Abstract
Background: Acute myeloid leukemia (AML) is an immunophenotypically heterogeneous malignant disease, in which CD34 positivity is associated with poor prognosis. Osteopontin (OPN) plays different roles in physiologic and pathologic conditions like: survival, metastasis and cell protection from cytotoxic and apoptotic stimuli. Due to anti-apoptotic effect of OPN in normal and malignant cells, silencing of OPN leads to elevation of sensitivity towards chemotherapeutic agents and attenuates cancer cells migration and invasion. Therefore, the aim of this study was to evaluate OPN roles in modulating curcumin-mediated growth inhibitory on leukemic stem cells (LSCs) colony forming potential and survival in AML cell lines and primary CD34+/CD38- bone marrow-derived AML cells.
Materials and Methods: Primary human CD34+/CD38- cells were isolated from bone marrow mononuclear cells of 10 AML patients at initial state of diagnosis, using a CD34 Multi sort kit. The growth inhibitory effects of curcumin (CUR) were evaluated by MTT and colony-formation assays. Apoptosis was analyzed by 7AAD assay in CD34+ KG-1, U937 cell lines and primary isolated cells. Short interfering RNA (siRNA) against OPN was used for OPN silencing in both cell lines and primary AML cells. Then, transfected cells were incubated with/without curcumin. The change in OPN gene expression was examined by Real-time PCR.
Results: CUR inhibited proliferation and induced apoptosis in both KG-1 and U937 cells and also primary isolated AML cells. OPN silencing by siRNA increased the susceptibility of KG-1, U937 and primary CD34+/CD38- AML cells to apoptosis. Moreover, soft agar colony assays revealed that silencing of OPN with siRNA significantly decreased colony numbers in LSCs compared with the non-targeting group. Furthermore, CD34+/CD38- populations as a main LSCs compartment through OPN overexpression towards CUR treatment might be nullified the inhibitory effects of OPN siRNA on their survival and colony forming potential.
Conclusion: Taken together, our results suggested that knockdown of OPN using OPN specific siRNA significantly decreased colony numbers in LSCs and this effect might be vetoed by LSCs via induction of OPN overexpressionin combination of CUR and siRNA.
Key Words: Curcumin, Osteopontin, Leukemic stem cell, Colony forming potential, SiRNA
Introduction
Acute myeloid leukemia (AML) is one of the most common leukemias in adults. AML is characterized by an accumulation of undifferentiated and heterogeneous populations of cells. 1 Overlay, any treatment that reduces the tumor burden less than 109 will result in a clinical complete remission (CR). Even with high-dose chemotherapy, only 30%-40% of AML patients survive, which is due mainly to relapse of the disease. 2 Hence, it is clear that there is a rare subset of malignant cells that are not effectively eradicated by current treatment regiments. 3 LSCs may justify this failure of the CR. 4 The phenotype of LSCs may be somehow variable from patient to patient and even, in some cases, more than one phenotypically distinct subpopulation may possess LSC activity. However, It is suggested that they mainly reside within the CD34+/CD38- compartment of leukemic clone and CD34+ CD38+ and CD34- populations are other fractions of LSCs. 5 , 6
Osteopontin (OPN), encoded by a single gene on chromosome 4q13, 7,8 is secreted by many cell types such as lymphocytes, osteoclasts, endothelial cells and tumoral cells. 9 OPN is elevated in stromal cells-mediated tumor microenvironment 10 and plays different roles in physiologic and pathologic conditions like: invasion, metastasis, survival, angiogenesis, tumorigenesis, tumor growth,11,12 regulation of bone hemostasis and cell protection from cytotoxic and apoptotic stimuli. 13 Since curative treatment of leukemia will most likely require the elimination of LSCs, new strategies need to be designed that overcome the drug resistance of these cells. In this respect, strategies that target a distinct Achilles' heel of the LSC biology appear to be particularly promising. Furthermore, most of the compounds that have been shown to successfully eradicate LSCs are known to be inhibitors of NF-kB. 14 In this regard, it seems curcumin (CUR) is a suitable choice to large extent. CUR, one of different molecules in Curcuma longa (diferuloylmethane) a member of the Zingiberacae (ginger) family, has several properties such as anti-inflammatory, antioxidant and antimicrobial activities. 15,16 Inhibition of cancer cell proliferation, invasion, metastasis, angiogenesis and also apoptosis induction 17 by disruption of molecular targets which inhibit the signaling pathways including NF-kB, 18 AKT/mTOR and HIF-1α molecules.17,19,20 Although CUR induces apoptosis in variety of AML cell lines, cytotoxic effects of CUR in LSCs remain unclear. Despite the well-defined functions for OPN in solid tumor and benign HSC biology, there is little data regarding OPN and leukemia. Recently, a few reports have been published in hematological malignancies.21,22 Due to anti-apoptotic effect of OPN in normal and malignant cells; 23-25 so, silencing of OPN that leads to elevation of sensitivity towards chemotherapeutic agents and attenuates cancer cells migration and invasion. 26,27 In this regard, based on the above mentioned, maybe CUR is probably considered choice. Therefore, the aim of present study was to evaluate the effect of CUR and OPN specific siRNA on LSCs survival and colony forming potential. In this study, we have demonstrated that LSCs via OPN overexpression at mRNA level towards CUR treatment might be vetoed the inhibitory effects of OPN siRNA on survival and colony forming potential.
MATERIALS AND METHODS
Reagents and antibodies
Curcumin (CUR) (Sigma, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO) to provide a 100 mM stock solution and stored at -20°C. 7AA Dassay kit (BD Biosciences; San Jose, CA, USA), CD34 Multi sort, Micro Bead kit (Miltenyi biotec, Auburn, CA, USA) and 3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) dye were obtained from Sigma-Aldrich (St Louis, MO, USA). CD34-RPE, CD38-FITC (BD Biosciences; San Jose, CA, USA) and Methocult semi-solid media purchased from (Stem Cell Technologies, Vancouver, BC, Canada).
Cell lines and cell culture
KG-1 and U937 cell lines were purchased from Pasteur institute of Iran. KG-1 cells were cultured in DMEM medium and supplemented with 20% FBS (Gibco; Invitrogen, USA), 2 mM L-glutamine, 100 units/ml penicillin and 100 µg/ml streptomycin. U937 cells were cultured in RPMI 1640 medium supplemented with 10% FBS (Gibco; Invitrogen, USA), 2 mM L-glutamine, 100 units/mL penicillin and 100 µg/mL streptomycin. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. For CUR treatment, a relevant amount of working solution (5 mM in DMSO) of CUR was added to culture medium to attain the concentrations of 20, 40, 60, 80 µM. Control cultures were received an equivalent amount of DMSO (0.1%) during treatment with CUR.
Cell separation, cell sorting and primary culture
Totally, 10 bone marrow aspirations were collected from patients at initial time of diagnosis and prior to any treatment during admission in Hematology-Oncology ward, Shariati Hospital. The study was approved by Ethical committee and informed consent was obtained from all of patients recruited in the study (Ethical code: Ir.tums.horcsct. 1394.103.5).
Diagnoses were based on standard hematological protocol and classification which was according to French-American-British classification. The clinical characteristic of leukemia patients has been shown in Table 1. Bone marrow mononuclear cells were obtained by Ficoll-Hypaque density gradient centrifugation. CD34+/CD38- cells were purified from these mononuclear cells by multi sort CD34 MACS Column Technology according to manufacturer protocol. The purity of enriched CD34+/CD38- was evaluated by staining with FITC-conjugated anti-CD34 and CD38-PE. The isolated CD34+/CD38- cells were treated with CUR and cultured in RPMI 1640 medium supplemented with 10% FBS (Gibco; Invitrogen, USA), 2 mM L-glutamine, 100 units/mL penicillin and 100 µg/mL streptomycin and incubated at 37°C in 5% CO2 incubator for indicated tests. Control cultures received an equivalent amount of DMSO (0.1%) during treatment with CUR.
Table 1.
Characteristic of patients
| Patient | Age/Sex | FAB | %CD34 in BMC | Source | Cytogenetic | FLT3 | WT-1 copy number |
|---|---|---|---|---|---|---|---|
| P1 | 35Y/M | M1 | 32% | BM | 46, XY [8] | Neg | 905 |
| P2 | 40Y/M | M0 | 38% | BM | 46, XY [20] | Neg | 1810 |
| P3 | 30Y/M | M1 | 43% | BM | 50, XY add (3) (p12), -5, +8, +21, +21, +marI [4]/51, XY, idem, +18, +marI [3]/50, XY, idem, +22 [2]/51, XY, idem, 22, marI [2]/52, xy, IDEM, +18, marI, +marII [4]/46, xy [5], 45, xx, -7 [49]/46, XX [1] | Neg | 701 |
| P4 | 50Y/F | M0 | 61% | BM | 45, XX, -7 [49]/46, XX [1] | Neg | 4302 |
| P5 | 40Y/M | M2 | 77% | BM | 45, XY, del (5) (q14;q34), der (15), t (15;17) (p11.1 ;q11.1), -17 [12]/46, XY [38] | Neg | 3066 |
| P6 | 28Y/M | M4 | 15% | BM | 45, XY, [20] | Neg | 780 |
| P7 | 38Y/M | M2 | 40% | BM | 45, XY, [20] | Neg | 7331 |
| P8 | 36Y/M | M2 | 45% | BM | 46, XY, t (6;9) (p23;q3), t (9;18) (q34;q21) [20] | Neg | 5080 |
| P9 | 49Y/M | M4 | 30% | BM | 46, XY, del (11q23) [10] | Neg | 28911 |
| P10 | 30Y/F | M2 | 43% | BM | 46, XX, [20] | Neg | 3532 |
P: Patient, Y: Year, M: Male, F: Female, FAB: French-American-British, BMC: Bone marrow cells, %CD34 in BMC: Percentage of CD34+ cells in bone marrow cells of AML patients before sorting, FLT3: Fms like tyrosine kinase, WT-1: Wilms Tumor-1
MTT assay
Micro culture Tetrazolium Test (MTT) assay was used to test the effect of CUR on viability of KG-1 and U937 cell lines. The cells at density of 5,000 cells/100 μl in 96-well culture plates (SPL Life sciences, Pocheon, Korea), were treated with desired concentration of CUR and incubated in a humidified tissue-culture chamber (370C, 5% CO2) for 24-48h. MTT solution (0.5 mg/ml) was added to each well and the plate was incubated at 37 C in 5% CO2 atmosphere. DMSO was added to each well and incubated for 4h to liquefy the dye crystals. Absorbance at a wavelength of 570 nm was gauged by an ELISA plate reader (Micro plate Reader; Bio-Rad). Cell treated with 0.1% DMSO was defined as the control group.
In vitro colony formation assay
Treated and untreated KG-1, U937 cell lines and primary CD34+/CD38- AML cells were suspended in RPMI 1640 medium. Colony formation assay was done in Methocult semi-solid media (Stem Cell Technologies, Vancouver, BC, Canada). The cell density was 2000/mL/plate and cells were incubated at 37°C for 14 days. Routine colony counts were evaluated by inverted microscope. Accumulation of 50 cells or more were scored as one colony and collection of 3~50 cells were as one cluster. Three independent experiments were carried out.
RNA isolation and Real-time PCR
Tripure isolation Reagent (Roche Applied Science, Germany) was used for total RNA extraction according to the manufacturer’s instruction. The quantity of RNA samples were assessed spectrophotometrically using Nanodrop ND-1000 (Nanodrop Technologies, Wilmington, DE). Complementary DNA (cDNA) synthesis was performed by using cDNA synthesis kit (Takara Bio Inc., Otsu, Japan). Real-Time PCR was performed with Step One Plus™ ABI instrument (Apply Bio systems, USA) using 2 μl of a 2-fold diluted cDNA, 0.5 µl of each forward and reverse primers (10 pMol) and SYBR Premix Ex Taq technology (Takara Bio Inc, Otsu, Japan) in a final volume of 20 μl. HPRT mRNA expression levels were used to estimate the relative expression levels. Thermal cycling conditions included a predenaturation step for 30s at 950C followed by 45 cycles including a denaturation step for 5s at 950C and a combined annealing/extension step for 20s at 600C. The specificity of PCR reactions was confirmed by melting curve analysis. The fold change for each mRNA in treated leukemic cell lines in comparison with untreated cells was computed by the 2-ΔΔCT method.28 The primers and their corresponding amplicon lengths were provided in Table 2.
Table 2.
Nucleotide sequences of primers used for Real-time RT-PCR
| Gene | Accession Number | Forward Primer | Reverse Primer | Size (bp) |
|---|---|---|---|---|
| HPRT | NM_000194 | TGGACAGGACTGAACGTCTTG | CCAGCAGGTCAGCAAAGAATTTA | 111 |
| OPN | NM_001251830 | ACCCTTCCAAGTAAGTCCAACG | GGTGAGAATCATCAGTGTCATCTAC | 139 |
OPN: Osteopontin, HPRT: Hypoxanthine-guanine phosphoribosyl transferase
Short interfering RNA (siRNA) transfection
The effect of OPN mRNA expression and also efficacy of curcumin in induction of apoptosis was tested by use of RNA interference in both cell lines and patient samples.
Small interfering RNA (siRNA) for OPN with a 21 base pair duplex as well as its control with the same base composition were designed against all variants of OPN gene and partial cds silencing in a random sequence (Table 3).
Table 3.
SiRNA sequence
| Name | Sequence |
|---|---|
| Sense (5’-3’) | GGAAUAUUACUGUGGGAAAdTdT |
| Antisense (5’-3’) | UUUCCCACAGUAAUAUUCCdTdT |
OPN specific as well as control siRNA were given in optimization experiments and maximal gene knockdown was attained 24h post-transfection by using siRNA at final concentration of 40 (pM). Impact of OPN gene knockdown on cell number and viability were evaluated on 96-well culture plates (SPL Life Sciences, Pocheon, Korea) for 24h after transfection. The siRNA were transfected using of Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer’s instruction.
Statistical analysis
SPSS 18 was used to perform statistical analysis. The significance of differences between experimental variables was determined by the use of two-tailed student's test to compare between the control and the experimental groups. All experiments were performed in triplicate and results have been expressed as the mean ± standard deviation (SD). One-way analysis of variance (One-way ANOVA) was used to determine statistical significances of difference. Statistical significance were defined at *p<0.05, **p<0.01 and ***p<0.001 compared to corresponding control.
Results
CUR inhibits cell proliferation
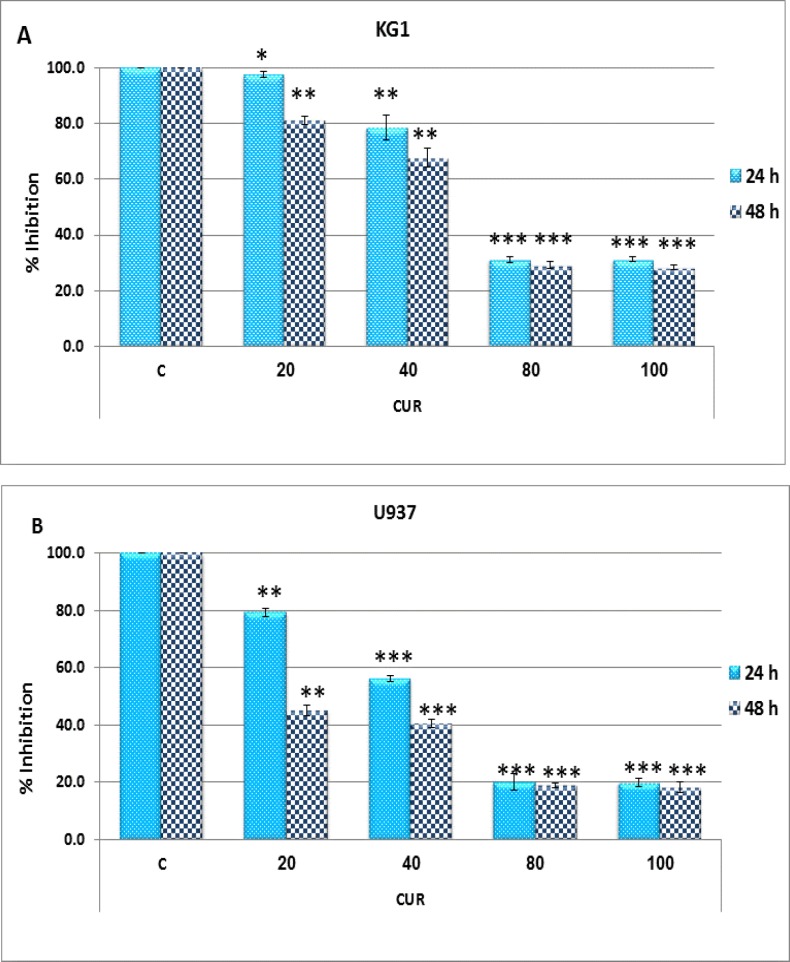
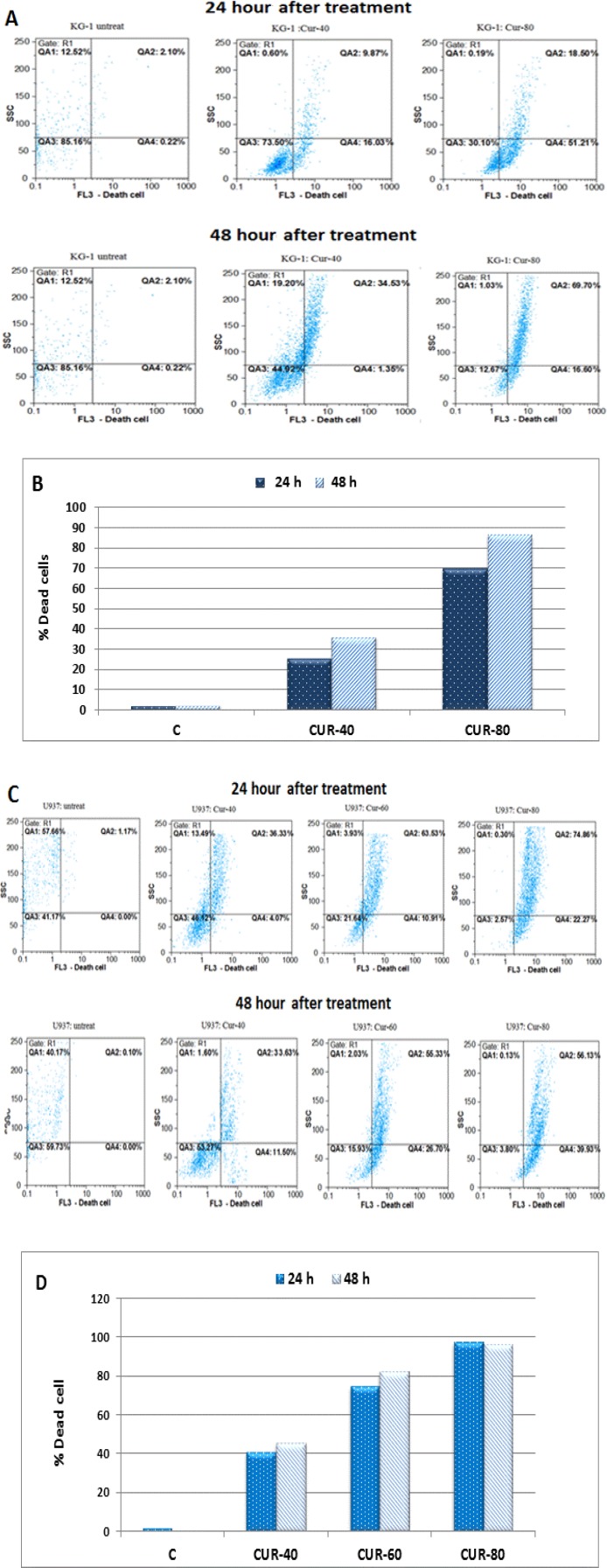
Initial studies were performed to elucidate the anti-survival effects of CUR on AML cell lines. After treatment of these cell lines with different concentrations of CUR for 24-48h, growth suppressive effect were assessed by MTT methods (Figure 1: A-B). Afterward, more sensitive 7AAD method was performed to confirm result of MTT assay. The result of 7AAD and MTT showed that CUR inhibited cell proliferation with lowest IC50 value for U937 cells (40 µM) in comparison to KG-1 cell lines (80 µM). Interestingly, drug exposure time (24-48h) had no significant effect on cell growth. CUR had a significant cytotoxic effect in all tested cell lines in dose dependent manners (Figure 1: A-B and Figure 2: A-D).
Figure 1.
Effects of curcumin with different concentration (0-100 µM) on cell viability. Anti-growth effect of curcumin was measured by MTT assay following 24-48h exposure to KG-1 (A) and U937 cell lines (B). Data are mean ± SD of three independent experiments. Statistical significance was defined at *p<0.05, **p<0.01 and ***p<0.001 compared to corresponding control.
Figure 2.
Results of 7AAD assay in KG-1 and U937 cell lines after 24-48h treatment with different concentration of curcumin (0-80 µM). (A): Flow cytometry histograms and (B) bar charts in KG-1 cell line. (C): Flow cytometry histograms and (D) bar charts in U937 cell line
Colony assay
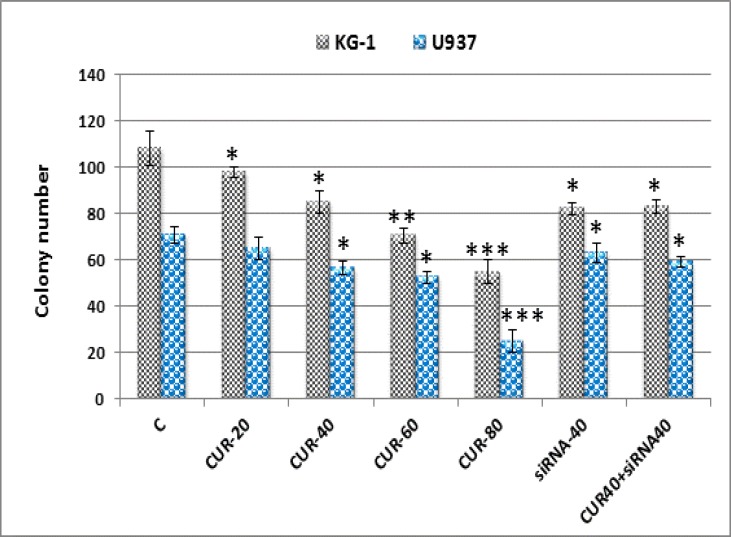
To assess the effects of CUR treatment, in vitro colony assays was used to determine whether CUR affected the colony forming potential of these cells. AML cell lines were first treated with 20-80 µM CUR, siRNA and/or combination of CUR and siRNA for 24 hours followed by analysis using methylcellulose colony assays. Figure 3 shows that AML colony-forming units (CFUs) were dramatically reduced by CUR and siRNA treatment alone but combination of CUR and siRNA have similar effect with CUR 40 µM alone.
Figure 3.
Effects of curcumin (40 µM) and siRNA (40 pM) on colony forming potential. Colony formation assay confirms great decrease in colony count by curcumin and siRNA in both cell lines. Values are given as mean ± SD of three independent experiments.
LSCs might be vetoed the inhibitory effects of OPN siRNA on colony forming potential via OPN overexpression towards CUR treatment
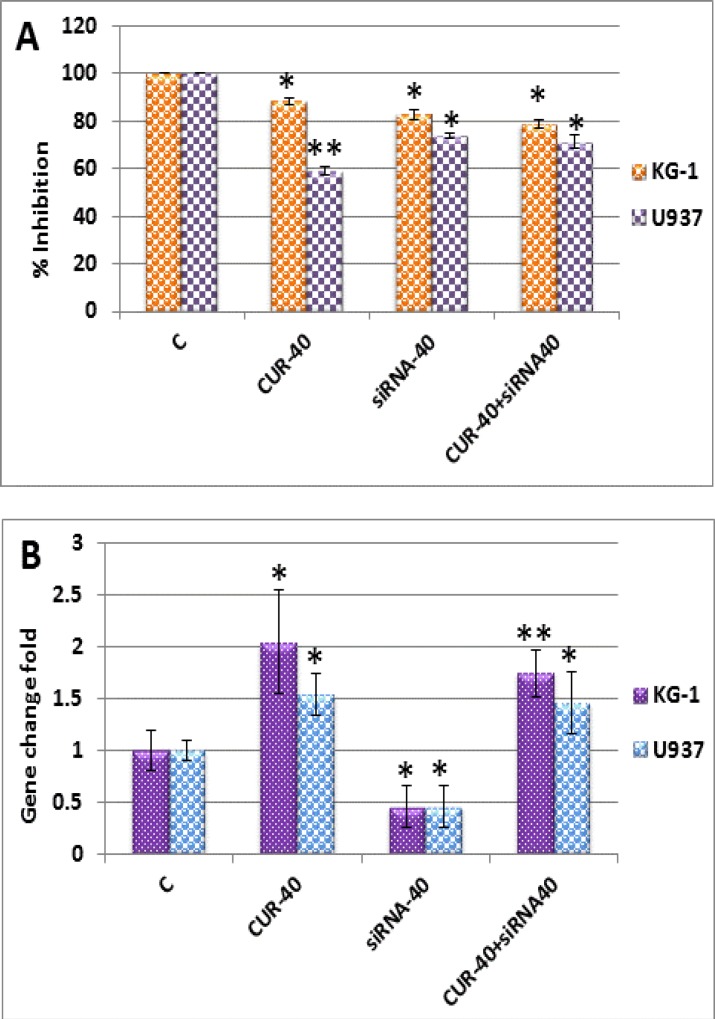
To directly examine whether change in OPN gene expression, plays role in nullifying of inhibitory potency of CUR and siRNA on AML cells colonization. OPN expression was suppressed by siRNA and also the effect of OPN knockdown on in vitro proliferation was examined by MTT assay; then, results in mRNA levels were confirmed by Real-time PCR (Figure 4: A-B). Suppression of OPN expression by siRNA alone increased the susceptibility of these cell lines to apoptosis (17 ± 2 in KG-1 cells vs. 26 ± 1% in U937 cells), compared to combination of curcumin with siRNA (21 ± 1.7% in KG-1 cells vs. 29 ± 2.8% in U937 cells) (Figure 4: A-B).
Figure 4.
Result of osteopontin gene transfection with siRNA (40 pM) and treatment with curcumin (40 µM) in KG-1 and U937 cell lines. (A): Viability was assessed by MTT after transfection in both cell lines. (B): Results of Real-time PCR after transfection with in both cell lines showed that osteopontin could preclude curcumin-induced apoptosis and has additive effect with siRNA. Data are mean ± S.D. of three similar experiments.
These results suggest that CUR with enhancement of OPN gene in mRNA level could preclude CUR induced apoptosis and nullify the colony forming potential of OPN specific inhibitor in AML cell lines.
Effect of curcumin on CD34+/CD38- isolated AML patient cells
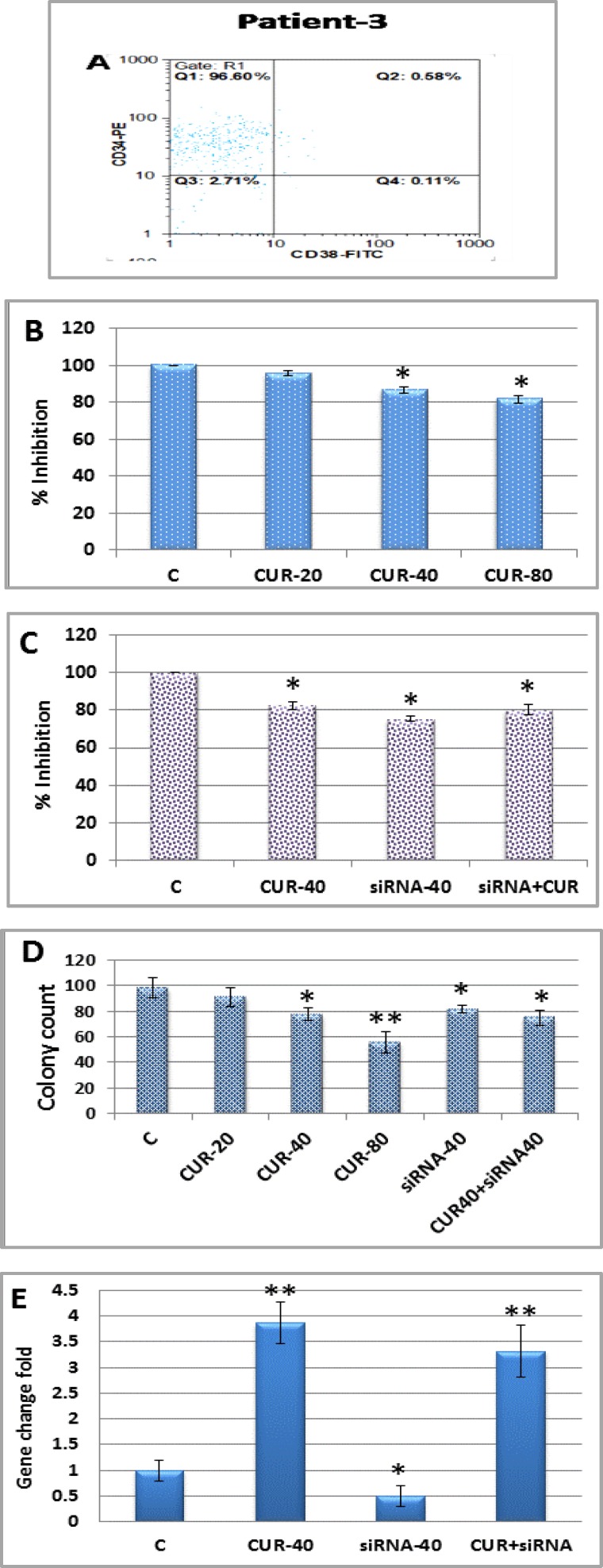
The cytotoxic effects of CUR on primary CD34+/CD38- as main phenotypes of AML LSCs were also examined. CD34+/CD38- cells were isolated from BMNCs of 10 AML patients by MACS Technology. The purity of isolated samples was more than 92% in most cases (Figure 5: A). CD34+/CD38- cells were treated with CUR (20, 40, 80 μM) for 24h and growth inhibitory effects were determined by MTT assay (Figure 5: B).
Figure 5.
Curcumin was effective against primary CD34+/CD38- AML cells. (A): Primary CD34+/CD38- cells isolated from BMMCs of 10 AML patients and subjected to flow cytometry to determine the purity of CD34+/CD38- cells. (B): MTT assay were performed for all isolated AML samples which treated with different concentrations of curcumin (0, 20, 40 and 80 μM) for 24h. (C): Effects of siRNA (40 pM) transfection and curcumin treatment on growth inhibitory were examined by MTT and (D): Colony assay. Result on OPN gene expression (E): was evaluated by Real-time PCR. Values are given as mean ± SD of three independent experiments.
In order to verify the role of OPN gene expression in LSCs colonization, OPN gene were silenced in all AML samples with siRNA followed by CUR treatment for 24h.
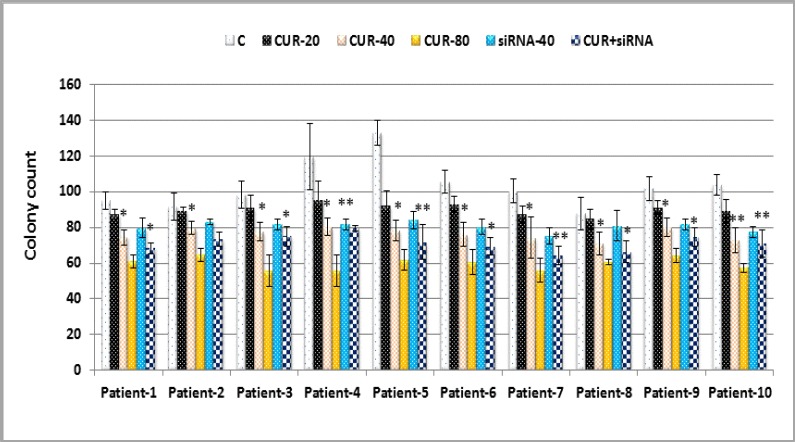
The effects of OPN knockdown on growth inhibitory were evaluated by MTT (Figure 5: C), colony assay (Figure 5: D and Figure 6) and in mRNA level assessed by Real-time PCR (Figure 5: E). Result showed that CUR inhibited proliferation and colonization potency of CD34+/CD38- LSCs depend on French-American-British (FAB) classifications, underlying cytogenetic and molecular changes. LSCs vetoed this effect via OPN overexpression.
Figure 6.
Effects of curcumin and siRNA on colony forming potential of primary CD34+/CD38- AML sample. Data are mean ± S.D. of three independent experiments. Statistical significance was defined at *p<0.05, **p<0.01 and ***p<0.001 compared to corresponding control
Discussion
In contrast to well-defined association between OPN and solid tumor progression and metastasis,27,29 there is paucity of research on OPN in leukemia. OPN is an important component of Hematopoietic Stem Cell (HSC) niche which participates in HSC location and as a physiologic-negative regulator of HSC proliferation. 9 Hence, deregulated expression of OPN may play an important pathogenic role in hematological malignancies.
Targeting OPN and OPN-related signaling pathways may represent an important avenue for the development of therapeutics strategy. 27 The results of present study demonstrated that CUR induced apoptosis and reduced colonization potency in both U937 cells and KG-1 cells, as well as in primary CD34+/CD38- AML cells.
Curcumin inhibited cell growth and also induced apoptosis in both cell lines and patients primary cell culture. The residual cells (CD34+ 38- as main phenotype of LSCs) remained after treatment with CUR has evolved to produce an increased level of OPN expression. This is evidenced by co-treatment of CUR and siRNA did not show any synergic effect on reduction of colonization potency in AML cells. These data provide the first proof of principle that how OPN can maintain LSCs dormancy towards CUR treatment. Our Real-time analyses demonstrated that the expression of OPN was up-regulated by CUR treatment in both AML cell lines and primary AML cells.
We hypothesized that the acquired over expression of OPN in survival signaling pathway may interpret acquired resistance of LSCs towards CUR induced apoptosis. Since OPN plays an important role in the mechanism underlying drug resistance to CUR in AML cells, these data may provide evidence that how LSC is able to attenuate CUR induced apoptosis in AML.
In present study, we demonstrated that CUR could enhance apoptosis and decrease colony count in both KG-1 and U937 cell lines, as well as in primary CD34+/CD38- AML cells. In line with our result, Rao and colleagues reported that CUR significantly inhibited proliferation and clonogenic growth in a dose-dependent manner in CD34+ AML cells. 30
CD44 is one of OPN receptors and also functions as an anti-apoptotic protein. 31,32 CD44 is an important determinant of tumor progression and metastasis in various human malignant tumors and recently, its role was demonstrated in tumor stem cell biology.33,34
In good agreement with our result, Subramaniam et al. in their research on HT-29 as a human colon cancer cell line which produce a large amount of CD44 protein showed that after CD44 silencing with siRNA, cell lines were less adhesive to hyaluronan and more susceptible to apoptosis induced by etoposide.35
They claimed that siRNA CD44 clones formed a lower number and size of colonies in soft agar assays .On the other hand, siRNA CD44 cell clone xenografted in nude mice generated tumors with a reduced tumor volume and wet weight, as compared to control vector clone.
CONCLUSION
Our results suggested that the knockdown of OPN inhibited apoptosis in LSCs. Also, soft agar colony assays revealed that silencing of OPN using siRNA against OPN variants and partial cds significantly decreased colony numbers in both AML cell lines and primary CD34+/CD38- AML cells. Furthermore, LSCs with promotion of OPN overexpression towards CUR treatment might be nullify the inhibitory effects of OPN siRNA on colony forming potential and also maintain their survival.
ACKNOWLEDGEMENT
This study was supported by Iranian Blood Transfusion Research Center, High Institute for Education and Research in Transfusion Medicine, Iran and also Hematology-Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran. Results of this study concluded from the thesis which was approved by High Institute for Research and Education in Transfusion Medicine.
CONFLICT OF INTEREST
None
References
- 1.Costello RT, Mallet F, Gaugler B, et al. Human acute myeloid leukemia CD34+/CD38- progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60(16):4403–11. [PubMed] [Google Scholar]
- 2.Roboz GJ, Guzman M. Acute myeloid leukemia stem cells: seek and destroy. Expert Rev Hematol. 2009;2(6):663–72. doi: 10.1586/ehm.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter RB, Appelbaum FR, Tallman MS, et al. Shortcomings in the clinical evaluation of new drugs: acute myeloid leukemia as paradigm. Blood. 2010;116(14):2420–8. doi: 10.1182/blood-2010-05-285387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 5.Martelli AM, Evangelisti C, Chiarini F, et al. The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget. 2010;1(2):89–103. doi: 10.18632/oncotarget.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eppert K, Takenaka K, Lechman ER, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17(9):1086–93. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- 7.Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90(10):1877–81. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16(2):79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106(4):1232–9. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Sharma P, Kumar D, et al. Functional characterization of stromal osteopontin in melanoma progression and metastasis. PloS one. 2013;8(7):e69116. doi: 10.1371/journal.pone.0069116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Z, Lin D, Yuan J, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res. 2005;11(13):4646–52. doi: 10.1158/1078-0432.CCR-04-2013. [DOI] [PubMed] [Google Scholar]
- 12.Weber GF, Lett GS, Haubein NC. Categorical meta-analysis of Osteopontin as a clinical cancer marker. Oncol Rep. 2011;25(2):433–41. doi: 10.3892/or.2010.1106. [DOI] [PubMed] [Google Scholar]
- 13.Boyerinas B, Zafrir M, Yesilkanal AE, et al. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood. 2013;121(24):4821–31. doi: 10.1182/blood-2012-12-475483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassane DC, Sen S, Minhajuddin M, et al. Chemical genomic screening reveals synergism between parthenolide and inhibitors of the PI-3 kinase and mTOR pathways. Blood. 2010;116(26):5983–90. doi: 10.1182/blood-2010-04-278044. [DOI] [PubMed] [Google Scholar]
- 15.Wilken R, Veena MS, Wang MB, et al. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24(12):651–4. [PubMed] [Google Scholar]
- 17.Hossain DM, Bhattacharyya S, Das T, et al. Curcumin: the multi-targeted therapy for cancer regression. Front Biosci (Schol Ed) 2012;4:335–55. doi: 10.2741/272. [DOI] [PubMed] [Google Scholar]
- 18.Bharti AC, Donato N, Singh S, et al. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101(3):1053–62. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 19.Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 2010;343(9):489–99. doi: 10.1002/ardp.200900319. [DOI] [PubMed] [Google Scholar]
- 20.Padhye S, Chavan D, Pandey S, et al. Perspectives on chemopreventive and therapeutic potential of curcumin analogs in medicinal chemistry. Mini Rev Med Chem. 2010;10(5):372–87. doi: 10.2174/138955710791330891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flamant S, Kortulewski T, Dugray A, et al. Osteopontin is upregulated by BCR-ABL. Biochem Biophys Res Commun. 2005;333(4):1378–84. doi: 10.1016/j.bbrc.2005.05.203. [DOI] [PubMed] [Google Scholar]
- 22.Vejda S, Piwocka K, McKenna SL, et al. Autocrine secretion of osteopontin results in degradation of I kappa B in Bcr-Abl-expressing cells. Br J Haematol. 2005;128(5):711–21. doi: 10.1111/j.1365-2141.2004.05355.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Zhang XF, Zhou HJ, et al. Expression and prognostic significance of osteopontin and caspase-3 in hepatocellular carcinoma patients after curative resection. Cancer Sci. 2010;101(5):1314–9. doi: 10.1111/j.1349-7006.2010.01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valcz G, Sipos F, Krenacs T, et al. Elevated osteopontin expression and proliferative/apoptotic ratio in the colorectal adenoma-dysplasia-carcinoma sequence. Pathol Oncol Res. 2010;16(4):541–5. doi: 10.1007/s12253-010-9260-z. [DOI] [PubMed] [Google Scholar]
- 25.Robertson BW, Chellaiah MA. Osteopontin induces beta-catenin signaling through activation of Akt in prostate cancer cells. Exp Cell Res. 2010;316(1):1–11. doi: 10.1016/j.yexcr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Chen JH, Cai D, et al. Osteopontin and integrin are involved in cholesterol gallstone formation. Med Sci Monit. 2012;18(1):BR16–23. doi: 10.12659/MSM.882194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell JA, Thomas D, Barry EF, et al. Expression profiling of a hemopoietic cell survival transcriptome implicates osteopontin as a functional prognostic factor in AML. Blood. 2009;114(23):4859–70. doi: 10.1182/blood-2009-02-204818. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using Real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008;111(12):5477–85. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao J, Xu DR, Zheng FM, et al. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J Transl Med. 2011;9:71. doi: 10.1186/1479-5876-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokosaki Y, Matsuura N, Sasaki T, et al. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274(51):36328–34. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 32.Katagiri YU, Sleeman J, Fujii H, et al. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59(1):219–26. [PubMed] [Google Scholar]
- 33.Chakraborty G, Jain S, Kale S, et al. Curcumin suppresses breast tumor angiogenesis by abrogating osteopontin-induced VEGF expression. Mol Med Rep. 2008;1(5):641–6. doi: 10.3892/mmr_00000005. [DOI] [PubMed] [Google Scholar]
- 34.Lin YH, Huang CJ, Chao JR, et al. Coupling of osteopontin and its cell surface receptor CD44 to the cell survival response elicited by interleukin-3 or granulocyte-macrophage colony-stimulating factor. Mol Cell Biol. 2000;20(8):2734–42. doi: 10.1128/mcb.20.8.2734-2742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramaniam V, Vincent IR, Gilakjan M, et al. Suppression of human colon cancer tumors in nude mice by siRNA CD44 gene therapy. Exp Mol Pathol. 2007;83(3):332–40. doi: 10.1016/j.yexmp.2007.08.013. [DOI] [PubMed] [Google Scholar]