Voltage-gated cation channels contain a single pore surrounded by four voltage-sensing domains (VSDs), each containing a critical component of voltage sensing, the S4 transmembrane segment. In response to membrane depolarization, the VSDs undergo a conformational change that results in positively charged residues within each of the S4 segments to move across the plane of the lipid bilayer, causing the channel’s activation gate to open. However, many questions about this mechanism remain. Why are there four VSDs per channel? Are all four VSDs needed to activate the channel? If not, how many, and which ones, need to move for channel activation? Do all VSDs contribute equally to the gating properties of the channel, or do different VSDs perform specialized tasks? In the past, these questions have been subject to intensive investigations in the potassium and sodium channel fields. However, pertinent evidence was scarce and mostly indirect for voltage-gated calcium channels (CaVs), until the research team of Riccardo Olcese at the University of California at Los Angeles introduced the voltage-clamp fluorometry method to the study of CaV channels (Pantazis et al., 2014). In this issue, Savalli et al. apply this powerful approach to determine which VSDs are involved in the modulation of CaV1.2 gating properties by the auxiliary α2δ-1 channel subunit (Savalli et al., 2016). Their results demonstrate that the VSDs that principally govern channel gating are also subject to modulation by α2δ-1.
CaVs transduce membrane depolarization into cellular functions such as secretion of hormones and neurotransmitters, contraction of striated and smooth muscles, and gene regulation. Currents through CaVs contribute to pacemaking and action potential shape in nerve and muscle cells, and calcium influx through CaV channels regulates the different signaling pathways involved in cell functions as diverse as fertilization, cell division, metabolism, differentiation, learning and memory, and even cell death. Accordingly, the different isoforms and splice variants of CaV channels operate within a much wider range of membrane potentials than voltage-gated sodium channels (Lipscombe et al., 2013), hence their subdivision into low-voltage– and high-voltage–activated channels (CaV3 and CaV1-2 channels, respectively). High-voltage–activated CaVs are multisubunit ion channel complexes composed of a pore-forming α1 subunit (CaV1.1-4 and CaV2.1-3) and several auxiliary channel subunits: α2δ1-4, β1-4, calmodulin, and, in skeletal muscle, the γ1 subunit and STAC3 (Campiglio and Flucher, 2015). The auxiliary subunits are involved in targeting the channel complexes to specific membranes and, in addition, different combinations of auxiliary subunits endow calcium channels with specific functional properties.
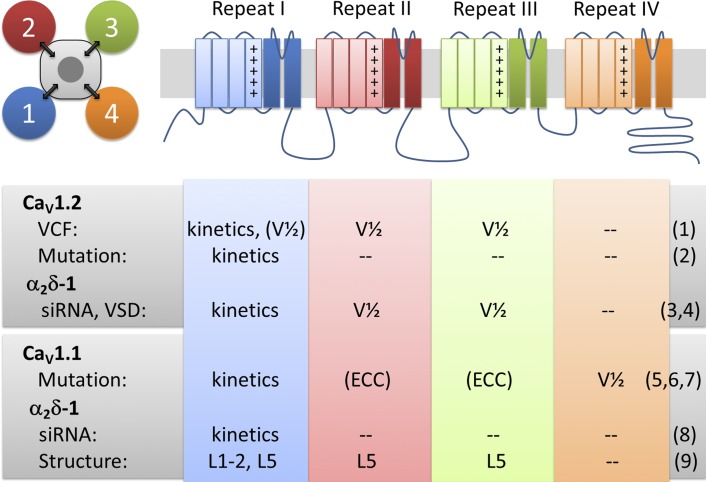
The α1 subunits of CaV (and NaV) channels are pseudotetrameric channels (Fig. 1). Each of the four homologous repeats contains six transmembrane helices (S1–S6), the first four of which (S1–S4) form a functional VSD, whereas S5 and S6 plus the connecting P-loop of all four repeats together form the channel pore containing the ion selectivity filter and the activation gate (Catterall, 2011). Previous work on the sodium channel, and isolated findings from the analysis of natural and experimentally introduced mutations in CaV channels, suggested that the four VSDs (I, II, III, and IV) contribute differently to activation of these cation channels. In sodium channels, combined evidence from classical biophysical work, mutagenesis, pharmacology, and voltage-clamp fluorometry indicated that VSDs I–III control channel activation, whereas VSD IV determines voltage-dependent inactivation (Ahern et al., 2016). For CaV channels, most of the information pertinent to this question comes from studies of L-type calcium channels, specifically CaV1.1 and CaV1.2. The latter is widely expressed in the nervous system, the cardiovascular system, and endocrine cells. In contrast, CaV1.1 is specifically expressed in skeletal muscle and, because of its unique properties, represents a striking example of the division of labor among the four VSDs of a pseudotetrameric channel.
Figure 1.
Pseudotetrameric domain structure of CaVs with functions associated to each VSD. Each of the four homologous repeats contains a functional VSD (first four transmembrane helices) and contributes one fourth of the channel pore. Voltage-clamp fluorometry (VCF), mutagenesis, siRNA knockdown, and structure studies provide evidence for distinct functions and interactions of each of the four VSDs of CaV1.2 and CaV1.1 channels and their modulation by α2δ-1. Kinetics and V1/2 refer to kinetics and voltage dependence of activation, respectively. L1-2 and L5 refer to extracellular loops connecting transmembrane helices S1 and S2 and helix S5 with the pore, respectively. References: (1) Pantazis et al. (2014); (2) Nakai et al. (1994); (3) Tuluc et al. (2007); (4) Savalli et al. (2016) in this issue; (5) Tuluc et al. (2016a); (6) Eltit et al. (2012); (7) Tuluc et al. (2009); (8) Obermair et al. (2005); (9) Wu et al. (2015).
The skeletal muscle CaV1.1 isoform is unique in the sense that its voltage sensors independently activate two distinct functions: excitation-contraction (EC) coupling and current conduction through the L-type calcium channel itself (Melzer et al., 1995). With physiological depolarizations, movement of the CaV1.1 gating charges directly activates the physically associated calcium release channel (type 1 ryanodine receptor) in the sarcoplasmic reticulum. In contrast, nonphysiologically long and strong depolarizations are required to activate relatively small calcium currents in skeletal muscle. These limitations on the speed and voltage dependence of CaV1.1 calcium current activation are controlled by VSD I and IV, respectively. Early chimera studies, in which sequences of VSD I were exchanged between CaV1.1 and CaV1.2, demonstrated that VSD I is important for determining the specific activation kinetics of these two CaV1 isoforms (Nakai et al., 1994; Tuluc et al., 2016a). Furthermore, alternative splicing of exon 29 in VSD IV of CaV1.1 was shown to give rise to two channel variants with greatly different voltage dependence of activation and reduced current amplitude (Tuluc et al., 2009), identifying VSD IV as a rate-limiting factor for the voltage dependence of current activation. These experiments clearly demonstrated that, in CaV1.1, VSDs I and IV are necessary for the activation of L-type calcium currents but contribute differentially to the kinetics and voltage dependence of activation. Conversely, because activation of EC coupling is faster than current activation and occurs at physiological voltages at which the current is not activated, it can be reasoned that activation of the slow VSD I and the voltage-insensitive VSD IV of CaV1.1 are not necessary for activation of EC coupling (Tuluc and Flucher, 2011). This conclusion is further supported by a disease mutation in VSD I (R174W) of CaV1.1, which ablated L-type calcium current without affecting EC coupling (Eltit et al., 2012).
The recent voltage-clamp fluorometry study of CaV1.2 provided for the first time a direct analysis of the kinetics and voltage dependence of individual VSDs in calcium channels (Pantazis et al., 2014). This technique had been used previously and extensively to analyze VSD movement in KV and NaV channels (Priest and Bezanilla, 2015; Ahern et al., 2016). To allow fluorescent labeling of the VSDs of CaV1.2, a cysteine was introduced into the extracellular flank of the S4 transmembrane helix of each of the four VSDs, one at a time. After expression of these CaV1.2 constructs in Xenopus oocytes, the cysteine was labeled with a thiol-reactive fluorophore. Upon depolarization, the positively charged S4 helix moves across the membrane in an outward direction, and this structural rearrangement of the VSD results in an altered extent of quenching of the fluorophore. Thus, any change (up or down) in the fluorescence signal provides a readout of conformational changes in the respective VSD. The observed rapid response of the fluorescence signal indicated that the conformational changes indeed reflect the response of the individual VSD to the changed membrane potential. Although the fluorometry signal does not provide information about the nature and absolute magnitude of the structural rearrangement, the kinetics and voltage dependence of the individual VSD can be faithfully recorded in parallel to the voltage-clamp recording. Finally, the slope of the voltage sensitivity curves is a measure of the effective charge moved across the electric field. Changes thereof can arise either from altered length and slope of the S4 trajectory across the membrane or from changes in the electric field resulting from a different distribution of hydrophilic and hydrophobic regions within the channel (Priest and Bezanilla, 2015).
The interpretation of voltage-clamp fluorometry data regarding the effects of VSD movement on pore opening is greatly facilitated by mathematical gating models. In the earlier study, Olcese’s group examined two types of models based on different assumptions (Pantazis et al., 2014). The obligatory models assume that each VSD is either necessary or not for activation of pore opening. The allosteric model assumes that all VSDs contribute to pore opening to different degrees, and therefore this model provides quantitative information on the contribution of each VSD.
In the human CaV1.2, all VSDs moved in response to depolarization, excluding the possibility that one or more VSDs remain locked in the activated or inactivated state during depolarization. The kinetics of VSDs I, II, and III were faster or similar to that of current activation (Pantazis et al., 2014). However, VSD IV moved much slower, indicating that movement of this VSD is not required for channel opening. The voltage dependence of VSDs II, III, and IV was more negative than that of ion conductance, whereas the voltage dependence of VSD I coincided with that of ion conductance. This suggests that VSD I may be rate limiting for the voltage dependence of channel opening. The data were reasonably well fit by a gating model that assumes obligatory activation of only VSDs II and III for pore opening. The allosteric model indicated that VSDs II and III together contributed ∼85%, and VSD I ∼15%, to channel gating. Importantly, the model indicated virtually no contribution of VSD IV to pore opening. As this VSD moved at very low (left-shifted) depolarizations, but with kinetics much slower than channel activation, its movement is probably not coupled to gate opening. In analogy with the situation in NaV channels (Ahern et al., 2016), it is tempting to speculate that the movement of this VSD might initiate voltage-dependent inactivation of CaV1.2 channels.
These experiments described the properties of the complete CaV1.2 α1:α2δ-1:β3 channel complex expressed in Xenopus oocytes (Pantazis et al., 2014). In the present study, Savalli et al. (2016) examine the role of the α2δ-1 subunit to channel gating by expressing CaV1.2:β3 without α2δ-1. First of all, the authors find that the absence of α2δ-1 specifically slows down the kinetics of VSD I to about half of the activation speed of the complete channel complex, whereas the kinetics of the other VSDs remain unaltered. This effect is consistent with the primary function of α2δ-1 in regulating the activation kinetics of CaV1.2 and CaV1.1 channels when expressed in a native muscle expression system. In reconstituted dysgenic (CaV1.1-null) muscle cells, siRNA knockdown of α2δ-1 decelerated activation kinetics of CaV1.2 and accelerated activation kinetics of CaV1.1 (Obermair et al., 2005; Tuluc et al., 2007). Apparently, the α2δ-1 subunit stabilizes the specific intrinsic activation properties of calcium channels as it makes the slow channel (CaV1.1) slow and the fast channel (CaV1.2) fast. Importantly, the voltage-clamp fluorometry experiments demonstrate that regulation of activation kinetics by α2δ-1 is exclusively accomplished by VSD I (Savalli et al., 2016). This finding is consistent with the critical role of VSD I in regulating activation kinetics that has previously been demonstrated with CaV1.1/CaV1.2 channel chimeras (Nakai et al., 1994; Tuluc et al., 2016a). If, however, VSD I with the help of α2δ-1 serves the critical role of determining activation kinetics, its movement/activation must be obligatory for pore opening. Therefore, these findings contradict the obligatory model III of Pantazis et al. (2014), in which only VSDs II and III are obligatory for channel activation. If its movement is irrelevant for pore opening, VSD I could not limit the speed of activation. In contrast, even if—as the allosteric model indicated—the energetic contribution of VSD I to activation may be minor, its activation can still be obligatory, and even rate limiting, for the speed of pore opening. The observation that the voltage dependence of VSD I most closely resembles that of ion conduction further supports this notion.
The most striking effect of the α2δ-1 subunit on the biophysical properties of calcium currents is a 50-mV shift of the voltage dependence of activation (V1/2) to less depolarizing potentials (Savalli et al., 2016). In the voltage-clamp fluorometry experiments, the dramatic right shift in V1/2 of current activation in the absence of α2δ-1 is accompanied by smaller but still substantial right shifts of VSD I, II, and III voltage dependence (Savalli et al., 2016). Also, the slope of the voltage dependence curves for VSDs I, II, and III decrease in the absence of α2δ-1, indicating a decrease of the effective charge moved in each of these VSDs. This affects the energetic contribution of these VSDs to pore opening. According to the allosteric model, the presence of α2δ-1 doubles the energetic contributions of VSDs I and III and triples that of VSD II.
Together, these findings demonstrate that the α2δ-1 subunit exerts its effects on three of the four VSDs by facilitating their intrinsic functions. α2δ-1 normalizes the speed of activation by increasing the activation kinetics of the one VSD (I) that determines kinetics, and it normalizes the voltage dependence of channel activation by left shifting the voltage dependence and increasing the voltage sensitivity of the three VSDs that govern pore opening (I, II, and III). Apparently, the α2δ-1 subunit does not endow any of the VSDs with a particular function or property of its own, but the association of the α2δ-1 with CaV1.2 appears to stabilize the channel complex in a conformation that brings about the most accurate movement of the VSDs in response to depolarization and coupling to pore opening.
Interestingly, in their earlier study (Pantazis et al., 2014), members of the Olcese group found that VSD IV does not at all contribute to pore opening (see discussion above), and here (Savalli et al., 2016) they show that the absence or presence of α2δ-1 does not alter the properties of VSD IV. In agreement with a low-resolution structure of CaV1.2 (Walsh et al., 2009), the authors speculate that the largely extracellular α2δ-1 protein might interact with the extracellular domain of VSDs I, II, and III but not of VSD IV. Indeed, the recent high-resolution structure of CaV1.1 revealed that α2δ-1 interacts with the L5 loops of repeats I–III and the loop connecting S1 and S2 of VSD I (Wu et al., 2015). This specific interaction with VSD I again is consistent with the unique role of VSD I in determining activation kinetics (Nakai et al., 1994; Tuluc et al., 2016a), as well as with the physiological role of α2δ-1 in shaping the specific kinetic properties of CaV1.1 and CaV1.2 (Obermair et al., 2005; Tuluc et al., 2007). Surprisingly, the other interactions of α2δ-1 with VSDs I, II, and III are with the L5 loops next to the pore-forming segments of the channel. These interactions likely affect the pore directly and only secondarily the movement of the VSDs. This suggests that the coupling between the pore region and VSD I, II, and III is capable of transmitting α2δ-1 modulation in both directions. Although interactions of α2δ-1 with the L1-2 loop of VSD I affect the speed of pore opening, interactions with the L5 loop in the pore-forming segments of repeats I–III might affect the coupling with the respective VSDs and thus their voltage dependence. Knowing the putative interaction domains, this hypothesis can now be tested by combining site-directed mutagenesis with voltage-clamp fluorometry.
How can the negligible contribution to pore opening of VSD IV, and the lack of its modulation by α2δ-1 in CaV1.2 channels (Pantazis et al., 2014; Savalli et al., 2016), be reconciled with the critical importance of VSD IV in determining the voltage dependence of current activation and channel open probability found in CaV1.1 channels (Tuluc et al., 2009, 2016a,b)? The simplest explanation would be that the VSDs in the two L-type calcium channels serve fundamentally different roles in controlling channel gating. In light of the fact that the VSDs in the skeletal muscle isoform CaV1.1 serve the additional function of activating EC coupling, a different assignment of the VSDs to channel gating is plausible. If that is so, the nonhomologous roles of the four VSDs in CaV1.2 would be more like those in NaV channels than those in its closest relative, CaV1.1. Another explanation would be specific interactions of this VSD with associated proteins. In fact, the high-resolution structure of the skeletal muscle CaV1.1 complex demonstrated that the γ1 subunit interacts with the S3 segment of VSD IV (Wu et al., 2015). This interaction would occur in the skeletal muscle expression system but not with recombinant expression of CaV1.2 in Xenopus oocytes. Alternatively, it is possible that inclusion of exon 29 in the adult CaV1.1a splice variant not only shifts the voltage dependence of VSD IV activation, but at the same time changes its functional link to the channel pore. In that case, the developmental isoform CaV1.1e (lacking exon 29) might function like CaV1.2, where VSD IV moves at low voltages but remains idle with respect to pore opening. Its activation at very low voltages is required for pore opening but does not contribute any energy to it. In contrast, in the mature isoform CaV1.1a, inclusion of exon 29 might stabilize VSD IV in the closed position and thus put a brake on pore opening, unless it becomes activated by nonphysiologically strong depolarizations. Future studies will be necessary to solve this problem. The recent mutagenesis and voltage-clamp fluorometry studies will provide the tools to do so.
In conclusion, both mutagenesis and voltage-clamp fluorometry studies have demonstrated that the four VSDs of CaV1 channels are nonhomologous with respect to their biophysical properties and functions (Fig. 1). On the one hand, they display distinct voltage sensitivity and kinetics of activation; on the other hand, they appear to be differentially coupled to the channel pore and thus contribute different amounts of energy to pore opening. Only if activation of a given VSD is obligatory, or contributes significantly to pore opening, can its biophysical properties and modulation by α2δ-1 influence the macroscopic current properties. VSDs II and III show intermediate voltage dependence and make the major energetic contribution to pore opening. VSD I makes a small contribution to pore opening, but because of the relatively right-shifted V1/2, it can be rate limiting for current kinetics. Modulation of these three VSDs (I, II, and III) explains the described modulation of gating properties by the α2δ-1 subunit. VSD IV is the first to respond to membrane depolarization but appears to make no contribution to pore opening in CaV1.2. However, upon insertion of exon 29 in adult CaV1.1, the voltage dependence of activation of this channel is substantially right-shifted, indicating that VSD IV becomes rate limiting and can prevent pore opening. Whether these distinct properties and functions of the individual VSDs are specific for the respective channel isoforms, or represent a general pattern for all voltage-activated calcium channels, remains to be shown.
ACKNOWLEDGMENTS
The experimental work from the Flucher Lab discussed in this commentary has been supported by grants from the Austrian Science Fund (FWF): P23479, P27031, and W1101.
The author declares no competing financial interests.
Eduardo Ríos served as editor.
References
- Ahern C.A., Payandeh J., Bosmans F., and Chanda B.. 2016. The hitchhiker’s guide to the voltage-gated sodium channel galaxy. J. Gen. Physiol. 147:1–24. 10.1085/jgp.201511492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campiglio M., and Flucher B.E.. 2015. The role of auxiliary subunits for the functional diversity of voltage-gated calcium channels. J. Cell. Physiol. 230:2019–2031. 10.1002/jcp.24998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W.A. 2011. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 3:a003947 10.1101/cshperspect.a003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltit J.M., Bannister R.A., Moua O., Altamirano F., Hopkins P.M., Pessah I.N., Molinski T.F., López J.R., Beam K.G., and Allen P.D.. 2012. Malignant hyperthermia susceptibility arising from altered resting coupling between the skeletal muscle L-type Ca2+ channel and the type 1 ryanodine receptor. Proc. Natl. Acad. Sci. USA. 109:7923–7928. 10.1073/pnas.1119207109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Andrade A., and Allen S.E.. 2013. Alternative splicing: functional diversity among voltage-gated calcium channels and behavioral consequences. Biochim. Biophys. Acta. 1828:1522–1529. 10.1016/j.bbamem.2012.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Herrmann-Frank A., and Lüttgau H.-C.. 1995. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1241:59–116. 10.1016/0304-4157(94)00014-5 [DOI] [PubMed] [Google Scholar]
- Nakai J., Adams B.A., Imoto K., and Beam K.G.. 1994. Critical roles of the S3 segment and S3-S4 linker of repeat I in activation of L-type calcium channels. Proc. Natl. Acad. Sci. USA. 91:1014–1018. 10.1073/pnas.91.3.1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermair G.J., Kugler G., Baumgartner S., Tuluc P., Grabner M., and Flucher B.E.. 2005. The Ca2+ channel α2δ-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of α1S or excitation-contraction coupling. J. Biol. Chem. 280:2229–2237. 10.1074/jbc.M411501200 [DOI] [PubMed] [Google Scholar]
- Pantazis A., Savalli N., Sigg D., Neely A., and Olcese R.. 2014. Functional heterogeneity of the four voltage sensors of a human L-type calcium channel. Proc. Natl. Acad. Sci. USA. 111:18381–18386. 10.1073/pnas.1411127112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest M., and Bezanilla F.. 2015. Functional site-directed fluorometry. Adv. Exp. Med. Biol. 869:55–76. 10.1007/978-1-4939-2845-3_4 [DOI] [PubMed] [Google Scholar]
- Savalli N., Pantazis A., Sigg D., Weiss J.N., Neely A., and Olcese R.. 2016. The α2δ-1 subunit remodels CaV1.2 voltage sensors and allows Ca2+ influx at physiological membrane potentials. J. Gen. Physiol. 148 10.1085/jgp.201611586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc P., and Flucher B.E.. 2011. Divergent biophysical properties, gating mechanisms, and possible functions of the two skeletal muscle CaV1.1 calcium channel splice variants. J. Muscle Res. Cell Motil. 32:249–256. 10.1007/s10974-011-9270-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc P., Kern G., Obermair G.J., and Flucher B.E.. 2007. Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel α2δ-1 subunit in cardiac excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 104:11091–11096. 10.1073/pnas.0700577104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc P., Molenda N., Schlick B., Obermair G.J., Flucher B.E., and Jurkat-Rott K.. 2009. A CaV1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys. J. 96:35–44. 10.1016/j.bpj.2008.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc P., Benedetti B., Coste de Bagneaux P., Grabner M., and Flucher B.E.. 2016a Two distinct voltage-sensing domains control voltage sensitivity and kinetics of current activation in CaV1.1 calcium channels. J. Gen. Physiol. 147:437–449. 10.1085/jgp.201611568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc P., Yarov-Yarovoy V., Benedetti B., and Flucher B.E.. 2016b Molecular interactions in the voltage sensor controlling gating properties of CaV calcium channels. Structure. 24:261–271. 10.1016/j.str.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.P., Davies A., Nieto-Rostro M., Dolphin A.C., and Kitmitto A.. 2009. Labelling of the 3D structure of the cardiac L-type voltage-gated calcium channel. Channels (Austin). 3:387–392. 10.4161/chan.3.6.10225 [DOI] [PubMed] [Google Scholar]
- Wu J., Yan Z., Li Z., Yan C., Lu S., Dong M., and Yan N.. 2015. Structure of the voltage-gated calcium channel Cav1.1 complex. Science. 350:aad2395 10.1126/science.aad2395 [DOI] [PubMed] [Google Scholar]