Abstract
Pulmonary sarcoidosis is reported to have complication of lymphoproliferative disease such as malignant lymphoma, but the complication of multicentric Castleman's disease (MCD) is rarely reported. In our case of a 60‐year‐old woman, bilateral hilar lymphadenopathy was noted in her chest X‐ray. We performed a transbronchial lung biopsy. She was diagnosed as having pulmonary sarcoidosis (Stage II). The shadow on chest X‐ray disappeared without treatment. However, after 8 years, swelling of the mediastinal and abdominal lymph node, thickened bronchovascular bundle, and multiple nodular shadows were identified, and a thoracoscopic lung biopsy was performed. Based on the histopathological findings and elevated serum interleukin‐6 level (75.7 pg/mL), she was diagnosed with pulmonary sarcoidosis complicated by MCD. When a change in chest X‐ray findings are found during monitoring of pulmonary sarcoidosis, it is important to proceed with a thoracoscopic lung biopsy, because of the possibility of the rare complication of MCD.
Keywords: Interleukin‐6, multicentric Castleman's disease, sarcoidosis, thoracoscopic lung biopsy
Introduction
In pulmonary sarcoidosis, there is a possibility of complications with lymphoproliferative disease such as malignant lymphoma 1, but reports of multicentric Castleman's disease (MCD) are rare 2, 3. We experienced a case of MCD after recognizing a change in an X‐ray image shadow during the monitoring of a case of pulmonary sarcoidosis and had a thoracoscopic lung biopsy to diagnose this complication. We present our findings here, with references to the current literature.
Case Report
The subject was a 60‐year‐old female with no issues of concern regarding her medical, family, or smoking history. An abnormal shadow on her chest X‐ray was discovered during a health exam in November 2005, and she was referred to our hospital. Her serum angiotensin converting enzyme (ACE) value was 21.9 (6.3–21.4) mU/mL, and interleukin‐6 (IL‐6) value was 5.4 pg/mL (<6.0) at that time. On her chest computed tomography (CT) scan, she had swelling of the mediastinum and hilar lymph nodes and multiple nodular shadows predominantly on the bilateral upper lobes. A transbronchial lung biopsy was performed, and the patient was diagnosed with sarcoidosis (Stage II). The X‐ray shadow disappeared without treatment. In December 2013, worsening was noted in the patient's chest and abdominal CT images. She was referred to our hospital for detailed examination and was admitted. Her peripheral capillary oxygen saturation (SpO2) was 95% (breathing normal air). Her superficial lymph node was not palpated, and adventitious sound was not detected by stethoscopic examination. Blood sampling showed anemia, with a hemoglobin value of 8.4 (12.1–14.6) g/dL and albumin of 1.8 (3.8–5.3) g/dL, and a C‐reative protein value of 20.6 (<0.3) pg/mL, as well as normal values for ACE. A polyclonal increase was found, with an immunoglobulin (Ig)G of 4415 (800–1600) mg/dL, IgA of 681 (100–350) mg/dL, IgM of 584 (33–190) mg/dL, and IL‐6 of 75.7 (<6.0) pg/mL. In the chest X‐ray taken, bilateral ground glass opacity and infiltration shadow were found on inferior pulmonary area. In the CT, swelling of the mediastinal and abdominal lymph node, thickened bronchovascular bundle, and multiple nodular shadows were recognized (Fig. 1). Thoracoscopy lymph node and lung biopsy (segments S4 and S8) were performed. The lung tissue of right middle lobe and S8 revealed marked infiltration of lymphoplasmacytic infiltration to the interlobular septa and alveolar walls with fibrosis and lymphoid follicles (Fig. 2A,B). Infiltrating plasma cells indicated no monoclonality by immunostaining of k and λ (Fig. 2C,D). Epithelioid cell granulomas were also observed in the interlobular septa with venous involvement (Fig. 2E). Based on the histopathological findings and elevated serum IL‐6 level, the diagnosis was determined to be sarcoidosis with a complication of MCD. Treatment with prednisolone 25 mg/day was started, and when verifying that the patient's condition was not aggravated, the dose was reduced and the patient is currently receiving 10 mg/day.
Figure 1.
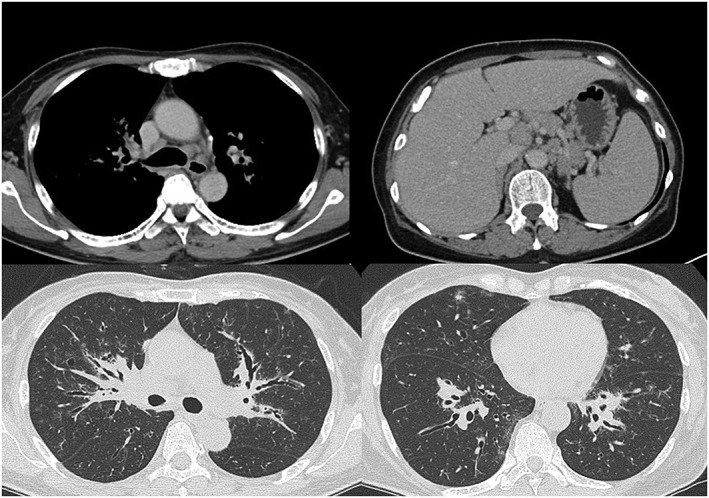
Chest and abdominal computed tomography on admission revealed swelling of the mediastinal and abdominal lymph node, thickened bronchovascular bundle, and multiple nodular shadows.
Figure 2.
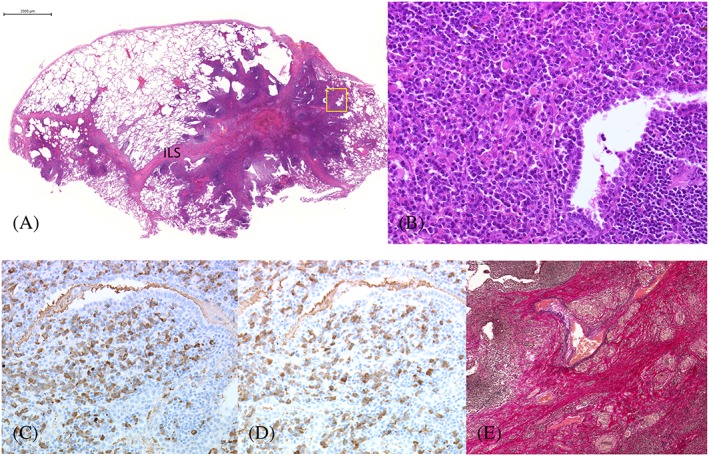
Histopathology of the right S4. (A) Panoramic view of the right S4. Marked lymphoplasmacytic infiltration in the interlobular septa and alveolar walls with fibrosis and lymphoid follicles. (Hematoxylin‐eosin, ×1, bar 2 mm). (B) Prominent infiltration of plasma cells in the alveoli with scattered Russell bodies. (Hematoxylin‐eosin, ×20) (square on A). (C) κ‐positive plasma cells. (D) λ‐positive plasma cells. (×20) There was no monoclonality. (E) Granulomatous involvement of interlobular vein with disruption of elastic fibers. (Elastic van Gieson, ×10).
Discussion
We identified a case of MCD while monitoring the progress of a patient with pulmonary sarcoidosis. The complication of pulmonary sarcoidosis and MCD is rare. Except for our patient, only two cases have been reported as complications or simultaneous occurrences of MCD with pulmonary sarcoidosis 2, 3. The relationship between these two diseases is not fully understood. Malignant lymphoma has been reported as a complication of pulmonary sarcoidosis 1. Brincker discussed that a complication of malignant lymphoma with pulmonary sarcoidosis is because of the immune‐induced inflammatory responses of sarcoidosis; there is a high possibility that abortive mitosis of the lymph node increases, lymph nodes deform, and get aggravated 1. In addition, the complication of MCD with malignant lymphoma has been reported 4, and genetic analysis of immunoglobulin suggests a monoclonal increase in B‐lymphocytes and suggests transferability to non‐Hodgkin's lymphoma. Although there is no clear relationship between pulmonary sarcoidosis and MCD, the complication of malignant lymphoma is found with both conditions. Therefore, it is important to collect additional case findings.
In our case, the X‐ray shadow naturally disappeared after the patient was diagnosed with sarcoidosis, but it was later aggravated. When it worsened, the X‐ray shadow pattern showed a thickened bronchovascular bundle, ground glass opacity, and an infiltrative shadow, which was significantly different from the shadow seen in the original diagnosis of pulmonary sarcoidosis (nodular shadows). Therefore, the possibility of the aggravation of sarcoidosis and differentiation from lymphoproliferative disease and carcinomatous lymphangiosis was considered necessary. Because it was necessary to consider complication with MCD, thoracoscopic mediastinal lymph node and lung biopsies were performed. These biopsy were useful for the diagnosis of MCD. It is necessary to have pathological diagnosis in order to have definite diagnosis of MCD. Eighty percent of MCD patients show swelling of superficial lymph nodes, and 40% of these patients display with a rash, and it is possible to diagnose MCD with tissue biopsy of the same area. However, our patient had no swelling of superficial lymph nodes or rash; in such cases, thoracoscopic lung biopsy is considered to be effective in diagnosis.
In this case, steroid therapy was initiated to treat the patient's MCD. Pulmonary sarcoidosis tends to react well to steroid treatment and immunosuppressant. On the other hand, the effect of corticosteroids and chemotherapy is often temporary for MCD, and the effectiveness of anti‐IL‐6 receptor antibody therapy has been reported 5. Based on the reasons earlier, it is important to differentiate the disease in order to initiate appropriate treatment.
In conclusion, it is important to proceed with testing via thoracoscopic lung biopsy to rule out the rare possibility of complication with MCD.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Sawata, T. , Bando, M. , Nakayama, M. , Mato, N. , Takemura, T. , and Sugiyama, Y. (2016) Multicentric Castleman's disease developing during follow‐up of sarcoidosis. Respirology Case Reports, 4 (4), e00168. doi: 10.1002/rcr2.168.
References
- 1. Brincker H, Wilbek E. 1974. The incidence of malignant tumors in patients with respiratory sarcoidosis. Br. J. Cancer 29:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rice BL, Farver CF, Pohlman B, et al. 2011. Concomitant Castleman's disease and sarcoidosis. Am. J. Med. Sci. 341:257–259. [DOI] [PubMed] [Google Scholar]
- 3. Awano N, Inomata M, Kondoh K, et al. 2012. Mixed‐type multicentric Castleman's disease developing during a 17‐year follow‐up of sarcoidosis. Intern. Med. 51:3061–3066. [DOI] [PubMed] [Google Scholar]
- 4. Frizzera G, Peterson BA, Bayrd ED, et al. 1985. A systemic lymphoproliferative disorder with morphologic features of Castleman's disease: clinical findings and clinicopathologic correlations in 15 patients. J. Clin. Oncol. 3:1202–1216. [DOI] [PubMed] [Google Scholar]
- 5. Nishimoto H, Kanakura Y, Aozasa K, et al. 2005. Humanized anti‐interleukin‐6 receptor antibody treatment of multicentric Castleman disease. Blood 106:2627. [DOI] [PubMed] [Google Scholar]


