Abstract
The DNA-alkylating agent temozolomide (TMZ) is an effective chemotherapeutic agent against malignant glioma, including glioblastoma multiforme (GBM). However, the clinical efficacy of TMZ is limited in many patients because of O6-methylguanine-DNA methyltransferase (MGMT)-driven resistance. Thus, new strategies to overcome TMZ resistance are urgently needed. Ursolic acid (UA) is a naturally derived pentacyclic triterpene acid that exerts broad anticancer effects, and shows capability to cross the blood-brain barrier. In this study, we evaluated the possible synergistic effect of TMZ and UA in resistant GBM cell lines. The results showed that UA prevented the proliferation of resistant GBM cells in a concentration-dependent manner. Compared with TMZ or UA treatment alone, the combination treatment of TMZ and UA synergistically enhanced cytotoxicity and senescence in TMZ-resistant GBM cells. This effect was correlated with the downregulation of MGMT. Moreover, experimental results with an in vivo mouse xenograft model showed that the combination treatment of UA and TMZ reduced tumor volumes by depleting MGMT. Therefore, UA as both a monotherapy and a resensitizer, might be a candidate agent for patients with refractory malignant gliomas.
Keywords: Ursolic acid, temozolomide, glioblastoma, O6-methylguanine-DNA methyltransferase, senescence
Introduction
Glioblastoma multiforme (GBM) is the most aggressive and most lethal primary malignant glioma in adults [1]. Malignant gliomas are highly proliferative, invasive, and difficult to resect completely through surgery; thus, its clinical effect had no encouraging change over the past 20 years [2]. The average survival time of patients with malignant gliomas is only 12-15 months [3,4]. Although temozolomide (TMZ) as a first-line alkylating agent exerts an antitumor activity in malignant glioma patients. Its efficacy is still unsatisfactory because of intrinsic resistance and acquired resistance to TMZ [5]. Overcoming drug resistance and exploring strategies for combining chemotherapy to reverse resistance have become attractive foci in human glioma research.
TMZ cytotoxicity is mainly due to DNA O6-G methylation in guanine-rich regions. During DNA replication, O6-methylaguanine mispairs with thymine and triggers the mismatch repair system that generates DNA strand breaks, triggering cell cycle arrest and apoptosis. O6-methylguanine-DNA methyltransferase (MGMT) removes methyl groups from the O6 position of guanine, and confers TMZ resistance [6,7]. Ongoing studies have intended to inhibit MGMT activity to enhance the therapeutic effect of TMZ [8]. One proposed approach involves the high-frequency administration of TMZ resulting in the rapid depletion of MGMT activity without unacceptable toxicity [9]. Another approach is to inactivate MGMT [10]. Many MGMT inhibitors, such as O6-benzylguanine (O6-BG), lomeguatrib and their corresponding derivatives, have been developed and proven to irreversibly deplete MGMT, and improve the efficacy of TMZ in vitro and in vivo [7]. However, the efficacy of MGMT inhibitors combined with alkylating agents in patients with glioblastoma remains controversial [10,11]. Therefore, other therapeutic agents that downregulate MGMT expression and attenuate TMZ resistance are highly desired.
Ursolic acid (3β-hydroxy-12-urs-12-en-28-oic acid, UA) is a naturally derived pentacyclic triterpene acid widely existing in food, medicinal herbs and other plants [12]. Its extensive pharmacological effects include hepatoprotective, antioxidant, anti-inflammatory, antiviral and cytotoxic activities. In recent years, UA has become a major focus in cancer research because of its broad anticancer effects and minimal toxicity. Although the concrete mechanisms are poorly understood, an increasing number of studies have found that UA can inhibit the proliferation, and induce the apoptosis of many tumor cell lines [13,14]. Moreover, many studies indicated that UA has a strong killing effect on multidrug-resistant cell lines [15]. UA induces apoptosis and factor-mediated killing in ADR-resistant human hepatoma cells [16]. UA can significantly improve the sensitivity of 5-FU to HCT15 cells, which are intrinsically resistant to 5-FU [17]. UA can also overcome apoptosis resistance in prostate cancer mediated by Bcl-2 [18]. However, whether or not UA could reverse TMZ resistance in GBM cells remains unknown.
In this study, we evaluated the possible synergistic anticancer efficacy of TMZ and UA, and hypothesized that UA partly mediates the effects of TMZ through the depletion of MGMT expression. In our experiments, we demonstrated that UA suppressed the expression of MGMT in TMZ-resistant cells, and enhanced its response to the cytotoxic effects of TMZ. Our results suggest that the combination treatment of TMZ and UA could be a potential strategy for patients with refractory malignant gliomas.
Materials and methods
Reagents
Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), typsin and penicillin/streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and G418 were purchased from Sigma (St. Louis, MO, USA), and polyvinylidene difluoride (PVDF) membrane was purchased from Millipore (Bedford, MA, USA). Annexin V-FITC/PI Apoptosis Detection Kit was purchased from BD Pharmingen (San Diego, CA, USA), and BCA protein assay was purchased from Pierce (Rockford, IL, USA). Rabbit monoclonal antibodies against MGMT and phospho-H2A.X (Ser 139) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse monoclonal antibody against β-actin was purchased from Santa Cruz Inc (Santa Cruz, CA, USA). IRDye-conjugated anti-rabbit or anti-mouse IgG antibodies were obtained from LI-COR Biosciences Inc (Lincoln, NE, USA). UA was supplied by Liyuanheng Pharmaceutical Co., Ltd (Wuhan, China) with purity over 98%, and TMZ was supplied by TASLY Holding Group Co., Ltd. (Tianjin, China) with purity greater than 98%.
Cell culture
Human GBM cell lines LN229, LN18 and T98G were obtained from the American Type Culture Collection (Manassas, Virginia, USA). These cells were maintained in DMEM culture medium supplemented with 10% FBS, 100 units/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere with 5% CO2 at 37°C.
Stable transfection of LN229 with human MGMT cDNA
Exogenous MGMT or vector control expression in LN229 cells was accomplished using a lentiviral expression system. In brief, the human MGMT cDNA was cloned into the pCDH-CMV-MCS-EF1-Puro lentiviral vector (Genewiz Inc, Suzhou, China) for lentivirus production. LN229 cells were selected with 2 μg/mL G418 at 48 h after MGMT or vector control virus infection for 2 weeks.
Cell proliferation assay
For the cell proliferation assays, GBM cells were seeded in 96-well culture plates at a density of 4×103 cells/well. After 24 h, various concentrations of UA, TMZ or dimethyl sulfoxide (DMSO) were added to the cells, which were treated for the indicated time periods. At the end of treatment, MTT (5 mg/mL) was added into each well for 4 h. The supernatants were carefully aspirated, and the formazan crystals were dissolved using DMSO. Absorbance was recorded at 570 nm using a microplate reader.
Apoptosis analysis
Cells were seeded at approximately 50% confluence in six-well cell culture plates. UA, TMZ or DMSO was added to the cells after 24 h, and the cells were collected after 72 h of incubation. Cell apoptosis was measured by Annexin V-FITC/PI staining. Cells were considered early apoptotic if Annexin V was positive and PI was negative, late apoptotic if both Annexin V and PI were positive and necrosis if Annexin V was negative and PI was positive.
Colonogenic survival assay
For colonogenic survival studies, 300 cells were seeded in twelve-well plates, exposed to different treatments for 48 h, and then observed for 10-14 days. The colonies were fixed for 6 min in cold methanol, and then stained with a 1% crystal violet solution for 30 min. Colonies of more than 50 cells were counted. Percent colony formation was calculated by assigning untreated cultures as 100%. The percent colony formation of treated cells was determined by (colony formation of treated cells/colony formation of untreated cells) ×100.
Western blot analysis
Subconfluent cells were incubated with UA or TMZ for the indicated time periods. Cells were washed with ice-cold phosphate-buffered saline, and lysed in 240 mmol/L Tris (pH 6.8), 2% sodium dodecyl sulfate (SDS), 0.5% glycerol, 5 mmol/L EDTA, 1 mg/L aprotinin, 1 mg/L leupeptin, 1 mg/L pepstatin A, 10 mmol/L β-glycerol phosphate, 1 mmol/L Na3VO4 and 1 mmol/L phenylmethylsulfonyl fluoride. Lysates were then spun at 10,000 rpm for 10 min to remove insoluble materials. The protein concentration was measured by BCA protein assay, and equal amounts of lysates were boiled with SDS sample buffer. Proteins were separated by SDS-polyacrylamide gel electrophoresis, and then electrotransferred onto PVDF membranes. Primary antibodies were diluted in accordance with the manufacturer’s instructions, and the membranes were incubated overnight at 4°C. After washing, the membranes were incubated with IRDye-conjugated anti-rabbit or anti-mouse IgG antibodies, and proteins were visualized with an Odyssey LI-COR infrared imaging system. The density of bands was quantified using ImageJ software (NIH, http://imagej.nih.gov/ij/) and the results were expressed as fold change relative to the control treatment.
Nuclear morphometric analysis
Nuclear morphometry was performed as previously described [19]. In brief, the cells were treated with different treatments for 48 h, and then fixed in 4% paraformaldehyde for 10 min at room temperature. The fixed cells were marked with a solution containing 5 μg/mL DAPI and 0.1% Triton X-100 for 30 min at room temperature, and then the images obtained with DAPI staining were quantified using Image J plugin. Data were presented as a plot of area versus nuclear irregularity index (NII). The percentages of normal, irregular, small, small and regular, small and irregular, large and regular, and large and irregular were determined as previously described.
Xenograft in vivo
Female BALB/c mice (four weeks old) were purchased from the Academy of Military Medical Sciences (Beijing, China). Mouse care and treatment was approved by the Animal Care Committee of Tianjin Medical University Cancer Institute and Hospital. The animals were maintained under standardized environmental conditions: 22-28°C, 60%-70% relative humidity, 12/12-hour dark/light cycle and water ad libitum. LN18 cells (5×106/mouse) were subcutaneously injected into the left hind flank of each mouse. Tumor sizes were assessed using the two largest perpendicular axes. Tumor volume was calculated using the formula V=(a×b 2)/2, where a is the length and b is the width. When the tumor volumes reached 100 mm3, the mice were randomized to treatment or vehicle groups (6 mice per group). TMZ was administered via intraperitoneal (i.p.) injection at a dose of 50 mg/kg in 10% DMSO in saline daily for 5 consecutive days. UA was administered via i.p. injection at a dose of 50 mg/kg in 0.5% sodium carboxymethyl cellulose daily for 21 consecutive days. Tumor growth was monitored and measured twice a week with a vernier caliper. Tumor-bearing mice were assessed for weight loss twice a week. Tumors were removed from mice at 21 days after treatment. The relative tumor volume (RTV) was calculated as the ratio between the tumor volume at time t and the tumor volume at the start of treatment. Inhibition rates were expressed as TRTV/CRTV% (RTV of the treatment groups versus RTV of the control group) by dividing the RTVs of the treatment groups by those of the control group, and then multiplying the quotient by 100.
Statistical analysis
Each experiment was done in triplicate, and values are presented as mean ± SD. Analyses were conducted using the SPSS 16.0 software. The comparisons between different groups were performed using the one-way ANOVA. Difference was considered significant at P < 0.05.
Results
UA inhibits the proliferation of MGMT-expressing GBM cells
LN229, LN18 and T98G cells were subjected to an MTT assay to determine cell viability, and consequently examine the cytotoxicity of TMZ in various GBM cells. The cells were treated with 0-1000 μM TMZ for 7 days. As shown in Figure 1A, TMZ inhibited cell viability in a dose-dependent manner in the three glioma cell lines. Moreover, the inhibitory effect of TMZ in the colonogenic survival assay were much stronger than those in the acute proliferation inhibition assays (Figure 1B). The results also showed a higher resistance to TMZ in LN18 and T98G cells than in LN229 cells. To determine the expression of MGMT in the three glioma cell lines, Western blot assay was performed to detect the levels of MGMT. LN18 and T98G cell lines showed strong immunoreactive bands at the predicted size of 21 kDa on immunoblots (Figure 1C). Thus, MGMT expression and resistance to TMZ in the survival assays were highly correlated.
Figure 1.
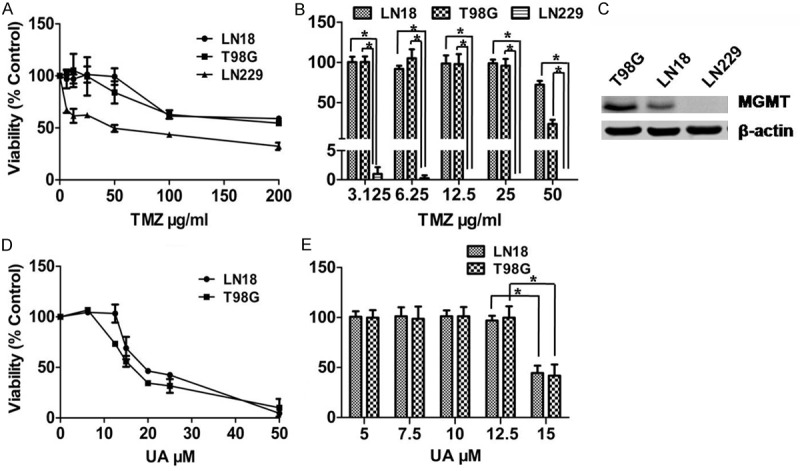
Effects of UA on MGMT-expressing GBM cells. A. LN18, T98G and LN229 cells were seeded in 96-well plates, and treated with TMZ for 7 days. Their responses were determined using MTT assay. B. LN18, T98G and LN229 cells were exposed to TMZ for 48 h. Colony-forming efficiency was determined 10-14 days later. C. Immunoblot analysis of MGMT in three GBM cell lines, namely, T98G, LN18 and LN229 (top). β-actin was used as a loading control (bottom). D. LN18 and T98G cells were seeded in 96-well plates and treated with UA for 3 days. Their responses were determined using MTT assay. E. LN18 and T98G cells were treated with UA for 48 h. Colony-forming efficiency was determined 10-14 days later. *P < 0.05.
To further investigate the effects of UA on MGMT-expressing GBM cells, the cytotoxic effects and inhibition of colony formation mediated by UA were conducted in T98G and LN18 cells. The results showed that UA inhibited cell viability and colony formation in a dose-dependent manner (Figure 1D and 1E).
UA downregulates MGMT expression in GBM cells
The expression of MGMT has been thought to be inversely related to TMZ cytotoxicity. To determine whether or not UA modulates MGMT expression, we treated LN18 and T98G cells with various concentrations of UA for 72 h, and performed Western blot assay to detect the level of MGMT. The results demonstrated that UA could downregulate MGMT expression in a dose-dependent manner in MGMT-expressing GBM cell lines (Figure 2A). Overexpression of MGMT in LN229 cells significantly enhanced resistance to TMZ (Figure 2B). However, UA downregulated MGMT expression in MGMT-overexpressing LN229 (LN229/MGMT) cells in a similar manner (Figure 2C), demonstrating that UA has the potential to enhance the efficacy of TMZ by MGMT depletion.
Figure 2.
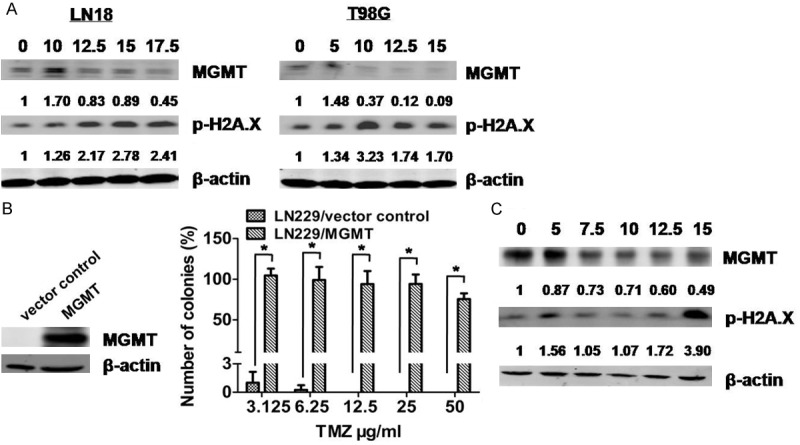
UA reduced the expression of MGMT in MGMT-expressing GBM cell lines. A. LN18 and T98G cells were treated with UA at varying concentrations for 48 h. MGMT and p-H2A.X levels were measured by immunoblot assay. B. Expression of MGMT determined by immunoblot assay of whole-cell protein extracts of LN229 cells, which were manipulated by stable transfection with vector control or MGMT. LN229/vector control and LN229/MGMT cells were exposed to TMZ for 48 h. Colony-forming efficiency was determined 10-14 days later. C. LN229/MGMT cells were treated with UA of varying concentrations for 72 h. MGMT and p-H2A.X levels were measured by immunoblot assay. *P < 0.05.
Phosphorylation of H2A.X were considered markers of DNA damage. The phospho-H2A.X levels in LN18, T98G and LN229/MGMT cells were elevated after UA treatment.
UA potentiates the cytotoxic effect of TMZ in GBM cells
We combined 12.5 μM UA with 0-1000 μM TMZ to treat LN18 and T98G cells for 72 h to verify the effect of UA on TMZ-mediated cytotoxicity in MGMT-expressing cells. Cell viability was lower in cells treated with a combination of 12.5 μM UA and various concentrations of TMZ than in cells treated with TMZ alone (Figure 3A). Furthermore, UA increased TMZ-induced apoptosis, necrosis and colonogenic inhibition, indicating that UA can sensitize MGMT-expressing GBM cells to TMZ (Figure 3B and 3C).
Figure 3.
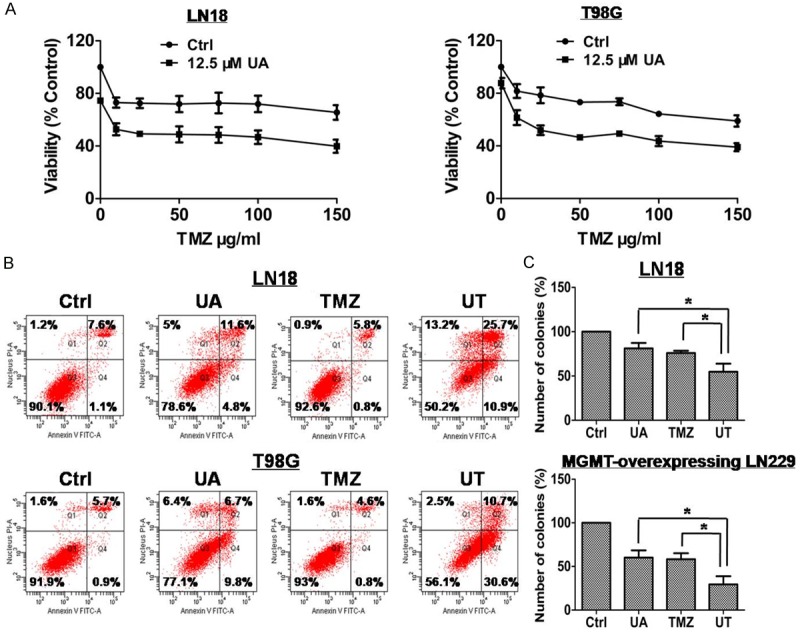
UA enhanced the cytotoxicity of TMZ in MGMT-expressing GBM cells. A. LN18 and T98G cells were seeded in 96-well plates, and treated with 12.5 μM UA, TMZ of varying concentrations, or UT for 3 days. Cell responses were determined using MTT assay. B. Induced overall cell deaths of T98G and LN18 cell lines were determined by flow cytometric analysis of Annexin V/PI double-stained cells 48 h after treatment with 12.5 μM UA, 25 μg/ml TMZ, or UT. C. LN18 cells were exposed to 12.5 μM UA, 25 μg/ml TMZ, or UT for 48 h. LN229/MGMT cells were exposed to 15 μM UA, 100 μg/ml TMZ, or UT for 48 h. Colony-forming efficiency was determined 10-14 days later. *P < 0.05.
UA attenuates TMZ resistance by downregulating MGMT expression
Furthermore, the effect of UA combined with TMZ (UT) on MGMT level was examined. The combination treatment produced a synergistic effect to reduce MGMT expression in T98G, LN18 and LN229/MGMT cells. Compared to TMZ or UA treatment alone, UT treatment did not significantly change the expression of phospho-H2A.X in LN18, T98G and LN229/MGMT cells (Figure 4A and 4B).
Figure 4.
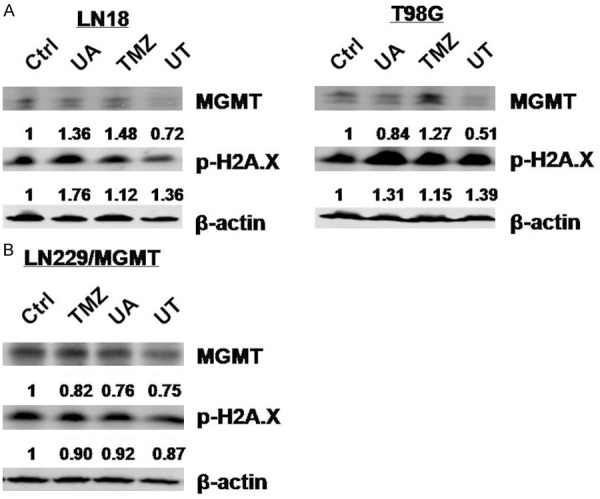
UT downregulates MGMT expression in MGMT-expressing cells. A. LN18 and T98G cells were treated with 12.5 μM UA, 25 μg/ml TMZ, or UT treatment for 72 h. The MGMT and p-H2A.X levels were measured by immunoblot assay. B. LN229/MGMT cells were treated with 15 μM UA, 100 μg/ml TMZ, or UT treatment for 72 h. The MGMT and p-H2A.X levels were measured by immunoblot assay.
UT mediates senescence
We observed that the UT-treated cells presented nuclear morphological changes. The percentage of large nuclei significantly increased in LN18 cells with UT treatment, indicating that UT induced senescence in TMZ-resistant GBM cells (Figure 5). Senescence induction was thought to be O6-G methylation dependent [20]. Thus, senescence induction and MGMT depletion with UT treatment were closely correlated.
Figure 5.
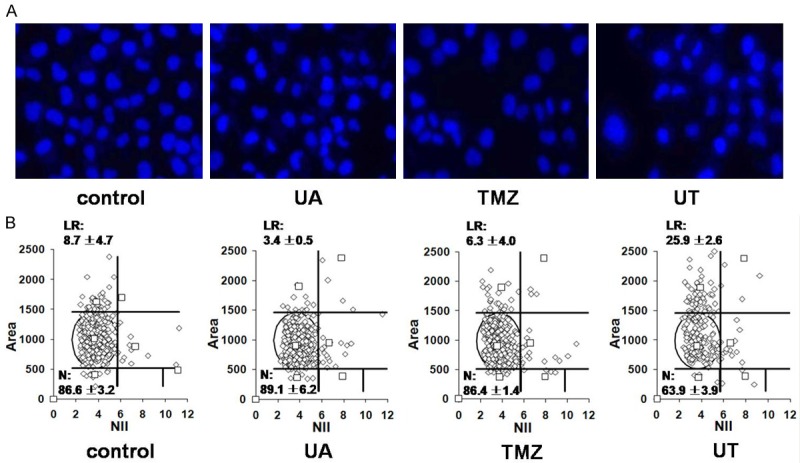
UT induces senescence in MGMT-expressing LN18 cells. A. Representative images of nuclei from LN18 cells subjected to 12.5 μM UA, 25 μg/ml TMZ, or UT treatment for 48 h, fixation, and then DAPI staining. B. DAPI-stained nuclei were analyzed for size and irregularity using NMA tool, as described in the Materials and methods section, and the percentages of normal (N) and large regular (LR) nuclear types are shown.
UA enhances the efficacy of TMZ in glioma xenografts
We conducted an antitumor study using a mouse xenograft model to determine the effect of UT on tumor growth in vivo. The inhibition rates for the UA, TMZ and UT groups were 92.4%, 83.2% and 39.9%, respectively. UT treatment significantly reduced tumor volume compared with TMZ and UA monotherapies (P < 0.05, Figure 6A), indicating that UA might have enhanced the cytotoxicity of TMZ in the xenograft model. No significant weight loss was observed in the treatment groups, suggesting that UA-associated toxicity was acceptable, as shown in Figure 6B. To verify the mechanism of UT, the proteins extracted from the tumor tissues were analyzed using the immunoblot assay. As shown in Figure 6C, TMZ induced the downregulation of MGMT expression, suggesting that UA potentiated the effect of TMZ by MGMT depletion. These results indicate that UA enhances the cytotoxicity of TMZ through the same pathway both in vitro and in vivo.
Figure 6.
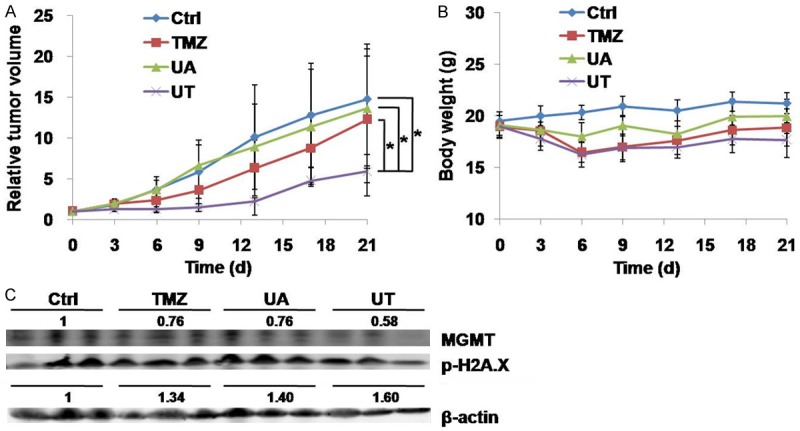
UA potentiates the efficacy of TMZ in a xenograft model. (A) Mice with LN18 xenograft were treated intraperitoneally with vehicle, i.e., TMZ (50 mg/kg), UA (50 mg/kg), or their combination for 21 days. (A) Tumor volume and (B) Body weight were measured as described in the Materials and methods section. (C) Lysates extracted from the tumor tissues were examined using immunoblot assay with anti-MGMT and anti-phospho-H2A.X antibody. β-actin was used as internal control. *P < 0.05.
Discussion
Despite the effectiveness of TMZ in treating malignant glioma, many patients still fail to benefit from this drug because of primary and acquired resistance. Several experimental agents, such as valproic acid [21], withaferin A [22], resveratrol [23], interleukin-24 [24], Interferon-alpha/beta [25], BC3EE2,9B [26], lipoic acid [27] and O6-BG derivatives, were developed to attenuate TMZ resistance in glioma cells. However, their clinical applications need to be further demonstrated.
At present, traditional medicine has become an important source of exploring novel antitumor drugs. Screening of high-efficiency and low-toxicity compounds from natural medicine for reversing drug resistance has become an attractive focus in cancer research. Previous studies demonstrated that UA induces notable antitumor effects and presents minimal toxicity [15]. More importantly, UA can pass through the blood-brain barrier in vivo [28], suggesting the potential of UA as a new candidate for GBM therapy.
In the present study, we showed for the first time that UA induced a potent cytotoxicity against TMZ-resistant GBM cells and enhanced the efficacy of TMZ in vitro and in vivo. Furthermore, the expression of MGMT was downregulated by UT, indicating that UA sensitized TMZ-resistant GBM cells to TMZ partly by downregulating MGMT expression.
Aside from apoptosis and autophagy, senescence is thought to be another cellular response to DNA damage [20]. Senescent cells are viable, but they cease to synthesize DNA and present morphological changes characterized by large nuclei. Given that senescence is dependent on O6-G methylation induction, which can be eliminated by the suicidal enzyme MGMT, senescence was not observed in MGMT-expressing LN18 cells when treated with 25 μg/mL TMZ for 48 h. However, TMZ combined with UA induced obvious senescence in LN18 cells, suggesting that UA promoted the accumulation of DNA damages resulting from O6-G methylation induced by depleting MGMT.
STAT3 activation is necessary for post-transcriptionally regulating MGMT in GBM cells. STAT3 inhibition could overcome TMZ resistance in GBM cells by downregulating MGMT expression [29]. A previous study showed that UA could inhibit STAT3 activity specifically in human multiple myeloma cells and improve the sensitivity of tumor cells to bortezomib and thalidomide therapies [30]. UA can significantly inhibit the growth of glioma xenografts in nude mice by blocking the STAT3 signaling pathway without weight loss and other adverse reactions [31]. In the present study, UA inhibited the phosphorylation of STAT3 in a concentration-dependent manner (data not shown). However, whether UA-induced MGMT depletion was mediated by STAT3 inhibition should be further investigated.
In conclusion, the current study demonstrated that UT combination treatment synergistically attenuates TMZ resistance and provides a novel strategy for the clinical application of UA to MGMT-driven resistant cell lines both as a cytotoxic agent and as an enhancer of TMZ efficacy. UA, as both a monotherapy and a resensitizer, might be a candidate agent for patients with refractory malignant glioma.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81402481), Tianjin Municipal Health Bureau fund (No. 2014KZ085) and Doctoral Foundation of Tianjin Medical University Cancer Institute and Hospital (No. B1312).
Disclosure of conflict of interest
None.
References
- 1.Theeler BJ, Groves MD. High-grade gliomas. Curr Treat Options Neurol. 2011;13:386–399. doi: 10.1007/s11940-011-0130-0. [DOI] [PubMed] [Google Scholar]
- 2.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 3.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 4.Batich KA, Sampson JH. Standard of care and future pharmacological treatment options for malignant glioma: an urgent need for screening and identification of novel tumor-specific antigens. Expert Opin Pharmacother. 2014;15:2047–2061. doi: 10.1517/14656566.2014.947266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messaoudi K, Clavreul A, Lagarce F. Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov Today. 2015;20:899–905. doi: 10.1016/j.drudis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Jiang G, Jiang AJ, Xin Y, Li LT, Cheng Q, Zheng JN. Progression of O(6)-methylguanine-DNA methyltransferase and temozolomide resistance in cancer research. Mol Biol Rep. 2014;41:6659–6665. doi: 10.1007/s11033-014-3549-z. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Stevens MF, Bradshaw TD. Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol. 2012;5:102–114. doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 8.Jiang G, Wei ZP, Pei DS, Xin Y, Liu YQ, Zheng JN. A novel approach to overcome temozolomide resistance in glioma and melanoma: Inactivation of MGMT by gene therapy. Biochem Biophys Res Commun. 2011;406:311–314. doi: 10.1016/j.bbrc.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 9.Balmaceda C, Peereboom D, Pannullo S, Cheung YK, Fisher PG, Alavi J, Sisti M, Chen J, Fine RL. Multi-institutional phase II study of temozolomide administered twice daily in the treatment of recurrent high-grade gliomas. Cancer. 2008;112:1139–1146. doi: 10.1002/cncr.23167. [DOI] [PubMed] [Google Scholar]
- 10.Kaina B, Margison GP, Christmann M. Targeting O(6)-methylguanine-DNA methyltransferase with specific inhibitors as a strategy in cancer therapy. Cell Mol Life Sci. 2010;67:3663–3681. doi: 10.1007/s00018-010-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn JA, Jiang SX, Reardon DA, Desjardins A, Vredenburgh JJ, Rich JN, Gururangan S, Friedman AH, Bigner DD, Sampson JH, McLendon RE, Herndon JE 2nd, Walker A, Friedman HS. Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J. Clin. Oncol. 2009;27:1262–1267. doi: 10.1200/JCO.2008.18.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 13.Prasad S, Yadav VR, Kannappan R, Aggarwal BB. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK. J Biol Chem. 2011;286:5546–5557. doi: 10.1074/jbc.M110.183699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Meng Y, Lin ZM, Ge N, Zhang DL, Huang J, Kong F. Ursolic Acid Induces Apoptosis of Prostate Cancer Cells via the PI3K/Akt/mTOR Pathway. Am J Chin Med. 2015;43:1471–1486. doi: 10.1142/S0192415X15500834. [DOI] [PubMed] [Google Scholar]
- 15.Shan JZ, Xuan YY, Ruan SQ, Sun M. Proliferation-inhibiting and apoptosis-inducing effects of ursolic acid and oleanolic acid on multi-drug resistance cancer cells in vitro. Chin J Integr Med. 2011;17:607–611. doi: 10.1007/s11655-011-0815-y. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Liu X, Lu Z, Yuet-Wa Chan J, Zhou L, Fung KP, Wu P, Wu S. Ursolic acid induces doxorubicin-resistant HepG2 cell death via the release of apoptosis-inducing factor. Cancer Lett. 2010;298:128–138. doi: 10.1016/j.canlet.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Xavier CP, Lima CF, Pedro DF, Wilson JM, Kristiansen K, Pereira-Wilson C. Ursolic acid induces cell death and modulates autophagy through JNK pathway in apoptosis-resistant colorectal cancer cells. J Nutr Biochem. 2013;24:706–712. doi: 10.1016/j.jnutbio.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YX, Kong CZ, Wang LH, Li JY, Liu XK, Xu B, Xu CL, Sun YH. Ursolic acid overcomes Bcl-2-mediated resistance to apoptosis in prostate cancer cells involving activation of JNK-induced Bcl-2 phosphorylation and degradation. J Cell Biochem. 2010;109:764–773. doi: 10.1002/jcb.22455. [DOI] [PubMed] [Google Scholar]
- 19.Filippi-Chiela EC, Oliveira MM, Jurkovski B, Callegari-Jacques SM, da Silva VD, Lenz G. Nuclear morphometric analysis (NMA): screening of senescence, apoptosis and nuclear irregularities. PLoS One. 2012;7:e42522. doi: 10.1371/journal.pone.0042522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knizhnik AV, Roos WP, Nikolova T, Quiros S, Tomaszowski KH, Christmann M, Kaina B. Survival and death strategies in glioma cells: autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS One. 2013;8:e55665. doi: 10.1371/journal.pone.0055665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu CH, Yoon WS, Park KY, Kim SM, Lim JY, Woo JS, Jeong CH, Hou Y, Jeun SS. Valproic acid downregulates the expression of MGMT and sensitizes temozolomide-resistant glioma cells. J Biomed Biotechnol. 2012;2012:987495. doi: 10.1155/2012/987495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grogan PT, Sarkaria JN, Timmermann BN, Cohen MS. Oxidative cytotoxic agent withaferin A resensitizes temozolomide-resistant glioblastomas via MGMT depletion and induces apoptosis through Akt/mTOR pathway inhibitory modulation. Invest New Drugs. 2014;32:604–617. doi: 10.1007/s10637-014-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CJ, Lee CC, Shih YL, Lin TY, Wang SH, Lin YF, Shih CM. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic Biol Med. 2012;52:377–391. doi: 10.1016/j.freeradbiomed.2011.10.487. [DOI] [PubMed] [Google Scholar]
- 24.Zheng M, Bocangel D, Ramesh R, Ekmekcioglu S, Poindexter N, Grimm EA, Chada S. Interleukin-24 overcomes temozolomide resistance and enhances cell death by down-regulation of O6-methylguanine-DNA methyltransferase in human melanoma cells. Mol Cancer Ther. 2008;7:3842–3851. doi: 10.1158/1535-7163.MCT-08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen D, Guo CC, Wang J, Qiu ZK, Sai K, Yang QY, Chen YS, Chen FR, Panasci L, Chen ZP. Interferon-alpha/beta enhances temozolomide activity against MGMT-positive glioma stem-like cells. Oncol Rep. 2015;34:2715–2721. doi: 10.3892/or.2015.4232. [DOI] [PubMed] [Google Scholar]
- 26.Chen CM, Syu JP, Way TD, Huang LJ, Kuo SC, Lin CT, Lin CL. BC3EE2,9B, a synthetic carbazole derivative, upregulates autophagy and synergistically sensitizes human GBM8901 glioblastoma cells to temozolomide. Int J Mol Med. 2015;36:1244–1252. doi: 10.3892/ijmm.2015.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goder A, Nagel G, Kraus A, Dorsam B, Seiwert N, Kaina B, Fahrer J. Lipoic acid inhibits the DNA repair protein O 6-methylguanine-DNA methyltransferase (MGMT) and triggers its depletion in colorectal cancer cells with concomitant autophagy induction. Carcinogenesis. 2015;36:817–831. doi: 10.1093/carcin/bgv070. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, Luo S, Zhang Y, Chen Z. Development of a liquid chromatography-mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: application to the pharmacokinetic and tissue distribution study. Anal Bioanal Chem. 2011;399:2877–2884. doi: 10.1007/s00216-011-4651-x. [DOI] [PubMed] [Google Scholar]
- 29.Kohsaka S, Wang L, Yachi K, Mahabir R, Narita T, Itoh T, Tanino M, Kimura T, Nishihara H, Tanaka S. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther. 2012;11:1289–1299. doi: 10.1158/1535-7163.MCT-11-0801. [DOI] [PubMed] [Google Scholar]
- 30.Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, Kadara H, Guha S, Sethi G, Aggarwal BB. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5:943–955. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 31.Shanmugam MK, Ong TH, Kumar AP, Lun CK, Ho PC, Wong PT, Hui KM, Sethi G. Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS One. 2012;7:e32476. doi: 10.1371/journal.pone.0032476. [DOI] [PMC free article] [PubMed] [Google Scholar]


