Abstract
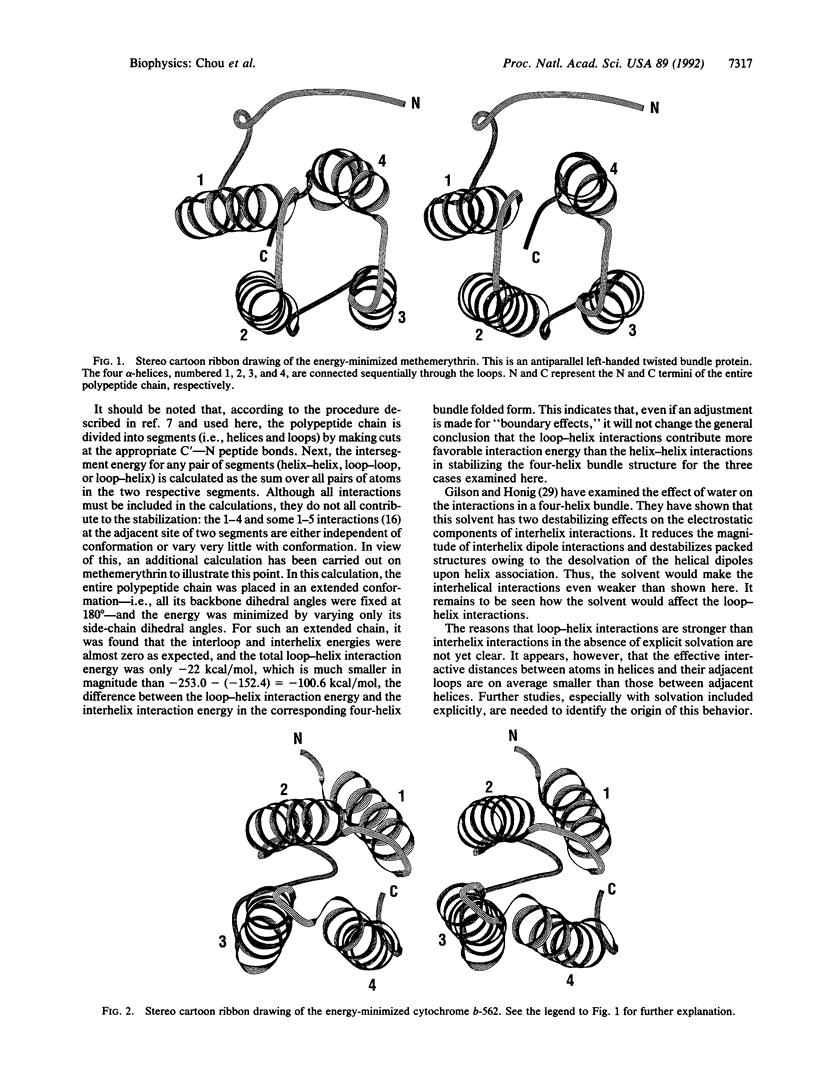
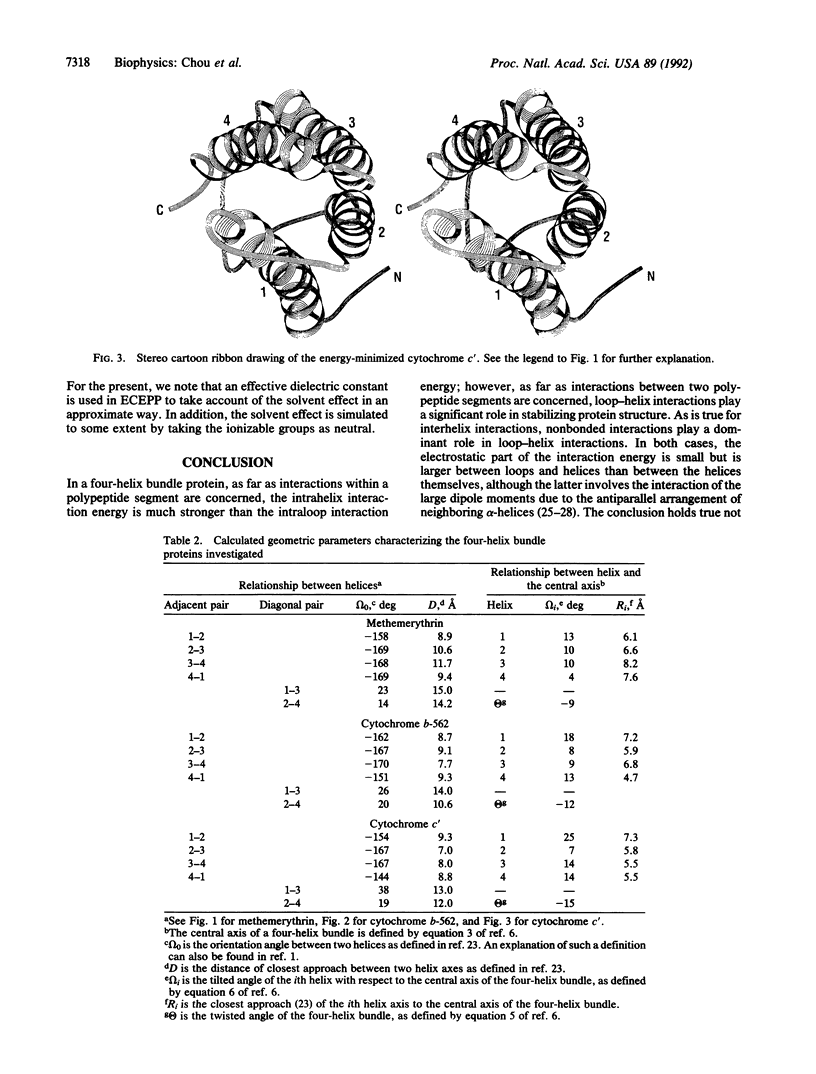
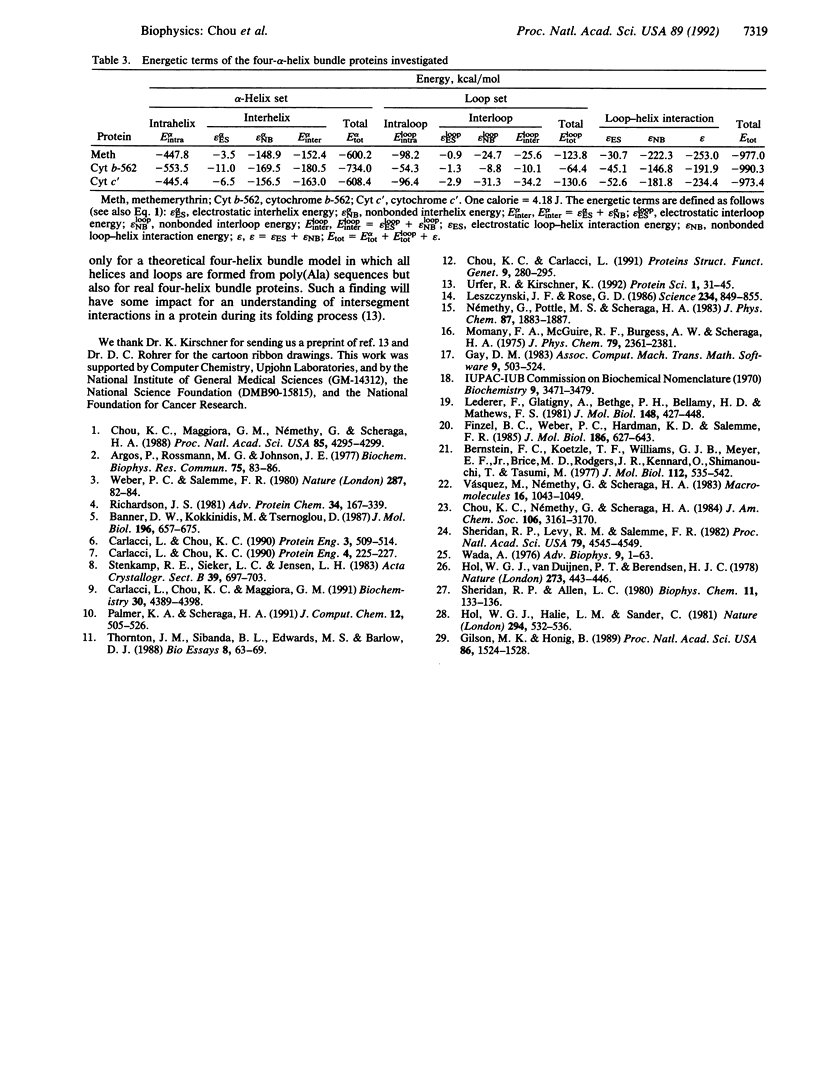
One of the critical issues regarding proteins with a four-helix bundle motif is which interactions play the major role in stabilizing this type of folded structure: the interaction among the four alpha-helices or the interaction between the loop and helix segments. To answer this question, an energetic analysis has been carried out for three proteins with a four-helix bundle--namely, methemerythrin, cytochrome b-562, and cytochrome c'. The structures on which the analysis has been made were derived from their respective crystallographic coordinates. All three proteins have long helices (16-26 residues) and most of their loops are short (3-5 residues). However, it was found in all three proteins that loop-helix interactions were stronger than helix-helix interactions. Moreover, not only the nonbonded component but also the electrostatic component of the interaction energy were dominated by loop-helix interactions rather than by interhelix interactions, although the latter involve favorable helix-dipole interactions due to the antiparallel arrangement of neighboring helices. The results of the energetic analysis indicate that the loop segments, whether they are in a theoretical model or in real proteins, play a significant role in stabilizing proteins with four-helix bundles.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Rossmann M. G., Johnson J. E. A four-helical super-secondary structure. Biochem Biophys Res Commun. 1977 Mar 7;75(1):83–86. doi: 10.1016/0006-291x(77)91292-x. [DOI] [PubMed] [Google Scholar]
- Banner D. W., Kokkinidis M., Tsernoglou D. Structure of the ColE1 rop protein at 1.7 A resolution. J Mol Biol. 1987 Aug 5;196(3):657–675. doi: 10.1016/0022-2836(87)90039-8. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Carlacci L., Chou K. C. Electrostatic interactions between loops and alpha-helices in four-helix bundle proteins. Protein Eng. 1990 Dec;4(2):225–227. doi: 10.1093/protein/4.2.225. [DOI] [PubMed] [Google Scholar]
- Carlacci L., Chou K. C. Energetic approach to the folding of four alpha-helices connected sequentially. Protein Eng. 1990 May;3(6):509–514. doi: 10.1093/protein/3.6.509. [DOI] [PubMed] [Google Scholar]
- Carlacci L., Chou K. C., Maggiora G. M. A heuristic approach to predicting the tertiary structure of bovine somatotropin. Biochemistry. 1991 May 7;30(18):4389–4398. doi: 10.1021/bi00232a004. [DOI] [PubMed] [Google Scholar]
- Chou K. C., Carlacci L. Energetic approach to the folding of alpha/beta barrels. Proteins. 1991;9(4):280–295. doi: 10.1002/prot.340090406. [DOI] [PubMed] [Google Scholar]
- Chou K. C., Maggiora G. M., Némethy G., Scheraga H. A. Energetics of the structure of the four-alpha-helix bundle in proteins. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4295–4299. doi: 10.1073/pnas.85.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzel B. C., Weber P. C., Hardman K. D., Salemme F. R. Structure of ferricytochrome c' from Rhodospirillum molischianum at 1.67 A resolution. J Mol Biol. 1985 Dec 5;186(3):627–643. doi: 10.1016/0022-2836(85)90135-4. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. Destabilization of an alpha-helix-bundle protein by helix dipoles. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1524–1528. doi: 10.1073/pnas.86.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol W. G., Halie L. M., Sander C. Dipoles of the alpha-helix and beta-sheet: their role in protein folding. Nature. 1981 Dec 10;294(5841):532–536. doi: 10.1038/294532a0. [DOI] [PubMed] [Google Scholar]
- Hol W. G., van Duijnen P. T., Berendsen H. J. The alpha-helix dipole and the properties of proteins. Nature. 1978 Jun 8;273(5662):443–446. doi: 10.1038/273443a0. [DOI] [PubMed] [Google Scholar]
- Lederer F., Glatigny A., Bethge P. H., Bellamy H. D., Matthew F. S. Improvement of the 2.5 A resolution model of cytochrome b562 by redetermining the primary structure and using molecular graphics. J Mol Biol. 1981 Jun 5;148(4):427–448. doi: 10.1016/0022-2836(81)90185-6. [DOI] [PubMed] [Google Scholar]
- Leszczynski J. F., Rose G. D. Loops in globular proteins: a novel category of secondary structure. Science. 1986 Nov 14;234(4778):849–855. doi: 10.1126/science.3775366. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Sheridan R. P., Allen L. C. The electrostatic potential of the alpha helix (electrostatic potential/alpha-helix/secondary structure/helix dipole). Biophys Chem. 1980 Apr;11(2):133–136. doi: 10.1016/0301-4622(80)80015-9. [DOI] [PubMed] [Google Scholar]
- Sheridan R. P., Levy R. M., Salemme F. R. alpha-Helix dipole model and electrostatic stabilization of 4-alpha-helical proteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4545–4549. doi: 10.1073/pnas.79.15.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J. M., Sibanda B. L., Edwards M. S., Barlow D. J. Analysis, design and modification of loop regions in proteins. Bioessays. 1988 Feb-Mar;8(2):63–69. doi: 10.1002/bies.950080205. [DOI] [PubMed] [Google Scholar]
- Urfer R., Kirschner K. The importance of surface loops for stabilizing an eightfold beta alpha barrel protein. Protein Sci. 1992 Jan;1(1):31–45. doi: 10.1002/pro.5560010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A. The alpha-helix as an electric macro-dipole. Adv Biophys. 1976:1–63. [PubMed] [Google Scholar]
- Weber P. C., Salemme F. R. Structural and functional diversity in 4-alpha-helical proteins. Nature. 1980 Sep 4;287(5777):82–84. doi: 10.1038/287082a0. [DOI] [PubMed] [Google Scholar]