Abstract
Background. Sauchinone has proved its anti-oxidant and anti-inflammatory properties in various animal tissues. This study sought to illustrate its regulatory nature on autophagy associated proteins (PI3K, ERK1/2, AMPK, and Beclin-1) during early cardiomyocyte ischemia and subsequent reperfusion. Methods. Cultured cardiomyocytes were subjected to simulated Ischemia/reperfusion with and without Sauchinone pretreatment and also in the presence of autophagy inhibitor (3-MA). Colorimetric analysis of CCK-8, LDH antibody assay as well as Western blot analysis were performed to observe the expressions of LC3B (II) and Beclin-1 protein (markers of autophagy), autophagy proteins (PI3K, ERK1/2 and AMPK) and apoptotic proteins (Bax and Bcl-2) and the results were quantified into their grey values and subjected to statistical analysis. Results. Sauchinone demonstrated cell survival enhancing properties with increase in CCK-8 (SD = 0.553±0.012) and decrease in LDH (SD = 0.183±0.054) expressions, both of which were best observed at test dose of 20 µmol/L. At this dose, there was increment in cellular autophagy as demonstrated by peaking of autophagy markers LC3B-II (p<0.05) and Beclin-1 (p<0.05) with strong correlations (r = 0.99). Similarly, the autophagy proteins, compared to control and I/R model, also showed a significant increased level with PI3K (p<0.0001), total p-ERK1/2 (p<0.0001) and p-AMPKα (p<0.0001). Simultaneously, a decrease in expressions of pro-apoptotic molecules Bax (r = 0.989, p<0.0001) with increment of in the anti-apoptotic protein Bcl-2 (r = 0.996, p<0.0001) was observed. The observed effects on cell density, viability and autophagy was abrogated in presence of 3-MA. Conclusions. Sauchinone enhances cell survival by promoting autophagy and inhibiting apoptosis in cardiomyocytes during early stages of Ischemia/reperfusion injury.
Keywords: Sauchinone, autophagy protein, ischemia-reperfusion injury, apoptosis
Introduction
Saururus chinensis-the parent plant
Saururus chinensis (Latin: Saururus = reptile’s tail, and chinensis = from china) is a perennial herb which was first described by Joao de Leureiro (Portuguese botanist) in 1790, in the book Flora Cochinchinensis. It is found in wet places of China, Taiwan, India, Japan, Korea, the Philippines and Vietnam. Various parts of the plant has been long used in Chinese folk medicine as- diuretic, purgative, laxative, anti-helminthic, anti-malarial and antiseptic. Sauchinone is one of the active lignan isolated from the plant that has documented antioxidant and anti-inflammatory properties in several physiopathological processes viz. -neuropathy [1,2], neuro-degeneration [3], hepatitis [4] airway allergic diseases [5], cancer, sepsis [6], inflammation [7,8] and bacterial infection [9]. In cardiovascular diseases, these properties have been demonstrated in varieties of pathologies associated with dilated cardiomyopathies [10,11], valvular heart diseases [12] and ischemia/reperfusion injuries (IRI) [13,14]. As such, explicit effects of this lignin have been the pinned down to its interaction with various intracellular processes chiefly- autophagy and apoptosis.
With regards to IRI, the intracellular events of ischemia (due to arterial occlusion) as well reperfusion (by opening up an occluded coronary vessel) are largely attributed to autophagy and accounts for cardiomyocyte loss. This loss is responsible for the subsequent ventricular dysfunction, ventricular arrhythmias and heart failure [15] and contributes to 40-50% of the final infarct size [16]. In the pretext of absent or slow cardiomyocyte regeneration rate with 1% renewal annually at the age of 25 that declines to 0.45% at the age of 75 [17], the life of individual cardiomyocyte is of paramount importance and autophagy decides the cell fate during I/R stress. Autophagy has been classified as type II cell death process [18] and shares similarities with other cell death processes regarding the final outcome. However, unlike other cell death processes it also comprises of intricate micro processes that help cells to survive. Thus the process of autophagy has been labelled as ‘a double edged sword’ in numerous researches. Further exploring the nature and mechanisms of key mediator proteins we find the actual purpose of autophagy as ensuring energy homeostasis, during stressful condition. On commencement of stress, the process initiates by degrading non-essential cellular organelles to generate substrates for recycling ATP, which replenishes the energy shortage. But with protracted or severe stress, protracted autophagy tries to meet the energy crisis by degrading whatever it finds and this leads to sacrifice of vital intracellular organelles that lead to cell death and functional impairment [19,20]. Thus we can perceive autophagy as a process of ‘cell death while trying to survive’. The mediator proteins of autophagy are the guiding force that either activate or inhibit pathways that lead to cell survival or cell death. These mediator proteins guide both the pathways through its representative machinery that passes through a sequence of phagophore induction, nucleation, autophagy vesicle formation, engulfment of organelles, transport of cargo, union with lysosomal membrane, fusion with the lysosomal membrane and final lysosomal enzyme mediated degradation of the cargo. The nature and availability of cargo also determines the ultimate cell fate. In this study we will be focusing on some of the mediator proteins, henceforth termed as survival proteins of autophagy (SPA), that strive toward cell survival namely phosphatidylinositol 3-phosphate kinase (PI3K), extra-cellular signal-regulated kinases (ERK), 5 ‘adenosine monophosphate- activated Protein kinase (AMPK) and Beclin-1 as studies conducted earlier have verified their cytoprotective roles during early ischemic stress [21]. Additionally, we shall also be discussing the role of beclin-1 during reperfusion which have proved to be detrimental to cardiomyocyte [22].
Sauchinone has been found to exert its beneficial effects by manipulation of different mediator molecules in extra cardiac tissues. However, in cardiovascular studies, only a handful of molecules have been explored that too pertaining to death signals generating mediator proteins. The cardioprotective effects of the drug has been demonstrated by impeding the death pathway that comprise p38 mitogen activated protein kinase (MAPK) and c-Jun N-terminal kinases (JNK) [23]. However, a serious lack in studies exists that explores its regulatory influences on survival enhancing autophagy proteins. Thus being plagued by sauchinone’s extensive antioxidant and anti-inflammatory properties, we sought to explore its’ possible linkage with SPA. Acknowledgement of the positive boost in the SPA levels, would definitely lead to cardiomyocyte protection during early stages of cardiac ischemia. This regulation would surely be important in limiting I/R injury to each cardiomyocyte, the key goal of this research. Our reinforcements to the proposed hypothesis- ‘sauchinone enhances expression of SPA (PI3K, total p-ERK 1/2, p-AMPKα and beclin-1) exponentially in cardiomyocyte labels itself as cardioprotective drug’ is expected to be informative and applicatory for future researches so as to curb IRI, an unmet clinical need.
Materials and methods
The procedural flow chart of the whole experiment that comprised of 8 steps is outline in Table 1
Table 1.
Procedural flow chart of the experiment
| Steps | Target Observation | Target | Reagent/Assay | Method | Interpretations |
|---|---|---|---|---|---|
| Step I | Culture rat cardiac cell line H9c2 | ||||
| Steps II | Group division into Control (A), I/R (B) and I/R + Sauchinone at different concentration (C) | ||||
| Step III | Cell Density | Optical density | CCK-8 | Colorimetry | Impact on overall cell density. Optimal dose of effects for subsequent experiments (Cn) |
| Cell Survival | LDH | Antibody | Colorimetry | Impact of Cn on cell survival | |
| Step IV | Autophagy | LC3B (II) | Antibody | WB | Impact of Cn on the markers of autophagy |
| Beclin-1 | Antibody | WB | |||
| Step V | Autophagy proteins | PI3K Total p-ERK p-AMPKα | Antibody | WB | Impact of Cn on autophagy mediator proteins |
| Step VI | Apoptotic proteins | Bax Bcl-2 | Antibody | WB | Impact of Cn on apoptosis |
| Step VII | Positive Control by 3-MA | PI3K, LC3B(II), Optical density LDH | Antibody | WB | All the effects demonstrated by Cn are inhibited in the presence of positive control or not? |
| Antibody | WB | ||||
| CCK-8 | Colorimetry | ||||
| Antibody | Colorimetry | ||||
| Step VIII | Statistical analysis, graphical presentation and pictorial illustration of the result. | ||||
The proteins under study are LC3B(II), Beclin-1, PI3K, total p-ERK (1/2), p-AMPKα, Bax and Bcl-2. Colorimetry and Western blot analysis are the laboratory methods used for conducting this experiment. Aside from CCK-8, antibodies are the reagents used. The experiment flows from step 1 to 8. [Note: the third step also determines the optimal dose of sauchinone (Cn) and the subsequent experiments are conducted upto this value so as to save time, effort and expenses].
Reagents
Sauchinone was purchased from Beijing Laiyao biotechnology, China. Cell counting kit-8 (CCK-8) and Lactate dehydrogenase (LDH) antibody was purchased from Nanjing Jian Cheng Bioengineering Institute, China. Rabbit polyclonal antibodies to PI3K p85 (phospho Y607), Beclin-1 (ab62557) and Bax (ab7977) were purchased from Abcam biotechnology, UK. Similarly Rabbit polyclonal antibodies against LC3B, phenyl-methanesulfonyl fluoride (PMSF) was purchased from Sigma Aldrich, USA. Rabbit polyclonal antibodies against phospho-AMPKα (Thr172) and Bcl-2 (BCL2L10), along with rabbit monoclonal antibodies to p-ERK 1/2 (phosphor-p44/42 MAPK) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH-14C10) were purchased from Cell Signaling Technology, Inc. Shanghai, China. Horseraddish peroxidase (HRP), sodium dodecyl sulfate-polyacrylamide gel elctrophoresis (SDS-PAGE) kit, 5×SDS loading buffer, dimethyl sulfoxide (DMSO) and mouse anti-goat (HRP) conjugated secondary antibody were purchased from Beyotime biotechnology, China while polyinylidene difluoride membranes (PVDF), bicinchoninic acid (BCA) kit, WB marker, enhanced chemiluminescence (ECL), Dulbecco minimum essential medium (DMEM) solution, fetal bovine serum (FBS) and pancreatin from Thermo Fisher scientific, Inc., USA. 3methyladenine (3-MA) was purchased from Selleck Chemicals Co. Ltd., USA.
Cell culture-I/R model
Rat cardiac cell lines, H9c2 (purchased from the Fu Xiang, Shanghai, China) were incubated in DMEM with high glucose content and 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin in culture flask at 37°C in an incubator with 5% CO2. Suspended cells were plated at a density of 1.0×105 cells/cm2 in multiple-well plates. H9c2 cardiac cells were randomized to control- group A, I/R model- group B and treatment model group-C (I/R + sauchinone at doses of (1, 5, 10, 15, 20, 25, 30, 35 µmol/L) and were subjected to 8 hours of hypoxia followed by 2 hours of reoxygenation at 37°C. To simulate ischemia, the cell culture medium of test groups were placed in Tyrode’s solution containing the following (in mmol/L): 130 NaCl, 5 KCl, 10 HEPES, 1 MgCl2, 1.8 CaCl2 at pH 7.4/37°C; Then the sample were exposed to hypoxia in a controlled hypoxic chamber by 95% Nitrogen and 5% CO2 gas mixture, flushing up to partial O2 pressure of 0.5% to 1%. Reoxygenation was executed in a normoxic incubator at 37°C by replacing the ischemia medium for 2 hours with normal cell culture medium. The control group was coped with normal situation for 14 hours consistently.
Cell viability, CCK-8 assay
Cultured cardioyocytes were plated in 96-well sterile plastic plates. All the 3 groups were then subjected to CCK-8 assay as well as LDH antibody assay. This revealed different cellular response to groups A, B and C. CCK-8 was added to each well of 10 µL after reoxygenation and incubated for 4 hours at 37°C before measuring. Absorbance at 490 nm was read spectrophotometrically using a microplate reader (Multiskan MK3, Thermo, America). The best positive anticipated cellular response visualized by optical density (OD) to the highest achievable dose of sauchinone (Cn) was determined and subsequent protein analysis by western blot analysis was conducted upto this Cn. Additionally, observations were also made in the presence of 3-MA (PI3K inhibitor) and the results deducted.
Western Blotting assay of different proteins
Cell lysate of cultured cardiomyocytes in the three groups (A, B and Cs) was prepared by homogenization of radioimmunoprecipitation assay (RIPA) buffer containing 100 mmol/L of 1% PMSF and the sample, lysed on ice for 30 minutes. These homogenates were then centrifuged at 12000 rpm for 30 minutes at 4°C. Protein amount of the supernatant was measured by BCA protein assay kit according to the product’s instruction. Protein samples were diluted with 4-fold 5×SDS loading buffer and then boiled for 10 minutes and stored at -20°C. Equal amounts of protein (30 or 40 μg) from each sample were resolved by SDS-PAGE and electro-blotted onto a PVDFM. Then these membranes were blocked with 5% bovine serum albumin in Tris-buffered saline for 3 hours at room temperature. Subsequently, the PVDFM were incubated with anti-LC3B (1:1000 dilution) and anti-Beclin1 (1:1000 dilution). Similarly, the membranes were incubated with anti-phosphorylated PI3K antibody (1:1000 dilution), anti-phosphorylated ERK antibody (1:500 dilutions), anti phospho AMPKα antibody (1:1000 dilutions) which were the autophagy associated proteins; Similarly following the same procedures, PVDF membranes were also incubated with anti-phosphorylated Bax antibody (1:1000 dilution), anti-Bcl2 antibody (1:500 dilution). All of these PVDF membranes incubated overnight at 4°C. The loading control used was anti GAPDH antibody at 1:1000 dilutions. Next day the membranes were again incubated with mouse anti-goat HRP conjugated secondary antibody (1:10000 dilutions) for 1 hour at room temperature. The signals in the PVDF membrane were enhanced using a high-sensitive ECL kit after washing thrice for 5 minutes in Tris-buffered saline. The obtained WB bands were quantified into their grey values using the Image J software which were subjected to statistical analysis. Further, similar procedures and results were additionally deducted for PI3K and LC3B (II) proteins in the presence of 3-MA. All of these experiments were repeated in three samples with at least three times each to ensure reproducibility of the result.
Statistical analysis
Inter group differences in sauchinone treatment and non-treatment groups as well as between subgroups were analyzed using unpaired t test or one- way ANOVA followed by Tuckey post-hoc testing as appropriate. All the obtained datas were represented as mean ± SD among treatment groups using the original data. The changes in protein expression was correlated between control, I/R and treatment group using Pearson correlation. The p values of <0.05 (two tailed) was considered to be statistically significant. All statistical analysis was performed with the SPSS software (Statistical Package for the Social Sciences, version 16.0, SPSS Inc., Chicago, IL, USA). Graph pad prism software version 6.0 was used to construct all the figures.
Results
In all controls, a low level of expressions of different proteins under study was observed signifying basal autophagy. Our observations were chiefly focused on comparison and correlation of the increments and decrements of different proteins among different groups in reference to sauchinone 20 µmol/L group.
Impact on cardiomyocyte viability
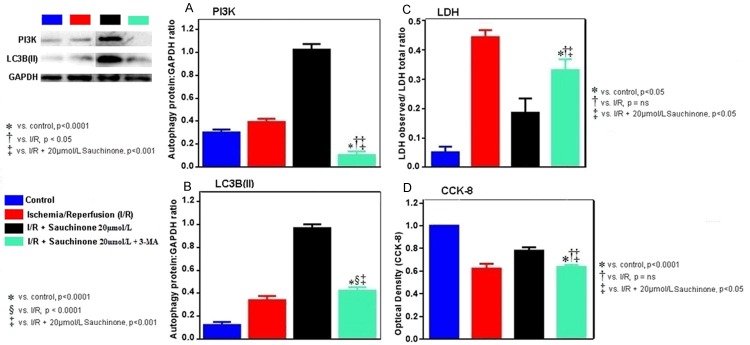
Figure 1A demonstrates colorimetric analysis of cardiomyocyte’s optical densities in different groups subjected to CCK-8 kit analysis. As compared to control mean (0.6632±0.0144), I/R model showed a decrease in OD mean (0.385±0.006) which however reversed with the treatment of sauchinone in a dose dependent manner (0.412±0.011 at 1 µmol/L, 0.435±0.021 at 5 µmol/L, 0.480±0.021 at 10 µmol/L, 0.501±0.015 at 15 µmol/L, 0.553±0.012 at 20 µmol/L) after which a decline in OD was noticed (0.487±0.014 at 25 µmol/L, 0.379±0.027 at 30 µmol/L, 0.351±0.025 at 35 µmol/L). The decrease in OD of I/R group compared to control (p<0.05) and the increment from I/R group to 20 µmol/L (p<0.05) were statistically significant and correlated strongly with lignan concentration (r = 0.996). Similarly the expressions of LDH demonstrated a reverse pattern (Figure 1B). As compared to control mean (0.052±0.019), the I/R model showed an increase in LDH levels (0.444±0.024), which decreased with the treatment in a dose dependent manner (0.421±0.019 at 1 µmol/L, 0.393±0.018 at 5 µmol/L, 0.341±0.014 at 10 µmol/L, 0.289±0.052 at 15 µmol/L and 0.183±0.054 at 20 µmol/L). Then progressive increments in the level of LDH was noticed (0.243±0.020 at 25 µmol/L, 0.282±0.025 at 30 µmol/L, 0.332±0.040 at 35 µmol/L). The increase in OD of I/R group compared to control (p<0.05) and the increment from I/R group to 20 µmol/L (p<0.05) were statistically significant and correlated strongly with lignan concentration (r = 0.971). Furthermore, the rise of LDH (cell death) from the control mean was 8.61 times towards I/R mean which subsequently fell 2.38 times with treatment. Similarly the calculated F value [(1.033, 2.066) = 88.10] of the test was found to be more than the critical value provided by the F-table. The 20 µmol/L drug concentration was used as the last reference value for subsequent experiments.
Figure 1.
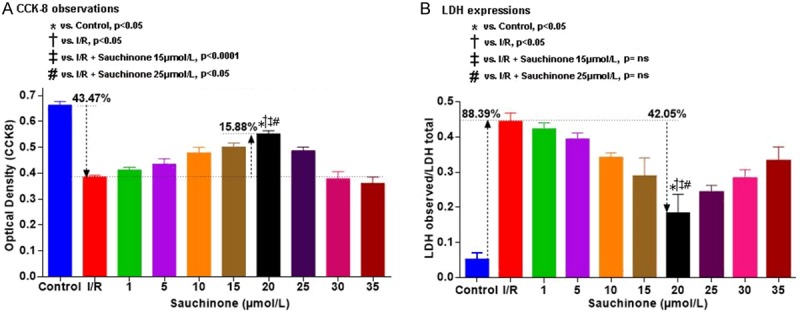
Graphical representation of colorimetric observation of CCK-8 (A) and LDH (B) expressions at different concentrations of sauchinone. (A) Demonstrates the initial decrement of optical densities of cells with I/R injury which on sauchinone treatment shows a dose dependent increment, attaining optimal concentration at 20 µmol/L followed by a decline. The maximal increment exhibited is statistically significant compared to the control (p<0.05), I/R group (p<0.05), 15 µmol/L group (p<0.0001) and the 25 µmol/L group (p<0.05). Out of the cell volume that is decreased (43.47%) with I/R, a rescue of 15.88% of the cell volume can be seen with the optimal dose. Similarly, (B) Depicts the expressions of LDH (cell death), where mean LDH level in I/R group rises 88.39% from the control means, out of which 42.05% of LDH is decreased with the optimal concentration of 20 µmol/L. The maximal decrements exhibited by the optimal dose is statistically significant compared to the control (p<0.05) and I/R (p<0.05) groups, while non-significant with the 15 µmol/L and 25 µmol/L groups.
Influence on autophagic markers LC3B(II) and Beclin-1 expression
It was observed that sauchinone boosts the expressions of LC3B(II) and Beclin-1 as depicted in Figure 2A and 2B respectively. As compared to control mean (0.123±0.026), LC3B(II) shows a small rise in I/R model (0.340±0.036) which sharply increases with treatment and peaks at 20 µmol/L (0.968±0.034). Similar increment in expression of beclin-1 was observed with means escalating from control (0.181±0.003) to I/R (0.49±0.032), attaining a peak at 20 µmol/L (0.97±0.033). Both the protein expressions within their groups were statistically significant (p<0.05) and the rise in means correlated strongly with the drug concentration (r = 1).
Figure 2.
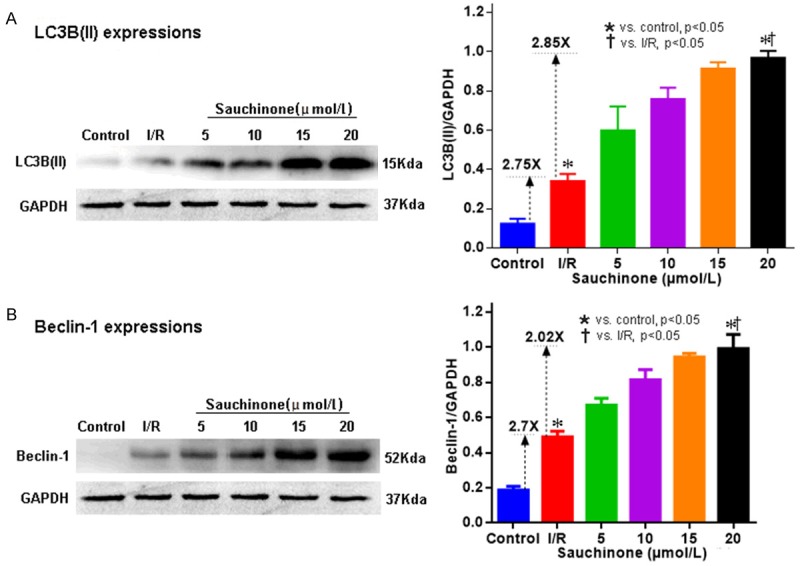
Western blot images and graphical representation of expressions of autophagy markers- LC3B(II) (A) and Beclin-1 (B), with sauchinone 20 µmol/L. (A) shows a sharp upsurge in the expressions of LC3B(II) with sauchinone 20 µmol/L treatment. Compared to the control mean, the rise in mean with I/R group is 2.75 X which further is propelled 2.85 X with optimal treatment. Similar results are seen with Beclin-1 (B), where the initial rise in mean of I/R group (2.7 X) from the control is further elevated (2.02 X) with optimal treatment. Increments of means seen at 20 µmol/L, of both proteins that signify autophagy enhancement, are statistically significant (p<0.05) when compared among respective subgroup means.
Effect on the expression of PI3K, total p-ERK, p-AMPKα proteins
Western blot assay with sauchinone treatment, as depicted in Figure 3A, revealed a rise in the expressions of PI3K (3B), p-ERK (1/2) (3C) and p-AMPKα (3D) as compared to their respective I/R and control groups. The mean observations measured between control: I/R: Sauchinone 20 µmol/L for PI3K, p-ERK (1/2) and p-AMPKα were- 0.0.302±0.026: 0.393±0.032: 1.025±0.048, 0.332±0.018: 0.418±0.015: 0.648±0.031 and 0.094±0.043: 0.198±0.035: 1.037±0.103 respectively.
Figure 3.

Western blot images (A) and graphical representation of the changes in expressions of autophagy proteins - p-PI3K (B), total p-ERK1/2 (C), p-AMPK (D), with sauchinone 20 µmol/L. (A-C) shows maximal change and upsurge in the expressions of autophagy proteins- PI3K, total p-ERK (1/2) and p-AMPKα respectively with sauchinone 20 µmol/L treatment. Compared to control, I/R and intra-sub groups, the increments observed have a statistical significant association (p<0.05). Moreover, the small rise noticed with mean values of respective I/R groups in all proteins, with the optimal dose administration, the expressions of PI3K, total p-ERK (1/2) and p-AMPKα were 2.61 X, 1.55 X and 5 X more respectively.
Moreover, the rise in the level of proteins as tabulated in Table 2, were statistically significant (p<0.001). The observed F values are more than the set critical limits for all 3 proteins, and the goodness of fit of datas (R2) as well as correlation with the changes in the variables are strong (approaching + 1).
Table 2.
Results of 1 way ANNOVA of different autophagy protein expression between control, I/R and sauchinone 20 µmol/L groups
| PI3K | Total p-ERK 1/2 | p-AMPKα | |
|---|---|---|---|
| F | 1.229 | (1.364, 12.28) = 1231 | (1.735, 15.62) = 540.1 |
| P value | <0.0001 | <0.0001 | <0.0001 |
| Statistically significant (P<0.05)? | Yes | Yes | Yes |
| R square | 0.9891 | 0.9927 | 0.9836 |
| Pearson (r) correlation | 0.997 | 0.996 | 0.989 |
Influence on apoptotic proteins Bax and Bcl-2 expression
During the experiment, we saw mixed results of this drug on apoptosis associated proteins. As illustrated in Figure 4 sauchinone at 20 µmol/L demonstrated an inhibitory effect on pro-apoptotic protein, Bax and promontory effect on anti-apoptotic protein, Bcl-2. As compared to control (0.139±0.029), Bax showed an increase in I/R model (0.985±0.091) which was sharply abrogated at 20 µmol/L (0.333±0.055). However, compared to control (0.385±0.017), Bcl-2 was significantly decreased in I/R model (0.333±0.023) but exhibited a sharp upsurge with treatment (0.736±0.024 at 20 µmol/L).
Figure 4.
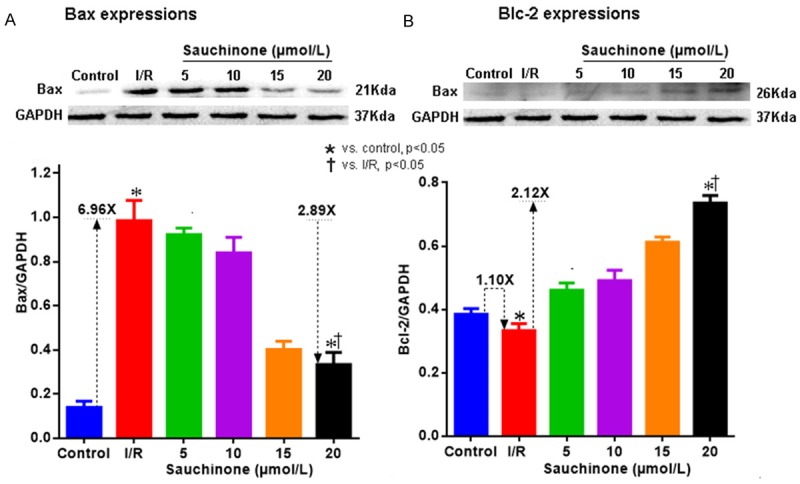
Western blot images and their respective graphical representations of apoptotic proteins -Bax (A) and Bcl-2 (B) with sauchinone 20 µmol/L. (A) Demonstrates increments of pro-apoptotic protein Bax in the I/R model group (increment of mean 6.96 times the control) which subsequently declines with Sauchinone 20 µmol/L treatment (decrement of mean 2.89 times from the peak I/R mean). Similarly (B) shows decrement of mean value of Bcl-2 in I/R group (1.10 times) which is revived and propelled 2.12 times more with optimal dose of sauchinone. All the observed values demonstrated a statistical significant association with the optimal levels of sauchinone (P<0.05 vs IR and P<0.05 vs control).
Moreover, as tabulated in Table 3, the rise in mean values between control, I/R and sauchinone group were statistically significant (p<0.0001), the observed F values were more than the set critical limits for the two proteins and the goodness of fit of datas (R2) as well as correlation with the changes in the variables were strong (approaching + 1). The observed F values are more than the set critical limits for the two proteins, signifying the validity of the hypothesis proposed-‘sauchinone 20 µmol/L inhibits pro-apoptotic and promotes anti-apoptotic protein expressions.’
Table 3.
Results of 1 way ANNOVA analysis of different apoptotic protein expression between control, I/R and sauchinone (20 µmol/L) groups
| Bax | Bcl-2 | |
|---|---|---|
| F | (1.125, 10.13) = 410.5 | (1.350, 12.15) = 1162 |
| P value | <0.0001 | <0.0001 |
| Statistically significance (p<0.05)? | Yes | Yes |
| R square | 0.979 | 0.992 |
| Pearson correlation | 0.989 | 0.996 |
Sauchinone’s activity in presence of 3MA (inhibitor of PI3K autophagy protein)
In the final part of the experiment, as seen in Figure 5, the effects of the lignin at 20 µmol/L and resultant autophagy was blunted by the addition of 3-MA. The decrease in means of LC3 (from 20 µmol/L (0.968±0.034) to sauchinone 20 µmol/L + 3-MA (0.422±0.031) approached that of I/R group (0.340±0.036) and PI3K decrements (1.025±0.048 at sauchinone 20 µmol/L to 0.104±0.032 at sauchinone 20 µmol/L + 3MA) also approached the levels of I/R group (0.393±0.028). As compared to rise of LC3B (II) and PI3K noticed earlier, the decrements were statistically significant (p<0.0001) compared to the control and sauchinone 20 µmol/L group, while as compared to the I/R groups, the drop in LC3B expression had lesser statistical association (p<0.05) than with PI3K expression (p<0.0001) and these changes strongly correlated (r = + 1) with the addition of 3MA. Similar abrogating effects of 3-MA was observed in the levels of optical densities (decrements of 0.636±0.032 from 0.780±0.052) as well as LDH levels (increments of 0.332±0.037 from 0.187±0.048). Additionally these changes were statistically insignificant compared to I/R groups. In relation to by increased LDH expressions, with the addition of 3-MA, statistical association was same with the control and suachinone treatment groups (p<0.05) while in regards to optical densities signifying the volume of live cells, statistical correlation was more with the control (p<0.0001) group than the treatment group (p<0.05)). There was no statistical association with the 3-MA group and I/R groups of both LDH and CCK-8 samples. These changes strongly correlated (r = + 1) with the changes observed after the addition of 3-MA.
Figure 5.
Graphical representation and western blot images of PI3K (A), LC-3 (B), Cellular volumes (C) and LDH (D) in presence of 3-MA (PI3K inhibitor) with sauchinone 20 µmol/L. (A and B) reveals the abrogating effect of 3-MA on the positive changes observed earlier with sauchinon 20 µmol/L on PI3K and LC3B (II) respectively. In regard to the changes observed on these two proteins, the mean values between the control and sauchinone 20 µmol/L groups demonstrate same statistical association with the levels of 3-MA group (p<0.001) while the expression of LC3B (II) is more statistically significant (p<0.0001) than the expressions of PI3K in the I/R model group (p<0.05). Similar inhibitory effects of 3-MA is seen in LDH expression (C) and cell volume (D). The restoration of LDH expression, signifying cell death with 3-MA is observed that is statistically same with the control as well as sauchinone 20 µmol/L (p<0.05) group. Likewise, the decrement in the optical density (meaning less cell) with 3-MA is statistically more significant in the control group (p<0.0001) than in the sauchinone 20 µmol/L group (p<0.05).
Discussion
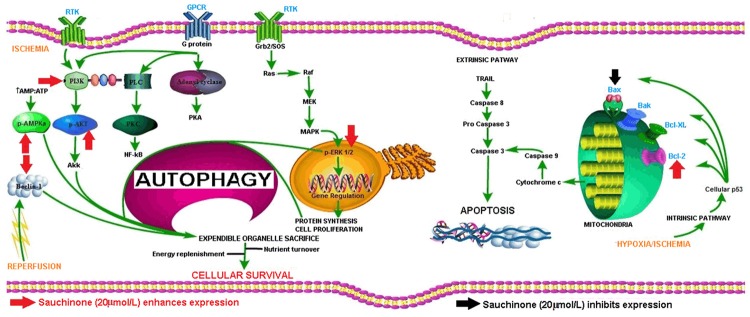
In the present study, we found that sauchinone enhanced the expression of beclin-1 and LC3, increased the levels of PI3K, total p-ERK, p-AMPKα and Bcl-2 and suppressed the expression of Bax in cultured myocytes. More importantly, it enhanced the cell viability in a dose dependent manner and the optimal dose that brought about the changes was 20 µmol/L. A pictorial illustration of the various steps of autophagy and sauchinone’s targets are presented in Figure 6.
Figure 6.
Overview of autophagy process, apoptosis process and the site of action of sauchinone. Autophagy process starts with energy depletion which leads to the activation of AMPK which is modulated by liver kinase B1 (LKB1), Ca2+/calmodulin-dependent kinase kinase β (CaMKKβ) and transforming growth factor β activated kinase-1 (TAK1). AMPK stimulates p27 gene that manufactures autophagy proteins, also inhibits mTORC1 (inhibitor of autophagy related protein 13) and activation of Beclin-1 through modulations of ULk1, ATG 13 and FIP200. Similarly Hypoxia promotes Beclin-1 and PI3K class III interaction that leads to induction of phagopohre, a building block of autophagosome. This interaction is also promoted by increased Ca2+ and ROS. Similarly RTK coupled activation of Grb2/SOS leads to activation in succession of Ras, Raf, MEK, MAPK and finally ERK1/2 which subsequently leads to regulation of DNA and synthesis of proteins as well as new cell turnover. These proteins are used for phagophore synthesis. Then by series of steps involving many mediator proteins, phagophore elongates and engulfs target organelles. After which the fully formed autophagosome transports the cargo upto the lysosome where fusion of its membrane with lysosomal membrane occurs with subsequent release of lysosomal degradative enzymes and breakdown of autophagosome as well as the contents into simpler sugars, fatty acids, amino acids and constitutive proteins, all of which are recycled back. Simultaneously, hypoxia and ischemia activates the intrinsic pathway of apoptosis with generation and activation of different proteins like Bax, Bak, Bcl-xl, Bcl-2. Bax after activation increases mitochondrial outer wall permeability with release of cytochrome c and subsequent activation of caspase 9 that ultimately activates caspase 3, the final effector of apoptosis. Also the extrinsic pathway can activate caspase 3 through activation of caspase 8. [Note-Red arrows and black arrows are the site of stimulatory and inhibitory actions respectively, Green arrows are the flow of reaction, red blunt arrow are the inhibitory pathway].
Impact on cell viability
Our study demonstrated sauchinone’s positive regulatory impact on cell density as well as in the amount of live cells. Cell density depends on the cell volume which is contributed by load of live cells that can be specifically traced by cell counting kit (CCK-8). Sauchinone’s cell density alleviating effect was noticed in this experiment. Similarly, LDH is an important marker that increases with increased number of injured cells and indirectly tells us about viable cell amount status. The upsurge of LDH in this study, seen with I/R stress and the drop in LDH seen with treatment signifies the inflation of viable cells. In a satellite view, we observed sauchinone’s capacity to maximize the number and volume of live cells in absence of any other confounding factors that could contribute to enhanced cellular sustenance. After witnessing this increments in cardiomyocyte’s longevity and entity, we further dwelled into finding an answer to ‘was this elaboration of cell viability due to autophagy?’
Impact on cardiac autophagy
It was seen that concomitant to enhanced cell vitality, the autophagy was taking place in both the control group and I/R group signifying basal autophagy and stressed autophagy respectively. The small rise noticed with I/R group when correlated with the cell survival parameters was occurring as attempts of survival were being made by the cell to some extent. This insufficient and insignificant attempts were seen to be amplified and boomed with treatment of sauchinone. The mean increments of markers of cellular autophagy [24] -LC3B (II) and beclin-1 from the control to I/R and to that of treatment groups were statistically significant and correlated strongly. Thus confirming the autophagy decorum as the target of sauchinone, we further explored the intricate autophagy proteins that were making these changes. We acknowledged amplications of autophagy proteins -PI3K-class III, total p-ERK, p-AMPKα, beclin-1. PI3K-class III [25] that in association with beclin-1 and vps 34 regulates phagophore induction (building block of autophagosome) while ERK1/2 influences autophagosome formation [26] and regulates gene expression for cellular turnover and autophagy protein synthesis. Sauchinone can be said to be cardioprotective as many studies have identified the mere activation of PI3K and ERK (1/2) is beneficial and cardioprotective in in vivo I/R models [27,28] while induction of autophagy alone can rescue heart functioning in IRI [29]. Similarly, AMPK is an intracellular energy deficit sensor that through a series of activations of ULK1, autophagy related protein -13 (ATG13) and 200KD focal adenosine kinase (FAK) family kinase-interacting protein (FIP200), activates Beclin-1 leading to phagophore induction and nucleation and address energy crisis [30] and ensure cardiomyocyte sustenance [31,32]. These proteins were actively working so as to execute autophagy and the overall cell survival and sauchinone was boosting their expressions. The direct link of sauchinone with these autophagy proteins were further established when an inhibitor of one of the proteins (PI3K)-3-MA was able to abrogate the overall effects of sauchinone on autophagy proteins, autophagy markers and cell survival as depicted in Figure 5. Furthermore, sauchinone was also seen to bolster and complement the expression of beclin-1, an important protein imperative for nucleation of phagophore as well as fusion of autophagosomal and lysosomal membranes at the terminal stages [33,34]. Beclin-1 however unlike other proteins, demonstrates an unusual rise with reperfusion signifying ongoing autophagy [35], which theoretically should have been silenced with reperfusion. This process of ongoing autophagy mediated by beclin-1 has been associated with cell death process [36-39]. Till date, the actual cause, trigger and explicit purpose for the latter rise and ongoing autophagy amid such nutrient plethora are still at large. Our study also witnessed a persistent inflation of beclin-1 during I/R model and with treatment. However, simultaneous to the rise in beclin-1 levels, the treatment group analogously demonstrated a drop in pro-apoptotic protein (discussed below) and a rise in the volume of live cells. This correlation has made us rethink about its explicit negative role during autophagy when placed alongside sauchinone. In this regards, we feel that sauchinone causes upsurge of beclin-1 for the alimentation of autophagy at levels that are compatible with cellular perpetuity rather than cessation. Our opinion regarding beclin-1 is also in alignment with similar possibility presented in recent studies [21]. Among the autophagy proteins we find one study that has highlighted results that contravenes with our results regarding p-ERK. Unlike the propellant effect of sauchinone on p-ERK noticed by us, they have demonstrated no such effect [36] and we feel that the differences could be attributed to - the effective optimal dose (20 µmol/L vs.10 mg/kg), choice of model (uninfected purified cell culture vs. associated chronic co-morbidity’s chronic influences) and the appreciation of a positive control. Thus we would cautiously suggest that the interactions of sauchinone and autophagy proteins needs to be translated into animal studies and the pharmacokinetic along with pharmacodynamics properties need to be explored more during IRI. Nonetheless, based on our study with cultured cardiomyocytes, we can say that sauchinone strengthens survival armory, boosts the vitality of a cardiomyocyte by enhancing expression of PI3K, total p-ERK, p-AMPKα and beclin-1 during IRI in a cell culture model.
Cardiac apoptosis
Exploring previous researches, we found that IRI activated apoptosis cell death process especially during prolonged periods of myocardial ischemia and reperfusion [40-42]. As depicted in Figure 6, it is a common knowledge that during hypoxia/ischemia bax protein (among others) is activated that results in release of cytochrome c, which subsequently activates caspase 9 while during reperfusion both caspases 8 and 9 are activated [43]. Both these caspases trigger caspase 3, the final effector of apoptosis. Our study appreciated the inhibitory effect of sauchinone on Bax protein thus stabilizing the outer mitochondrial membrane and hindering the release of Cytochrome c and resultant apoptosis. Furthermore, by impeding the release of caspases, the lignan relieved its oppressive actions on autophagy related proteins viz., Beclin-1, vps34, Atg3, Atg4D, autophagy/Beclin-1 regulator 1 (AMBRA1) and p62 [44], further aiding in autophagy. Similarly, sauchinone was seen to boost the expression of Bcl-2 protein (that in physiology sequesters Bax and Bak proteins) and led to stabilization of outer mitochondrial membrane [45] and cytochrome c release, further inhibiting apoptosis. Further studies have demonstrated enhanced cell survival by inhibition of Bax [46] expressions as well as reduction in cardiac cell death [47] and infarct size [48] with increased Bcl2 expressions during IRI. Thus by curbing the expression of pro-apoptotic molecules-Bax and contemplating the elucidation of Bcl-2, sauchinone directly hinders apoptosis process and indirectly aids autophagy process, both protecting a cardiomyocyte against IRI.
Limitations
Direct counting of cell was not done and viability observations were based on turbidometry and optical densities. Bacterial contamination is a common occurrence with cell cultures that can influence the viability of cells and molecules. The loading buffer GAPDH although considered inert during oxidative stress, can itself initiate apoptosis [49]. Similarly the Statistical analysis, statistical association and correlation cannot be linked to causation theory and F test used to justify the hypothesis can give rise to type I error (that is detecting an effect that is not present). This study is also limited to a cell culture model due to the explorative in the background of unknown influences of the drug that needs translation into animal live models and harvested tissues before gaining access to a trial. Further identification of intermediary proteins or intracellular receptors or the amino acid domains of PI3K, ERK, AMPK or Beclin-1 could specifically make its therapeutic application reliable. This would require a multidisciplinary coordinated research so as to combat IRI and ensure cardio-protection.
Translational outlook
IRI stems down to molecular mediators and different pathways of autophagy and apoptosis. Activation of RISK pathways as well as phagophore forming influences like Beclin-1, AMPK results in survival efforts while inducement of JNK and MAPK kinases along with Bax results in cellular death. Sauchinone had positive influences on autophagy for cell sustenance and suppression of apoptosis. Administration of sauchinone during the initial stages of IRI in H9c2 cardiac cell is found beneficial in this studies and we plan to carry this experiment in live tissues harvested from animal and observe morphological changes in animal heart. This research lies in the continuum of seeking remedy for IRI and sauchinone shows promising preliminary results in helping each cardiomyocyte sustain their livelihood in the background of terminally differentiated cell pool.
In summary, best effects of sauchinone that are observed at concentrations of 20 µmol/L, increases autophagy proteins, promotes the process of autophagy and inhibits cardiomyocyte apoptosis. With these beneficial properties, sauchinone could be used as a therapeutic drug that offers cardioprotection from early ischemia-reperfusion injury.
Acknowledgements
Supported by Grants-in-aid for scientific research (No. 1508085MH145) from the Natural Scientific Research foundation of Anhui Province, Hefei-230032, Anhui, China.
Disclosure of conflict of interest
None.
References
- 1.Choi IY, Yan H, Park YK, Kim WK. Sauchinone reduces oxygen-glucose deprivation-evoked neuronal cell death via suppression of intracellular radical production. Arch Pharm Res. 2009;32:1599–1606. doi: 10.1007/s12272-009-2113-1. [DOI] [PubMed] [Google Scholar]
- 2.Jang EY, Park KA, Lee JR, Yang CH, Hwang M. Protective effect of sauchinone on methamphetamine-induced neurotoxicity in mice. J Pharmacol Sci. 2012;118:531–536. doi: 10.1254/jphs.11207sc. [DOI] [PubMed] [Google Scholar]
- 3.Song SY, Jung YY, Hwang CJ, Lee HP, Sok CH, Kim JH, Lee SM, Seo HO, Hyun BK, Choi DY, Han SB, Ham YW, Hwang BY, Hong JT. Inhibitory effect of ent-Sauchinone on amyloidogenesis via inhibition of STAT3-mediated NF-κB activation in cultured astrocytes and microglial BV-2 cells. J Neuroinflammation. 2014;11:118. doi: 10.1186/1742-2094-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YW, Lee SM, Shin SM, Hwang SJ, Brooks JS, Kang HE, Lee MG, Kim SC, Kim SG. Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury. Free Radic Biol Med. 2009;47:1082–1092. doi: 10.1016/j.freeradbiomed.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Min HJ, Won HY, Kim YC, Sung SH, Byun MR, Hwang JH, Hong JH, Hwang ES. Suppression of Th2-driven, allergen-induced airway inflammation by sauchinone. Biochem Biophys Res Commun. 2009;385:204–209. doi: 10.1016/j.bbrc.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 6.Seo CS, Lee YK, Kim YJ, Jung JS, Jahng Y, Chang HW, Song DK, Son JK. Protective effect of lignans against sepsis from the roots of Saururus chinensis. Biol Pharm Bull. 2008;31:523–526. doi: 10.1248/bpb.31.523. [DOI] [PubMed] [Google Scholar]
- 7.Hwang BY, Lee JH, Jung HS, Kim KS, Nam JB, Hong YS, Paik SG, Lee JJ. Sauchinone, a lignan from Saururus chinensis, suppresses iNOS expression through the inhibition of transactivation activity of RelA of NF-kappaB. Planta Med. 2003;69:1096–1101. doi: 10.1055/s-2003-45189. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Lee DS, Choi HG, Kim KS, Kang DG, Lee HS, Jeong GS, Kim YC. Sauchinone suppresses pro-inflammatory mediators by inducing heme oxygenase-1 in RAW264.7 macrophages. Biol Pharm Bull. 2011;34:1566–1571. doi: 10.1248/bpb.34.1566. [DOI] [PubMed] [Google Scholar]
- 9.Jeong KM, Choi JI, Lee SH, Lee HJ, Son JK, Seo CS, Song SW, Kwak SH, Bae HB. Effect of sauchinone, a lignan from Saururus chinensis, on bacterial phagocytosis by macrophages. Eur J Pharmacol. 2014;728:176–182. doi: 10.1016/j.ejphar.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 10.Knaapen MW, Davies MJ, De Bie M, Haven AJ, Martinet W, Kockx MM. Apoptotic versus autophagic cell death in heart failure. Cardiovasc Res. 2001;51:304–312. doi: 10.1016/s0008-6363(01)00290-5. [DOI] [PubMed] [Google Scholar]
- 11.Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 12.Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 13.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsasser A, Vogt AM, Nef H, Kostin S, Möllmann H, Skwara W, Bode C, Hamm C, Schaper J. Human hibernating myocardium is jeopardized by apoptotic and autophagic cell death. J Am Coll Cardiol. 2004;43:2191–2199. doi: 10.1016/j.jacc.2004.02.053. [DOI] [PubMed] [Google Scholar]
- 15.Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circ Res. 2008;103:1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulda S. Autophagy and cell death. Autophagy. 2012;8:1250–1251. doi: 10.4161/auto.20669. [DOI] [PubMed] [Google Scholar]
- 19.Platini F, Perez-Tomas R, Ambrosio S, Tessitore L. Understanding autophagy in cell death control. Curr Pharm Des. 2010;16:101–113. doi: 10.2174/138161210789941810. [DOI] [PubMed] [Google Scholar]
- 20.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 22.Sciarretta S, Hariharan N, Monden Y, Zablocki D, Sadoshima J. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr Cardiol. 2011;32:275–281. doi: 10.1007/s00246-010-9855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SJ, Jeong CW, Bae HB, Kwak SH, Son JK, Seo CS, Lee HJ, Lee J, Yoo KY. Protective effect of sauchinone against regional myocardial ischemia/reperfusion injury: inhibition of p38 MAPK and JNK death signaling pathways. J Korean Med Sci. 2012;27:572–575. doi: 10.3346/jkms.2012.27.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;9:5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Ravingerova T, Matejikova J, Neckar J, Andelova E, Kolar F. Differential role of PI3K/Akt pathway in the infarct size limitation and antiarrhythmic protection in the rat heart. Mol Cell Biochem. 2007;297:111–120. doi: 10.1007/s11010-006-9335-z. [DOI] [PubMed] [Google Scholar]
- 28.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouysségur J, Pagès G, De Windt LJ, Doevendans PA, Molkentin JD. The MEK1-ERK2 signaling pathway protects the myocardium from ischemic damage in vivo. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 29.Gottlieb RA, Mentzer RM Jr. Cardioprotection through autophagy: ready for clinical trial? Autophagy. 2011;7:434–435. doi: 10.4161/auto.7.4.14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardie DG. The AMP-activated protein kinase pathway-new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, Lopez BL, Christopher TA, Tian R, Koch W, Ma XL. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119:835–844. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 33.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 34.Oyabu J, Yamaguchi O, Hikoso S, Takeda T, Oka T, Murakawa T, Yasui H, Ueda H, Nakayama H, Taneike M, Omiya S, Shah AM, Nishida K, Otsu K. Autophagy-mediated degradation is necessary for regression of cardiac hypertrophy during ventricular unloading. Biochem Biophys Res Commun. 2013;441:787–792. doi: 10.1016/j.bbrc.2013.10.135. [DOI] [PubMed] [Google Scholar]
- 35.Matsui Y, Kyoi S, Takagi H, Hsu CP, Hariharan N, Ago T, Vatner SF, Sadoshima J. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo R, Berliocchi L, Adornetto A, Varano GP, Cavaliere F, Nucci C, Rotiroti D, Morrone LA, Bagetta G, Corasaniti MT. Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2011;2:e144. doi: 10.1038/cddis.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 38.Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy. 2011;7:1115–1131. doi: 10.4161/auto.7.10.16608. [DOI] [PubMed] [Google Scholar]
- 39.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2003;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Dumont EA, Hofstra L, van Heerde WL, van den Eijnde S, Doevendans PA, DeMuinck E, Daemen MA, Smits JF, Frederik P, Wellens HJ, Daemen MJ, Reutelingsperger CP. Cardiomyocyte death induced by myocardial ischemia and reperfusion: measurement with recombinant human Annexin-V in a mouse model. Circulation. 2000;102:1564–1568. doi: 10.1161/01.cir.102.13.1564. [DOI] [PubMed] [Google Scholar]
- 42.Dumont EA, Reutelingsperger CPM, Smits JF, Daemen MJAP, Doevendans PAF, Wellens HJJ, Hofstra L. Real-time imaging of apoptotic cell-membrane changes at the single-cell level in the beating murine heart. Nat Med. 2001;7:1352–1355. doi: 10.1038/nm1201-1352. [DOI] [PubMed] [Google Scholar]
- 43.Stephanou A, Brar BK, Liao Z, Scarabelli TM, Knight RA, Latchman DS. Distinct initiator caspases are required for the induction of apoptosis in cardiac myocytes during ischaemia versus reperfusion injury. Cell Death Differ. 2001;8:434–435. doi: 10.1038/sj.cdd.4400846. [DOI] [PubMed] [Google Scholar]
- 44.Norman JM, Cohen GM, Bampton ET. The in vitro cleavage of the hAtg proteins by cell death proteases. Autophagy. 2010;6:1042–1056. doi: 10.4161/auto.6.8.13337. [DOI] [PubMed] [Google Scholar]
- 45.Jacobson MD, Burne JF, King MP, Miyashita T, Reed JC, Raff MC. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993;361:365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- 46.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 47.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 48.McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H1923–H1935. doi: 10.1152/ajpheart.00935.2003. [DOI] [PubMed] [Google Scholar]
- 49.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]