Abstract
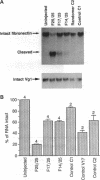
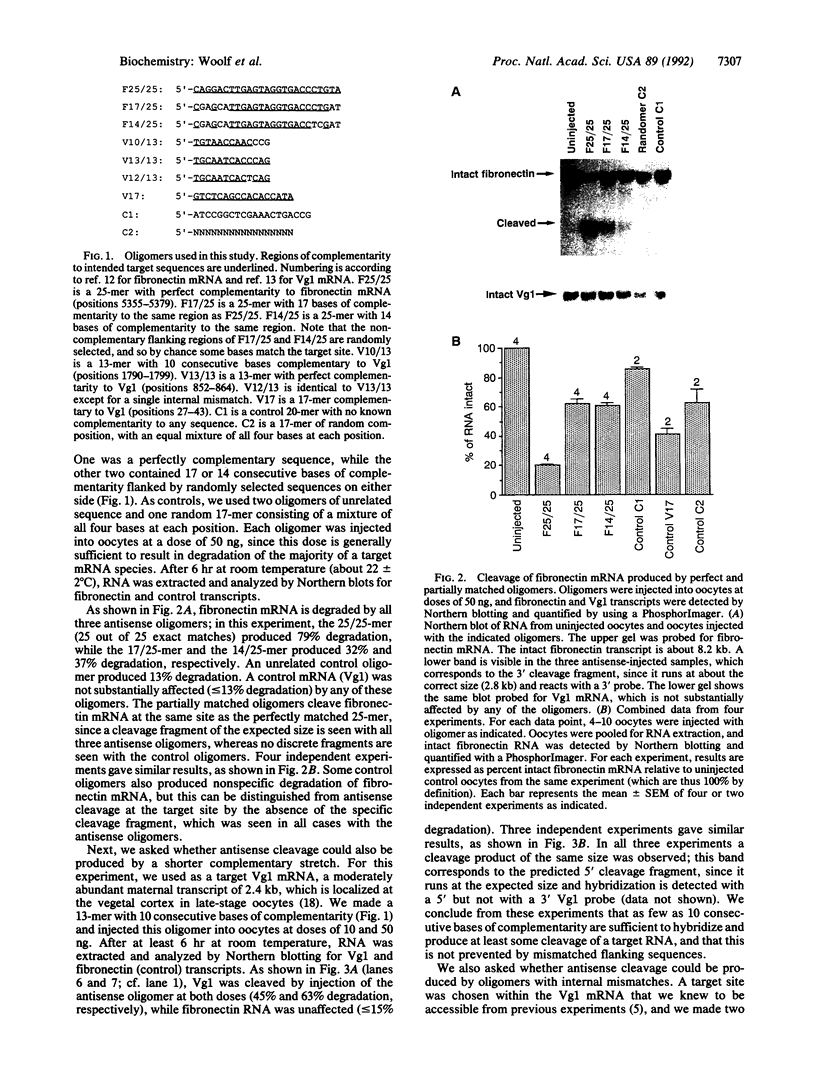
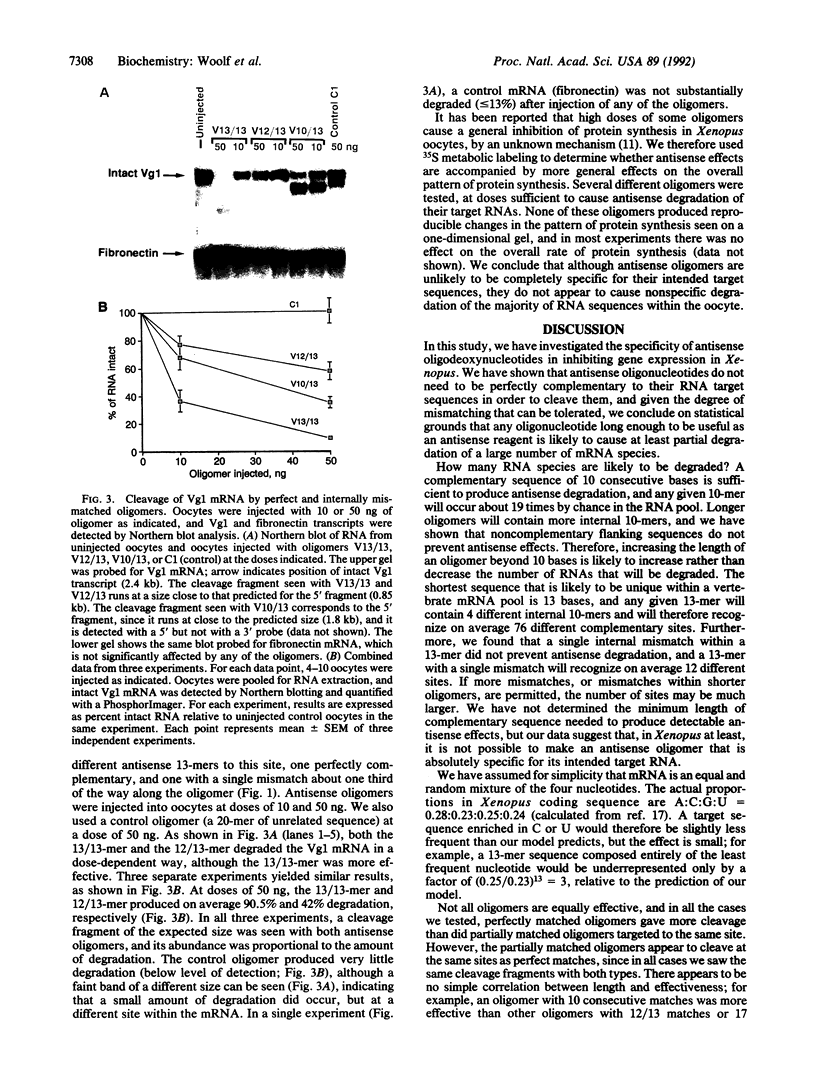
Antisense oligonucleotides are widely used as inhibitors of gene expression in cultured cells and have been proposed as potential therapeutic agents, but it is not known to what extent they are specific for their intended target RNAs. Statistical considerations indicate that if oligonucleotides can form hybrids with mRNA molecules in vivo by means of short or imperfect regions of complementarity, then the specificity of oligonucleotides as antisense reagents will be greatly compromised. We have used Xenopus oocytes as a model system in which to investigate the potential specificity of antisense oligonucleotides in vivo. We injected perfect and partially matched antisense oligonucleotides into oocytes and measured the resulting degradation of the target RNA in each case. On the basis of the extent to which antisense oligonucleotides can cause cleavage of RNAs at imperfectly matched target sites, we conclude that in this system it is probably not possible to obtain specific cleavage of an intended target RNA without also causing at least the partial destruction of many nontargeted RNAs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aota S., Gojobori T., Ishibashi F., Maruyama T., Ikemura T. Codon usage tabulated from the GenBank Genetic Sequence Data. Nucleic Acids Res. 1988;16 (Suppl):r315–r402. doi: 10.1093/nar/16.suppl.r315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Chevrier M., Nguyen T. T., Hélène C. Rate of degradation of [alpha]- and [beta]-oligodeoxynucleotides in Xenopus oocytes. Implications for anti-messenger strategies. Nucleic Acids Res. 1987 Dec 23;15(24):10507–10521. doi: 10.1093/nar/15.24.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R. Ribozymes and their medical implications. JAMA. 1988 Nov 25;260(20):3030–3034. [PubMed] [Google Scholar]
- Chang E. H., Miller P. S., Cushman C., Devadas K., Pirollo K. F., Ts'o P. O., Yu Z. P. Antisense inhibition of ras p21 expression that is sensitive to a point mutation. Biochemistry. 1991 Aug 27;30(34):8283–8286. doi: 10.1021/bi00098a001. [DOI] [PubMed] [Google Scholar]
- Dash P., Lotan I., Knapp M., Kandel E. R., Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone D. W., Norton P. A., Hynes R. O. Identification and characterization of alternatively spliced fibronectin mRNAs expressed in early Xenopus embryos. Dev Biol. 1992 Feb;149(2):357–369. doi: 10.1016/0012-1606(92)90291-n. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Herschlag D. Implications of ribozyme kinetics for targeting the cleavage of specific RNA molecules in vivo: more isn't always better. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6921–6925. doi: 10.1073/pnas.88.16.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S. Quantitative hybridization-arrest of mRNA in Xenopus oocytes using single-stranded complementary DNA or oligonucleotide probes. Nucleic Acids Res. 1985 Jul 11;13(13):4991–5004. doi: 10.1093/nar/13.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M., Miller M., Carrasco A. E., Eastman E., Etkin L. The maternal store of the xlgv7 mRNA in full-grown oocytes is not required for normal development in Xenopus. Development. 1989 Dec;107(4):899–907. doi: 10.1242/dev.107.4.899. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A. Translocation of a localized maternal mRNA to the vegetal pole of Xenopus oocytes. Nature. 1987 Jul 2;328(6125):80–82. doi: 10.1038/328080a0. [DOI] [PubMed] [Google Scholar]
- Pan Z. Q., Prives C. Assembly of functional U1 and U2 human-amphibian hybrid snRNPs in Xenopus laevis oocytes. Science. 1988 Sep 9;241(4871):1328–1331. doi: 10.1126/science.2970672. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Engstrom L., Mahowald A. P. Zygotic lethals with specific maternal effect phenotypes in Drosophila melanogaster. I. Loci on the X chromosome. Genetics. 1989 Feb;121(2):333–352. doi: 10.1093/genetics/121.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner M. H., De Santo R. J., Arnheiter H., Staudt L. M. Oct-3 is a maternal factor required for the first mouse embryonic division. Cell. 1991 Mar 22;64(6):1103–1110. doi: 10.1016/0092-8674(91)90265-z. [DOI] [PubMed] [Google Scholar]
- Rosner M., De Santo R. J., Arnheiter H., Staudt L. M. Retraction: Oct-3 is a maternal factor required for the first mouse embryonic division. Cell. 1992 May 29;69(5):724–724. doi: 10.1016/0092-8674(92)90284-j. [DOI] [PubMed] [Google Scholar]
- Shuttleworth J., Colman A. Antisense oligonucleotide-directed cleavage of mRNA in Xenopus oocytes and eggs. EMBO J. 1988 Feb;7(2):427–434. doi: 10.1002/j.1460-2075.1988.tb02830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth J., Matthews G., Dale L., Baker C., Colman A. Antisense oligodeoxyribonucleotide-directed cleavage of maternal mRNA in Xenopus oocytes and embryos. Gene. 1988 Dec 10;72(1-2):267–275. doi: 10.1016/0378-1119(88)90152-7. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Bement W. M., Dersch M. A., Dworkin-Rastl E., Dworkin M. B., Capco D. G. Nonspecific effects of oligodeoxynucleotide injection in Xenopus oocytes: a reevaluation of previous D7 mRNA ablation experiments. Development. 1990 Nov;110(3):769–779. doi: 10.1242/dev.110.3.769. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Woolf T. M., Jennings C. G., Rebagliati M., Melton D. A. The stability, toxicity and effectiveness of unmodified and phosphorothioate antisense oligodeoxynucleotides in Xenopus oocytes and embryos. Nucleic Acids Res. 1990 Apr 11;18(7):1763–1769. doi: 10.1093/nar/18.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Wagner R. W. Hybridization and dissociation rates of phosphodiester or modified oligodeoxynucleotides with RNA at near-physiological conditions. Nucleic Acids Res. 1991 May 11;19(9):2463–2470. doi: 10.1093/nar/19.9.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Baradi T., Bouwmeester T., Giltay R., Pieler T. The maternal store of zinc finger protein encoding mRNAs in fully grown Xenopus oocytes is not required for early embryogenesis. EMBO J. 1991 Jun;10(6):1407–1413. doi: 10.1002/j.1460-2075.1991.tb07661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]