Abstract
Objective: This study aimed to investigate the nanobacteria (NB) induced damage to human tubular epithelial HK-2 cells and the potential role of NB in the kidney stone formation. Methods: Serum sample from 15 patients with kidney stone was collected. Four groups were included: control, NB group, nanograde hydroxyapatite (nHAP) and calcium oxalate monohydrate (COM) group. Catalase (CAT), malonaldehyde (MDA) and Na+/K+ ATPase activity was detected in the supernatant at 12 and 24 h. At 12 and 24 h, COM was added. Results: At 12 h and 24 h, the CAT in NB group was significantly higher than in control group and nHAP group (P<0.01). CAT at 24 h was significantly higher than in COM group (P<0.01). At 12 h and 24 h, the MDA in NB group was significantly higher than in control group and nHAP group (P<0.01) and significantly lower than in COM group (P<0.01). At 12 h, the Na+/K+ ATPase activity in NB group and nHAP group was significantly lower than in control group, but dramatically increased as compared to COM group (P<0.01). At 24 h, the Na+/K+ ATPase activity in NB group and nHAP group was significantly lower than in control group (P<0.01). Conclusion: NB may induce lipid peroxidation in HK-2 cells and cause adhesion of HK-2 cells to COM in a time-dependent manner, resulting in damage to HK-2 cells. This injury-causing capability of NB is more potent than nHAP and might be involved in the pathogenesis of kidney stone formation.
Keywords: Nanobacteria, HK-2 cells, nanograde hydroxyapatite, calcium oxalate monohydrate
Introduction
Urinary lithogenesis is a common disease with high morbidity, and its incidence is increasing in recent years [1]. Although a variety studies have been conducted to investigate the pathogenesis and therapy of urinary lithogenesis, the specific mechanism is still poorly understood. Thus, the prevention of urinary lithogenesis has been a challenge in clinical practice.
The tubular epithelial cell injury is one of important cause of kidney stone formation. After injury, the basement membrane is exposed to the tubular fluid, which may cause the retention and growth of microcrystals, forming crystallization nest. In addition, membrane fragments in the supersaturated tubular fluid may induce heterogeneous nucleation or even tubular obstruction, which may create an environment for the crystal growth, resulting in kidney stone formation [2,3]. In recent years, numerous studies have been conducted to investigate the calcium oxalate monohydrate (COM) induced damage to the epithelial cells in the proximal tubules and collecting tubules. Results reveal that a large amount of COM is adherent to tubular epithelial cells under electron microscope after cells are incubated with medium containing COM for 8 h [4,5]. The adhesion between crystals and tubular epithelial cells is an early event and a key step in the kidney stone formation [6-8].
In recent years, some investigators have developed new tools and methods to investigate the role of nanobacteria (NB) in the pathogenesis of urinary lithogenesis. NB were first identified by the Finnish scientist Kajander in 1988 and named in 1990 [9,10]. In 95% of patients with kidney stones, NB are detectable in the blood, urine and kidney stone. NB may infect any type of tissues and cells in human body and are toxic to them. They may cause vacuolization in cells, induce tissue inflammation and edema and are closely related to the pathogenesis of infections and pathological calcification in human [11,12]. A variety of studies in rodents and rabbits have indicated that NB have the tropism to the kidney. Circulating NB may be secreted into the urine via the kidney and then they become adherent to the injured cells in the collecting tubule and renal papillae. Ciftcioglu et al [13] proposed that NB could cause damage to the collecting tubules and renal papillae and induce the formation of the core of calcium phosphate stones via biomineralization, resulting in kidney stone formation.
In the present study, cell immunostaining, H&E staining, transmission electron microscopy and laser scanning confocal microscopy were employed to investigate the NB induced damage to HK-2 cells and the adhesion of COM to injured HK-2 cells as well as explore the potential mechanism underlying the adhesion of COM to HK-2 cells.
Materials and methods
Culture and identification of NB
Blood was collected from 15 patients with clinically proven kidney stones and serum was harvested after centrifugation. The serum was sterilized via filtration. NB were maintained in 1640 medium containing bacteria free serum at 37°C in an environment with 5% CO2. The medium was refreshed once every 30-45 days. The NB growth was observed under an inverted phase contrast microscope. The monoclonal antibody against NB (Nanobac, Finland) was used to identify the NB by immunohistochemistry.
Grouping
There were 4 groups in this study: Control group (serum and HK-cells), NB group (NB solution was used to treat HK-2 cells), nHAP group (nHAP solution was used to HK-2 cells), COM group (HK-2 cells were incubated with 5 mmol/L COM). The NB solution was the serum from 15 patients with kidney stones. Nhap was purchased from Beijing Dk Nano technology Co., LTD and COM was from Beijing Hengye Zhongyuan chemical Co., LTD. The nHAP solution and COM solution were irradiated with high dose γ-ray at 25-35 kGy for 26 h for sterilization and inhibition of NB growth. For the investigation of adhesion of HK-2 cells to COM, HK-2 cells were seeded into 24-well plates before detection.
Detections
After incubation for 12 h and 24 h, cells were centrifuged for smearing, H&E staining and transmission electron microscopy. At 12 h and 24 h, the catalase (CAT), malonaldehyde (MDA) content and Na+/K+ ATPase activity were detected in 15 samples in each group.
Hematoxylin-eosin (H&E) staining
At different time points, cells were collected by digestion in 0.25% trypsin. Then, 100 μl of cell suspension was collected and centrifuged for smearing, followed by washing in PBS and drying. Sections were deparaffinized and hydrated, followed by Harris hematoxylin staining for 1 min. Sections were then washed with flowing water for 3 min and treated with 1% hydrochloric acid for 10 s. Sections were treated with 1% eosin for 5 min. After dehydration, transparentization, mounting was performed with neutral gum. Cells were observed under a light microscope (×600).
Cell processing for transmission electron microscopy
Cells were digested, centrifuged and then transferred into a microfuge tube, followed by incubation with PBS for 10 min. Then, cells were fixed in 3% glutaraldehyde for 1-2 h, followed by washing in PBS thrice. After fixation in 1% osmium tetroxide for 1-2 h, cells were washed in PBS twice and then dehydrated in series of ethanol (50%, 70% and 90% for each) and then in 100% ethanol thrice. After incubation in Epon812 solution in 100% propylene oxide (1:1), cells were embedded and polymerized at 80°C over night. Ultrathin sections were obtained and subjected to uranium and lead staining. Cell morphology and structure were observed under an electron microscope at 4000× and 20000×.
Measurement of CAT
The supernatant (300 μl/well) was collected from 24-well plates, and colorimetry was done to detect the hydrogen peroxide in the supernatant with corresponding kit.
Measurement of MDA
The supernatant (400 μl) was collected from 24-well plates and the MDA was measured by colorimetry with corresponding kit.
Measurement of Na+/K+ ATPase
At the different time points, the supernatant was removed and 0.25% trypsin was added to digest cells. Then, cells were transferred into EP tubes, followed by homogenization in PBS (400 μl) with ultrasound. Colorimetry was done to measure the ATPase activity. All the above detections were done according to the manufacturer’s instructions (Nanjing Jiancheng Institute of Biotechnology).
Adhesion between cells and COM
HK-2 cells were seeded into plates pre-coated with coverslips and cells were incubated for 12 h and 24 h. At different time points, COM (0.6 mg/well) was added at a final concentration 200 μg/ml. The plates were slightly shaken for 5 s to allow the COM crystals to deposit and contact with cells. Three minutes later, the medium was removed, and cells were washed in saturated sodium oxalate thrice to remove residual COM crystals.
Cell processing for laser scanning confocal microscopy
After COM treatment, coverslips were collected and cells fixed in 4% paraformaldehyde for 20 min. Then, cells were washed in PBS twice (5 min for each) and 5 μg/ml phalloidin-FITC (50 μl) was added, followed by incubation at room temperature for 18 min in dark. After washing in PBS twice (5 min for each), mounting was done with glycerin. Cells were observed under a fluorescence microscope with the wavelength of 488 nm for the cells with green fluorescence and 633 nm for the COM crystals with red fluorescence. Photographs were captured at 200× and 600×.
Statistical analysis
Data of CAT, MDA and ATPase activity were statistically analyzed with SPSS version l3.0. Homogeneity of variance was tested. Homogeneity of variance was defined if a value of P>0.05 was present. Comparisons were performed with one way analysis of variance, followed by LSD method for comparisons between groups. A value of P<0.05 was considered statistically significant.
Results
Culture and identification of NB
After culture for 4-6 weeks, NB with diffuse distribution were observed under light microscope. After culture for 4-6 months, NB mass formed by small, round granules were found. They had even density and were light yellow, with small granules around them. In addition, NB were positive for monoclonal antibody against NB. These findings confirmed that there were NB in the serum from patients with kidney stones.
H&E staining
At 12 h, cells in control group had normal morphology. Some were arranged in tube form, the nucleus and cytoplasm were dense and no obvious abnormality was observed (Figure 1A). In NB group, some cells became swollen, with loose nucleus and cytoplasm. A fraction of nuclear membrane was lysed or nucleolus was absent, and vacuolar degeneration was found in a few cells (Figure 1B). In nHAP group, the cell state was similar to that in NB group, but cells with nuclear membrane lysis and nucleolus disappearance were fewer. In COM group, the nucleus and cytoplasm were loose, cells with lysed nuclear membrane increased, and COM crystals were distributed in diffuse manner in some cells or some cells were adherent to COM crystals.
Figure 1.
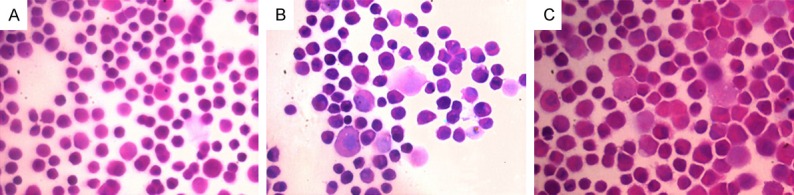
A: H&E staining at 12 h in control group (600×); B: H&E staining at 12 h in NB group (600×); C: H&E staining at 24 h in NB group (600×).
At 24 h, cell density increased in different groups. In control group, the cell morphology was regular and largely remained unchanged. In NB group, cells were larger than in control group, some had irregular morphology, cells with vacuoles increased, and near 50% of cells had lysed nuclear membrane (Figure 1C). In nHAP group, cells had irregular morphology, and the vacuolar degeneration and nuclear membrane lysis were the same to those in NB group. In COM group, only a few cells displayed near normal morphology, and COM crystals were found between cells in a diffuse formed, or some cells were adherent to COM crystals.
Transmission electron microscopy
At 12 h, the cell membrane was intact, there was typical brush border at the luminal side of tubular epithelial cells, there was no vacuolar degeneration in the cytoplasm, the mitochondria were normal and had no swelling, and the nuclear membrane was intact (Figure 2A). In NB group, the cell membrane was intact and continuous, but brush border was absent in a few cells or cells were irregularly arranged, there were diffuse vacuoles with similar size in the cytoplasm, NB and mineralized shell were observable. NB were in the division phase, but there were no abnormalities in the mitochondria and nucleus (Figure 2B). In nHAP group, cell membrane was relatively intact, with disordered brush border. There were vacuoles with different sizes in the cytoplasm. The mitochondria had no evident swelling, with a little nHAP granules found in the cytoplasm, and the nuclear membrane was complete (Figure 2C). In COM group, the brush border was towering or stiff, with a large amount of vacuoles of different sizes in the cytoplasm. The mitochondria had slight swelling, and the nuclear membrane was unclear in some cells (Figure 2D).
Figure 2.
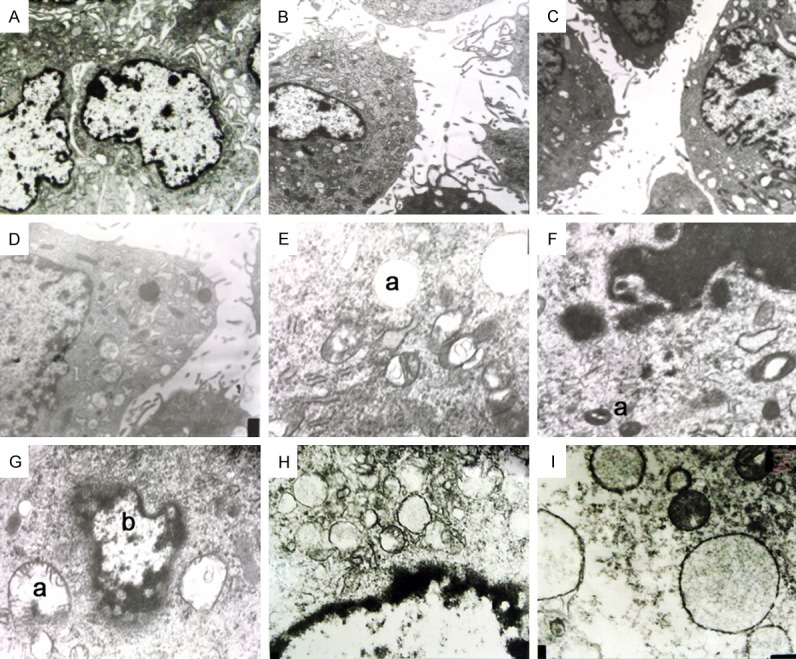
A: TEM at 12 h in control group, Normal brush border (4000×); B: TEM at 12 h in NB group. The brush border displayed disordered arrangement (4000×); C: TEM at 12 h in nHAP group: similar to NB group. The brush border displayed disordered arrangement (4000×); D: TEM at 12 h in COM group. The brush border was bare or stiff (4000×); E: TEM at 24 h in NB group. a: Vacuole with larger size (20000×); F: TEM at 24 h in NB group. a: Nanobacterias in division phase (20000×); G: TEM at 24 h in NB group. a: Swollen mitochondria, b: Dissolving karyotheca (20000×); H: TEM at 24 h in nHAP group. There were diffuse vacuoles in the cytoplasm and the nuclear membrane was unclear (20000×); I: TEM at 24 h in COM group: There were a large amount of diffuse vacuoles in the cytoplasm, the nuclear membrane was lysed, and the nucleolus disappeared (20000×).
At 24 h, the findings in control were similar to above mentioned. In NB group, the cell membrane was intact, with stiff and disordered brush border. There were large vacuoles in the cytoplasm (Figure 2E). NB and mineralized shell were observable (Figure 2F), the mitochondrial swelling became evident, and the nuclear membrane was lysed or being lysed in some cells (Figure 2G). In nHAP group, the cell membrane was intact, and the brush border displayed disordered arrangement. There were diffuse vacuoles in the cytoplasm, without evident mitochondrial swelling. nHAP granules were found in the cytoplasm and intercellular space, and the nuclear membrane was unclear in some cells (Figure 2H). In COM group, about 50% of cells had towering brush border. There were a large amount of vacuoles in the cytoplasm. The mitochondria had characteristics abovementioned, the nuclear membrane was lysed, and the nucleolus was absent in some cells (Figure 2I).
Detection of CAT, MDA and Na+/K+ ATPase activity
There was significant difference among 4 groups (P<0.01). Paired comparison with LSD method showed the CAT in NB group was significantly higher than in control group and nHAP group at 12 h and 24 h (P<0.01). The CAT in NB group was similar to that in COM group at 12 h (P>0.05), but significantly higher than in COM group at 24 h (P<0.01). In NB group, the MDA at 12 h and 24 h was significantly higher than in control group and nHAP group (P<0.01) and significantly lower than in COM group (P<0.01). At 12 h, the Na+/K+ ATPase activity in NB group and nHAP group was significantly lower than in control group (P<0.01), but there was no significant difference between NB group and nHAP group (P>0.05). The Na+/K+ ATPase activity at 12 h in NB group was significantly higher than in COM group (P<0.01). At 24 h, the Na+/K+ ATPase activity in NB group and nHAP group was significantly lower than in control group (P<0.01), but there was no significant difference among NB group, nHAP group and COM group (P>0.05) (Table 1).
Table 1.
CAT, MDA and Na+/K+ ATPase activity at different time points
| Group | CAT (U/mL) | MDA (nmol/ml) | Na+/K+ ATPase activity | |||
|---|---|---|---|---|---|---|
|
| ||||||
| 12 h | 24 h | 12 h | 24 h | 12 h | 24 h | |
| CK | 11.47±2.15 | 9.83±3.34 | 9.95±2.52 | 9.23±1.72 | 13.11±2.56 | 4.23±2.06 |
| NB | 15.27±3.12* | 18.22±4.15*,# | 14.52±1.23* | 25.46±5.72*,# | 7.12±0.62#,$ | 1.15±0.32$ |
| nHAP | 12.08±1.89 | 8.27±2.73 | 10.28±3.12 | 8.12±1.24 | 6.89±3.12 | 2.17±2.82 |
| COM | 16.16±3.35 | 10.72±1.95 | 87.27±4.47 | 68.16±7.79 | 2.32±0.35 | 1.43±1.17 |
P<0.05 vs CK and nHAP groups;
P<0.05 vs COM group;
P<0.05 vs CK group.
Findings from laser scanning confocal microscopy
At 12 h, cell adhesion to COM was not found in control group and nHAP group (Figure 3A and 3B). In NB group, a small amount of COM crystals were adherent to cells (Figure 3C). In COM group, COM crystals adherent to cells increased, and some crystals entered the cytoplasm of cells (Figure 3D).
Figure 3.
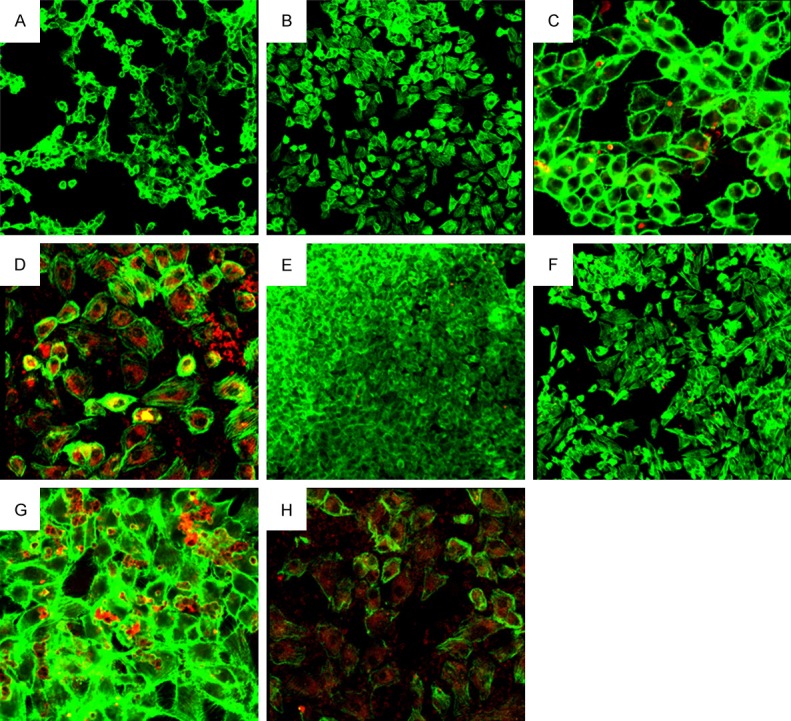
A: Cell adhesion to COM shown by laser scanning confocal microscope at 12 h in control group (200×); B: Cell adhesion to COM shown by laser scanning confocal microscope at 12 h in nHAP group (200×); C: Cell adhesion to COM shown by laser scanning confocal microscope at 12 h in NB group (600×); D: Cell adhesion to COM shown by laser scanning confocal microscope at 12 h in COM group (600×); E: Cell adhesion to COM shown by laser scanning confocal microscope at 24 h in control group (200×); F: Cell adhesion to COM shown by laser scanning confocal microscope at 24 h in nHAP group (200×); G: Cell adhesion to COM shown by laser scanning confocal microscope at 24 h in NB group (600×); H: Cell adhesion to COM shown by laser scanning confocal microscope at 24 h in COM group (600×).
At 24 h, the cell density increased. In NB group, the COM adhesion to cells was similar to that at 12 h (Figure 3G). In COM group, the COM adhesion to cells was similar to that at 12 h, but a large amount of COM crystals entered the cytoplasm or were adherent to cells (Figure 3H). In control group and nHAP group, only a little COM crystals were found to be adherent to cells, which was similar to that at 12 h (Figure 3E, 3F).
Discussion
A large amount of studies have confirmed that the kidney stone formation is a complex process with involvement of multiple factors including urine supersaturation, formation of small crystals and growth and maturation of crystals. Of them, the adhesion of small crystals to urinary epithelial cells has been found as a key and essential step in the kidney stone formation [14,15]. In the present study, nHAP was introduced, aiming to compare with the NB induced HK-2 cell injury, and COM served as a positive control. Thamilselvan et al [16] employed scanning electron microscopy and detection of ATPase to investigate the COM (5 mmol/L) induced damage to rat tubular epithelial cells. Thus, COM (5 mmol/L) induced damage to tubular epithelial cells has been widely accepted and served as a positive control group in the present study. In our study, H&E staining, transmission electron microscopy and detection of ATPase were employed to evaluate the NB induced damage to cell membrane. In addition, CAT and MAD were also measured to elucidate whether there was lipid peroxidation in NB induced injury to tubular epithelial cells. Our findings indicated that NB could cause damage with similar extent to HK-2 cells as in nHAP group (transmission electron microscopy and detection of ATPase activity). At 24 h, cells with mitochondrial swelling, nuclear membrane lysis and nucleolus disappearance increased significantly in NB group as compared to nHAP group. This indicates that NB induced damage to HK-2 cells does not merely reflect the hydroxyapatite shell induced injury. The NB or nHAP induced damage to cell membrane was characterized by disordered arrangement of brush border, and the continuity and integrity of cell membrane were not significantly affected. However, COM caused a severe injury to cell membrane, which was characterized by towering brush border and even incomplete cell membrane.
Thamilselvan et al [17] found LLC-PK1 cells from proximal tubular cells of Hampshire pig and distal tubular cells of Benny Cox dog displayed significant increase in MDA after incubation with 1.0 mmol/ml oxalic acid and 500 mg/ml COM for 120 min or 240 min, but it reduced dramatically in the presence of radical scavengers---catalase or superoxide dismutase. In addition, a lot of studies have reported that COM induced damage to HK-2 cells had involvement of pro-inflammatory cytokines including IL-6 [18,19]. In the present study, CAT and MDA were measured to evaluate whether lipid peroxidation was involved in the NB induced damage to tubular epithelial cells. Results showed that NB induced damage to tubular epithelial cells was accompanied by significant elevation of MDA and CAT, which was not observed in nHAP group. This indicates that lipid peroxidation is involved and a key step in the NB induced damage to tubular epithelial cells, but nHAP induced damage to cells has no involvement of lipid peroxidation. However, the target and the specific mechanism of NB induced peroxidation are required to be further studied in depth.
Yasui et al [20] treated NRK-52E cells with 195 μg/ml COM crystals for 1 h and scanning electron microscopy showed the adhesion of COM crystals to cells. In this study, laser scanning confocal microscopy was employed to observe the adhesion between COM crystals and cells. Results showed the more cells in NB group were adherent to COM crystals at 12 h and 24 h as compared to control group and nHAP group, and there was no marked difference between control group and nHAP group at both time points. In addition, NB induced damage to HK-2 cell membrane was similar to that caused by nHAP, but the adhesion of injured cells to COM crystals was significantly different between NB group and nHAP group. This might be ascribed to the differences in the extent of injury of the cell membrane at the luminal side and the change in the charge of luminal fluid after cell injury. In recent years, factors affecting the adhesion of tubular epithelia cells to COM crystals have been a focus in studies on the kidney stone formation. Lieske et al [21] found the concentrations of Na+, Ca2+ and H+ were important factors affecting the adhesion of tubular epithelial cells to COM crystals. Thus, to inhibit the adhesion of tubular cells to crystals after injury has been a challenge in clinical practice and our result will provide a new strategy for the prevention of kidney stone formation.
Disclosure of conflict of interest
None.
References
- 1.Sheng X, Ward MD, Wesson JA. Adhesion between molecules and calcium oxalate crystals: critical interactions in kidney stone formation. J Am Chem Soc. 2003;125:2854–2855. doi: 10.1021/ja029575h. [DOI] [PubMed] [Google Scholar]
- 2.Lieske JC, Toback FG. Regulation of renal epithelial cell endocytosis of calcium oxalate monohydrate crystals. Am J Physiol. 1993;264:F800–807. doi: 10.1152/ajprenal.1993.264.5.F800. [DOI] [PubMed] [Google Scholar]
- 3.Ciftcioglu N, Miller-Hjelle MA, Hjelle JT, Kajander EO. Inhibition of nanobacteria by antimicrobial drugs as measured by a modified microdilution method. Antimicrob Agents Chemother. 2002;46:2077–2086. doi: 10.1128/AAC.46.7.2077-2086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigelow MW, Wiessner JH, Kleinman JG, Mandel NS. Calcium oxalate-crystal membrane interactions: dependence on membrane lipid composition. J Urol. 1996;155:1094–1098. [PubMed] [Google Scholar]
- 5.Schellens JH, Malingre MM, Kruijtzer CM, Bardelmeijer HA, van Tellingen O, Schinkel AH, Beijnen JH. Modulation of oral bioavailability of anticancer drugs: from mouse to man. Eur J Pharm Sci. 2000;12:103–110. doi: 10.1016/s0928-0987(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 6.Hammes MS, Lieske JC, Pawar S, Spargo BH, Toback FG. Calcium oxalate monohydrate crystals stimulate gene expression in renal epithelial cells. Kidney Int. 1995;48:501–509. doi: 10.1038/ki.1995.320. [DOI] [PubMed] [Google Scholar]
- 7.Koul HK, Menon M, Chaturvedi LS, Koul S, Sekhon A, Bhandari A, Huang M. COM crystals activate the p38 mitogen-activated protein kinase signal transduction pathway in renal epithelial cells. J Biol Chem. 2002;277:36845–36852. doi: 10.1074/jbc.M200832200. [DOI] [PubMed] [Google Scholar]
- 8.Asselman M, Verhulst A, De Broe ME, Verkoelen CF. Calcium oxalate crystal adherence to hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys. J Am Soc Nephrol. 2003;14:3155–3166. doi: 10.1097/01.asn.0000099380.18995.f7. [DOI] [PubMed] [Google Scholar]
- 9.Kajander EO, Ciftcioglu N, Aho K, Garcia-Cuerpo E. Characteristics of nanobacteria and their possible role in stone formation. Urol Res. 2003;31:47–54. doi: 10.1007/s00240-003-0304-7. [DOI] [PubMed] [Google Scholar]
- 10.Rahman NU, Meng MV, Stoller ML. Infections and urinary stone disease. Curr Pharm Des. 2003;9:975–981. doi: 10.2174/1381612033455125. [DOI] [PubMed] [Google Scholar]
- 11.Umekawa T, Chegini N, Khan SR. Oxalate ions and calcium oxalate crystals stimulate MCP-1 expression by renal epithelial cells. Kidney Int. 2002;61:105–112. doi: 10.1046/j.1523-1755.2002.00106.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller C, Kennington L, Cooney R, Kohjimoto Y, Cao LC, Honeyman T, Pullman J, Jonassen J, Scheid C. Oxalate toxicity in renal epithelial cells: characteristics of apoptosis and necrosis. Toxicol Appl Pharmacol. 2000;162:132–141. doi: 10.1006/taap.1999.8835. [DOI] [PubMed] [Google Scholar]
- 13.Ciftcioglu N, Haddad RS, Golden DC, Morrison DR, McKay DS. A potential cause for kidney stone formation during space flights: enhanced growth of nanobacteria in microgravity. Kidney Int. 2005;67:483–491. doi: 10.1111/j.1523-1755.2005.67105.x. [DOI] [PubMed] [Google Scholar]
- 14.Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS. Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int. 2001;59:637–644. doi: 10.1046/j.1523-1755.2001.059002637.x. [DOI] [PubMed] [Google Scholar]
- 15.Schepers MS, Asselman M, Duim RA, Romijn JC, Schroder FH, Verkoelen CF. Pericellular matrix formation by renal tubule epithelial cells in relation to crystal binding. Nephron Exp Nephrol. 2003;94:e103–112. doi: 10.1159/000072028. [DOI] [PubMed] [Google Scholar]
- 16.Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res. 2003;31:3–9. doi: 10.1007/s00240-002-0286-x. [DOI] [PubMed] [Google Scholar]
- 17.Thamilselvan S, Byer KJ, Hackett RL, Khan SR. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol. 2000;164:224–229. [PubMed] [Google Scholar]
- 18.Huang MY, Chaturvedi LS, Koul S, Koul HK. Oxalate stimulates IL-6 production in HK-2 cells, a line of human renal proximal tubular epithelial cells. Kidney Int. 2005;68:497–503. doi: 10.1111/j.1523-1755.2005.00427.x. [DOI] [PubMed] [Google Scholar]
- 19.Hesse A, Heimbach D. Causes of phosphate stone formation and the importance of metaphylaxis by urinary acidification: a review. World J Urol. 1999;17:308–315. doi: 10.1007/s003450050152. [DOI] [PubMed] [Google Scholar]
- 20.Yasui T, Fujita K, Tozawa K, Asai K, Soji T, Kato T, Kohri K. Calcium oxalate crystal attachment to cultured rat kidney epithelial cell, NRK-52E. Urol Int. 2001;67:73–76. doi: 10.1159/000050949. [DOI] [PubMed] [Google Scholar]
- 21.Lieske JC, Farell G, Deganello S. The effect of ions at the surface of calcium oxalate monohydrate crystals on cell-crystal interactions. Urol Res. 2004;32:117–123. doi: 10.1007/s00240-003-0391-5. [DOI] [PubMed] [Google Scholar]


