Abstract
Background
One to five years of therapy of chronic hepatitis B with oral nucleoside analogues result in significant clinical improvements, but effects of more prolonged therapy are not well defined.
Aims
To describe outcomes of chronic hepatitis B with long-term lamivudine therapy.
Methods
42 patients with chronic hepatitis B treated with lamivudine were followed for 3.2 to 19.5 (median = 16.1) years. Therapy was switched to other agents (n=16) if patients developed lamivudine resistance and relapse of disease.
Results
Among 22 HBeAg-positive patients, 17 (77%) became HBeAg negative, of whom 5 (23%) subsequently cleared HBsAg. Among 20 HBeAg-negative patients, 10 (50%) cleared HBsAg. The time to HBsAg clearance ranged from 0.9 to 16.8 (median = 9.3) years. Lamivudine resistance arose in 24 patients (57%) of whom 6 (25%) lost HBsAg. HBsAg clearance was not always accompanied by seroconversion; anti-HBs appearing concurrently in only 5 patients (33%). Nevertheless, HBsAg loss allowed for stopping therapy in all patients, none re-developing HBsAg or suffering relapse; all having normal ALT levels and no (n=13) or unquantifiable HBV DNA levels (n=2) when last seen. In contrast, 7 of 27 patients (26%) who remained HBsAg-positive died of liver disease or liver cancer or underwent liver transplantation, all of whom had cirrhosis.
Conclusion
Long-term viral suppression with nucleoside analogues leads to HBsAg loss in a substantial proportion of patients, particularly if HBeAg-negative. Serious outcomes during the first 10–20 years of treatment occur largely among patients with pre-existing cirrhosis who do not clear HBsAg with therapy.
Introduction
Oral nucleoside analogues with potent activity against the hepatitis B virus (HBV) have become the mainstay of therapy of chronic hepatitis B. Therapy with these agents result in a prompt decline in HBV DNA levels and subsequent improvements in serum biochemical tests and liver histology.1, 2 Therapy for one or two years can be accompanied by clearance of hepatitis B e antigen (HBeAg) and seroconversion to antibody (anti-HBe) in 10% to 30% of patients.3–9 Long-term therapy has been associated with improvement in hepatic fibrosis and, in some instances, clearance of hepatitis B surface antigen (HBsAg) and development of antibody to HBsAg (anti-HBs).10, 11 What remains unclear is how long patients should be treated and what criteria should be used to stop therapy. Relapse upon stopping therapy even after loss of HBeAg is common. Furthermore, oral nucleoside therapy is extremely well tolerated usually without significant serious side effects. For these reasons, therapy of hepatitis B with oral antiviral agents is generally given long-term with no clear basis or criteria for stopping treatment other than HBsAg loss.2
In 1995, we initiated an open label trial of lamivudine at the Clinical Center of the National Institutes of Health.12 At the time, lamivudine was not approved for use in hepatitis B although it was widely used for human immunodeficiency virus (HIV) infection. Initially planned for 5 years, the study was later extended for patients to continue on lamivudine (100 mg daily) indefinitely or until they became HBsAg-negative or developed clinically apparent antiviral resistance. Those who developed lamivudine resistance with rising levels of HBV DNA and serum alanine aminotransferase (ALT) levels were offered treatment with other agents, if available. Until 2001, the only other option for therapy of hepatitis B was interferon alfa. Thereafter, adefovir dipivoxil became available (2002) and later entecavir (2005) and tenofovir disoproxil fumarate (2008). In recent years, trials of approaches to promote clearance of HBsAg were initiated and some of these subjects entered studies of antiviral therapy withdrawal (2012) or addition of a six-month course of peginterferon (2015). In 2015, we conducted a long-term follow up evaluation on all patients who were enrolled to assess long-term outcomes in those who remained on lamivudine as well as those who were switched to other agents.
Materials and Methods
Initial Protocol
An open-label prospective study of lamivudine therapy was initiated in 1995 at the Clinical Center of the National Institutes of Health.12 Lamivudine was provided under a Clinical Trial Agreement with GlaxoSmithKline. Adult patients (age 18 or above) with biopsy proven chronic hepatitis B were eligible. All patients were required to have hepatitis B surface antigen (HBsAg) and HBV DNA in serum (in levels above 105 copies/mL as detected by branched chain hybridization assays13 available at that time). Both HBeAg positive and negative patients were included. Patients also had to have elevations in serum alanine or aspartate aminotransferase (ALT or AST) and a liver biopsy showing chronic hepatitis with necrosis and active inflammation. Patients with compensated cirrhosis were eligible but those with decompensated cirrhosis (Childs Pugh B and C) were not. Other exclusion criteria included antiviral therapy within the previous six months, chronic infection with hepatitis C or D virus, human immunodeficiency virus (HIV) infection, need for chronic immunosuppressive therapy and significant renal impairment (serum creatinine above 1.5 mg/dL). Two patients were enrolled on a compassionate-use basis who were 14 and 17 years of age for whom no other therapeutic options were available, and another was enrolled despite not having a recent liver biopsy because of a low platelet count due to cirrhosis (54,000/µL).
Follow-up Protocol
The initial protocol called for therapy to continue for 5 years with repeat liver biopsies at 1 and 4 years (95-DK-0199: NCT 00001457). The protocol was later amended to continue therapy for 8 years after which patients were switched to an omnibus, natural history study of patients with chronic liver disease (91-DK-0214: NCT00001971). Therapy was continued indefinitely. Patients who developed lamivudine resistance and recurrence of liver injury were switched to other agents if available. Some patients were later eligible for a study of adefovir with or without lamivudine (01-DK-0246: NCT 00023309)13 and subsequently a similar study of tenofovir with or without emtricitabine (07-DK-0207: NCT 00524173). Other patients were treated “off-protocol” with other medications approved for therapy of hepatitis B such as interferon, adefovir, entecavir and tenofovir. Patients who became HBsAg negative were continued on treatment for another 6 months and then withdrawn. Some patients were continued on treatment for longer than 6 months due to irregularity of follow up or resistance to stopping the medication so soon after loss of HBsAg. One patient stopped therapy on their own at year 4. Data on patients who were enrolled in studies of withdrawal of antiviral therapy (11-DK-0151: NCT01581554) or addition of peginterferon in attempts to induce clearance of HBsAg (15-DK-0082: NCT02364336) were censored at the time of enrollment in those studies.
Monitoring
Patients were monitored on therapy at once monthly intervals for the first year and at three monthly intervals thereafter. Liver biopsies were repeated at 1 year and again at 4 and 8 years in consenting patients if still on therapy. Regular monitoring included routine liver tests, complete blood counts, HBsAg, HBeAg, anti-HBe and HBV DNA levels. All details of these protocols were approved by the NIDDK institutional review board and all patients provided written, informed consent.
Laboratory testing
The assays used for detection of HBV DNA varied over the period of the initial study and follow up, being initially direct branched-chain hybridization (expressed as copies/mL with a lower limit of detection of ~100,000 copies/mL)14 as well as an in-house, experimental polymerase chain reaction (PCR) and later quantitative PCR-based assays, most recently COBAS® AmpliPrep/COBAS® TaqMan® HBV Test, v2.0 (Roche Molecular Systems, Inc., Branchburg, NJ) with a lower limit of detection of 20 IU/mL.15 Hepatitis B virus genotyping was done by line-probe hybridization assay.16 Selected stored specimens were tested for HBsAg concentration using the ADVIA Centaur HBsAg quantitative assay (Siemens Healthcare Diagnostics, USA), and results were expressed as IU/mL.17 Virologic resistance to lamivudine was tested using a restriction fragment-length polymorphism assay initially (12) and later by line probe hybridization, INNO-LiPA HBV Genotyping assay (Innogenetics N.V., Ghent, Belgium). Selected stored serum specimens were retested for genotype and HBV DNA levels using more recently developed assays. Monitoring also included hepatic imaging by ultrasound done every six months in patients with cirrhosis or advanced fibrosis and every one to two years in those without significant fibrosis. Liver histology was assessing using a modification of the histology activity index and Ishak fibrosis scores.18
Statistical analysis
Mann-Whitney U test was used for continuous variables (Age, ALT, AST, total bilirubin, prothrombin time, albumin, HAI score, Ishak score, HBV DNA and HBsAg). Fischer’s exact test was used to compare frequencies between two groups for categorical variables (race, genotype, sex, cirrhosis, HBeAg status, lamivudine resistance). All calculated P values were 2-tailed and compared with a significance level of 0.05. Serum HBV DNA levels and HBsAg titers were logarithmically transformed for analysis. Kaplan-Meier analysis was applied to estimate and compare the rates of loss of surface antigen between HBeAg-positive and HBeAg-negative patients. SAS v. 9.4 (Cary, NC) was used for data analysis.
Results
Patients enrolled
Between 1995 and 2002, 42 patients with chronic hepatitis B were enrolled in an open-label study of lamivudine therapy. The 42 patients were mostly men (86%), ages 14 to 71 years, of whom 22 (52%) were non-Hispanic whites, 17 (40%) Asian Americans and 3 (7%) African-Americans or black. All patients were positive for HBsAg and 22 had HBeAg, the remaining 20 being HBeAg-negative and anti-HBe-positive. The baseline clinical, biochemical, histologic and virologic features of the cohort comparing those with HBeAg to those without are shown in Table 1. The two groups had similar clinical and demographic features, serum aminotransferase elevations and histological features. As expected, patients with HBeAg had higher levels of HBV DNA and HBsAg. Genotype A was most common in the HBeAg positive group, and genotypes D and E were found only among the HBeAg-negative group. Three HBeAg positive and one HBeAg negative patients had glomerulonephritis suspected to be due to hepatitis B. Thirteen patients (31%) had cirrhosis (Ishak fibrosis score 5 or 6 or clinically apparent cirrhosis without biopsy). Results of lamivudine treatment in the initial 27 patients enrolled in this study were published in 2000.12
Table 1.
Patient Baseline Characteristics
| Characteristic | HBeAg positive (n=22) |
HBeAg negative (n=20) |
p value |
|---|---|---|---|
| Age (years)* | 47 (14–67) | 48 (17–71) | 0.85 |
| Male Sex | 20 (91%) | 16 (80%) | 0.40 |
| Race: White or Caucasian | 13 | 9 | |
| Black or African-American | 1 | 2 | 0.56 |
| Asian-American | 8 | 9 | |
| Source: Sexual | 10 | 3 | |
| Maternal-Infant | 7 | 15 | 0.03 |
| Transfusion | 2 | 0 | |
| Other or Unknown | 3 | 2 | |
| ALT (U/L)* | 106.5 (28–524) | 99.5 (39–336) | 0.65 |
| AST (U/L)* | 69 (25–331) | 64.5 (25–171) | 0.42 |
| Bilirubin (mg/dL)* | 0.6 (0.3–3.0) | 0.5 (0.3–1.8) | 0.45 |
| Albumin (g/dL)* | 4.1 (1.7–4.6) | 4.1 (2.9–4.7) | 0.90 |
| Prothrombin time (sec)* | 12.6 (11.3–13.6) | 13.4 (11.8–15.0) | 0.01 |
| Platelet count (per µL)* | 187 (113–346) | 174.5 (33–328) | 0.43 |
| APRI* | 1.3 (0.2–6.2) | 1.4 (0.3–12.9) | 0.74 |
| HBV DNA (log IU/mL)* | 9.2 (7.3–10.4) | 7.4 (5.3–9.5) | <0.001 |
| HBsAg concentration (log IU/mL)* | 3.9 (2.0–5.3) | 2.9 (1.9–4.0) | 0.02 |
| Genotype: A | 15 | 6 | |
| C | 7 | 8 | 0.008 |
| D | 0 | 5 | |
| E | 0 | 1 | |
| HAI score (0–18)* | 10.5 (3–17) | 12.0 (5–14) | 0.92 |
| Ishak score (0–6)* | 4 (0–6) | 3 (0–6) | 0.05 |
| Cirrhosis (Ishak 5 or 6) | 8 (36%) | 5** (25%) | 0.51 |
Median (range).
Imputed for one case.
Follow up
The 42 patients enrolled were continued on lamivudine for 1.5 to 19.4 (median = 7.4) years and have been followed for 3.2 to 19.5 (median = 16.1) years. At the time of the last attempt at contact (February 2016), 5 patients had been lost to follow up and 8 had died or undergone liver transplantation. Of the remaining 29 patients, 12 were no longer taking antiviral therapy, 7 were still on lamivudine alone, and 10 were on various other antiviral regimens for hepatitis B including entecavir (n=4), tenofovir (n=2) and the combination of tenofovir and emtricitabine (Truvada, n=4).
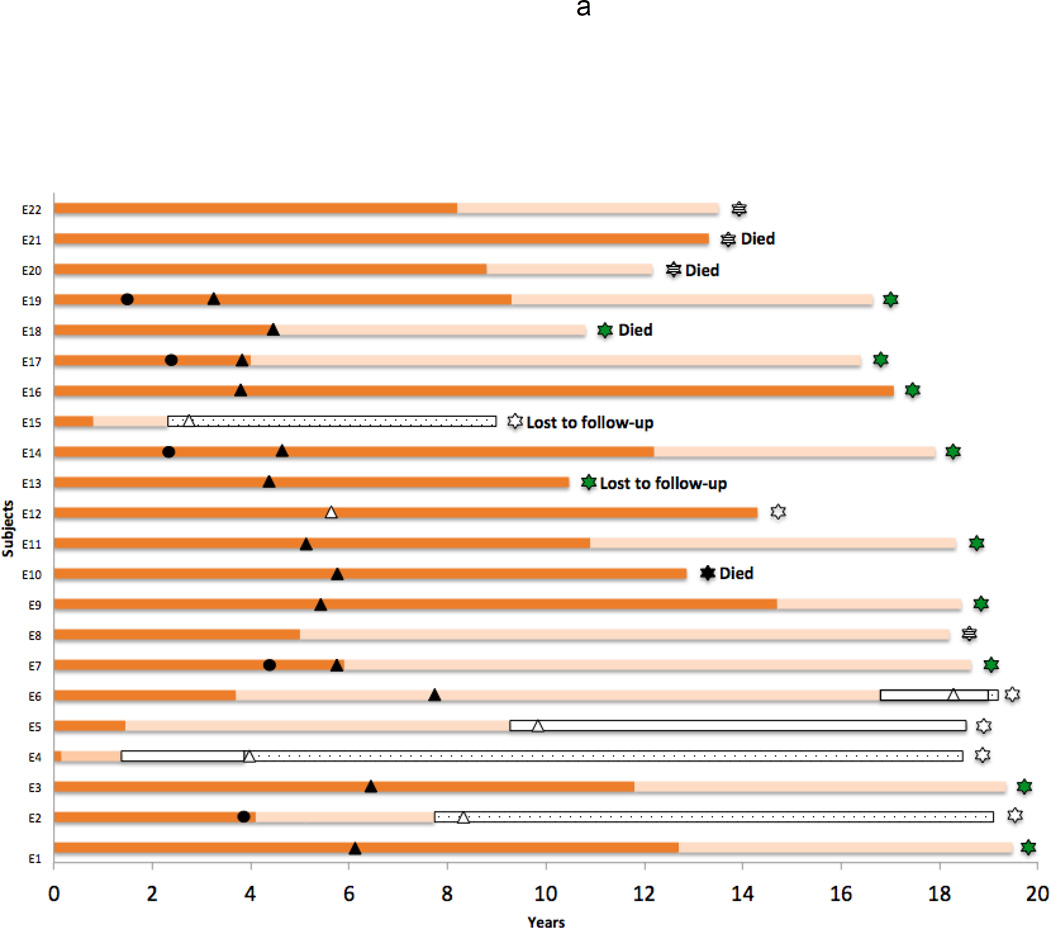
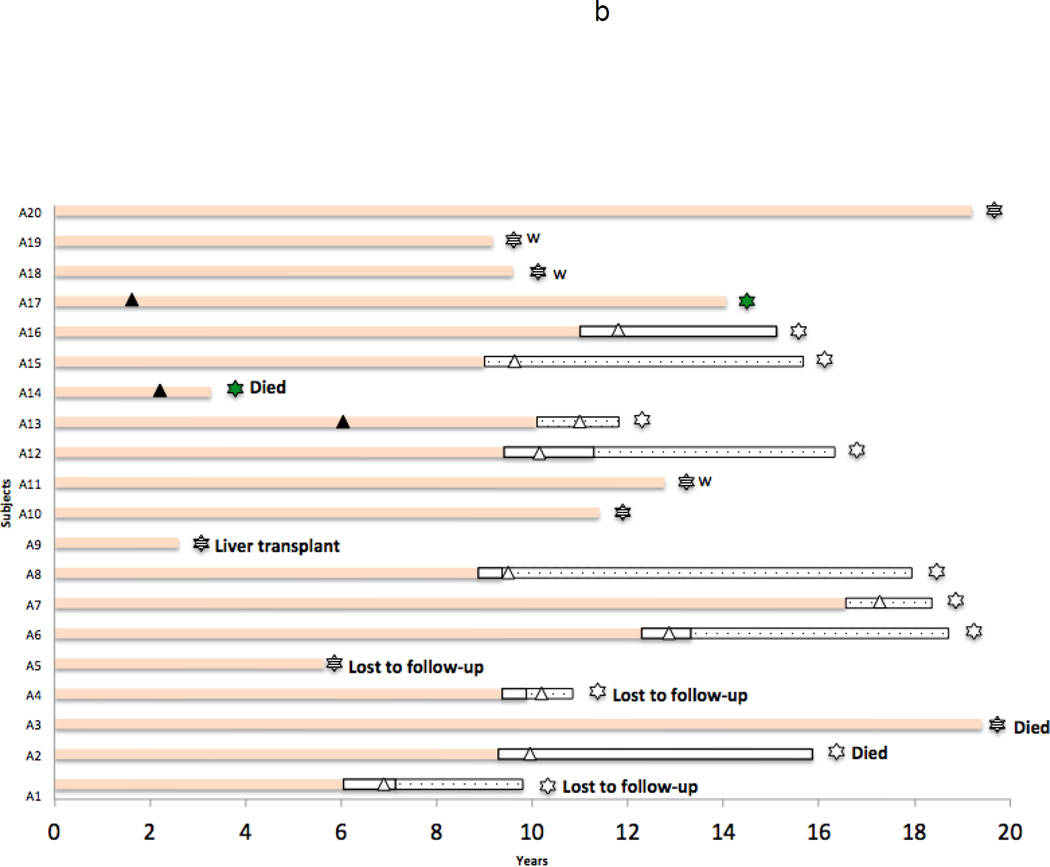
The duration of therapy, serological responses and outcome of each patient is displayed in Figure 1a (HBeAg-positive group: E1-E22) and 1b (Anti-HBe-positive group: A1-A20). Among the 22 HBeAg-positive patients, 17 (77%) became HBeAg-negative and 5 (23%) subsequently cleared HBsAg as well. Among the 20 subjects who were initially HBeAg-negative, 10 (50%) lost HBsAg.
Figure 1.
a: Therapy and serologic status of each of the HBeAg-positive patients (E1–E22) who were enrolled in a clinical study of lamivudine therapy of chronic hepatitis B. Dark orange bar = HBeAg and HBsAg positive; Pastel orange bar = HBeAg negative but HBsAg positive; Open bar = HBsAg negative; Stippled open bar = HBsAg negative and anti-HBs positive; Dark triangle = point at which therapy was changed from lamivudine to another agent; Open triangle = point at which all antiviral therapy was stopped; Striped star = Patient still on lamivudine at time of last visit; Green star = Patient still on other nucleosides at the time of last visit; Open star = Patient not on antiviral therapy at time of last visit. Dark circle = time of addition of a 4–6 month course of interferon to lamivudine therapy (E2, D7, D14, D17, D19). Times at which patients died or were lost to follow up are indicated as well.
b: Therapy and serologic status of each of the HBeAg-negative [and anti-HBe positive] patients (A1–A22) who were enrolled in a clinical study of lamivudine therapy of chronic hepatitis B. Pastel orange bar = HBeAg negative but HBsAg positive; Open bar = HBsAg negative; Stippled open bar = HBsAg negative and anti-HBs positive; Dark triangle = point at which therapy was changed from lamivudine to another agent; Open triangle = point at which all antiviral therapy was stopped; Striped star = Patient still on lamivudine at time of last visit; Green star = Patient still on other nucleosides at the time of last visit; Open star = Patient not on antiviral therapy at time of last visit. W = Time at which patient data was censored and they were enrolled in a study of deliberative withdrawal of antiviral therapy (A11, A18 and A19). Times at which patients died or were lost to follow up are indicated as well.
Death and Liver Transplantation
A total of 7 patients died and one underwent liver transplantation 2.6 to 19.4 (median = 9.1) years after start of antiviral therapy. All outcomes except one (A2: cancer of pancreas) were HBV-related that included 2 from end-stage liver disease and 4 from hepatocellular carcinoma. These six patients with HBV-related outcomes all had cirrhosis at the time of enrollment (n=5) or on subsequent liver biopsies (n=1) and all were still HBsAg-positive and on antiviral therapy at the time of death. The patient who underwent liver transplantation was HBsAg-positive at the time of transplant and has remained HBsAg positive, although HBV DNA negative thereafter, and is still on antiviral therapy 18 years later (initially lamivudine and hepatitis B immune globulin, later lamivudine alone, currently tenofovir alone). A comparison of the 7 patients who underwent liver transplantation or died due to a complication of chronic hepatitis B to the 35 other patients is given in Table 2. While patients who developed severe hepatic outcomes were more likely to have cirrhosis and often had lamivudine resistance, some had a seemingly beneficial virological response, two having cleared HBeAg with treatment and 4 had long-term suppression of HBV DNA levels. In 5 of the 7 patients, serial liver biopsies indicated a decrease in hepatic fibrosis during treatment, and 3 did not have cirrhosis on the last available biopsy (Ishak scores that were initially 5, decreased to 4, 3 and 1). No HBV-related outcomes were observed in patients who lost HBsAg, although 4 had pre-existing cirrhosis.
Table 2.
Comparison of Baseline Characteristics of 7 Patients who died or underwent Liver Transplant for HBV related Liver Disease to the 35 Patients who did not.
| Characteristic | Survival (n=35) |
Death or Liver Tx (n=7) |
p value |
|---|---|---|---|
| Age (years)* | 47 (14–71) | 50 (34–58) | 0.15 |
| Male Sex | 31 (89%) | 5 (71%) | 0.26 |
| Race: White or Caucasian | 19 | 3 | |
| Black or African-American | 2 | 0 | 0.77 |
| Asian-American | 14 | 4 | |
| ALT (U/L)* | 107 (28–524) | 91 (64–156) | 0.35 |
| AST (U/L)* | 67 (25–331) | 71 (52–145) | 0.82 |
| Bilirubin (mg/dL)* | 0.6 (0.3–3.0) | 0.8 (0.3–1.8) | 0.45 |
| Albumin (g/dL)* | 4.1 (1.7–4.7) | 3.9 (2.7–4.4) | 0.06 |
| Prothrombin time (sec)* | 13.1 (11.3–15.0) | 13.1 (12.2–14.3) | 0.51 |
| Platelet count (per µL)* | 191 (96–346) | 135 (33–169) | 0.001 |
| APRI* | 1.0 (0.2–6.2) | 1.5 (1.1–12.9) | 0.13 |
| HBV DNA (log IU/mL)* | 8.3 (5.4–10.4) | 7.7 (6.4–9.7) | 0.95 |
| HBsAg concentration (log IU/mL)* | 3.4 (1.9–5.3) | 3.0 (2.1–5.1) | 0.48 |
| Genotype: A | 18 | 3 | |
| C | 11 | 4 | 0.59 |
| D | 5 | 0 | |
| E | 1 | 0 | |
| HAI score (0–18)* | 11 (3–17) | 13 (7–16) | 0.31 |
| Ishak score (0–6)* | 4 (0–6) | 5 (4–6) | 0.004 |
| Cirrhosis | 7 (20%) | 6** (86%) | 0.002 |
| HBeAg positivity | 18 (51%) | 4 (57%) | 1.00 |
Median (range).
Imputed for one case.
Loss of HBeAg and HBsAg
In the initial HBeAg-positive group of 22 patients, 17 became HBeAg negative, 0.2 to 14.7 (median = 5.9) years after starting therapy. Eight of these patients were taking lamivudine as monotherapy at the time of loss of HBeAg while the remaining 9 had been switched to other antiviral agents. Among the 17 patients who lost HBeAg, 5 subsequently lost HBsAg as well, 1.7 to 17.2 years after starting therapy and 0.8 to 13.1 years after loss of HBeAg.
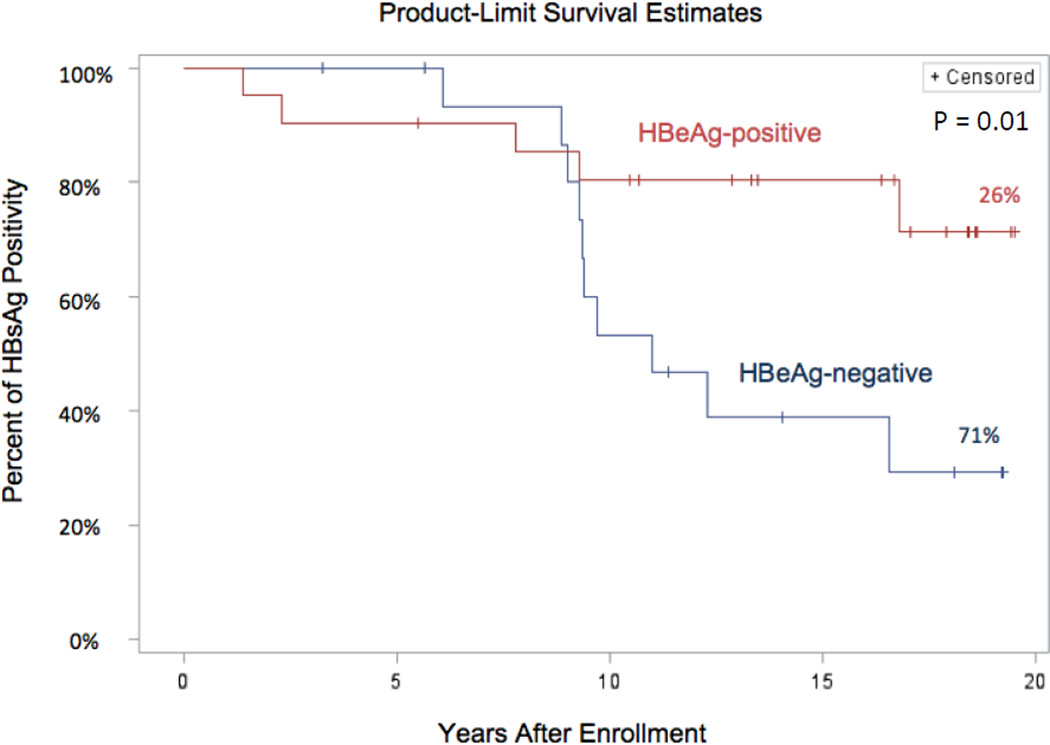
In the 20 patients who were initially HBeAg-negative, 10 (50%) subsequently lost HBsAg, which occurred 6.1 to 16.6 (median = 9.5) years after starting therapy. A Kaplan-Meier analysis of the time to loss of HBsAg in the HBeAg-positive and -negative cohorts is shown in Figure 2. During the first 5 years of therapy, loss of HBsAg was uncommon, occurring in only 2 of the HBeAg-positive and in none of the HBeAg-negative group. Thereafter, however, an increasing proportion of patients became HBsAg-negative, ultimately being more frequent in the HBeAg-negative cohort. By 10 years, 4 (18%) of the HBeAg-positive and 6 (30%) of the HBeAg-negative cohorts were HBsAg negative. The final life-table estimates demonstrated a 26% HBsAg loss in the HBeAg-positive cohort during an average follow up of 16 years and 71% HBsAg loss in the anti-HBe positive cohort with an average follow up of 13.6 years. All 15 patients who became HBsAg-negative were withdrawn from antiviral therapy after continuing for at least 6 months after the initial negative HBsAg result. During follow up ranging from 0.7 to 14.6 (median = 6) years, none redeveloped HBsAg reactivity or had a flare of disease activity after withdrawal. Transient HBV DNA positivity was documented in 8 patients although peak levels were low (186 to 1780 IU/mL) and the presence of viral DNA detectable in serum was generally short-lived (1 to 6 months). Minor serum aminotransferase elevations were intermittently present in 2 subjects that were attributed to nonalcoholic fatty liver.
Figure 2.
Kaplan-Meier analysis curves comparing the time to loss of HBsAg in the HBeAg-positive and the HBeAg-negative groups.
Seroconversion to anti-HBe
Among the 17 patients who lost HBeAg, 7 became anti-HBe positive at the same time as the antigen was first found to be undetectable and 2 others became positive after a delay of 1.0 and 5.9 years. During subsequent follow up, 3 patients lost anti-HBe reactivity and were negative for both HBeAg and anti-HBe when last seen. The remaining 8 patients never seroconverted to anti-HBe positivity despite 3.3 to 19.4 (median = 7.1) years of follow up. The absence of anti-HBe did not appear to have clinical or virological consequences. Subsequent loss of HBsAg occurred equally among those who became anti-HBe positive (3 of 9 patients) and those who remained negative for both HBeAg and antibody (2 of 8 patients).
Seroconversion to anti-HBs
Among the 15 patients who lost HBsAg, 5 (33%) seroconverted to anti-HBs positivity on the specimen when the antigen was first negative. During follow up, another 7 patients became anti-HBs positive after a delay of 0.5 to 2.9 years, but 6 had been given HBV vaccine (1 to 4 doses) in an attempt to induce anti-HBs reactivity. The final 3 patients remained anti-HBs negative during follow up of 4.1, 6.6 and 9.4 years despite being given multiple doses of HBV vaccine. The absence of seroconversion to anti-HBs positivity did not appear to have clinical or virologic significance. No patient who became HBsAg-negative developed evidence of liver decompensation or hepatocellular carcinoma, and at the time of last follow up, serum ALT levels were persistently normal in all, and serum HBV DNA levels were undetectable in 13 and present but below the level of quantification in 2 (<20 IU/mL).
Antiviral Resistance
Development of antiviral resistance was frequent during lamivudine therapy, ultimately being detected in 18 (81%) of the HBeAg-positive and 6 (30%) of the HBeAg-negative group. The appearance of lamivudine resistance was generally, but not invariably, followed by an exacerbation of disease with rise in previously normal or near-normal serum aminotransferase levels (12). Antiviral resistance led to switching therapy in 14 patients. Subsequently, 1 of the 14 (7%) patients who were switched to other agents became HBsAg-negative, compared to 5 of 10 (50%) patients with resistance who remained on lamivudine therapy alone.
Factors associated with loss of HBsAg
Comparisons of the 15 patients who became HBsAg-negative to the 27 who remained positive to the time of last follow-up are shown in Table 3. The two groups were similar in most respects including age, sex, race, ALT levels and histological features including cirrhosis. The patients in the two groups were followed for a similar period of time (14.3 vs 16.3 years). Those who lost HBsAg were more likely to be HBeAg-negative at the start of therapy and to have lower levels of serum HBV DNA and HBsAg but differences were only marginal. Those who remained HBsAg positive were more likely to have developed detectable lamivudine resistance than those who became HBsAg negative (67% vs 40%: p = 0.09). None of the HBsAg-negative patients died of an HBV-related condition, compared to 7 of the 27 (26%) who remained HBsAg-positive despite the fact that a similar proportion had cirrhosis at the time of enrollment.
Table 3.
Patients who Remained HBsAg-positive vs those who Became HBsAg-negative
| Characteristic | HBsAg-positive (n=27) |
HBsAg negative (n=15) |
p value |
|---|---|---|---|
| Age (years)* | 47 (14–71) | 48 (31–68) | 0.48 |
| Male Sex | 22 (81%) | 14 (93%) | 0.39 |
| Race: White or Caucasian | 12 | 10 | |
| Black or African-American | 2 | 1 | 0.38 |
| Asian-American | 13 | 4 | |
| ALT (U/L)* | 96 (28–524) | 167 (50–488) | 0.19 |
| APRI * | 1.2 (0.2–12.9) | 1.6 (0.3–6.2) | 0.32 |
| HBV DNA (log IU/mL)* | 8.5 (5.5–10.1) | 7.8 (5.3–10.4) | 0.08 |
| HBsAg concentration (log IU/mL)* | 3.5 (2.0–5.3) | 2.9 (1.9–4.4) | 0.08 |
| HBeAg positivity | 17 (63%) | 5 (33%) | 0.11 |
| Genotype: A | 12 | 9 | |
| C | 12 | 3 | 0.27 |
| D | 2 | 3 | |
| E | 1 | 0 | |
| HAI score (0–18)* | 10 (3–16) | 12 (5–17) | 0.51 |
| Ishak score (0–6)* | 4 (0–6) | 4 (1–5) | 0.64 |
| Cirrhosis (Ishak 5 or 6) | 9** (33%) | 4 (27%) | 0.73 |
| Duration of follow up (years)* | 14.3 (3.3–19.5) | 16.3 (7.1–19.1) | 0.59 |
| Lamivudine resistance | 18 (67%) | 6 (40%) | 0.09 |
| Death or Transplant, liver-related | 7 (26%) | 0 (0%) | 0.04 |
Median (range).
Includes one imputed case
Discussion
This analysis has demonstrated the range of long-term outcomes in a cohort of patients enrolled in an early study of lamivudine therapy for chronic hepatitis B starting 20 years ago. The study was initiated before the availability of oral nucleoside therapies of hepatitis B and long before the availability of the more potent agents with a higher barrier to resistance such as tenofovir and entecavir. Lamivudine was found to have good activity, lowering HBV DNA levels in virtually all patients with subsequent improvements in serum enzyme levels and hepatic histology.3, 4 A major shortcoming, however, was the development of antiviral resistance after which HBV DNA levels generally rose and the biochemical and histologic features worsened. In this cohort, 57% of patients developed lamivudine resistance, most of these within the first two years.12 If resistance occurred and disease relapsed, patients in this cohort were switched to other therapies, although the choices of alternative treatments were initially limited to interferon alfa and peginterferon until the approval of adefovir in 2002, entecavir in 2005 and tenofovir in 2008. Nevertheless, the majority of patients who were enrolled in this study did well; liver transplants and deaths from end-stage liver disease or hepatocellular carcinoma being limited to those who had cirrhosis or advanced fibrosis before starting therapy. Indeed, during long term follow up, the majority of patients had not only excellent viral suppression and biochemical and histologic improvement but also loss of HBsAg that allowed for discontinuation of treatment without relapse including 4 patients with pre-existing cirrhosis. Even though liver related outcomes were seen in patients with advanced fibrosis or cirrhosis who were still HBsAg positive, a recent meta analysis suggested that hepatocellular carcinoma can develop in patients even after HBsAg seroclearance in patients with cirrhosis or age more than 50 years at the time of HBsAg loss.19, 20
Because of their broad efficacy and safety, oral nucleoside analogues have become a standard approach to management of hepatitis B and continuation of treatment is largely indefinite. In patients with HBeAg, therapy is sometimes discontinued once there is clearance of HBeAg, treatment being continued for at least six months after seroconversion.21 In this cohort, loss of HBeAg was not used as a criterion for discontinuation, largely because of earlier, limited experience of the frequency of relapse when nucleosides were stopped after loss of HBeAg22, combined with the common lack of actual seroconversion and development of anti-HBe.
Perhaps a greater problem occurs in HBeAg-negative patients with chronic hepatitis B, in whom there are no clear indications of viral eradication and the ability to stop treatment. In a study from Greece, patients who had been treated with adefovir for 5 years were withdrawn from therapy among whom almost half relapsed and had to be retreated.23 Among the other half, however, the disease did not return and many subsequently became HBsAg-negative. In the current study, most patients with HBeAg-negative chronic hepatitis B were continued on treatment beyond five years and, as shown in Figure 2, the majority ultimately became HBsAg-negative. Based upon the report from Greece, a study of deliberate withdrawal of antiviral therapy was initiated in 2011. Data from patients in this cohort who were enrolled in that withdrawal study were censored at the time of the withdrawal.
A striking finding in this cohort was that the loss of HBeAg or HBsAg was not always followed by seroconversion to antibody reactivity. Almost half of persons who became HBeAg-negative never made detectable levels of anti-HBe and many of those who produced anti-HBe later became negative. The absence of anti-HBe, however, did not appear to have major clinical significance. More concerning was that the majority of patients who became HBsAg-negative did not become anti-HBs positive in the months following loss of the antigen. The absence of spontaneous development of antibody led to attempts to promote its production by vaccination. While this intervention appeared to induce detectable anti-HBs in most patients, several remained negative despite multiple attempts and long-term monitoring. As with anti-HBe, the absence of anti-HBs did not appear to have important clinical significance in regard to relapse, persistent viremia or serum aminotransferase elevations.
The strengths of this study were the duration of follow up and the careful monitoring of biochemical and virologic features, as well as strict adherence to a regimen of management, continuing therapy until loss of HBsAg, rather than stopping after loss of HBeAg or after a set period of time. The weaknesses of the study were the small number of patients, single center, lack of an untreated control group, and the evolution of alternative therapies and diagnostic tests during the course of the study. Lamivudine, for instance, is now rarely used as a first-line treatment for chronic hepatitis B and interferon is rarely used as an alternative therapy to oral nucleoside analogues.
Despite these issues, these analyses suggest that indefinite treatment with oral nucleoside therapy of chronic hepatitis B is an appropriate approach to management, using the loss of HBsAg as a surrogate endpoint that indicates the safety of stopping treatment. Therapy can be continued for a minimum of 6 months after loss of HBsAg and cirrhotic patients will need regular follow up screening for HCC even after the therapy is discontinued. Therapy should be initiated before the development of cirrhosis or advanced fibrosis as these factors predicted poor outcomes despite treatment. Whether a similar high rate of loss of HBsAg occurs with therapy using the more potent oral nucleosides for hepatitis B requires further long-term follow up of cohorts of patients. In this study, only a few patients became HBsAg negative during the first 5 years of treatment. Also, whether there are features that might predict which patients can be withdrawn from treatment after a set period despite being HBeAg-negative still HBsAg-positive will require larger, prospective long-term studies. Of course, an important need is for adjunctive therapies that would speed or promote loss of HBsAg. Also further studies are needed to show that loss of HBsAg is a reliable surrogate that predicts lack of long-term consequences of chronic HBV infection.
Acknowledgments
Dr. Lau has received research grants from Gilead Sciences, Merck and Bristol-Myers Squibb. She has served in scientific advisory board for Gilead Sciences, Bristol-Myers Squibb, Editas, Janssen and Abbvie.
Financial Support: Lamivudine was supplied for the initial 10 years of the open-label study of lamivudine for chronic hepatitis B by GlaxoSmithKline through a Clinical Trial Agreement with the National Institutes of Health. This study was supported in part by the Intramural Division of NIDDK.
Abbreviations
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NIH
National Institutes of Health
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- anti-HBs
antibody to HBsAg
- HBeAg
hepatitis B e antigen
- anti-HBe
antibody to HBeAg
- ALT
alanine aminotransferase
- ULN
upper limit of normal
Footnotes
Authorship Statement:
(i) Guarantor of the article: Jay H. Hoofnagle
(ii) Specific author contributions: Jay Hoofnagle and Shilpa Lingala performed the research, collected and analysed the data and wrote the paper. Daryl Lau and Marc Ghany contributed to the design and writing the paper. Sungyoung Auh and Christopher Koh contributed by analysing the data.
(iii) All authors approved the final version of the manuscript.
Conflicts of Interest: Drs. Lingala, Koh, Auh, Ghany and Hoofnagle have no conflicts of interest to report
Contributor Information
Shilpa Lingala, Email: shilpa.lingala@nih.gov.
Daryl T-Y Lau, Email: dlau@bidmc.harvard.edu.
Christopher Koh, Email: christopher.koh@nih.gov.
Sungyoung Auh, Email: sungyoung.auh@nih.gov.
Marc G. Ghany, Email: marcg@intra.niddk.nih.gov.
Jay H. Hoofnagle, Email: Hoofnaglej@extra.niddk.nih.gov.
References
- 1.Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doo EC, Hoofnagle JH, Rodgers GP. NIH consensus development conference: management of Hepatitis B. Introduction. Hepatology. 2009;49(5 Suppl):S1–S3. doi: 10.1002/hep.22993. [DOI] [PubMed] [Google Scholar]
- 3.Dienstag JL, Schiff ER, Mitchell M, et al. Extended lamivudine retreatment for chronic hepatitis B: maintenance of viral suppression after discontinuation of therapy. Hepatology. 1999;30(4):1082–1087. doi: 10.1002/hep.510300427. [DOI] [PubMed] [Google Scholar]
- 4.Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341(17):1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 5.Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348(9):808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 6.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348(9):800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 7.Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354(10):1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 8.Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354(10):1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 9.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 10.Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140(1):132–143. doi: 10.1053/j.gastro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 12.Lau DT, Khokhar MF, Doo E, et al. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32(4 Pt 1):828–834. doi: 10.1053/jhep.2000.17912. [DOI] [PubMed] [Google Scholar]
- 13.Ghany MG, Feld JJ, Zhao X, et al. Randomised clinical trial: the benefit of combination therapy with adefovir and lamivudine for chronic hepatitis B. Aliment Pharmacol Ther. 2012;35(9):1027–1035. doi: 10.1111/j.1365-2036.2012.05059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urdea MS. Synthesis and Characterization of Branched DNA (Bdna) for the Direct and Quantitative Detection of Cmv, Hbv, Hcv, and Hiv. Clinical Chemistry. 1993;39(4):725–726. [Google Scholar]
- 15.Pyne MT, Vest L, Clement J, et al. Comparison of Three Roche Hepatitis B Virus Viral Load Assay Formats. Journal of Clinical Microbiology. 2012;50(7):2337–2342. doi: 10.1128/JCM.00746-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuyver I, Rossau R, Maertens G. Line probe assay for the detection of hepatitis B and C virus genotypes. Antiviral Ther. 1996;1:54–57. [Google Scholar]
- 17.Chen D, Kaplan L, Liu Q. Evaluation of two chemiluminescent immunoassays of ADVIA Centaur for hepatitis B serology markers. Clin Chim Acta. 2005;355(1–2):41–45. doi: 10.1016/j.cccn.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Mani H, Kleiner DE. Liver biopsy findings in chronic hepatitis B. Hepatology. 2009;49(5 Suppl):S61–S71. doi: 10.1002/hep.22930. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Wang XW, Chen L, et al. Systematic review with meta-analysis: development of hepatocellular carcinoma in chronic hepatitis B patients with hepatitis B surface antigen seroclearance. Aliment Pharmacol Ther. 2016 doi: 10.1111/apt.13634. [DOI] [PubMed] [Google Scholar]
- 20.Chen YC, Jeng WJ, Chien RN, Chu CM, Liaw YF. Clinical outcomes after spontaneous and nucleos(t)ide analogue-treated HBsAg seroclearance in chronic HBV infection. Aliment Pharmacol Ther. 2016;43:1311–1318. doi: 10.1111/apt.13630. [DOI] [PubMed] [Google Scholar]
- 21.Chang ML, Liaw YF, Hadziyannis SJ. Systematic review: cessation of long-term nucleos(t)ide analogue therapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Aliment Pharmacol Ther. 2015;42:243–257. doi: 10.1111/apt.13272. [DOI] [PubMed] [Google Scholar]
- 22.Song BC, Suh DJ, Lee HC, Chung YH, Lee YS. Hepatitis B e antigen seroconversion after lamivudine therapy is not durable in patients with chronic hepatitis B in Korea. Hepatology. 2000;32(4 Pt 1):803–806. doi: 10.1053/jhep.2000.16665. [DOI] [PubMed] [Google Scholar]
- 23.Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143(3):629–636. e1. doi: 10.1053/j.gastro.2012.05.039. [DOI] [PubMed] [Google Scholar]