Abstract
Aberrant expression of programmed death-1 (PD-1) receptor/PD-1 ligand (PD-L1) proteins alters human immunoresponse and promotes tumor development and progression. We assessed the expression status of PD-1 and PD-L1 in spinal chordoma tissue specimens and their association with clinicopathological characteristics of patients. Formalin-fixed paraffin-embedded tumor samples from 54 patients with spinal chordoma were collected for immunohistochemical analysis of PD-1 and PD-L1 expression. The association of the expression levels of PD-1 and PD-L1 with clinicopathological variables and survival data were statistically analyzed. Lymphocyte infiltrates were present in all 54 patient samples. Of 54 samples, 37 (68.5%) had both positive PD-1 and PD-L1 expression in tumor cell membrane. Moreover, 38 (70.4%) and 12 (22.2%) had positive PD-1 and PD-L1 expression in tumor-infiltrating lymphocytes (TILs), respectively. Tumors with positive PD-L1 expression were significantly associated with advanced stages of chordoma (p = 0.041) and TIL infiltration (p = 0.005), and had a borderline association with tumor grade (p = 0.051). However, positive tumor PD-L1 expression was not significantly associated with local recurrence-free survival (LRFS) or overall survival (OS). PD-1 expression in TILs was associated with poor LRFS (χ2 = 10.051, p = 0.002, log-rank test). Multivariate analysis showed that PD-L1 expression only in TILs was an independent predictor for LRFS (HR = 0.298, 95% CI: 0.098-0.907, p = 0.033), and OS (HR = 0.188, 95% CI: 0.051-0.687, p = 0.011) in spinal chordoma patients. In conclusion, PD-L1 expression in TILs was an independent predictor for both LRFS and OS in spinal chordoma patients. Our findings suggest that the PD-1/PD-L1 pathway may be a novel therapeutic target for the immunotherapy of chordoma.
Keywords: Spinal chordoma, tumor-infiltrating lymphocytes, PD-1, PD-L1, prognosis
Introduction
Chordoma is a very rare mesenchymal tumor with a low to intermediate malignant grade [1], and accounts for 1-4% of all bone malignancies with an incidence rate of less than 1 case per million [2,3]. Clinically, chordoma usually responds poorly to chemotherapeutic agents [4,5] and conventional radiotherapy [6-8]. To date, treatment of chordoma with en bloc resection and wide resection margins offers the best chance of the long-term disease control [1,5,6,8,9]. However, to accomplish a radical surgical resection of chordoma lesions remains technically challenging, as the tumor is often adjacent to vital neurovascular structures with poor margins and invasion into surrounding soft tissues [9]. Importantly, surgery has the potential to lead to a high risk of tumor recurrence [5,10,11] and 5-40% of patients develop metastases following surgery [12-16]. Patients with a metastatic disease will have only about 1-year median survival time [12,17]. Thus, the development of novel therapeutic strategies is highly needed for these patients.
Programmed cell death 1 (PD-1), expressed in various immune cells, is a member of the B7-CD28 receptor family and can negatively regulate T-cell function, survival, and expansion [18-20]. This inhibitory signal is mediated by interaction with the co-stimulatory PD-1 ligands (PD-L) [21], PD-L1 and PD-L2, which represents a mechanism allowing tumor cells to escape the host’s immune response [22,23]. Previous studies have shown that PD-L1 is expressed in different cancers [24-28] and its expression is associated with poor prognosis of cancer patients [29,30]. Furthermore, tumor-infiltrating lymphocytes (TILs) express PD-1 and are associated with a high level of PD-L1 in various types of cancers [24,31-34]. In addition, intratumoral infiltration of PD1-positive T-cells is positively associated with tumor progression [21]. Previous clinical trials showed promising results by targeting the PD-1/PD-L1 signaling pathway using monoclonal antibodies in several human cancers [35-37]. Emerging data indicate that the PD-1/PD-L1 pathway may be deregulated in chordoma [31,38], suggesting that targeting the PD-1/PD-L1 signaling pathway is a promising strategy for the treatment of chordoma. However, to avoid bias [39], statistical analysis of the multivariate adjustment of the expression status of PD-1/PD-L1 with clinical outcome is essential. Furthermore, it is important to systematically investigate the level of intratumoral and TILs-associated PD-1 expression, and their association with clinicopathological features or outcome of chordoma patients.
In this study, we assessed PD-1 and PD-L1 expression in chordoma tissue specimens and in TILs as well as their association with clinicopathological data and local relapse-free survival (LRFS) and overall survival (OS) of chordoma patients.
Material and methods
Patients and tissue samples
In this study, we collected 54 tissue specimens from spinal chordoma patients who were surgically treated in the Department of Spine Surgery, The Second Xiangya Hospital, Central South University (Changsha, China) between June 2002 and April 2015. We retrospectively reviewed the clinicopathological characteristics, including age, gender, tumor size, location, tumor grade, stage, surrounding muscle invasion, preoperative recurrence, type of resection, tumor hemorrhage and necrosis, from patients’ medical records (Table 1). Regarding that previous treatment other than surgery may influence the expression of the proteins of interest, we reviewed the complete patients’ medical records, excluding those who had previously received any types of tumor-specific therapy, such as chemotherapy or radiotherapy. Tumor grade and stage were evaluated according to the Enneking staging system for the surgical staging of malignant bone and soft tissue tumors [1,8,40]. Resected tumor specimens were evaluated by anatomic pathologists and recorded as Enneking appropriate or Enneking inappropriate according to the Enneking principles [41]. Tumor-muscle invasion was confirmed by preoperative magnetic resonance images and histology [42]. Tumor recurrence was recorded in patients who had previously received tumor resection and relapsed on admission.
Table 1.
Characterization of patients
| Characteristic | Number of patients (%) |
|---|---|
| Age (range) in years | 55.6 (23-79) |
| Gender | |
| Male | 35 (64.8) |
| Female | 19 (35.2) |
| Tumor size (cm) | 6.3 (3-12) |
| Tumor location | |
| Sacral vertebra | 42 (77.8) |
| Cervical vertebra | 6 (11.1) |
| Thoracic vertebra | 4 (7.4) |
| Lumbar vertebra | 2 (3.7) |
| Surrounding muscle invasion | |
| Yes | 35 (64.8) |
| No | 19 (35.2) |
| Preoperative recurrence | |
| Yes | 11 (20.4) |
| No | 43 (79.6) |
| Tumor grade | |
| High | 38 (70.4) |
| Low | 16 (29.6) |
| Tumor stage | |
| IA | 13 (24.1) |
| IB | 6 (11.1) |
| IIA | 4 (7.4) |
| IIB | 27 (50) |
| III | 4 (7.4) |
| Type of resection | |
| EI | 17 (31.5) |
| EA | 37 (68.5) |
| Tumor hemorrhage | |
| No | 10 (18.5) |
| Yes | 44 (81.5) |
| Tumor necrosis | |
| Absent | 13 (24.1) |
| Mild | 18 (33.3) |
| Moderate | 15 (27.8) |
| Severe | 8 (14.8) |
| Level of TILs | |
| Absent | 0 (0) |
| Rare/few | 23 (42.6) |
| Moderate | 15 (27.8) |
| Prominent | 16 (29.6) |
| Ki-67 staining index | |
| Low | 25 (46.3) |
| High | 29 (53.7) |
EI, Enneking inappropriate; EA, Enneking appropriate; TILs, tumor-infiltrating lymphocytes.
Immediately following surgery, tissue samples from these patients were immediately fixed in 10% buffered formalin and embedded in paraffin. Formalin-fixed paraffin-embedded blocks from these 54 chordoma patients were retrieved from the Department of Pathology and sectioned to 4-µm thick tissue sections for immunohistochemistry. Tumor diagnosis was made on histological examination of hematoxylin and eosin-stained tissue sections according to criteria described in a previous study [43], and performed by two pathologists who confirmed the diagnosis of chordoma of the conventional subtype in all patients. This study was approved by the hospital ethical committee of The Second Xiangya Hospital, Central South University, and informed consent was obtained from each patient before participation in this study.
Follow-up of patients
The patients received clinical and radiographical follow-up at three-month intervals over the first two years, then every six months for three years, and annually thereafter. Local recurrence of tumor was diagnosed from clinical manifestations and imaging finding during follow-up or histology of the second surgery [44]. Events were defined as the first evidence of local recurrence for LRFS or death related to any cause for OS. All patients were followed until September 2015 and observations were censored when a patient was tumor free (LRFS analysis) or was alive (OS analysis) at the time of last clinical follow-up (September 2015).
Immunohistochemistry
Paraffin sections were deparaffinized twice in xylene and rehydrated in a series of ethanol and then subjected to antigen retrieval in microwave in 0.01 M citrate buffer, pH 6.0 at 121°C for 15 min. Next, the sections were incubated in 3% H2O2 in methanol for 15 min to quench potential endogenous peroxidase activity. After being rinsed with phosphate buffer solution (PBS), the section were incubated with 10% normal goat serum to block non-specific binding at room temperature for 30 min and then with primary anti-PD-1 antibody (#ab137132, Abcam, Cambridge, MA, USA) at a dilution of 1:400, anti-PD-L1 antibody (#ab174838, Abcam) at a dilution of 1:50, and anti-Ki-67 (#ab16667, Abcam) at a dilution of 1:100 at 4°C overnight. On the next day, the sections were washed with PBS thrice and incubated with biotinylated goat anti-rabbit immunoglobulin and subsequently with a streptavidin-peroxidase conjugate (Auragene, Changsha, Hunan, China). Antibody binding was visualized using 3,3’-diaminobenzidine solution, counterstained briefly with hematoxylin and mounted. Negative control sections were incubated with PBS instead of the primary antibody, and positive control sections were from human tonsil tissues according to manufacturer instructions.
Evaluation of immunohistochemistry
The immunostained sections were reviewed and scored under a microscope by two well-experienced pathologists (JY and SXL) who had no any prior information on clinical data. For PD-1 and PD-L1 expression in tumor tissues, positive staining was scored if tumor cells with membrane staining constituted ≥ 5% of all tumor cells, as previously described [27]. TILs were evaluated in hematoxylin and eosin-stained sections and scored as absent (0), rare/few (1), moderate (2), and prominent (3), similar to previous studies [27,31]. The tissue specimens were classified as negative (score 0-1) and positive (score 2-3). PD-L1 and PD-1 expression was then scored in TILs using the same scoring scale (0-3) and samples with a score of 2-3 were considered PD-L1-positive or PD-1-positive according to the methods described in a previous study [27].
Ki-67 expression index in tumor tissues was scored as percentage of cells with cell nuclear staining and divided into low (< 10% positivity) and high expression (≥ 10%) according to a previous study [42].
Statistical analysis
All statistical analyses were carried out using SPSS 17.0 (SPSS, Chicago, IL, USA). The χ2 test or Wilcoxon rank sum test were used to analyze the association of PD-1 and PD-L1 expression in tumor cell membrane or TILs with clinicopathological characteristics from spinal chordoma patients, where appropriate. LRFS and OS were calculated by the Kaplan-Meier method, and univariate survival analysis was performed by the log-rank test. Multivariate analysis was performed with a Cox proportional hazard model to assess whether PD-1 or PD-L1 expression independently predicted the outcome with the inclusion of factors determined to be significant by univariate analysis. All tests were two-sided and a p value ≤ 0.05 was considered statistically significant.
Results
Patient characteristics
All clinicopathological data are summarized in Table 1. Briefly, 35 were males and 19 females with a mean age of 55.59 years old (ranging between 23 and 79 years old). There were 43 patients with primary chordoma and 11 with recurrent chordoma (Table 1). All patients were followed up until September 2015, with a mean follow-up period of 42.39 months (ranging between 5 and 158 months).
All spinal chordoma tissue specimens have tumor-infiltrated lymphocytes
Tumor-infiltrated lymphocytes (TILs) in tumor lesions were assessed in tissue sections after HE staining and showed positive in all of these 54 cases of patients (Figure 1). The levels of TILs were as follows: rarely or a few in 23 (42.6%), moderate in 15 (27.8%), and prominent in 16 (29.6%) cases (Table 1).
Figure 1.
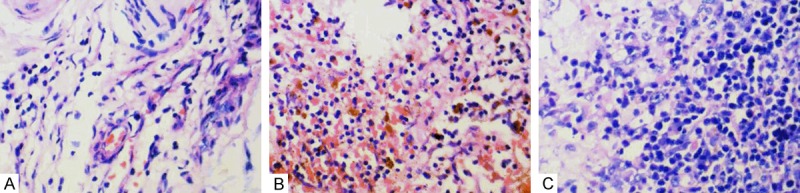
Representative illustration of tumor-infiltrating lymphocytes (TILs) in chordoma tissues (HE × 400). A. Rare/few TILs; B. Moderate TILs; C. Prominent TILs.
Expression of PD-1 and PD-L1 in tumor cells and in TILs
PD-1 was not expressed in the tumor cells of 17 patients (31.5%) and expressed in 37 patients (68.5%) (Table 2 and Figure 2A, 2B). In contrast, PD-1 was expressed in TILs in all 54 cases. Specifically, PD-1 level in TILs was scored as rare/few (1) in 15 patients (27.8%), moderate (2) in 20 patients (37.0%), and prominent (3) in 19 patients (35.2%) (Figure 2C-E). In summary, PD-1 expression level in TILs was considered negative (0 or 1) in 16 (29.6%) and positive (2 or 3) in 38 (70.4%) cases (Table 2).
Table 2.
Association between PD-1/PD-L1 expression and clinicopathological features of 54 spinal chordoma patients
| Clinicopathological factors | PD-1 in tumor | PD-1 in TIL | PD-L1 in tumor | PD-L1 in TIL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Negative (n = 17) | Positive (n = 37) | p-value | Negative (n = 16) | Positive (n = 38) | p-value | Negative (n = 17) | Positive (n = 37) | p-value | Negative (n = 42) | Positive (n = 12) | p-value | |
| Age (years) | ||||||||||||
| ≤ 50 | 9 | 14 | 0.29 | 9 | 11 | 0.058 | 7 | 16 | 0.88 | 15 | 8 | 0.056 |
| > 50 | 8 | 23 | 7 | 27 | 10 | 21 | 27 | 4 | ||||
| Sex | ||||||||||||
| Male | 8 | 27 | 0.064 | 8 | 27 | 0.13 | 12 | 23 | 0.54 | 28 | 7 | 0.84 |
| Female | 9 | 10 | 8 | 11 | 5 | 14 | 14 | 5 | ||||
| Tumor size | ||||||||||||
| ≤ 5 cm | 6 | 15 | 0.71 | 7 | 14 | 0.63 | 6 | 15 | 0.71 | 17 | 4 | 0.91 |
| > 5 cm | 11 | 22 | 9 | 24 | 11 | 22 | 25 | 8 | ||||
| Tumor location | ||||||||||||
| Sacral vertebra | 13 | 29 | 1.00 | 11 | 31 | 0.49 | 14 | 28 | 0.84 | 32 | 10 | 0.89 |
| Cervical or thoracic or lumbar vertebra | 4 | 8 | 5 | 7 | 3 | 9 | 10 | 2 | ||||
| Surrounding muscle invasion | ||||||||||||
| Yes | 9 | 27 | 0.14 | 8 | 28 | 0.092 | 11 | 25 | 0.83 | 29 | 7 | 0.72 |
| No | 8 | 10 | 8 | 10 | 6 | 12 | 13 | 5 | ||||
| Preoperative recurrence | ||||||||||||
| Yes | 5 | 6 | 0.45 | 4 | 7 | 0.85 | 6 | 5 | 0.13 | 6 | 5 | 0.095 |
| No | 12 | 31 | 12 | 31 | 11 | 32 | 36 | 7 | ||||
| Grade | ||||||||||||
| High | 12 | 26 | 0.98 | 12 | 26 | 0.87 | 15 | 23 | 0.051 | 27 | 11 | 0.14 |
| Low | 5 | 11 | 4 | 12 | 2 | 14 | 15 | 1 | ||||
| Stagea | ||||||||||||
| IA | 5 | 8 | 0.97 | 4 | 9 | 0.38 | 2 | 11 | 0.041 | 12 | 1 | 0.23 |
| IB | 2 | 4 | 1 | 5 | 0 | 6 | 6 | 0 | ||||
| IIA | 0 | 4 | 0 | 4 | 3 | 1 | 2 | 2 | ||||
| IIB | 8 | 19 | 9 | 18 | 9 | 18 | 18 | 9 | ||||
| III | 2 | 2 | 2 | 2 | 3 | 1 | 4 | 0 | ||||
| Type of resection | ||||||||||||
| EI | 4 | 14 | 0.30 | 3 | 15 | 0.14 | 5 | 13 | 0.67 | 17 | 1 | 0.083 |
| EA | 13 | 23 | 13 | 23 | 12 | 24 | 25 | 11 | ||||
| Tumor hemorrhage | ||||||||||||
| No | 4 | 6 | 0.79 | 4 | 6 | 0.68 | 3 | 7 | 1.00 | 7 | 3 | 0.81 |
| Yes | 13 | 31 | 12 | 32 | 14 | 30 | 35 | 9 | ||||
| Tumor necrosisb | ||||||||||||
| Absent | 4 | 9 | 0.42 | 3 | 10 | 0.64 | 4 | 9 | 0.41 | 10 | 3 | 0.34 |
| Mild | 8 | 10 | 6 | 12 | 5 | 13 | 16 | 2 | ||||
| Moderate | 3 | 12 | 4 | 11 | 3 | 12 | 11 | 4 | ||||
| Severe | 2 | 6 | 3 | 5 | 5 | 3 | 5 | 3 | ||||
| Extent of TILs | ||||||||||||
| Negative | 9 | 14 | 0.29 | 10 | 13 | 0.055 | 12 | 11 | 0.005 | 16 | 7 | 0.21 |
| Positive | 8 | 23 | 6 | 25 | 5 | 26 | 26 | 5 | ||||
| Ki-67 index | ||||||||||||
| Low | 10 | 15 | 0.21 | 8 | 17 | 0.72 | 8 | 17 | 0.93 | 19 | 6 | 0.77 |
| High | 7 | 22 | 8 | 21 | 9 | 20 | 23 | 6 | ||||
EI, Enneking inappropriate; EA, Enneking appropriate; TILs, tumor-infiltrating lymphocytes; PD-1, programmed cell death 1; PD-L1, programmed cell death-1 ligand 1.
Wilcoxon rank sum test.
Wilcoxon rank sum test.
Figure 2.
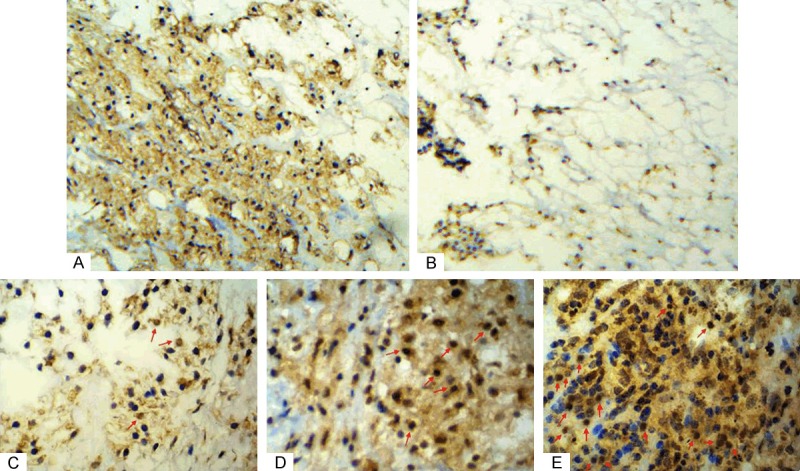
Immunohistochemical analysis of PD-1 expression in tumor cells and TILs of chordoma tissue specimens. A. PD-1 protein on tumor cell membrane; B. Lack of PD-1 expression on tumor cell membrane; C. Rare/few level of TILs that expressed PD-1 in chordoma tissues (red arrow); D. Moderate level of TILs that expressed PD-1 in chordoma tissues (red arrow); E. Prominent level of TILs that expressed PD-1 in chordoma tissues (red arrow) (×400).
PD-L1 protein was not expressed in tumor cells in 17 patients (31.5%), but was expressed in 37 patients (68.5%) (Table 2 and Figure 3A, 3B). PD-L1 expression level in TILs was scored as absent (0) in 0 (0%), rare/few (1) in 24 (44.4%), moderate (2) in 18 (33.3%), and prominent (3) in 12 (22.2%) cases (Figure 3C-E). In summary, PD-L1 expression level in TILs was negative (0 or 1) in 42 (77.8%) and positive (2 or 3) in 12 (22.2%) cases (Table 2).
Figure 3.
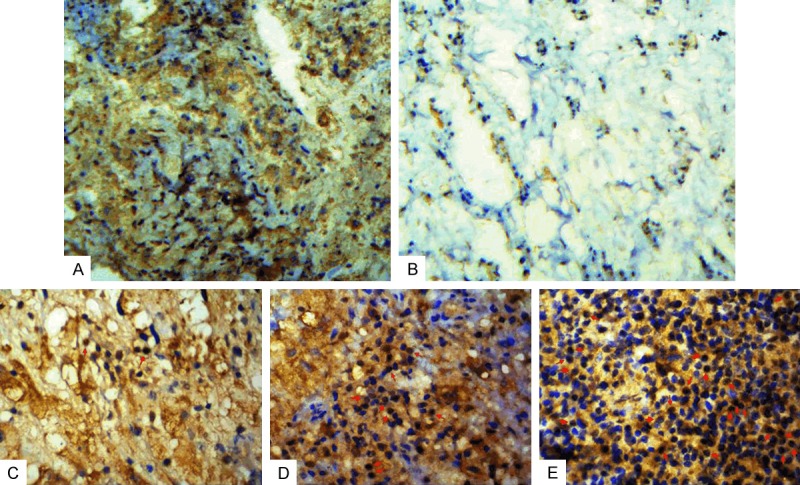
Immunohistochemical analysis of PD-L1 expression in tumor cells and TILs of chordoma tissue specimens. A. PD-L1 protein on tumor cell membrane; B. Lack of PD-L1 expression on tumor cell membrane; C. Rare/few level of TILs that expressed PD-L1 in chordoma tissues (red arrow); D. Moderate level of TILs that expressed PD-L1 in chordoma tissues (red arrow); E. Prominent level of TILs that expressed PD-L1 in chordoma tissues (red arrow) (×400).
Association of PD-1/PD-L1 expression with TILs in chordoma tissue specimens
Tumors with PD-L1 positive expression were more likely to have lymphocytes tumor infiltration (OR = 5.673, 95% CI: 1.611-19.981, p = 0.005; Table 2). There was a borderline trend for patients with intratumoral PD-1-positive immune cells to have lymphocyte tumor infiltration (OR = 3.205, 95% CI: 0.952-10.790, p = 0.055; Table 2). However, there was no statistically significant association between tumor PD-1 or PD-L1 expression in TILs and the level of TILs in chordoma samples (Table 2).
Association of PD-1/PD-L1 expression with clinicopathological data from chordoma patients
Chordoma with positive PD-L1 expression was significantly associated with advanced stages (p = 0.041). Moreover, patients with positive PD-L1 expression in tumor cells demonstrated a tendency of higher pathological grade at presentation, though such an association did not reached a statistical significance (OR = 1.015, 95% CI: 0.288-3.577, p = 0.051; Table 2). However, there was no statistically significant association of PD-1/PD-L1 expression in tumor cells or TILs with other clinicopathological data (Table 2).
Association of PD-1/PD-L1 expression with LRFS and OS of spine chordoma patients
During the follow-up period, 41 patients (75.93%) showed local recurrence and 24 patients (44.44%) were deceased. Kaplan-Meier analysis showed that TILs with positive PD-L1 expression was statistically associated with better LRFS (χ2 = 8.792, p = 0.003 by the log-rank test; Table 3 and Figure 4A) and OS (χ2 = 7.007, p = 0.008 by the log-rank test; Table 4 and Figure 4B) of spinal chordoma patients. LRFS was also statistically associated with Ki-67 staining, age, tumor muscle invasion, hemorrhage, and type of resection (Table 3). Furthermore, we found that PD-1 expression in TILs was statistically associated with LRFS (χ2 = 10.051, p = 0.002 by the log-rank test; Table 3 and Figure 4C). In addition, tumor muscle invasion, Enneking inappropriate resection and tumor stage were significantly associated with poor OS (Table 4).
Table 3.
Univariate and multivariate Cox proportional hazard analyses of prognostic factors for local recurrence-free survival of spinal chordoma patients
| Factors | Categories | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
|
| |||||
| χ2 | p-value | p-value | HR (95% CI) | ||
| Sex | Male/Female | 2.949 | 0.086 | ||
| Age | ≤ 50/> 50 | 6.560 | 0.010 | 0.25 | 1.693 (0.686-4.177) |
| Tumor size | ≤ 5 cm/> 5 cm | 0.278 | 0.59 | ||
| Tumor location | Sacral vertebra/Cervical or thoracic or lumbar vertebra | 0.003 | 0.95 | ||
| Preoperative recurrence | Yes/No | 0.954 | 0.32 | ||
| Surrounding muscle invasion | Yes/No | 24.585 | < 0.001 | 0.003 | 3.660 (1.544-8.675) |
| Grade | High/Low | 0.231 | 0.631 | ||
| Stage | IA/IB/IIA/IIB/III | 0.960 | 0.916 | ||
| Type of resection | EI/EA | 16.472 | < 0.001 | 0.30 | 1.607 (0.645-4.004) |
| Tumor hemorrhage | Yes/No | 4.031 | 0.045 | 0.430 | 1.523 (0.536-4.329) |
| Tumor necrosis | Absent/mild/moderate/severe | 0.189 | 0.97 | ||
| Extent of TILs | Negative/Positive | 4.104 | 0.043 | 0.93 | 1.034 (0.472-2.263) |
| Ki-67 index | High/Low | 14.163 | < 0.001 | 0.002 | 3.806 (1.627-8.907) |
| PD-1 expression on tumor cells | Negative/Positive | 3.406 | 0.065 | ||
| PD-L1 expression on tumor cells | Negative/Positive | 1.035 | 0.30 | ||
| PD-1 expression in TILs | Negative/Positive | 10.051 | 0.002 | 0.391 | 1.537 (0.576-4.102) |
| PD-L1 expression in TILs | Negative/Positive | 8.792 | 0.003 | 0.033 | 0.298 (0.098-0.907) |
EI, Enneking inappropriate; EA, Enneking appropriate; TILs, tumor-infiltrating lymphocytes; PD-1, programmed cell death 1; PD-L1, programmed cell death-1 ligand 1; HR, hazard ratio; CI, confidence interval.
Figure 4.
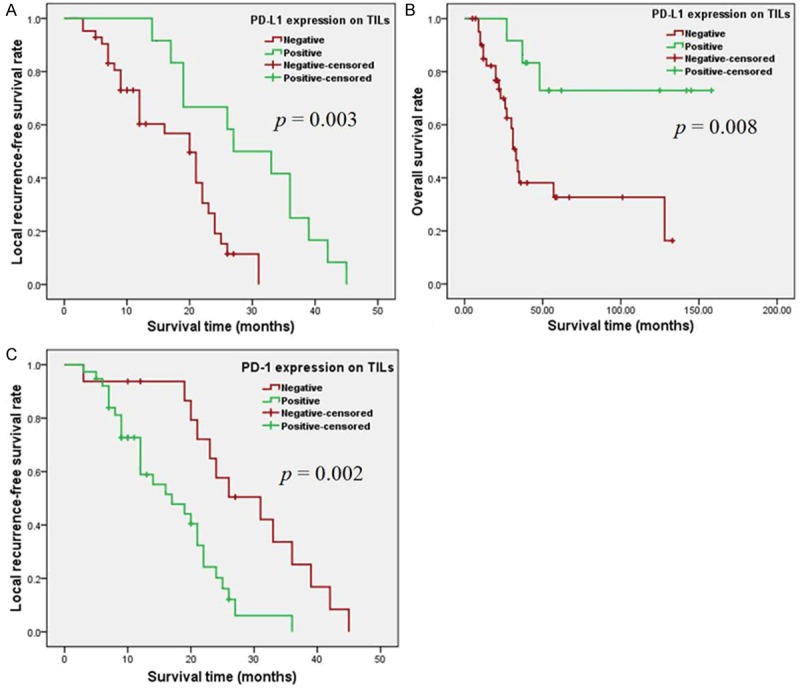
Kaplan-Meier curve analysis of the local recurrence-free survival (LRFS) and overall survival (OS) of cordoma patients. A. Kaplan-Meier curves of LRFS stratified by PD-L1 expression in TILs (p = 0.003 via log-rank test); B. Kaplan-Meier curves of OS stratified by PD-L1 expression in TILs (p = 0.008); C. Kaplan-Meier curves of LRFS stratified by PD-1 expression in TILs (p = 0.002).
Table 4.
Univariate and multivariate Cox proportional hazard analyses of prognostic factors for overall survival of spinal chordoma patients
| Factors | Categories | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
|
| |||||
| χ2 | p-value | p-value | HR (95% CI) | ||
| Sex | Male/Female | 1.540 | 0.21 | ||
| Age | ≤ 50/> 50 | 1.459 | 0.22 | ||
| Tumor size | ≤ 5 cm/> 5 cm | 0.127 | 0.72 | ||
| Tumor location | Sacral vertebra/Cervical or thoracic or lumbar vertebra | 0.132 | 0.71 | ||
| Preoperative recurrence | Yes/No | 0.546 | 0.46 | ||
| Surrounding muscle invasion | Yes/No | 6.793 | 0.009 | 0.70 | 1.222 (0.434-3.439) |
| Grade | High/Low | 0.641 | 0.42 | ||
| Stage | IA/IB/IIA/IIB/III | 22.303 | < 0.001 | 0.10 | 1.372 (0.941-1.999) |
| Type of resection | EI/EA | 5.898 | 0.015 | 0.36 | 1.519 (0.619-3.724) |
| Tumor hemorrhage | Yes/No | 0.319 | 0.57 | ||
| Tumor necrosis | Absent/mild/moderate/severe | 4.032 | 0.25 | ||
| Extent of TILs | Negative/Positive | 0.003 | 0.95 | ||
| Ki-67 index | High/Low | 2.045 | 0.15 | ||
| PD-1 expression on tumor cells | Negative/Positive | 1.047 | 0.30 | ||
| PD-L1 expression on tumor cells | Negative/Positive | 0.005 | 0.94 | ||
| PD-1 expression in TILs | Negative/Positive | 1.772 | 0.18 | ||
| PD-L1 expression in TILs | Negative/Positive | 7.007 | 0.008 | 0.011 | 0.188 (0.051-0.687) |
EI, Enneking inappropriate; EA, Enneking appropriate; TILs, tumor-infiltrating lymphocytes; PD-1, programmed cell death 1; PD-L1, programmed cell death-1 ligand 1; HR, hazard ratio; CI, confidence interval.
Then we performed univariate and multivariate analyses using a Cox proportional hazards model (Tables 3 and 4). The results showed that PD-L1 expression in TILs was an independent predictor for both LRFS (HR = 0.298, 95% CI: 0.098-0.907, p = 0.033, Table 3) and OS (HR = 0.188, 95% CI: 0.051-0.687, p = 0.011, Table 4). A high Ki-67 index (HR = 3.806, 95% CI: 1.627-8.907, p = 0.002, Table 3) and tumor muscle invasion (HR = 3.660, 95% CI: 1.544-8.675; p = 0.006, Table 3) were also independent predictors for poor LRFS.
Discussion
In the current study, we determined the levels of PD-1 and PD-L1 proteins in tumor cells and TILs in spinal chordoma and the association of their expression with the clinicopathological features and survival of the patients. We found that PD-1 and PD-L1 were differentially expressed in tumor cells and TILs of chordoma tissues. PD-L1 expression in tumor cells was significantly associated with advanced chordoma stage and increased TIL infiltration, and had a borderline association with higher tumor grades but was not associated with LRFS or OS. Moreover, PD-1 expression in TILs was associated with poor LRFS. Importantly, PD-L1 expression in TILs, but not in tumor cells, was an independent predictor for both LRFS and OS of spinal chordoma patients. This study revealed that PD-1 and PD-L1 were expressed in the microenvironment components of chordoma and associated with survival of patients, suggesting that targeting the PD-1/PD-L1 pathway might be a novel immunotherapeutic strategy to treat chordoma. However, further studies are needed to define the role of PD-L1 expression in TILs as a predictive and prognostic biomarker in spinal chordoma.
An increasing number of molecular biomarkers have been evaluated for their association with prognosis of spinal chordoma, such as aberrant expression of different proteins or epigenetic dysregulation in tumor tissues [45-48]. However, most of these investigations of prognostic biomarkers in spinal chordoma did not adjust for confounding factors using multivariate analysis, which may distort the usefulness of prognostic biomarkers [39,45]. Thus, it is important to identify additional biologic markers as potential indicators of prognosis for this disease.
Recently, blockade of the PD-1/PD-L1 axis has resulted in substantive and durable clinical responses in patients with several advanced human cancers [36,37]. In the current study, we aimed to profile the status of PD-1 and PD-L1 expression in both tumor cells and TILs in spinal chordoma tissues and to assess the association of immune checkpoint with spinal chordoma prognosis, after adjusting for other clinicopathological parameters by multivariate analysis. We found that PD-L1 was expressed in tumor cell membrane in most chordoma specimens (68.5%), which is consistent with a previous report of PD-L1 positive immunoreactivity in 94.9% of 78 chordoma samples [31]. Thus far, the precise mechanism of upregulation of PD-L1 in chordoma remains unclear. Previous studies demonstrated that loss of tumor suppressor phosphatase and tensin homolog (PTEN) expression was able to upregulate PD-L1 expression in several human malignancies including chordoma [49-53]. A recent study showed that knockdown of PTEN expression using a short hairpin RNA in triple-negative breast cancer could induce expression of PD-L1 mRNA and protein [54]. It will be interesting to investigate whether PTEN suppress the expression of PD-L1 via PI3K-AKT dependent or independent mechanism in chordoma.
Furthermore, our current study showed that TILs expressing PD-L1 were widely present in chordoma tissues, which is consistent with a previous study [38]. Our findings are also supported by another recent study that showed PD-L1 expression in tumor-infiltrating mononuclear cells in 58 of 143 patients with urothelial carcinoma [27]. Actually, activated T-cells secrete cytokines to induce PD-L1 expression on surrounding immune and tumor cells [27]. The expression of PD-L1 on TILs as observed in this study suggests that these intratumoral lymphocytes are chordoma antigen-specific. Moreover, previous studies have shown that TILs express PD-1 in various cancers, including chordoma [24,33,38]. Similarly, we showed PD-1 expression in TILs in chordoma. Because the interaction of PD-1 with PD-L1 represents a major pathway that is often hijacked by tumors to suppress immune control, the correlated expression of PD-1 by TILs and PD-L1 in tumor cells may play a crucial role in chordoma evasion from host immunity. Indeed, for the first time, we unraveled PD-1 expression in chordoma tumor cells. This result is consistent with a recent study showing positive PD-1 expression in 43 of 122 non-small-cell lung cancer specimens [55]. Our finding suggests that the PD-1/PD-L1 pathway may be a novel therapeutic target for chordoma.
To characterize the association between the expression of PD-1 or PD-L1 proteins and clinical behavior, we correlated PD-1 or PD-L1 expression with patient survival rates and clinicopathological parameters and found a statistically significant association between PD-L1 expression on tumor cells and advanced chordoma stage or tumor grade. Previous studies reported that PD-L1 expression in tumor cells was associated with poor tumor differentiation, high grade, risk of recurrence and advanced clinical or pathologic stage in various human cancer types [21,56-59]. In chordoma, a recent study showed PD-L1 expression was associated with chordoma metastasis [31]. These data suggest that chordoma cells may escape from attack by immune cells through immune checkpoints, like PD-L1 [60]. Consistent with previous studies [24,27,31-34], our current data showed that tumor PD-L1 expression was associated with TILs infiltration in chordoma. This finding can be explained by the fact that TILs can induce PD-L1 expression by upregulation of cytokines [61]. It was reported that TIL infiltration was able to facilitate melanoma metastasis [33]; our results indicated the potential role of TILs in chordoma progression. In addition, we found that tumor-infiltrating PD-1-positive lymphocytes were statistically associated with survival, similar to the results of two previous studies [21,62]. However, our current data provided the first evidence that only higher PD-L1 expression in TILs was associated with longer LRFS and OS. This association was also observed in urothelial carcinoma [27]. Moreover, a recent phase I clinical trial evaluating the efficacy of MPDL3280A, an anti-PD-L1 mAb, revealed that patients with PD-L1 expression in immune cells exhibited higher overall response rate compared to those without PD-L1 expression [63]. These data may support the rationale for use of PD-L1 expression in immune cells as a potential predictive biomarker for immunotherapy in spinal chordoma. However, it should be noted that patients with PD-L1-negative tumor also responded to anti-PD-L1 therapy, highlighting the need for better biomarkers to predict responses of agents targeting this pathway. Nevertheless, unlike studies of other types of cancers [27,29,30,64], our analyses failed to show any association of tumor PD-L1 expression or the presence of TILs with the survival of spinal chordoma patients. This inconsistency may be attributed to the retrospective nature of the current study and our small number of patients, leading to a correspondingly low statistical power.
Our study does have some limitations: for example, it is retrospective, which may imply a potential selection bias. Moreover, it remains to be determined how PD-L1 expression in TILs of spinal chordoma tissues impacts survival of patients. Finally, the potential heterogeneity of PD-1 or PD-L1 expression within and between tumor tissues may limit the ability to adequately assess the data as a previous study suggested [27].
In summary, we demonstrated that PD-L1 expression in tumor cells was significantly associated with advanced chordoma stage and increased TIL infiltration; PD-L1 expression in TILs was an independent predictor for both LRFS and OS in spinal chordoma patients; PD-1 expression in TILs was associated with poor LRFS. Our findings suggest that targeting the PD-1/PD-L1 pathway might be a novel immunotherapeutic strategy to treat chordoma.
Acknowledgements
We would like to thank Auragene Bioscience (Changsha, China) for technical support and Medjaden Bioscience Limited (Hong Kong, China) for assistance in preparation of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Samson IR, Springfield DS, Suit HD, Mankin HJ. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Joint Surg Am. 1993;75:1476–1484. doi: 10.2106/00004623-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Bydon M, Papadimitriou K, Witham T, Wolinsky JP, Bydon A, Sciubba D, Gokaslan Z. Novel therapeutic targets in chordoma. Expert Opin Ther Targets. 2012;16:1139–1143. doi: 10.1517/14728222.2012.714772. [DOI] [PubMed] [Google Scholar]
- 3.Bompas E, Le Cesne A, Tresch-Bruneel E, Lebellec L, Laurence V, Collard O, Saada-Bouzid E, Isambert N, Blay JY, Amela EY, Salas S, Chevreau C, Bertucci F, Italiano A, Clisant S, Penel N. Sorafenib in patients with locally advanced and metastatic chordomas: a phase II trial of the French Sarcoma Group (GSF/GETO) Ann Oncol. 2015;26:2168–2173. doi: 10.1093/annonc/mdv300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzarelli A, Quagliuolo V, Cerasoli S, Zucali R, Bignami P, Mazzaferro V, Dossena G, Gennari L. Chordoma: natural history and treatment results in 33 cases. J Surg Oncol. 1988;37:185–191. doi: 10.1002/jso.2930370311. [DOI] [PubMed] [Google Scholar]
- 5.York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA, Gokaslan ZL. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44:74–79. doi: 10.1097/00006123-199901000-00041. discussion 79-80. [DOI] [PubMed] [Google Scholar]
- 6.Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC Jr. Lumbosacral chordoma. Prognostic factors and treatment. Spine (Phila Pa 1976) 1999;24:1639–1645. doi: 10.1097/00007632-199908150-00004. [DOI] [PubMed] [Google Scholar]
- 7.Catton C, O’Sullivan B, Bell R, Laperriere N, Cummings B, Fornasier V, Wunder J. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41:67–72. doi: 10.1016/s0167-8140(96)91805-8. [DOI] [PubMed] [Google Scholar]
- 8.Kayani B, Hanna SA, Sewell MD, Saifuddin A, Molloy S, Briggs TW. A review of the surgical management of sacral chordoma. Eur J Surg Oncol. 2014;40:1412–1420. doi: 10.1016/j.ejso.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Kayani B, Sewell MD, Tan KA, Hanna SA, Williams R, Pollock R, Skinner J, Briggs TW. Prognostic factors in the operative management of sacral chordomas. World Neurosurg. 2015;84:1354–1361. doi: 10.1016/j.wneu.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Arıel IM, Verdu C. Chordoma: an analysis of twenty cases treated over a twenty-year span. J Surg Oncol. 1975;7:27–44. doi: 10.1002/jso.2930070106. [DOI] [PubMed] [Google Scholar]
- 11.Rich TA, Schiller A, Suit HD, Mankin HJ. Clinical and pathologic review of 48 cases of chordoma. Cancer. 1985;56:182–187. doi: 10.1002/1097-0142(19850701)56:1<182::aid-cncr2820560131>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Baratti D, Gronchi A, Pennacchioli E, Lozza L, Colecchia M, Fiore M, Santinami M. Chordoma: natural history and results in 28 patients treated at a single institution. Ann Surg Oncol. 2003;10:291–296. doi: 10.1245/aso.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–2216. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 14.Yonemoto T, Tatezaki S, Takenouchi T, Ishii T, Satoh T, Moriya H. The surgical management of sacrococcygeal chordoma. Cancer. 1999;85:878–883. [PubMed] [Google Scholar]
- 15.Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122–2134. doi: 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Osaka S, Kodoh O, Sugita H, Osaka E, Yoshida Y, Ryu J. Clinical significance of a wide excision policy for sacrococcygeal chordoma. J Cancer Res Clin Oncol. 2006;132:213–218. doi: 10.1007/s00432-005-0067-3. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill P, Bell BA, Miller JD, Jacobson I, Guthrie W. Fifty years of experience with chordomas in southeast Scotland. Neurosurgery. 1985;16:166–170. doi: 10.1227/00006123-198502000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Park HJ, Kusnadi A, Lee EJ, Kim WW, Cho BC, Lee IJ, Seong J, Ha SJ. Tumor-infiltrating regulatory T cells delineated by upregulation of PD-1 and inhibitory receptors. Cell Immunol. 2012;278:76–83. doi: 10.1016/j.cellimm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22:265–268. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 20.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, Park HS, Lee H, Moon WS, Chung MJ, Kang MJ, Jang KY. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One. 2013;8:e82870. doi: 10.1371/journal.pone.0082870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 24.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wöhrer A, Dieckmann K, Filipits M, Brandstetter A, Weller M, Kurscheid S, Hegi ME, Zielinski CC, Marosi C, Hainfellner JA, Preusser M, Wick W. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17:1064–1075. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, Zoubeidi A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2015;6:234–242. doi: 10.18632/oncotarget.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, Tokito T, Kinoshita T, Kage M, Hoshino T. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10:426–430. doi: 10.1097/JTO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 27.Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, Taplin ME, Choueiri TK, Hodi FS, Freeman GJ, Signoretti S. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26:812–817. doi: 10.1093/annonc/mdv009. [DOI] [PubMed] [Google Scholar]
- 28.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One. 2015;10:e0131403. doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Kang S, Shen J, He J, Jiang L, Wang W, Guo Z, Peng G, Chen G, He J, Liang W. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: a meta-analysis. Medicine (Baltimore) 2015;94:e515. doi: 10.1097/MD.0000000000000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Y, Shen J, Gao Y, Liao Y, Cote G, Choy E, Chebib I, Mankin H, Hornicek F, Duan Z. Expression of programmed cell death ligand 1 (PD-L1) and prevalence of tumor-infiltrating lymphocytes (TILs) in chordoma. Oncotarget. 2015;6:11139–11149. doi: 10.18632/oncotarget.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berghoff AS, Ricken G, Widhalm G, Rajky O, Dieckmann K, Birner P, Bartsch R, Holler C, Preusser M. Tumour infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology. 2015;66:289–299. doi: 10.1111/his.12537. [DOI] [PubMed] [Google Scholar]
- 34.D’Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin LX, Carvajal RD, Dickson MA, Gounder M, Keohan ML, Schwartz GK, Tap WD. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. 2015;46:357–365. doi: 10.1016/j.humpath.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathios D, Ruzevick J, Jackson CM, Xu H, Shah S, Taube JM, Burger PC, McCarthy EF, Quinones-Hinojosa A, Pardoll DM, Lim M. PD-1, PD-L1, PD-L2 expression in the chordoma microenvironment. J Neurooncol. 2015;121:251–259. doi: 10.1007/s11060-014-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 40.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 41.Fisher CG, Saravanja DD, Dvorak MF, Rampersaud YR, Clarkson PW, Hurlbert J, Fox R, Zhang H, Lewis S, Riaz S, Ferguson PC, Boyd MC. Surgical management of primary bone tumors of the spine: validation of an approach to enhance cure and reduce local recurrence. Spine (Phila Pa 1976) 2011;36:830–836. doi: 10.1097/BRS.0b013e3181e502e5. [DOI] [PubMed] [Google Scholar]
- 42.Zhou M, Chen K, Yang H, Wang G, Lu J, Ji Y, Wu C, Chen C. Expression of insulin-like growth factor II mRNA-binding protein 3 (IMP3) in sacral chordoma. J Neurooncol. 2014;116:77–82. doi: 10.1007/s11060-013-1274-4. [DOI] [PubMed] [Google Scholar]
- 43.Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13:e69–76. doi: 10.1016/S1470-2045(11)70337-0. [DOI] [PubMed] [Google Scholar]
- 44.Meng T, Yin H, Li B, Li Z, Xu W, Zhou W, Cheng M, Wang J, Zhou L, Yang X, Liu T, Yan W, Song D, Xiao J. Clinical features and prognostic factors of patients with chordoma in the spine: a retrospective analysis of 153 patients in a single center. Neuro Oncol. 2015;17:725–732. doi: 10.1093/neuonc/nou331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou MX, Huang W, Wang XB, Li J, Lv GH, Deng YW. Prognostic factors in spinal chordoma: A systematic review. Clin Neurol Neurosurg. 2015;139:110–118. doi: 10.1016/j.clineuro.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Osaka E, Kelly AD, Spentzos D, Choy E, Yang X, Shen JK, Yang P, Mankin HJ, Hornicek FJ, Duan Z. MicroRNA-155 expression is independently predictive of outcome in chordoma. Oncotarget. 2015;6:9125–9139. doi: 10.18632/oncotarget.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang K, Chen H, Zhang B, Sun J, Lu J, Chen K, Yang H. Overexpression of Raf-1 and ERK1/2 in sacral chordoma and association with tumor recurrence. Int J Clin Exp Pathol. 2015;8:608–614. [PMC free article] [PubMed] [Google Scholar]
- 48.Zou MX, Huang W, Wang XB, Li J, Lv GH, Wang B, Deng YW. Reduced expression of miRNA-1237-3p associated with poor survival of spinal chordoma patients. Eur Spine J. 2015;24:1738–1746. doi: 10.1007/s00586-015-3927-9. [DOI] [PubMed] [Google Scholar]
- 49.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 50.Song M, Chen D, Lu B, Wang C, Zhang J, Huang L, Wang X, Timmons CL, Hu J, Liu B, Wu X, Wang L, Wang J, Liu H. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS One. 2013;8:e65821. doi: 10.1371/journal.pone.0065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, Reibel JB, Walton Z, Ji H, Watanabe H, Jänne PA, Castrillon DH, Rustgi AK, Bass AJ, Freeman GJ, Padera RF, Dranoff G, Hammerman PS, Kim CF, Wong KK. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25:590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Zhang J, Xu K, Xiao Z, Sun J, Xu J, Wang J, Tang Q. PTEN/PI3K/mTOR/B7-H1 signaling pathway regulates cell progression and immuno-resistance in pancreatic cancer. Hepatogastroenterology. 2013;60:1766–1772. [PubMed] [Google Scholar]
- 53.Le LP, Nielsen GP, Rosenberg AE, Thomas D, Batten JM, Deshpande V, Schwab J, Duan Z, Xavier RJ, Hornicek FJ, Iafrate AJ. Recurrent chromosomal copy number alterations in sporadic chordomas. PLoS One. 2011;6:e18846. doi: 10.1371/journal.pone.0018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A, Curran M, Hwu P, Sharma P, Litton JK, Molldrem JJ, Alatrash G. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, Tibaldi C, Minuti G, Salvini J, Coppi E, Chella A, Fontanini G, Filice ME, Tornillo L, Incensati RM, Sani S, Crinò L, Terracciano L, Cappuzzo F. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng L, Huang D, Liu J, Qian Y, Deng J, Li D, Hu Z, Zhang J, Jiang G, Zheng S. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol. 2008;134:1021–1027. doi: 10.1007/s00432-008-0364-8. [DOI] [PubMed] [Google Scholar]
- 58.Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, Leibovich BC, Kwon ED, Frank I. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res. 2008;14:4800–4808. doi: 10.1158/1078-0432.CCR-08-0731. [DOI] [PubMed] [Google Scholar]
- 59.Pan ZK, Ye F, Wu X, An HX, Wu JX. Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2015;7:462–470. doi: 10.3978/j.issn.2072-1439.2015.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, Fradet Y, Lacombe L, Kwon ED. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 61.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 63.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 64.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, Sato E. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]


