashima K, Rokudai S, Tsuruo T. Acceleration of apoptotic cell death after the cleavage of Bcl-XL protein by caspase-3-like proteases. Oncogene. 1998;17:1295–1304. doi: 10.1038/sj.onc.1202065. [DOI] [PubMed] [Google Scholar]
- 34.D’Amelio M, Cavallucci V, Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 2010;17:1104–1114. doi: 10.1038/cdd.2009.180. [DOI] [PubMed] [Google Scholar]
- 35.Pan Y, Guo M, Nie Z, Huang Y, Peng Y, Liu A, Qing M, Yao S. Colorimetric detection of apoptosis based on caspase-3 activity assay using unmodified gold nanoparticles. Chem Commun (Camb) 2012;48:997–999. doi: 10.1039/c1cc15407a. [DOI] [PubMed] [Google Scholar]
- 36.Giffard RG, Han RQ, Emery JF, Duan M, Pittet JF. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology. 2008;109:339–348. doi: 10.1097/ALN.0b013e31817f4ce0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- 38.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, Dai AY, Tao K, Xiao Q, Huang ZL, Gao M, Li H, Wang X, Cao WX, Feng WL. Heat shock protein-70 neutralizes apoptosis inducing factor in Bcr/Abl expressing cells. Cell Signal. 2015;27:1949–1955. doi: 10.1016/j.cellsig.2015.06.006. [DOI] [PubMed] [Google Scholar]


