Voltage-sensing domains (VSDs) in voltage-gated calcium channels sense the potential difference across membranes and interact with the pore to open it. Savalli et al. find that the accessory subunit α2δ-1 increases the sensitivity of VSDs I–III and also their efficiency of coupling to the pore.
Abstract
Excitation-evoked calcium influx across cellular membranes is strictly controlled by voltage-gated calcium channels (CaV), which possess four distinct voltage-sensing domains (VSDs) that direct the opening of a central pore. The energetic interactions between the VSDs and the pore are critical for tuning the channel’s voltage dependence. The accessory α2δ-1 subunit is known to facilitate CaV1.2 voltage-dependent activation, but the underlying mechanism is unknown. In this study, using voltage clamp fluorometry, we track the activation of the four individual VSDs in a human L-type CaV1.2 channel consisting of α1C and β3 subunits. We find that, without α2δ-1, the channel complex displays a right-shifted voltage dependence such that currents mainly develop at nonphysiological membrane potentials because of very weak VSD–pore interactions. The presence of α2δ-1 facilitates channel activation by increasing the voltage sensitivity (i.e., the effective charge) of VSDs I–III. Moreover, the α2δ-1 subunit also makes VSDs I–III more efficient at opening the channel by increasing the coupling energy between VSDs II and III and the pore, thus allowing Ca influx within the range of physiological membrane potentials.
INTRODUCTION
Calcium influx through voltage-activated calcium (CaV) channels translates electrical signals into a variety of physiological outcomes such as cell contraction, neurotransmitter or hormonal release, and gene expression (Catterall, 2011; Zamponi et al., 2015). The specificity of the Ca2+ signal relies on the activity of the CaV channel complex being perfectly tuned to voltage signals. CaV channels are multimeric proteins formed by the pore-forming α1 subunit and at least three auxiliary subunits, β, α2δ, and calmodulin, in a 1:1:1:1 stoichiometry, resulting in an asymmetric structural architecture (Fig. 1; Findeisen and Minor, 2010; Catterall, 2011; Dolphin, 2013; Ben-Johny and Yue, 2014; Neely and Hidalgo, 2014; Campiglio and Flucher, 2015; Wu et al., 2015). The α2δ auxiliary subunit is a large (∼170 kD), mostly extracellular protein with a single membrane-anchoring segment (Davies et al., 2010) that binds to the α1 subunit from the extracellular side (Cassidy et al., 2014). α2 and δ proteins are the products of the same gene as a preprotein that is posttranslationally proteolysed and then linked by a disulfide-bond to form the mature α2δ protein (Calderón-Rivera et al., 2012). Four genes (CACNA2D1–4) encode for distinct α2δ isoforms (α2δ-1–4), which are all expressed in the brain (Dolphin, 2013). In addition to brain tissue, α2δ-1 is strongly expressed in cardiac, smooth, and skeletal muscles, whereas α2δ-4 is found in endocrine tissues and the retina. Mutations in the α2δ-1 gene can lead to Brugada (Burashnikov et al., 2010) and short QT (Templin et al., 2011; Bourdin et al., 2015) syndromes and are associated with epilepsy and mental disability (Vergult et al., 2015). In mice, naturally occurring mutations in the α2δ-2 gene lead to ataxia and epilepsy (Barclay et al., 2001), whereas the α2δ-3 protein is important for synaptic morphogenesis (Kurshan et al., 2009) and nociception (Neely et al., 2010). Mutations in α2δ-4 can result in night blindness (Wycisk et al., 2006). Moreover, α2δ-1 and -2 have been identified as the molecular targets of gabapentinoid drugs (such as gabapentin and pregabalin), mediating their analgesic action in neuropathic pain (Field et al., 2006; Hendrich et al., 2008; Uchitel et al., 2010). Finally, it has been shown that α2δ proteins also play an important role in synapse formation (Eroglu et al., 2009).
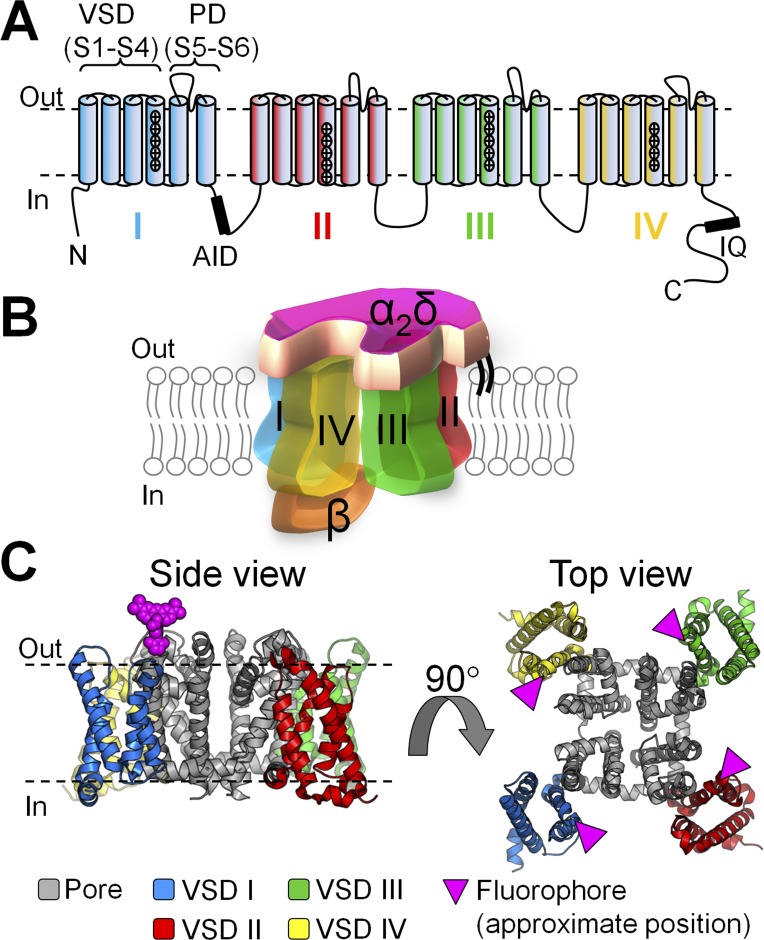
Figure 1.
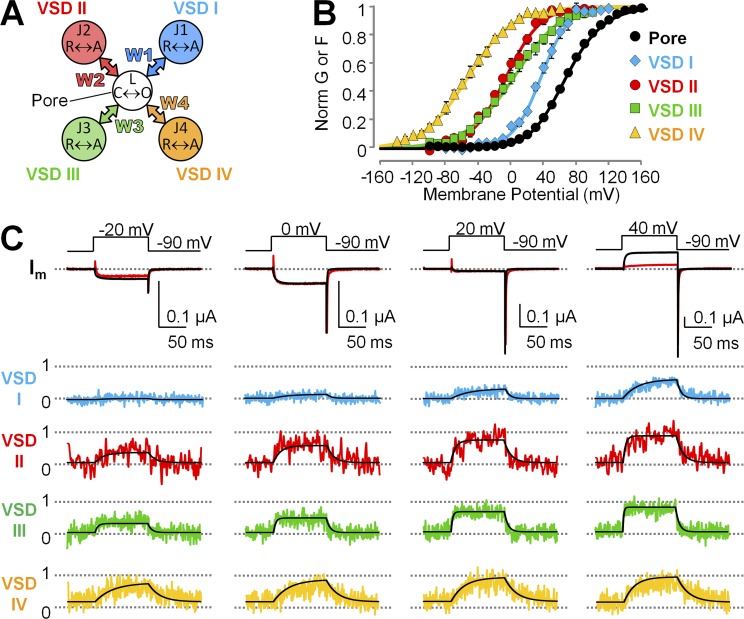
CaV1.2 channel topology and subunit composition. (A) In CaV1.2, the pore-forming α1C subunit consists of four tandem repeats (I–IV), each crossing the membrane six times (S1–S6). Helices S1–S4 form the VSD, and helices S5 and S6 form the pore domain (PD). The intracellular loop between repeats I and II encompasses the binding site for the auxiliary β subunit (α-interacting domain [AID]), whereas the C terminus includes the IQ region, where calmodulin binds. (B) CaV1.2 channels are multimeric proteins composed of the α1C pore-forming subunit, a mostly extracellular α2δ subunit, and an intracellular β subunit. In this cartoon representation, the four repeats that constitute the α1C subunit are arranged clockwise, as in a recent cryo-EM structure of related α1S (CaV1.1) channel (Wu et al., 2015): repeats III (green) and IV (yellow) are in the front, whereas I (blue) and II (red) are in the back. (C) Side and top views of the atomic structure of a voltage-gated Na+ channel (NaVAb; PDB accession no. 4EKW; Payandeh et al., 2012), shown as putative structural representation of a CaV α1 channel. S4 helices were fluorescently labeled at their extracellular flank, one VSD at a time (pink triangles).
Several studies report that the interaction of α2δ-1 with the pore-forming α1C subunits (L-type CaV1.2) favors channel activation, as manifested by a hyperpolarizing shift of channel opening (Felix et al., 1997; Platano et al., 2000; Bourdin et al., 2015) and an accelerated time course of activation (Bangalore et al., 1996). Accordingly, channel activation is diminished with α2δ-1 down-regulation (Tuluc et al., 2007; Fuller-Bicer et al., 2009) and enhanced with α2δ-1 up-regulation (Li et al., 2006). Thus, by facilitating channel opening, α2δ-1 allows CaV1.2 channels to operate at physiological membrane potentials. However, the molecular mechanism by which α2δ-1 facilitates CaV1.2 activation is as yet poorly understood.
Because α2δ-1 modulates the voltage-dependent properties of CaV1.2 channels (Felix et al., 1997; Platano et al., 2000; Bourdin et al., 2015) and associates with α1C subunits asymmetrically (Walsh et al., 2009a), we hypothesized that α2δ-1 differentially modulates each of the four voltage-sensing domains (VSDs), as well as their contribution to channel opening. In fact, the pore-forming α1C subunit consists of four homologous, but nonidentical, concatenated repeats (I–IV), each composed of a VSD (transmembrane helices S1–S4) and a quarter of the pore domain (S5–S6; Catterall, 2011). Using voltage clamp fluorometry (VCF), we have recently revealed the functional heterogeneity of the four CaV1.2 VSDs, whereby each undergoes structural changes during channel activation, with unique voltage- and time-dependent properties, such that the activation of VSDs II and III, and to a lesser extent VSD I, energetically contributes to channel opening (Pantazis et al., 2014). VCF is a powerful investigative tool that allows for simultaneous measurements of ionic current kinetics and structural rearrangements occurring within specific protein domains, the latter tracked using environmentally sensitive fluorophores (Claydon and Fedida, 2007; Gandhi and Olcese, 2008; Talwar and Lynch, 2015; Zhu et al., 2016). VCF has been a successful approach in the study of numerous voltage-sensitive proteins (Mannuzzu et al., 1996; Cha et al., 1999; Smith and Yellen, 2002; Savalli et al., 2006; Kohout et al., 2008; Osteen et al., 2010; Tombola et al., 2010), transporters (Meinild et al., 2002; Larsson et al., 2004; Ghezzi et al., 2009), and receptors (Dahan et al., 2004; Lörinczi et al., 2012). Using VCF, auxiliary subunit modulation of VSDs has also been detected in ion channels, such as BK channels (Savalli et al., 2007) or KV7.1 (Ruscic et al., 2013). However, VCF has only recently been adapted to investigate CaV channels (Pantazis et al., 2014).
In this study, by using VCF and a structurally relevant allosteric model of CaV1.2 activation, we show that the α2δ-1 auxiliary subunit (a) facilitates the voltage-dependent activation of CaV1.2 VSDs I–III; (b) accelerates VSD I kinetics; and (c) increases the energetic contribution of VSDs I–III to pore opening. These results unravel the molecular mechanisms by which α2δ-1 exerts its modulation on CaV1.2 channel activation, allowing for Ca2+ influx to occur in excitable cells at physiological membrane potentials.
MATERIALS AND METHODS
Molecular biology
Human α1C-77 subunits (GenBank accession no. CAA84346; Soldatov, 1992) of CaV1.2 channels, with a Cys substituted at an extracellular position in the S3–S4 linker of each VSD at a time, were used (F231C, L614C, V994C, or S1324C for VSDs I–IV, respectively) as previously described (Pantazis et al., 2014). Single-point mutations were generated using the QuikChange Site-Directed Mutagenesis kit (Agilent Technologies) and confirmed by sequencing. Auxiliary subunits α2δ-1 (UniProt accession no. P13806) and β3 (UniProt accession no. P54286) were also coexpressed. The cRNA of the different subunits was transcribed in vitro (mMESSAGE MACHINE; Ambion) and injected into stage VI Xenopus laevis oocytes (50 nl at 0.1–0.5 µg/µl).
VCF
3–4 d after injection, oocytes were incubated with thiol-reactive fluorophores sensitive to environmental changes (10 µM tetramethylrhodamine-5-maleimide [TMRM] for VSD II and 20 µM 2-((5(6)-tetramethyl-rhodamine)carboxylamino)ethyl methanethiosulfonate [MTS-TAMRA] for VSD I, III, or IV) in a depolarizing solution (120 mM K-methanesulfonate [MES], 2 mM Ca(MES)2, and 10 mM HEPES, pH 7.0). Subsequently, oocytes were voltage clamped using the cut-open oocyte technique (Stefani and Bezanilla, 1998; Pantazis and Olcese, 2013). Fluorescence changes and ionic currents were acquired simultaneously from the same membrane area (Gandhi and Olcese, 2008; Pantazis and Olcese, 2013). External solution was 2 mM Ba(MES)2, 120 mM NaMES, and 10 HEPES, pH 7.0, supplemented with 0.1 ouabain to eliminate charge movement from Na/K ATPase (Neely et al., 1994). Internal solution was 120 mM K-glutamate and 10 mM HEPES, pH 7.0. Pipette solution was 2.7 M Na-MES, 10 mM NaCl, and 10 mM Na-HEPES, pH 7.0. Before experiments, oocytes were injected with 10 mM BAPTA•4K, pH 7.0, to prevent activation of native Ca2+- and Ba2+-dependent Cl− channels (Barish, 1983).
Data analysis
The voltage dependence of ionic conductance (G(V), estimated from the peaks of the tail currents) and fluorescence changes (F(V)) were empirically characterized by fitting to one or two Boltzmann functions as f(V) = {1 + exp([q · (Vhalf − Vm)(F/RT)])}−1, where q is the effective charge, Vhalf is the half-activation potential, Vm is the membrane potential, T is the absolute temperature, and F and R are the Faraday and Gas constants, respectively. F(V) curves can be satisfactorily described by single Boltzmann functions both the absence and the presence of the α2δ-1 subunit (see Figs. 4 and 5).
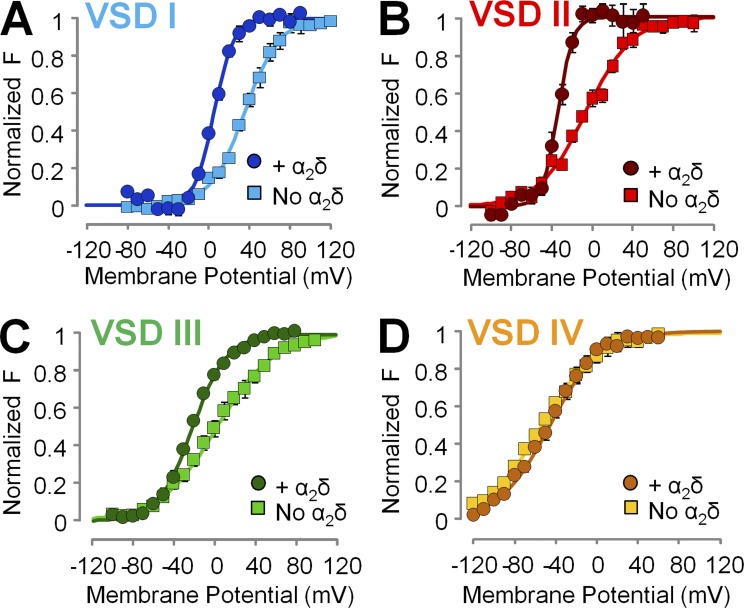
Figure 4.
The α2δ-1 subunit facilitates the voltage-dependent activation of CaV1.2 VSDs I–III, whereas VSD IV is unperturbed. (A–D) Mean voltage dependence of VSD activation constructed from experiments as in Fig. 3. The α2δ-1 subunit facilitated the activation of VSDs I–III, as revealed by a more hyperpolarized voltage dependence of VSD activation, although to a different extent, whereas VSD IV was unaffected. Boltzmann fitting parameters are reported in Table 2.
Figure 5.
The α2δ-1 subunit remodels VSDs I–III. (A and B) Mean normalized G(V) and F(V) data points from α1C + β3 (A) or α1C + β3 + α2δ-1 (B) channels and the corresponding Boltzmann fits are shown superimposed (mean ± SEM). These results suggest that the facilitation of voltage-dependent pore activation by α2δ-1 subunits is likely mediated by the remodeling of VSDs I–III.
The time course of fluorescence onset (VSD activation) was fitted in background-subtracted fluorescence traces to the sum of two exponential components:
where B is the baseline, A is the amplitude, t is time, and τ is the time constant. Fitting was performed in Matlab (MathWorks) by least squares (Optimization Toolbox). Only traces with sufficient signal-to-noise ratio (S:N >2) were included in the kinetics statistics. S:N is defined as mean signal amplitude divided by the root mean square. Fractional amplitude–weighted time constants (τavg) were calculated using
where
Data are reported as mean ± SEM; statistical analysis was performed using Excel (Microsoft).
Allosteric model
Modeling CaV1.2 kinetics and activation curves through a five-particle allosteric scheme was performed as described previously (Pantazis et al., 2014).In brief, equilibrium states were determined from the values of five particle equilibrium constants (L, J1–4) and four VSD–pore coupling constants D1–4 using the channel partition function:
The pore particle (L) derives its voltage dependence through a gating charge displacement ΔqL and a characteristic midpoint voltage VL; for example, L = exp(ΔqL(V − VL)/kT). Similar expressions applied to J1–4 were used to describe intrinsic VSD activation. The four allosteric factors D1–4 are related to VSD–pore interaction energies W1–4 through Di = exp(–Wi/kT).
The equilibrium curves for the five gating particles are easily derived from the partition function through the relations which were used to fit the experimental conductance (G(V)) and fluorescence (F(V)) curves.
A kinetic model of channel activation that reduces to the thermodynamic model under equilibrium conditions was obtained by assigning two additional variables for each particle transition: a frequency factor ν and a fractional position x of the transition barrier between resting and active states (Sigg, 2014). The forward and backward rate constants for the transition between a configuration i and any of the accessible configurations j after the activation of one of the five gating particles were expressed as
and
where k refers to the transitioning particle and Zi and Zj are the configuration-specific contributions to the overall partition function Z (obtained by expanding the earlier expression of Z into its 32 terms).
The channel kinetics were solved by integrating , which describes state probabilities of all states (p). Q is the standard rate matrix as described in Colquhoun and Hawkes (1981). The initial condition p(0) for the holding potential was obtained from The time dependence of a quantity of interest A (ionic current or fluorescence) was obtained from
where ai is the value of the desired quantity at configuration i.
To find the set of parameters that best described the data, several approaches were used such as Marquadt–Levenberg, implemented in Berkeley Madonna, and Nedler–Mead, developed in Matlab, minimizing the error function “ssq” generated by the weighed sum of 10 error functions, five for steady-state voltage dependencies and five for time-dependent signals. Each individual error function corresponds to the sum of the squares of the difference between the experimental and simulated datasets normalized by n and the square of the maximum value.
To test for the uniqueness of the solution and estimating the 95% credible interval of each parameter, we used a Bayesian approach using Markov chain Monte Carlo (MCMC) sampling as in Hines et al. (2014), but instead of using likelihood ratio to test for high posterior probabilities, we used
Then, transitions of the Markov chain were accepted with probability a, as described in Li (2012). To further constrain the solution space and take better advantage of the time-dependent data, we added a set of penalty functions as further explained in the supplementary figure legends.
Online supplemental material
Fig. S1 shows representative Ba2+ current traces from Xenopus oocytes expressing CaV1.2 channel complexes formed by α1C + β3 subunits. Fig. S2 shows histograms of posterior distributions of 14 parameters obtained from a 100,000-trial MCMC run. Fig. S3 shows histograms of posterior distributions of parameters xi and ni (i = L,1,2,3,4). Online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201611586/DC1.
RESULTS
α2δ-1 facilitates the voltage-dependent activation of human CaV1.2 channels
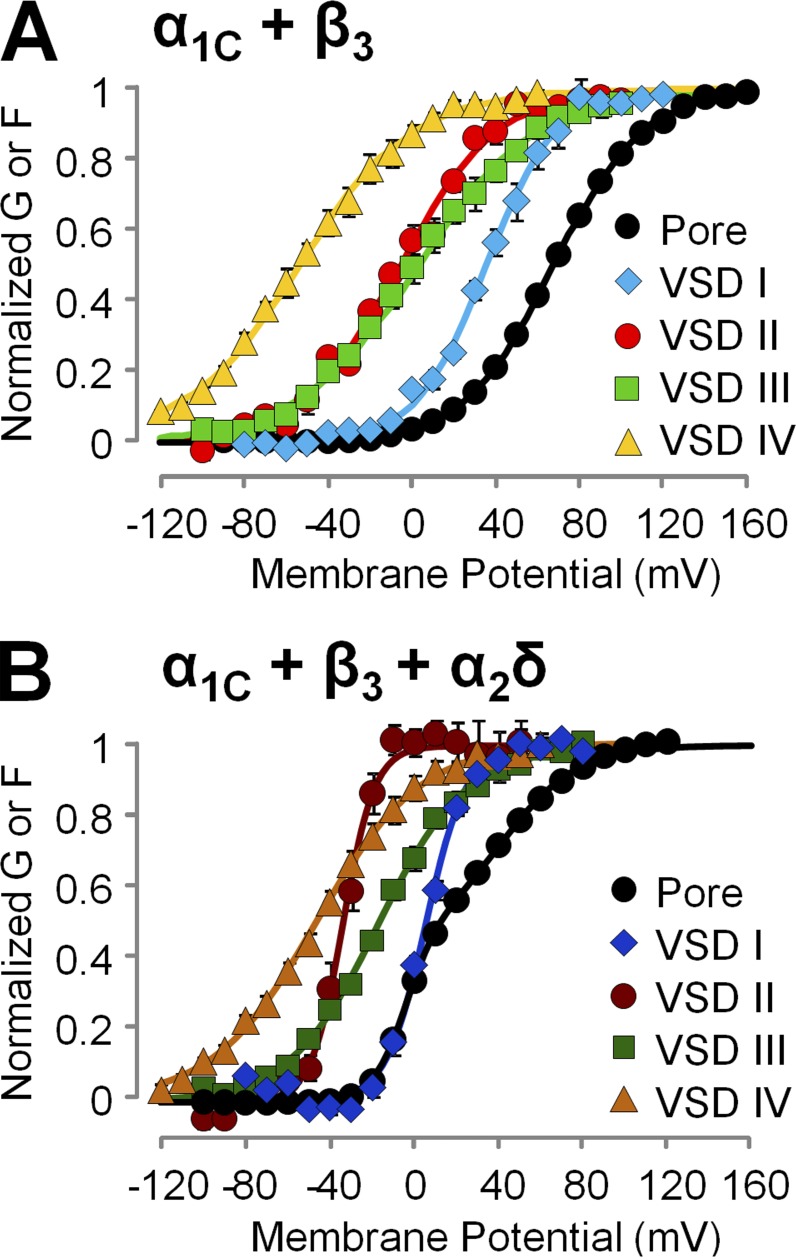
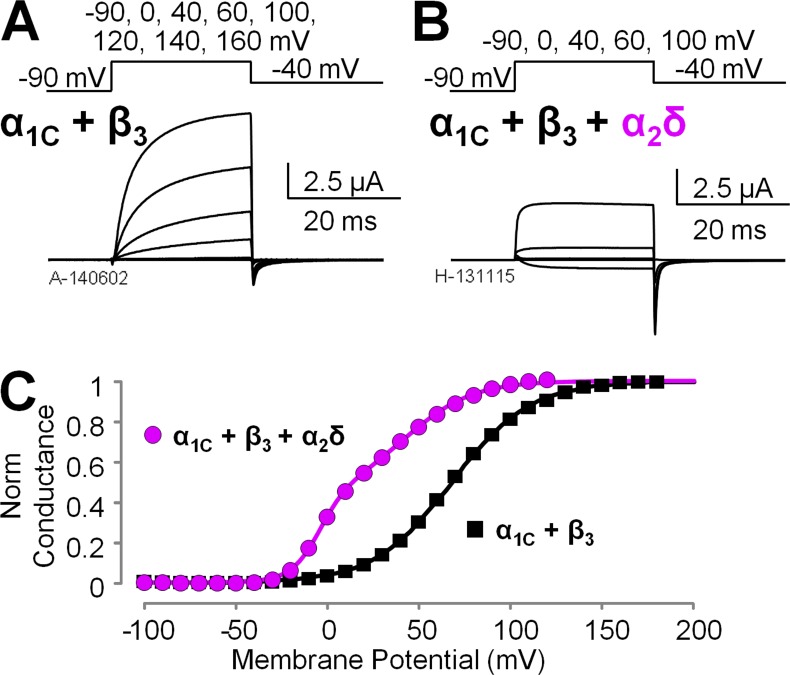
To understand the mechanism of α2δ-1 subunits modulation of human CaV1.2 channels, we expressed in Xenopus oocytes CaV1.2 channels consisting of α1C and β3 subunits, in the presence or the absence of α2δ-1 proteins. We voltage clamped the cells using the cut-open oocyte voltage clamp technique (Stefani and Bezanilla, 1998; Pantazis and Olcese, 2013) and recorded ionic currents (Fig. 2, A and B) using Ba2+ as the charge carrier to prevent calcium-dependent inactivation (Peterson et al., 1999; Qin et al., 1999). Because CaV1.2 channels lacking α2δ-1 subunits activate slowly (Fig. 2 A), relatively longer depolarizations were necessary for ionic current to reach quasi–steady state (Fig. S1), a condition necessary to construct conductance versus voltage relationships (G(V)) from tail currents. We observed that α2δ-1 coexpression strongly facilitated channel opening in human CaV1.2 channels by shifting the CaV1.2 half-activation potential (Vhalf) of the G(V) curves by ∼50 mV toward more hyperpolarized potentials (Fig. 2 C), in agreement with data previously obtained from the rabbit isoforms (Felix et al., 1997; Platano et al., 2000; Bourdin et al., 2015). G(V) curves obtained from channels expressed with the full complement of auxiliary subunits were well described by the sum of two Boltzmann functions with distinct voltage-dependent properties (Vhalf1 = −4.02 ± 0.38 mV, z1 = 3.46 ± 0.11 e0, G1 = 57.56 ± 4.82%, Vhalf2 = 42.56 ± 1.87 mV, z2 = 1.27 ± 0.04 e0, G2 = 42.44 ± 4.82%; n = 4; Fig. 2 C), alluding to a complex voltage-dependent activation mechanism with more than one voltage-dependent opening transitions; in contrast, G(V) curves for channels lacking α2δ-1 were well accounted for by a single Boltzmann distribution (Vhalf = 68.01 ± 1.32 mV and z = 1.19 ± 0.01 e0; n = 7), which is a tentative (yet tantalizing) indication that these channels gate in a two-state process. We further mechanistically evaluated this premise using an allosteric model of voltage-dependent CaV activation.
Figure 2.
The α2δ-1 subunit facilitates CaV1.2 channel opening. (A and B) Representative Ba2+ current traces from Xenopus oocytes expressing human CaV1.2 channel complexes with different subunit composition: α1C + β3 subunits (A) or α1C + β3 + α2δ-1 subunits (B). The voltage protocol is reported above the current traces. (C) Mean conductance versus voltage (G(V)) relationships were constructed from tail currents as in B and Fig. S1 (mean ± SEM; error bars are within the symbols; n = 7 for α1C + β3 and n = 4 for α1C + β3 + α2δ-1). The α2δ-1 auxiliary subunit facilitates channel opening, as manifested by a hyperpolarizing shift of the conductance voltage dependence by ∼50 mV.
α2δ-1 increases the rate of VSD I activation
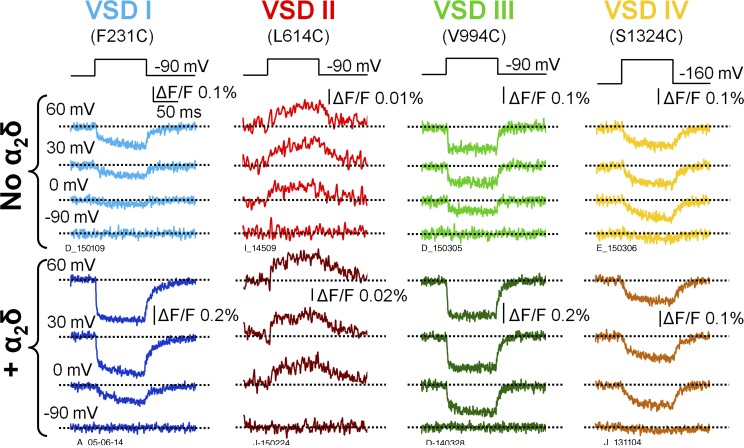
Given the large difference (>50 mV) in the voltage dependence of CaV1.2 activation in the presence or absence of α2δ-1 subunits (Fig. 2), we tested the hypothesis that α2δ-1 association with α1C induces a functional remodeling of one or more VSDs, altering their gating properties. We used the VCF technique (Mannuzzu et al., 1996; Cha and Bezanilla, 1997; Gandhi and Olcese, 2008) to track the molecular rearrangements of the individual VSDs of human CaV1.2 channels in the presence or absence of α2δ-1. Briefly, this involves the introduction of a cysteine residue one at a time at a strategic and specific position extracellular to the S4 helix in each CaV1.2 VSD, as shown in our previous study (Pantazis et al., 2014). In voltage-gated ion channels, the S4 segment typically contains the positively charged amino acids effectively responsible for voltage sensing and undergoes structural rearrangements during depolarizations (Tombola et al., 2006; Bezanilla, 2008; Chanda and Bezanilla, 2008; Swartz, 2008; Catterall, 2010; Palovcak et al., 2014). CaV1.2 channels (α1C + β3 subunits) were expressed with or without α2δ-1 subunits in Xenopus oocytes. After labeling of the cysteines with thiol-reactive fluorophores that are sensitive to the environment, we used VCF to simultaneously study the voltage-dependent activation of the pore (ionic current) and each of the four VSDs (fluorescence) in conducting CaV1.2 channels. The labeling positions and fluorophores used here were the same as in our previous work (Pantazis et al., 2014). The effect of α2δ-1 subunits on the kinetics of VSD activation was quantified for 100-ms depolarizations to 20 mV (Table 1). The activation of VSD I was accelerated by approximately twofold by the α2δ-1 subunit, increasing the fractional amplitude of the fast component of activation (Fig. 3 and Table 1). This result suggests that the acceleration of ionic current by the α2δ-1 subunit (Fig. 2, A and B; Felix et al., 1997; Platano et al., 2000; Tuluc et al., 2007) may result from a faster VSD I, consistent with the findings of Nakai et al. (1994), who demonstrated a relevant role for VSD I in controlling CaV1.2 current kinetics by transferring CaV1.1 VSD I sequences into the corresponding location in CaV1.2. In contrast, the kinetics of VSDs II–IV were practically unaffected by α2δ-1 (Fig. 3 and Table 1).
Table 1. Effect of the α2δ-1 subunit on CaV1.2 (α1C/β3) VSD activation kinetics (100-ms depolarizations to 20 mV) .
| VSD | Parameter | No α2δ | With α2δ-1 |
|---|---|---|---|
| VSD I | τ1 (ms) | 5.48 ± 1.57 (n = 4) | 3.8 ± 0.37 (n = 3) |
| α1 (%) | 47.2 ± 3.7 | 63.9 ± 7.7 | |
| τ2 (ms) | 56.3 ± 7.9 | 29.6 ± 6.0 | |
| τavg (ms) | 31.4 ± 2.3 | 13.6 ± 3.7 | |
| VSD II | τ1 (ms) | 0.83 ± 0.32 (n = 3) | 1.04 ± 0.39 (n = 3) |
| α1 (%) | 43.1 ± 2.0 | 66.2 ± 8.6 | |
| τ2 (ms) | 28.3 ± 12.5 | 30.7 ± 5.7 | |
| τavg (ms) | 16.5 ± 7.2 | 11.9 ± 3.6 | |
| VSD III | τ1 (ms) | 2.39 ± 0.44 (n = 6) | 2.0 ± 0.17 (n = 4) |
| α1 (%) | 91.8 ± 5.5 | 83.5 ± 9.7 | |
| τ2 (ms) | 37.9 ± 7.9 | 32.0 ± 9.0 | |
| τavg (ms) | 4.6 ± 1.1 | 5.09 ± 0.98 | |
| VSD IV | τ1 (ms) | 21.5 ± 1.9 (n = 6) | 16.5 ± 2.1 (n = 3) |
| α1 (%) | 100 | 100 | |
| τ2 (ms) | NA | NA | |
| τavg (ms) | 21.5 ± 1.9 | 16.5 ± 2.1 |
NA, not applicable.
Figure 3.
Voltage-dependent rearrangements of the individual CaV1.2 VSDs in the presence and absence of the α2δ subunit. Fluorescence traces reporting the structural rearrangements of each CaV1.2 VSD in the absence (top) or presence (bottom) of the α2δ-1 subunit are shown. The channels were expressed in Xenopus oocytes and fluorescently labeled at extracellular S4 positions in VSD I (F231C), VSD II (L614C), VSD III (V994C), or VSD IV (S1324C). The voltage protocol is reported above the traces. The holding potential was –90 mV. Because VSD IV was partially activated at –90 mV (Fig. 4 D), oocytes expressing S1324C channels underwent a prepulse to –160 mV to allow VSD IV to return to its resting state before each depolarization.
α2δ-1 facilitates the voltage-dependent activation of VSDs I, II, and III
To assess how the voltage dependence of the individual VSDs was affected by α2δ-1 subunit association, we constructed activation curves (F(V)) from the corresponding fluorescence intensity at the end of 100-ms pulses over a wide range of membrane potentials (Fig. 4). The F(V) values of VSDs I–III were shifted to more negative potentials in the presence of the α2δ-1 subunit, whereas VSD IV activation was unaffected (Fig. 4, A–D). Moreover, VSDs I–III exhibited a steeper slope of voltage-dependent activation: the effective charge q increased by approximately twofold (Fig. 4, A–C; and Table 2). Overall, in the absence of the α2δ-1 subunit, the activation curves of the four VSDs were highly disparate, spread over a voltage range spanning ∼90 mV (Fig. 5 A and Table 2), while the variance of the Vhalf values was 1,300 mV2. The association of α2δ-1 subunit narrowed the range of membrane potentials at which VSDs activated (∼50 mV; Table 2, Vhalf variance: 520 mV2) and shifted the G(V) such that VSD voltage dependence was closer to pore opening (Fig. 5 B). A separation of the half activation potential of VSD activation and channel opening can be interpreted as decreased coupling between VSD activation and pore gating (Sigg, 2014). Taken together, these results are consistent with the view that α2δ-1 is required to increase the coupling between VSDs I–III and the CaV1.2 pore. In addition, because the effective charge q is the summed displacements of residue charges across the membrane potential profile, we cannot exclude that α2δ-1 association also increases CaV1.2 voltage sensitivity by altering the shape of the profile (for example, making it steeper in the region of charge translation). To discriminate among these mechanisms (increased coupling, increased effective charge, or both), we modeled CaV1.2 activation with an allosteric model used previously (Pantazis et al., 2014).
Table 2. Fitting parameters for the Boltzmann functions fitting the fluorescence data from each VSD ( Fig. 4 ) .
| VSD | Parameter | No α2δ | With α2δ−1 |
|---|---|---|---|
| VSD I | q (e0) | 1.6 ± 0.1 (n = 5) | 2.8 ± 0.1 (n = 5) |
| Vhalf (mV) | 36.5 ± 3.1 | 6.1 ± 1.3 | |
| VSD II | q (e0) | 1.2 ± 0.1 (n = 6) | 2.7 ± 0.2 (n = 3) |
| Vhalf (mV) | −6.7 ± 1.8 | −30.8 ± 3.9 | |
| VSD III | q (e0) | 0.9 ± 0.1 (n = 6) | 1.5 ± 0.09 (n = 5) |
| Vhalf (mV) | 0.9 ± 4.1 | −22.0 ± 1.7 | |
| VSD IV | q (e0) | 0.9 ± 0.04 (n = 7) | 1.1 ± 0.1 (n = 4) |
| Vhalf (mV) | −51.4 ± 4 | −48.5 ± 2.5 |
α2δ-1 facilitates CaV1.2 activation by increasing the energetic contribution of VSDs I–III to pore opening
We analyzed the VCF data with the 32-state allosteric model for CaV1.2 activation (Pantazis et al., 2014), consisting of five gating elements (one pore, four VSDs; Fig. 6 A) and therefore relevant to CaV1.2 architecture. Pore and VSDs can exist in two states: closed-open and resting-active, each undergoing voltage-dependent transitions. Thus, in this model, the pore as well as the VSDs are intrinsically voltage dependent (half-activation potential V and charge displacement q). The activation of one or more VSDs stabilizes the open state of the pore through energy coupling with magnitude Wi (i = 1–4).
Figure 6.
An allosteric structure-based model of voltage-dependent CaV1.2 activation accounts for time- and voltage-dependent properties of CaV1.2 channels lacking the α2δ-1 subunit. (A) Scheme of the model used to simultaneously fit current and optically tracked conformational changes from each of the four CaV1.2 VSDs. Each VSD and the pore are modeled as two-state particles that can undergo restingactive or closedopen voltage-dependent transitions, respectively. Activation of a VSD allosterically stabilizes the open pore state by energy W. (B) Mean normalized G(V) and F(V) data points from α1C + β3 channels are shown superimposed with the model predictions (curves). (C) Ionic currents (top) and fluorescence traces from each VSD (normalized to VSD activation; bottom) from α1C + β3 channels. The simultaneous model fits are shown superimposed as black lines. Fitting parameters are reported in Table 3.
Kinetic and quasi-equilibrium data from the pore (ionic currents) and each VSD (fluorescence) were simultaneously fitted in the absence of α2δ-1 with no assumption or constraint. The model accurately accounts for the voltage- and time-dependent properties of channels composed of α1C + β3 subunits (Fig. 6, B and C). The most salient feature of the fitted quantities is that the energetic contribution to pore opening (W) of each VSD is small (<1 kT or 25 meV). A comparison of W values with and without the α2δ-1 subunit demonstrates a doubling of the energetic contribution to pore opening by VSDs I and III (W1 and W3) and an approximately threefold increase of W2 in the presence of α2δ-1, whereas the contribution of VSD IV (W4) was practically unchanged (Table 3; parameters with α2δ-1 are from Pantazis et al., 2014 and reported here for clarity). In addition to enhancing VSD I–III energetic contributions to pore opening, α2δ-1 increased the charge displacement (q) of VSDs I and II by ∼140% and ∼200%, respectively (Table 3). In contrast, the intrinsic pore parameters qL and VL varied minimally with the addition of α2δ-1 to the channel. Thus, α2δ-1 modulates the CaV1.2 channel by exerting its effect on the VSDs (VSDs I–III) rather than on the pore.
Table 3. Fitting parameters for the model predictions in Fig. 6.
| Parameter | No α2δ | With α2δ-1a | |
|---|---|---|---|
| Pore | qL (e0) | 0.99 | 0.76 |
| VL (mV) | 103 | 140 | |
| x L | 1.0 | 0.49 | |
| νL (s−1) | 1,356 | 670 | |
| VSD I | q1 (e0) | 1.4 | 2.0 |
| V1 (mV) | 37 | 8.5 | |
| x 1 | 0.72 | 0.89 | |
| ν1 (s−1) | 17 | 110 | |
| VSD II | q2 (e0) | 1.2 | 2.5 |
| V2 (mV) | −6.9 | −27 | |
| x 2 | 0.78 | 0.99 | |
| ν2 (s−1) | 35 | 44 | |
| VSD III | q3 (e0) | 0.83 | 1.0 |
| V3 (mV) | 3.7 | −11 | |
| x 3 | 1.0 | 0.93 | |
| ν3 (s−1) | 145 | 160 | |
| VSD IV | q4 (e0) | 0.92 | 1.1 |
| V4 (mV) | −54 | −52 | |
| x 4 | 0.35 | 0.55 | |
| ν4 (s−1) | 16 | 11 | |
| Energetic interaction | W1 (meV) | −8.0 | −16 |
| W2 (meV) | −16 | −50 | |
| W3 (meV) | −19 | −45 | |
| W4 (meV) | 4.1 | −0.87 |
Parameters in this column are from Pantazis et al., 2014.
The uniqueness of the solution and the 95% credible interval of each parameter were obtained with a Bayesian approach using MCMC sampling as in Hines et al. (2014). The results are reported in Figs. S2 and S3.
DISCUSSION
The α1 subunit of voltage-gated CaV channels is a modular, pseudotetrameric protein consisting of a central pore domain coupled to four homologous but not identical VSDs. Several auxiliary subunits, including α2δ and β, associate with the α1 subunit in a 1:1:1 ratio (Catterall, 2011; Wu et al., 2015) to regulate channel trafficking and biophysical properties (Fang and Colecraft, 2011; Dolphin, 2012; Neely and Hidalgo, 2014; Campiglio and Flucher, 2015). Using VCF to optically track the individual VSDs in a human CaV1.2 α1 + β3 complex with and without the auxiliary α2δ-1 subunits, we gained a mechanistic understanding of α2δ-1–mediated facilitation of CaV1.2 activation. We found that α2δ-1 alters the biophysical properties of three VSDs (I–III). The association of α2δ-1 with α1C increases the coupling of VSDs I–III to the channel pore, allowing the CaV1.2 channel to operate in the range of physiological membrane potentials found in excitable cells.
The physical nature of α1C/α2δ-1 association
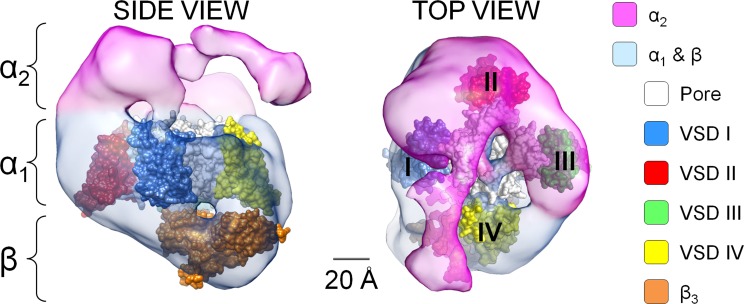
The association of α2δ-1 with α1C resulted in a substantial change in the intrinsic voltage-sensing properties of VSDs I–III (Fig. 4 and Table 3). This effect suggests either a direct physical or long-range allosteric interaction of α2δ-1 with these VSDs, causing their structural rearrangement. Our results are consistent with coimmunoprecipitation experiments showing that α2δ subunits bind to extracellular loops of repeat III and possibly to other extracellular loops of α1 (Gurnett et al., 1997). On the other hand, VSD IV remained completely unaffected, suggesting that α2δ-1 and VSD IV do not physically interact and consistent with the findings by Tuluc et al. (2009), who showed that the deletion of S3-S4 loop in VSD IV affects channel gating in CaV1.1 but not its modulation by α2δ-1 subunits. The findings that three VSDs out of four are remodeled by α2δ-1 is in agreement with a low-resolution structure of CaV1.2 channel complexes, which demonstrates that the α2δ-1 subunit forms a cap that embraces ~3/4 of the extracellular surface of the α1C subunit (Walsh et al., 2009a,b). Taking these data together, we propose that the α2δ-1 encapsulates a part of α1C that comprises VSDs I–III, whereas the exposed quarter of the α1C subunit is VSD IV (Fig. 7). Finally, we favor a physical interaction between α2δ-1 and VSDs I–III because of a recent cryo-electron microscopy (cryo-EM) structure of CaV1.1 channels showing that the α2δ-1 subunit interacts with the extracellular loops of repeats I–III (Wu et al., 2015), suggesting a common α1/α2δ topology in CaV1.1 (α1S) and CaV1.2 (α1C) channels. However, contribution of long-range allosteric interactions cannot be ruled out.
Figure 7.
The α2δ subunit covers ∼3/4 of the CaV1.2 pore-forming subunit, excluding VSD IV. Side and top views of the CaV1.2 (α1C/α2/β) channel volume (pink and light blue) modified from cryo-EM data from Walsh et al. (2009a) are shown. The atomic structures of NaVAb (PDB accession no. 4EKW; Payandeh et al., 2012), with subunits arranged clockwise, and the atomic structure of AID-associated β3 subunit (PDB accession no. 1VYT; Chen et al., 2004) were manually positioned in the cryo-EM volume. Because VSD IV is not perturbed by α2δ-1, we propose that the resolved α1C volume not covered by α2 is occupied by VSD IV, whereas VSDs I–III are encompassed by the α2δ-1 subunit, which alters their biophysical properties and, in the case of VSDs II and III, enhances their coupling to the channel pore.
α2δ-1 association alters the intrinsic voltage-sensing properties of VSDs I–III
The hyperpolarizing shifts in the F(V) values of VSDs I–III (Fig. 4) indicates that the active state of these voltage sensors is favored in the presence of α2δ-1 subunits. In VSD-gated channels, the activation of charge-bearing S4 segments is facilitated by the formation of salt bridges between positively charged S4 residues and negatively charged residues in adjacent VSD helices (Papazian et al., 1995; Wu et al., 2010; DeCaen et al., 2011; Tuluc et al., 2016). Association of the α2δ-1 subunit may facilitate the formation of such bonds by physically remodeling the spatial organization of the transmembrane helices of VSDs, altering their relative positions. Interestingly, we found that the VSDs’ sensitivity to changes in the membrane potential, i.e., the effective charge or slope of the F(V) curves (q), is almost equal among the four VSDs in the absence of the α2δ-1 subunit (q ≈ 1 e0), whereas q increases by approximately twofold for VSDs I–III in the presence of α2δ-1. The effective charge q of a voltage-sensing residue is given by the product zδ, where z is the valence number and δ is the electrical distance or fraction of membrane potential traversed by the residue. Because it is very unlikely that α2δ-1 association adds voltage-sensing charges, the increased apparent charge observed for VSD I–III suggests that their charged S4 helices move across a greater electrical distance, through either a larger spatial translation (e.g., moving at a steeper angle) or more concentrated electric field lines. Indeed, in VSD-endowed proteins, the local electric field can be tremendously enhanced by the existence of aqueous crevices separated by hydrophobic gaskets comprised of aromatic residue side chains (Asamoah et al., 2003; Starace and Bezanilla, 2004; Ahern and Horn, 2005; Chanda et al., 2005; Long et al., 2007; Tao et al., 2010; Lacroix and Bezanilla, 2011).
The α2δ-1 subunit enhances the coupling of VSDs I–III to the pore
Do the observed changes in VSD voltage-sensing properties account for the facilitation of CaV1.2 activation by α2δ-1 subunit? To answer this question, we used our allosteric model for CaV1.2 channel activation, which predicts the time- and voltage-dependent properties of each VSD and the pore (Pantazis et al., 2014). This model successfully accounted for the effects of α2δ-1 binding by both increasing the energetic contributions of VSDs I–III to pore opening and increasing the effective charges of VSDs I and II (Fig. 6 and Table 3). Specifically, in channels lacking the α2δ-1 subunit, VSDs I–III make a weak contribution to channel opening (W > −20 meV, equivalent to 0.8 kT or an allosteric factor of 2.2). The striking outcome of this study is that the energetic contribution of the activation of VSDs II and III to pore opening in channels lacking α2δ is greatly reduced. This is in contrast to channels containing α2δ-1, where VSDs II and III contribute two to three times as much energy toward channel opening (~−95 meV, ∼3.7 kT) or, in an alternative interpretation, their activation is obligatory for pore opening (Pantazis et al., 2014). The diminished VSD–pore conformational coupling in channels lacking α2δ is also supported by the good approximation of the G(V) by a Boltzmann distribution (Fig. 2 C), which implies a single voltage-dependent opening transition without significant input from VSDs. Our previous work on α2δ-containing CaV1.2 channels revealed a surprising disparity in the VSD voltage dependencies, greater than that observed in related pseudotetrameric NaV channels: VSD activations (Vhalf values) spanned 50 mV. The functional heterogeneity of the four VSDs was attributed to (a) the different amino acid composition of each VSD and (b) the structural asymmetry of the channel complex arising from its 1:1:1 α1/β/α2δ subunit stoichiometry. Interestingly, in this work, we found that increasing CaV1.2 structural symmetry (by excluding α2δ-1 subunits) in fact made the VSD voltage dependencies even more disparate, spanning ∼90 mV (Fig. 5). Our current model interpreted this finding as a result of direct modification of VSD voltage-sensing properties and reduced coupling of VSDs I–III to the pore by α2δ-1. Another possible explanation is that α2δ-1 acts as an allosteric center (in addition to the pore), increasing the coupling between voltage sensors. However, this possibility implies that α2δ-1 also undergoes conformational changes, for which there is yet no experimental evidence. Perhaps future studies could explore the possibility.
Conclusions
In summary, we have used VCF to optically track the molecular rearrangements of the individual VSDs of a human CaV1.2 channel in the presence or absence of α2δ-1. VCF is now a well-established method to assess voltage-dependent conformational changes, allowing us to track the movement of individual VSDs and to resolve slow conformational changes (as those observed in VSD I) that are extremely difficult to capture by gating current measurements. In this work, we have not systematically recorded gating currents, as they could not reveal the individual contributions of each VSD to CaV1.2 activation. Perhaps the most important advantage of VCF is that all recordings could be performed in conducting channels, whereby ionic currents and VSD movements were sampled simultaneously, without the use of pore-blockers. We found that the α2δ-1 auxiliary subunit significantly alters the voltage dependence of VSDs I–III, facilitating their activation, but not that of VSD IV. A 32-state allosteric model, consistent with the CaV1.2 molecular architecture, predicts the major kinetic and steady-state features of the experimental data, revealing that the association of α2δ-1 with α1C (in the presence of β3) specifically increased the coupling energy of VSDs I–III to the pore, as well as effective gating charge in segments I and II. Without the enhanced gating properties brought about by α2δ-1 association, CaV1.2 channels could not operate at physiological membrane potentials.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Ashraf Kitmitto for sharing the cryo-EM volumes of CaV α1C/α2δ (Walsh et al., 2009a). The human α1C-77 clone was a gift from Nicolaj Soldatov. We thank the members of the Olcese laboratory for insightful discussion and Jing Gao for the weekly preparation of the Xenopus oocytes.
This work was supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute grant P01HL078931 to J.N. Weiss and R. Olcese; NIH/National Institute of General Medical Sciences grant R01GM110276 to R. Olcese; American Heart Association Scientist Development grant 14SDG20300018 to A. Pantazis and postdoctoral fellowship 16POST27250284 to N. Savalli; and Chilean government grants FONDECYT 1161672 and ACT1104 to A. Neely. The Centro Interdisciplinario de Neurociencia de Valparaíso is a Millennium Institute supported by the Millennium Scientific Initiative of the Chilean Ministry of Economy.
The authors declare no competing financial interests.
Eduardo Rios served as editor.
Footnotes
Abbreviations used:
- cryo-EM
- cryo-electron microscopy
- MES
- methanesulfonate
- VCF
- voltage clamp fluorometry
- VSD
- voltage-sensing domain
References
- Ahern, C.A., and Horn R.. 2005. Focused electric field across the voltage sensor of potassium channels. Neuron. 48:25–29. 10.1016/j.neuron.2005.08.020 [DOI] [PubMed] [Google Scholar]
- Asamoah, O.K., Wuskell J.P., Loew L.M., and Bezanilla F.. 2003. A fluorometric approach to local electric field measurements in a voltage-gated ion channel. Neuron. 37:85–98. 10.1016/S0896-6273(02)01126-1 [DOI] [PubMed] [Google Scholar]
- Bangalore, R., Mehrke G., Gingrich K., Hofmann F., and Kass R.S.. 1996. Influence of L-type Ca channel alpha 2/delta-subunit on ionic and gating current in transiently transfected HEK 293 cells. Am. J. Physiol. 270:H1521–H1528. [DOI] [PubMed] [Google Scholar]
- Barclay, J., Balaguero N., Mione M., Ackerman S.L., Letts V.A., Brodbeck J., Canti C., Meir A., Page K.M., Kusumi K., et al. 2001. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 21:6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish, M.E. 1983. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J. Physiol. 342:309–325. 10.1113/jphysiol.1983.sp014852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Johny, M., and Yue D.T.. 2014. Calmodulin regulation (calmodulation) of voltage-gated calcium channels. J. Gen. Physiol. 143:679–692. 10.1085/jgp.201311153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla, F. 2008. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 9:323–332. 10.1038/nrm2376 [DOI] [PubMed] [Google Scholar]
- Bourdin, B., Shakeri B., Tétreault M.P., Sauvé R., Lesage S., and Parent L.. 2015. Functional characterization of CaVα2δ mutations associated with sudden cardiac death. J. Biol. Chem. 290:2854–2869. 10.1074/jbc.M114.597930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov, E., Pfeiffer R., Barajas-Martinez H., Delpón E., Hu D., Desai M., Borggrefe M., Häissaguerre M., Kanter R., Pollevick G.D., et al. 2010. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 7:1872–1882. 10.1016/j.hrthm.2010.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Rivera, A., Andrade A., Hernández-Hernández O., González-Ramírez R., Sandoval A., Rivera M., Gomora J.C., and Felix R.. 2012. Identification of a disulfide bridge essential for structure and function of the voltage-gated Ca(2+) channel α(2)δ-1 auxiliary subunit. Cell Calcium. 51:22–30. 10.1016/j.ceca.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campiglio, M., and Flucher B.E.. 2015. The role of auxiliary subunits for the functional diversity of voltage-gated calcium channels. J. Cell. Physiol. 230:2019–2031. 10.1002/jcp.24998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy, J.S., Ferron L., Kadurin I., Pratt W.S., and Dolphin A.C.. 2014. Functional exofacially tagged N-type calcium channels elucidate the interaction with auxiliary α2δ-1 subunits. Proc. Natl. Acad. Sci. USA. 111:8979–8984. 10.1073/pnas.1403731111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall, W.A. 2010. Ion channel voltage sensors: Structure, function, and pathophysiology. Neuron. 67:915–928. 10.1016/j.neuron.2010.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall, W.A. 2011. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 3:a003947. 10.1101/cshperspect.a003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, A., and Bezanilla F.. 1997. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron. 19:1127–1140. 10.1016/S0896-6273(00)80403-1 [DOI] [PubMed] [Google Scholar]
- Cha, A., Ruben P.C., George A.L. Jr., Fujimoto E., and Bezanilla F.. 1999. Voltage sensors in domains III and IV, but not I and II, are immobilized by Na+ channel fast inactivation. Neuron. 22:73–87. 10.1016/S0896-6273(00)80680-7 [DOI] [PubMed] [Google Scholar]
- Chanda, B., and Bezanilla F.. 2008. A common pathway for charge transport through voltage-sensing domains. Neuron. 57:345–351. 10.1016/j.neuron.2008.01.015 [DOI] [PubMed] [Google Scholar]
- Chanda, B., Asamoah O.K., Blunck R., Roux B., and Bezanilla F.. 2005. Gating charge displacement in voltage-gated ion channels involves limited transmembrane movement. Nature. 436:852–856. 10.1038/nature03888 [DOI] [PubMed] [Google Scholar]
- Chen, Y.H., Li M.H., Zhang Y., He L.L., Yamada Y., Fitzmaurice A., Shen Y., Zhang H., Tong L., and Yang J.. 2004. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 429:675–680. 10.1038/nature02641 [DOI] [PubMed] [Google Scholar]
- Claydon, T.W., and Fedida D.. 2007. Voltage clamp fluorimetry studies of mammalian voltage-gated K(+) channel gating. Biochem. Soc. Trans. 35:1080–1082. 10.1042/BST0351080 [DOI] [PubMed] [Google Scholar]
- Colquhoun, D., and Hawkes A.G.. 1981. On the stochastic properties of single ion channels. Proc. R. Soc. Lond. B Biol. Sci. 211:205–235. 10.1098/rspb.1981.0003 [DOI] [PubMed] [Google Scholar]
- Dahan, D.S., Dibas M.I., Petersson E.J., Auyeung V.C., Chanda B., Bezanilla F., Dougherty D.A., and Lester H.A.. 2004. A fluorophore attached to nicotinic acetylcholine receptor βM2 detects productive binding of agonist to the αδ site. Proc. Natl. Acad. Sci. USA. 101:10195–10200. 10.1073/pnas.0301885101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, A., Kadurin I., Alvarez-Laviada A., Douglas L., Nieto-Rostro M., Bauer C.S., Pratt W.S., and Dolphin A.C.. 2010. The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc. Natl. Acad. Sci. USA. 107:1654–1659. 10.1073/pnas.0908735107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaen, P.G., Yarov-Yarovoy V., Scheuer T., and Catterall W.A.. 2011. Gating charge interactions with the S1 segment during activation of a Na+ channel voltage sensor. Proc. Natl. Acad. Sci. USA. 108:18825–18830. 10.1073/pnas.1116449108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin, A.C. 2012. Calcium channel auxiliary α2δ and β subunits: Trafficking and one step beyond. Nat. Rev. Neurosci. 13:542–555. 10.1038/nrn3317 [DOI] [PubMed] [Google Scholar]
- Dolphin, A.C. 2013. The α2δ subunits of voltage-gated calcium channels. Biochim. Biophys. Acta. 1828:1541–1549. 10.1016/j.bbamem.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Eroglu, C., Allen N.J., Susman M.W., O’Rourke N.A., Park C.Y., Ozkan E., Chakraborty C., Mulinyawe S.B., Annis D.S., Huberman A.D., et al. 2009. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 139:380–392. 10.1016/j.cell.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, K., and Colecraft H.M.. 2011. Mechanism of auxiliary β-subunit-mediated membrane targeting of L-type (Ca(V)1.2) channels. J. Physiol. 589:4437–4455. 10.1113/jphysiol.2011.214247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, R., Gurnett C.A., De Waard M., and Campbell K.P.. 1997. Dissection of functional domains of the voltage-dependent Ca2+ channel alpha2delta subunit. J. Neurosci. 17:6884–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, M.J., Cox P.J., Stott E., Melrose H., Offord J., Su T.Z., Bramwell S., Corradini L., England S., Winks J., et al. 2006. Identification of the α2-δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc. Natl. Acad. Sci. USA. 103:17537–17542. 10.1073/pnas.0409066103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeisen, F., and Minor D.L. Jr. 2010. Progress in the structural understanding of voltage-gated calcium channel (CaV) function and modulation. Channels (Austin). 4:459–474. 10.4161/chan.4.6.12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Bicer, G.A., Varadi G., Koch S.E., Ishii M., Bodi I., Kadeer N., Muth J.N., Mikala G., Petrashevskaya N.N., Jordan M.A., et al. 2009. Targeted disruption of the voltage-dependent calcium channel α2/δ-1-subunit. Am. J. Physiol. Heart Circ. Physiol. 297:H117–H124. 10.1152/ajpheart.00122.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi, C.S., and Olcese R.. 2008. The voltage-clamp fluorometry technique. Methods Mol. Biol. 491:213–231. 10.1007/978-1-59745-526-8_17 [DOI] [PubMed] [Google Scholar]
- Ghezzi, C., Murer H., and Forster I.C.. 2009. Substrate interactions of the electroneutral Na+-coupled inorganic phosphate cotransporter (NaPi-IIc). J. Physiol. 587:4293–4307. 10.1113/jphysiol.2009.175596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett, C.A., Felix R., and Campbell K.P.. 1997. Extracellular interaction of the voltage-dependent Ca2+ channel alpha2delta and alpha1 subunits. J. Biol. Chem. 272:18508–18512. 10.1074/jbc.272.29.18508 [DOI] [PubMed] [Google Scholar]
- Hendrich, J., Van Minh A.T., Heblich F., Nieto-Rostro M., Watschinger K., Striessnig J., Wratten J., Davies A., and Dolphin A.C.. 2008. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc. Natl. Acad. Sci. USA. 105:3628–3633. 10.1073/pnas.0708930105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines, K.E., Middendorf T.R., and Aldrich R.W.. 2014. Determination of parameter identifiability in nonlinear biophysical models: A Bayesian approach. J. Gen. Physiol. 143:401–416. 10.1085/jgp.201311116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout, S.C., Ulbrich M.H., Bell S.C., and Isacoff E.Y.. 2008. Subunit organization and functional transitions in Ci-VSP. Nat. Struct. Mol. Biol. 15:106–108. 10.1038/nsmb1320 [DOI] [PubMed] [Google Scholar]
- Kurshan, P.T., Oztan A., and Schwarz T.L.. 2009. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat. Neurosci. 12:1415–1423. 10.1038/nn.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix, J.J., and Bezanilla F.. 2011. Control of a final gating charge transition by a hydrophobic residue in the S2 segment of a K+ channel voltage sensor. Proc. Natl. Acad. Sci. USA. 108:6444–6449. 10.1073/pnas.1103397108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, H.P., Tzingounis A.V., Koch H.P., and Kavanaugh M.P.. 2004. Fluorometric measurements of conformational changes in glutamate transporters. Proc. Natl. Acad. Sci. USA. 101:3951–3956. 10.1073/pnas.0306737101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. 2012. MOMCMC: An efficient Monte Carlo method for multi-objective sampling over real parameter space. Comput. Math. Appl. 64:3542–3556. 10.1016/j.camwa.2012.09.003 [DOI] [Google Scholar]
- Li, C.Y., Zhang X.L., Matthews E.A., Li K.W., Kurwa A., Boroujerdi A., Gross J., Gold M.S., Dickenson A.H., Feng G., and Luo Z.D.. 2006. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 125:20–34. 10.1016/j.pain.2006.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, S.B., Tao X., Campbell E.B., and MacKinnon R.. 2007. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 450:376–382. 10.1038/nature06265 [DOI] [PubMed] [Google Scholar]
- Lörinczi, É., Bhargava Y., Marino S.F., Taly A., Kaczmarek-Hájek K., Barrantes-Freer A., Dutertre S., Grutter T., Rettinger J., and Nicke A.. 2012. Involvement of the cysteine-rich head domain in activation and desensitization of the P2X1 receptor. Proc. Natl. Acad. Sci. USA. 109:11396–11401. 10.1073/pnas.1118759109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzzu, L.M., Moronne M.M., and Isacoff E.Y.. 1996. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science. 271:213–216. 10.1126/science.271.5246.213 [DOI] [PubMed] [Google Scholar]
- Meinild, A.K., Hirayama B.A., Wright E.M., and Loo D.D.. 2002. Fluorescence studies of ligand-induced conformational changes of the Na(+)/glucose cotransporter. Biochemistry. 41:1250–1258. 10.1021/bi011661r [DOI] [PubMed] [Google Scholar]
- Nakai, J., Adams B.A., Imoto K., and Beam K.G.. 1994. Critical roles of the S3 segment and S3-S4 linker of repeat I in activation of L-type calcium channels. Proc. Natl. Acad. Sci. USA. 91:1014–1018. 10.1073/pnas.91.3.1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely, A., and Hidalgo P.. 2014. Structure-function of proteins interacting with the α1 pore-forming subunit of high-voltage-activated calcium channels. Front. Physiol. 5:209. 10.3389/fphys.2014.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely, A., Olcese R., Wei X., Birnbaumer L., and Stefani E.. 1994. Ca(2+)-dependent inactivation of a cloned cardiac Ca2+ channel alpha 1 subunit (alpha 1C) expressed in Xenopus oocytes. Biophys. J. 66:1895–1903. 10.1016/S0006-3495(94)80983-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely, G.G., Hess A., Costigan M., Keene A.C., Goulas S., Langeslag M., Griffin R.S., Belfer I., Dai F., Smith S.B., et al. 2010. A genome-wide Drosophila screen for heat nociception identifies α2δ3 as an evolutionarily conserved pain gene. Cell. 143:628–638. 10.1016/j.cell.2010.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteen, J.D., Gonzalez C., Sampson K.J., Iyer V., Rebolledo S., Larsson H.P., and Kass R.S.. 2010. KCNE1 alters the voltage sensor movements necessary to open the KCNQ1 channel gate. Proc. Natl. Acad. Sci. USA. 107:22710–22715. 10.1073/pnas.1016300108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palovcak, E., Delemotte L., Klein M.L., and Carnevale V.. 2014. Evolutionary imprint of activation: The design principles of VSDs. J. Gen. Physiol. 143:145–156. 10.1085/jgp.201311103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis, A., and Olcese R.. 2013. Cut-open oocyte voltage clamp technique. In Encyclopedia of Biophysics. Roberts G.C.K., editor. Springer, Berlin, Heidelberg. 406–413. 10.1007/978-3-642-16712-6_371 [DOI] [Google Scholar]
- Pantazis, A., Savalli N., Sigg D., Neely A., and Olcese R.. 2014. Functional heterogeneity of the four voltage sensors of a human L-type calcium channel. Proc. Natl. Acad. Sci. USA. 111:18381–18386. 10.1073/pnas.1411127112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian, D.M., Shao X.M., Seoh S.A., Mock A.F., Huang Y., and Wainstock D.H.. 1995. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron. 14:1293–1301. 10.1016/0896-6273(95)90276-7 [DOI] [PubMed] [Google Scholar]
- Payandeh, J., Gamal El-Din T.M., Scheuer T., Zheng N., and Catterall W.A.. 2012. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 486:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, B.Z., DeMaria C.D., Adelman J.P., and Yue D.T.. 1999. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 22:549–558. 10.1016/S0896-6273(00)80709-6 [DOI] [PubMed] [Google Scholar]
- Platano, D., Qin N., Noceti F., Birnbaumer L., Stefani E., and Olcese R.. 2000. Expression of the alpha(2)delta subunit interferes with prepulse facilitation in cardiac L-type calcium channels. Biophys. J. 78:2959–2972. 10.1016/S0006-3495(00)76835-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, N., Olcese R., Bransby M., Lin T., and Birnbaumer L.. 1999. Ca2+-induced inhibition of the cardiac Ca2+ channel depends on calmodulin. Proc. Natl. Acad. Sci. USA. 96:2435–2438. 10.1073/pnas.96.5.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscic, K.J., Miceli F., Villalba-Galea C.A., Dai H., Mishina Y., Bezanilla F., and Goldstein S.A.. 2013. IKs channels open slowly because KCNE1 accessory subunits slow the movement of S4 voltage sensors in KCNQ1 pore-forming subunits. Proc. Natl. Acad. Sci. USA. 110:E559–E566. 10.1073/pnas.1222616110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savalli, N., Kondratiev A., Toro L., and Olcese R.. 2006. Voltage-dependent conformational changes in human Ca(2+)- and voltage-activated K(+) channel, revealed by voltage-clamp fluorometry. Proc. Natl. Acad. Sci. USA. 103:12619–12624. 10.1073/pnas.0601176103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savalli, N., Kondratiev A., de Quintana S.B., Toro L., and Olcese R.. 2007. Modes of operation of the BKCa channel beta2 subunit. J. Gen. Physiol. 130:117–131. 10.1085/jgp.200709803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigg, D. 2014. Modeling ion channels: Past, present, and future. J. Gen. Physiol. 144:7–26. 10.1085/jgp.201311130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, P.L., and Yellen G.. 2002. Fast and slow voltage sensor movements in HERG potassium channels. J. Gen. Physiol. 119:275–293. 10.1085/jgp.20028534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatov, N.M. 1992. Molecular diversity of L-type Ca2+ channel transcripts in human fibroblasts. Proc. Natl. Acad. Sci. USA. 89:4628–4632. 10.1073/pnas.89.10.4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starace, D.M., and Bezanilla F.. 2004. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 427:548–553. 10.1038/nature02270 [DOI] [PubMed] [Google Scholar]
- Stefani, E., and Bezanilla F.. 1998. Cut-open oocyte voltage-clamp technique. Methods Enzymol. 293:300–318. 10.1016/S0076-6879(98)93020-8 [DOI] [PubMed] [Google Scholar]
- Swartz, K.J. 2008. Sensing voltage across lipid membranes. Nature. 456:891–897. 10.1038/nature07620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar, S., and Lynch J.W.. 2015. Investigating ion channel conformational changes using voltage clamp fluorometry. Neuropharmacology. 98:3–12. 10.1016/j.neuropharm.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Tao, X., Lee A., Limapichat W., Dougherty D.A., and MacKinnon R.. 2010. A gating charge transfer center in voltage sensors. Science. 328:67–73. 10.1126/science.1185954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templin, C., Ghadri J.R., Rougier J.S., Baumer A., Kaplan V., Albesa M., Sticht H., Rauch A., Puleo C., Hu D., et al. 2011. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6). Eur. Heart J. 32:1077–1088. 10.1093/eurheartj/ehr076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola, F., Pathak M.M., and Isacoff E.Y.. 2006. How does voltage open an ion channel? Annu. Rev. Cell Dev. Biol. 22:23–52. 10.1146/annurev.cellbio.21.020404.145837 [DOI] [PubMed] [Google Scholar]
- Tombola, F., Ulbrich M.H., Kohout S.C., and Isacoff E.Y.. 2010. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat. Struct. Mol. Biol. 17:44–50. 10.1038/nsmb.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc, P., Kern G., Obermair G.J., and Flucher B.E.. 2007. Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel α2δ-1 subunit in cardiac excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 104:11091–11096. 10.1073/pnas.0700577104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc, P., Molenda N., Schlick B., Obermair G.J., Flucher B.E., and Jurkat-Rott K.. 2009. A CaV1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys. J. 96:35–44. 10.1016/j.bpj.2008.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc, P., Yarov-Yarovoy V., Benedetti B., and Flucher B.E.. 2016. Molecular interactions in the voltage sensor controlling gating properties of CaV calcium channels. Structure. 24:261–271. 10.1016/j.str.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchitel, O.D., Di Guilmi M.N., Urbano F.J., and Gonzalez-Inchauspe C.. 2010. Acute modulation of calcium currents and synaptic transmission by gabapentinoids. Channels (Austin). 4:490–496. 10.4161/chan.4.6.12864 [DOI] [PubMed] [Google Scholar]
- Vergult, S., Dheedene A., Meurs A., Faes F., Isidor B., Janssens S., Gautier A., Le Caignec C., and Menten B.. 2015. Genomic aberrations of the CACNA2D1 gene in three patients with epilepsy and intellectual disability. Eur. J. Hum. Genet. 23:628–632. 10.1038/ejhg.2014.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, C.P., Davies A., Butcher A.J., Dolphin A.C., and Kitmitto A.. 2009a. Three-dimensional structure of CaV3.1: Comparison with the cardiac L-type voltage-gated calcium channel monomer architecture. J. Biol. Chem. 284:22310–22321. 10.1074/jbc.M109.017152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, C.P., Davies A., Nieto-Rostro M., Dolphin A.C., and Kitmitto A.. 2009b. Labelling of the 3D structure of the cardiac L-type voltage-gated calcium channel. Channels (Austin). 3:387–392. 10.4161/chan.3.6.10225 [DOI] [PubMed] [Google Scholar]
- Wu, D., Delaloye K., Zaydman M.A., Nekouzadeh A., Rudy Y., and Cui J.. 2010. State-dependent electrostatic interactions of S4 arginines with E1 in S2 during Kv7.1 activation. J. Gen. Physiol. 135:595–606. 10.1085/jgp.201010408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., Yan Z., Li Z., Yan C., Lu S., Dong M., and Yan N.. 2015. Structure of the voltage-gated calcium channel Cav1.1 complex. Science. 350:aad2395. 10.1126/science.aad2395 [DOI] [PubMed] [Google Scholar]
- Wycisk, K.A., Zeitz C., Feil S., Wittmer M., Forster U., Neidhardt J., Wissinger B., Zrenner E., Wilke R., Kohl S., and Berger W.. 2006. Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am. J. Hum. Genet. 79:973–977. 10.1086/508944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi, G.W., Striessnig J., Koschak A., and Dolphin A.C.. 2015. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 67:821–870. 10.1124/pr.114.009654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W., Varga Z., and Silva J.R.. 2016. Molecular motions that shape the cardiac action potential: Insights from voltage clamp fluorometry. Prog. Biophys. Mol. Biol. 120:3–17. 10.1016/j.pbiomolbio.2015.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.