Abstract
Recombinant (r) butyrylcholinesterase (rBChE) produced in CHO cells is being developed as a prophylactic countermeasure against neurotoxicity resulting from exposure to organophosphates (OPs) in the form of pesticides and nerve agents. To evaluate the efficacy of a parenteral pretreatment, a PEGylated macaque (Ma) form of rBChE was administered into homologous animals to ensure good plasma retention without immunogenicity. Thus, macaques were administered PEG-rMaBChE at either 5 or 7 mg/kg intravenously (i.v.) and exposed subcutaneously to 12 µg/kg of the potent pesticide paraoxon (Px) at 1 hr or at 1 and 72 hr respectively. Protection was measured by the ability of rBChE prophylaxis to prevent the inhibition of circulating acetylcholinesterase on red blood cells (RBC-AChE). In rBChE-pretreated animals, no inhibition of RBC-AChE activity after the first Px exposure and only a 10–20% reduction after the second exposure were observed as compared to a 75% RBC-AChE inhibition usually obtained without pretreatment. In addition, these studies raised other interesting issues. The lipophilic nature of Px, appears to result in early and transient inhibition of RBC-AChE as a result of transfer of OP bound to RBC even in BChE-pretreated animals. The protection by a single injection of rBChE against two administrations of Px represents the first example of protection by an i.v. rBChE pretreatment against a pesticide such as Px and bodes well for a parenteral rHuBChE pretreatment as an OP countermeasure in humans.
Keywords: butyrylcholinesterase, aerosol delivery, paraoxon, protection, monkey model
1. Introduction
Currently, plasma-derived human butyrylcholinesterase (HuBChE) is the most advanced candidate for a pre-exposure antidotal treatment for exposure to organophosphates (OPs) in the form of nerve agents and pesticides. By sequestering and neutralizing the OP toxicants in the blood, exogenously administered BChE can prevent the inhibition of acetylcholinesterase (AChE) on red blood cells (RBC) and in the CNS and any subsequent cholinergic crises [1–3]. Thus, the use of BChE as a bioscavenger will find application as a pretreatment for military, first responder or civilian personnel against deliberate or occupational exposure to nerve agents and pesticides, in addition to its use as a post-exposure treatment in cases of pesticide exposure, apnea and cocaine overdose [4, 5].
Due to the highly toxic nature of nerve agents and pesticides, which may cause impairment of critical tasks as well as death, testing of pretreatments in humans is precluded on ethical grounds and might require the Animal Rule (21CFR 601.90 for biological products) for regulatory approval. For this reason, the efficacy of protection by parenteral i.m. or i.v. administration of plasma–derived HuBChE has thus been studied against multiple LD50s of the nerve agents, in many animal models including rodents, minipigs, and monkeys [6–8]. However, since plasma–derived BChE is costly to purify and has limited availability, focus has switched to recombinant (r) forms of primate BChE molecules that have, to date, been successfully produced in mammalian cell lines, goat milk, and plants and have proven to be functionally equivalent to the native forms in terms of their reactivity with OPs [9–12]. It should be noted however, that without post-translational modification, clearance of rBChE from the circulation is very rapid following administration by parenteral injection; protection only being achieved when PEG-conjugation of the rBChE increases the molecular size, reduces renal clearance and permits increased plasma retention [9–13].
In addition to rHuBChE, macaque (Ma) BChE has also been produced in CHO cells and plants in order to specifically assess pharmacokinetics and efficacy in monkeys; the homologous system (rMaBChE into macaques) offering the advantage of permitting accurate pharmacokinetic (PK) and efficacy studies in the absence of any potential anti-BChE immune response. Thus i.v. injections of PEG-rMaBChE exhibit PK parameters similar to the macaque native forms with no immunogenicity even after three injections [9,14]; the 8–12 day half life being similar to that observed following transfusions of human plasma or daily administrations of partially purified HuBChE for several weeks into humans [15,16]. More recently, pretreatment with aerosolized (aer) forms of both CHO-derived rMaBChE and rHuBChE at 5–10 mg/kg, has been shown to protect against the aerosolized OP pesticide paraoxon (aer-Px), administered 1–40 hr later [17]. Importantly, this protection was demonstrated using unmodified forms of rBChE, which are sufficiently large to be retained in the lung without PEGylation.
The aim of the present study was to assess protection afforded by a single i.v. injection of PEG-rMaBChE, against one or two sc injections of Px given at one and 72 hr later, to further demonstrate the flexibility of a CHO-produced rBChE bioscavenger in preventing toxicity when administered by parenteral as well as the pulmonary routes. These studies permit analysis of inhibition, reactivation and protection following rBChE pretreatment in vivo and in doing so, may also provide data for model validation for pesticide and more potent neurotoxic nerve agent exposure in humans [18,19].
2. Materials and Methods
Animal studies were conducted in compliance with the Animal Welfare Act and other federal statutes and regulations stated in the Guide for the Care and Use of Laboratory Animals (NRC Publication, 1996). Procedures with animals received prior approval by Institutional Animal Care and Use Committees at Bioqual (macaque studies) and were performed at Bioqual, Rockville, MD, which are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
2.1. Chemicals
All chemicals were obtained from Sigma Aldrich, St Louis, MO). Paraoxon (99.1% pure) was obtained from ChemService Inc. (West Chester, PA), diluted in sterile water to obtain a stock solution of 1 mg/ml and maintained at RT protected from light. Inoculation doses were prepared in 1 ml PBS pH 6.8, 12–24 hr prior to use. Handling of Px and loading of syringes were performed in a biological safety cabinet. The Px residues were disposed into a container with 0.1N NaOH and autoclaved prior to elimination.
2.2. Production of PEG-rMaBChE
Recombinant tetrameric MaBChE proteins were expressed using CHO-K1 cells, purified using procainamide-Sepharose (Sigma Aldrich, St Louis, MO) affinity chromatography and conjugated with polyethylenelglycol (PEG) as previously described [10]. The most efficient lines producing tetrameric rMaBChE (clone T58, >25 U/ml) were used in these studies; the specific activity of MaBChE being 900 U/mg. The catalytic parameters for the purified CHO-derived PEG-rMaBChE have been shown to be similar to native MaBChE and HuBChE (unpub. obs).
2.3. AChE and BChE activity assay
Assays for plasma BChE and RBC-AChE activity were performed as described previously [17] using a modified Ellman assay [20]. Briefly, whole blood samples (20 µl) from macaques were diluted in 180 µl of water and tested for BChE and AChE activity using 1mM butyrylthiocholine and 1 mM acetylthiocholine as substrates, respectively. In the AChE assay, 20 µM ethopropazine was used as a BChE-specific inhibitor. AChE and BChE activities were measured by monitoring the change in absorbance of 5,5-dithiobis 2-nitrobenzoic acid at 412 nm for 5 min at 22 °C. One unit (U) of enzyme activity is defined as the amount that hydrolyzes one µmol of substrate in one min. Assays were performed in triplicate and repeated three times. Background levels in macaques were 3–5 U/ml for AChE and 5–6 U/ml for BChE. The inhibition constant for the classical active-site inhibitor ethopropazine (39 ± 25 nM) was similar to that for the native enzyme (48 ± 34 nM).
2.4. Efficacy of PEG-rMaBChE in macaques
These studies were performed at Bioqual, Rockville, MD. To assess efficacy, eight 3–5 kg Chinese rhesus macaques were used in two studies. In the first study, four macaques were injected i.v. with 7 mg/kg of PEG-rMaBChE one hr prior to a subcutaneous (sc) dose of 12 µg/kg of Px. In the second study, four macaques were pretreated with 5 mg/kg of PEG-rMaBChE and exposed twice to 12 µg/kg of Px one and 72 hr later. This dose of Px is known to result in ~75% inhibition of RBC-AChE and ~70% inhibition of plasma BChE 1–2 hr later. Blood was drawn before enzyme administration and at 0.5, 1, 2, 4, 8, 24, 48, 72 hr after Px administration, and diluted blood samples were kept at 4°C until they were assayed for AChE and BChE activity.
The BChE activity levels (mean ± SEM) in the four 5 mg/kg PEG-rMaBChE-pretreated macaques following Px administration were compared to the pharmacokinetic clearance profiles of PEG-rMaBChE in 8 naïve macaques (mean+/−SEM) previously reported [9,12].
2.5. In vitro assays to demonstrate the binding of Px to macaque RBC
One ml of blood (prebleed-PB) was obtained from a macaque and diluted in 19 ml of water to produce control lysed RBC designated as PB-RBC. This macaque was given a s.c. injection of Px (12 µg/kg), blood was drawn at 0.5, 1, 2, 4, 8, and 48 hrs, and 40 µl of blood was diluted 1/10 either in water (lysed Px-RBC) or in PBS (intact Px-RBC). The Px-RBC were washed three times with PBS to eliminate serum proteins. To perform mixing studies, equal volumes of PB-RBC were mixed with either lysed Px-RBC or intact Px-RBC from each time point. The PB-RBC, lysed Px-RBC and the intact Px-RBC were assayed for AChE and BChE activity either separately or combined in mixtures. In one experiment, Px-RBC were pre-incubated with or without 1.8 ng/µl organophosphorus hydrolase (OPH) for 10 min before mixing with the PB-RBC. The E. coli-derived OPH was a kind gift from Dr. Tony Reeves (Southwest Research Institute, TX).
3. Results
3.1. Inhibition of RBC-AChE and plasma BChE by a subcutaneous dose of Px
To assess protection, Px was administered at 12 µg/kg which, in the absence of a BChE pretreatment, resulted in ~75% inhibition of RBC-AChE and ~70% inhibition of plasma BChE without clinical toxicity; peak inhibition occurring at 1–2 hr and returning to background levels at 120–150 hr post exposure (inset Fig.1).
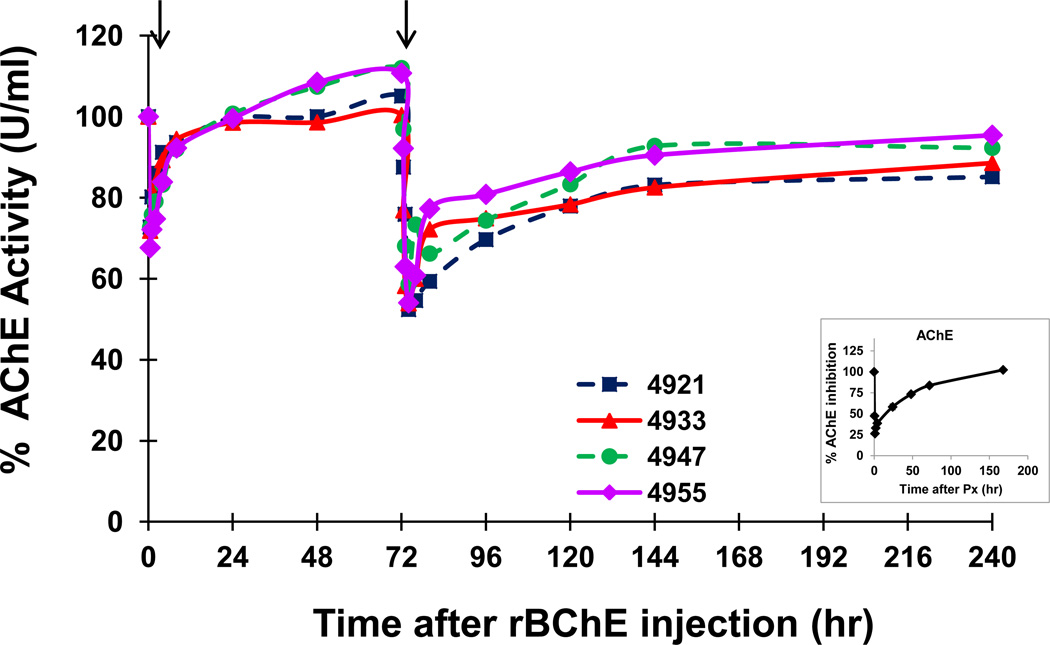
Figure 1.
Protection by a 5 mg/kg pretreatment of PEG-rMaBChE against two s.c. injections of Px (12 µg/kg) given at 1 h and 72 hr post pretreatment. Results are presented as percent RBC-AChE activity. Individual monkey numbers are indicated. The inset indicates average RBC-AChE inhibition following s.c. administered Px in two control macaques that did not receive PEG-rMaBChE pretreatment.
3.2. Ability of a single administration of PEG-rMaBChE to protect macaques against one or two subcutaneous injections of Px
In an initial protection study, 4 macaques were pretreated i.v. with 7 mg/kg of PEG-rMaBChE 1 hr prior to a single s.c. exposure with 12 µg/kg of Px; inhibition of RBC-AChE and plasma BChE activity being monitored at various times before and after Px exposure and percent inhibition calculated. In this study, after an initial rapid and transient inhibition (<20%) lasting only a few hrs, all macaques maintained control levels of RBC-AChE (~100% activity) with levels in two of the animals actually increasing to >120% by 7 days post-Px. As expected, the rapid sequestration of the Px by the injected BChE led to the rapid reduction of BChE levels of 25–30 U; remaining stable for 48–72 hr before declining further (not shown).
To confirm and expand on these results, an additional 4 macaques were pretreated with a 5 mg/kg dose of PEG-rMaBChE and exposed twice to 12 µg/kg Px injected s.c. at one and 72 hr post pretreatment. Since good protection was observed using a 7 mg/kg BChE pretreatment in the previous study, the dose was reduced to 5 mg/kg in this study. As shown in Fig. 1, the results were essentially the same as the first experiment. Thus, following the first Px injection, RBC-AChE activity in each individual monkey was transiently reduced and then rapidly normalized to ~100% activity levels indicating full protection; levels rebounding to higher than 100% activity by 48 hr in two macaques. Immediately after the second Px injection at 72 hr, a small transient reduction in RBC-AChE again occurred after which the activity returned to 80–90% of the initial pretreatment levels. The monkeys that exhibited the highest activity after the first exposure, similarly exhibited the highest levels after the second injection. The inset (Fig. 1) shows the RBC-AChE inhibition following s.c. injection of a single dose of Px in the absence of rBChE pretreatment.
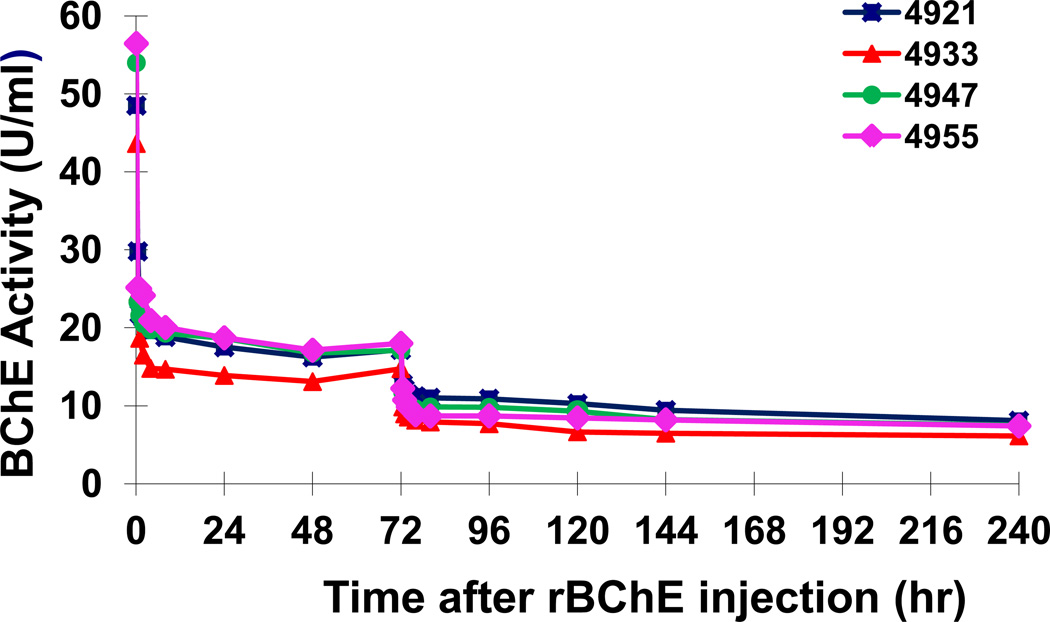
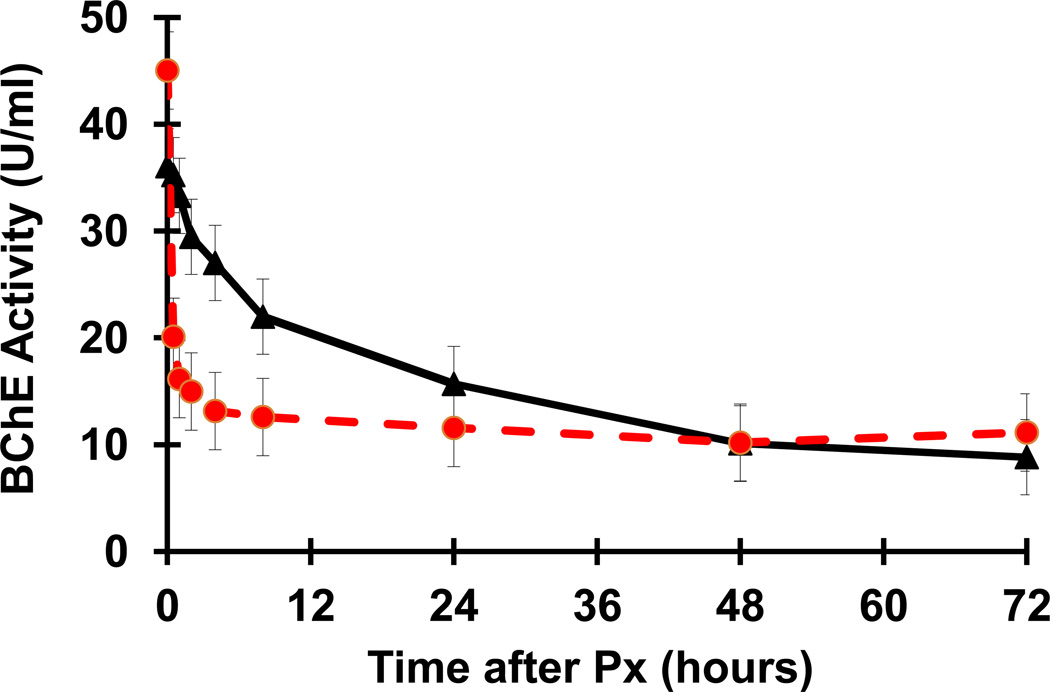
As expected, protection of RBC-AChE in circulation following the first injection of Px occurred concomitantly with a sharp decline in the activity of the exogenously administered PEG-rMaBChE from 45 ± 2.67 U/ml to 15 ± 1.4 U/ml by two hr, which remained stable until the second Px injection at 72 hr (Fig. 2). One macaque #4933 consistently exhibited lower levels than the other 3 animals in the group. It should be noted that these levels of BChE activity (mean +/− SEM) following Px in the four pretreated macaques were different from the normal plasma clearance profiles observed previously following i.v. injection of ~3 mg/kg PEG-rBChE in naïve macaques (n=8) [9,12](Fig. 3). After the second Px exposure, BChE levels only dropped from 11.14 ± 0.49 U/ml to 5 ± 0.49 U/ml despite the presence of ~17 U/ml BChE (made up of the remaining 11.14 U/ml of exogenous PEG-rMaBChE plus 5.62 U/ml of the endogenous MaBChE). This was surprising since it was anticipated that inhibition of RBC-AChE after the second injection would not have been observed until all the circulating BChE had been sequestered by Px.
Figure 2.
Plasma BChE activity in macaques pretreated with 5 mg/kg PEG-rMaBChE prior to two s.c. injections of Px (12 µg/kg) given 1 h and 72 hr post pretreatment. Endogenous BChE background levels were not subtracted.
Figure 3.
Comparison of the plasma BChE levels in PEG-rMaBChE-pretreated (5 mg/kg) macaque plasma following Px exposure (12 µg/kg s.c.)(n=4, dashed) with the pharmacokinetic clearance profiles of i.v. injected PEG-rMaBChE (~3mg/kg) of in naïve macaques (n=8) [10,13]. Data are presented as mean ± SEM.
3.3. Rapid transient inhibition of RBC-AChE and plasma BChE following Px injection
In the first hrs following either a s.c. injection of Px in PEG-rMaBChE-pretreated macaques (Fig 1) or the administration of aer-Px into aer-rBChE pretreated macaques [17], an unexpected transient inhibition of both RBC-AChE and plasma BChE was observed immediately after Px exposure even in protected monkeys. In the aer-Px studies, this early in vivo inhibition appeared to be dependent on the dose of aer-rBChE used and was inversely related to the level of protection, suggesting a role for residual Px in the circulation. Since OP pesticides are known to be lipophilic, the possibility existed that during dilution of blood samples for ChE assay, Px bound to RBC in vivo was potentially transferred to AChE molecules on RBC that had not been inactivated by Px.
To ascertain this, mixing experiments were performed in vitro, in which normal control PB-RBC were assayed for RBC-AChE and BChE activity alone or after the addition of either lysed Px-RBC or intact Px-RBC, which were obtained from macaques at 0.5, 1, 2, 4 and 8 hr after a s.c. injection of 12 µg/kg of Px. Results shown in Table 1 clearly indicate that the observed combined AChE activity of mixtures on the control and Px-exposed RBC (both lysed RBC and intact washed RBC) was ~50% lower than the predicted activity based on the sum of each alone and was consistent with the presence of Px in the samples (Table 1). To confirm this, RBC samples from the Px-injected macaques were first incubated with OPH [21] before mixing with the control samples and assaying for AChE activity. OPH is known to hydrolyze Px and would prevent the transfer and inhibition of AChE in this assay. In this experiment, the ~30% inhibition observed with both the Px-exposed RBC ghosts (Table 2) and intact cells (not shown) was prevented in the presence of OPH, with AChE activity in the mixtures increasing from ~70% (without OPH) to 100% (with OPH).
Table 1.
The transfer of lipophilic Px on RBC from Px-exposed macaques can inhibit AChE activity on control RBC in vitro.
| PB- RBC (U/ml) |
Px- RBC (U/ml). |
Hr post Px |
PB-RBC + lysed Px RBC (U/ml) |
*AChE activity (%) |
PB-RBC + intact Px- RBC (U/ml) |
*AChE activity (%) |
||
|---|---|---|---|---|---|---|---|---|
| Predicted | Observed | Predicted | Observed | |||||
| 4.21 | - | 0 | ||||||
| 1.29 | 0.5 | 5.50 | 2.51 | 46% | 5.50 | 3.03 | 55% | |
| 0.64 | 1 | 4.85 | 2.32 | 50% | 4.65 | 2.41 | 52% | |
| 1.09 | 2 | 5.30 | 2.78 | 52% | 5.30 | 2.33 | 44% | |
| 1.61 | 4 | 5.82 | 2.82 | 48% | 5.82 | 2.48 | 43% | |
| 1.88 | 8 | 6.09 | 3.22 | 53% | 6.09 | 3.00 | 49% | |
Percent activity was calculated by observed AChE activity /predicted AChE activity.
Table 2.
Preincubation of Px-exposed RBC with OPH prevents the inhibition of control RBCAChE activity.
| PB-RBC (U/ml) |
Px-RBC (U/ml). |
Hr post Px |
Predicted# | Observed# No OPH |
AChE activity (%)* |
Observed# Plus OPH |
AChE Activity (%)* |
|---|---|---|---|---|---|---|---|
| 3.718 | |||||||
| 1.026 | 0.5 | 4.744 | 3.405 | 72% | 5.069 | 106.8% | |
| 0.806 | 1 | 4.524 | 3.027 | 67% | 4.676 | 103.4% | |
| 1.024 | 2 | 4.724 | 3.315 | 70% | 4.826 | 102.6% | |
| 1.216 | 4 | 4.934 | 3.833 | 78% | 5.257 | 106.5% | |
| 1.579 | 8 | 5.297 | 4.215 | 80% | 5.714 | 107.9% | |
| 2.135 | 48 | 5.853 | 4.723 | 81% | 5.935 | 101.4% |
Predicted and observed values are given as U/ml.
Percent activity was calculated by observed
AChE activity /predicted AChE activity.
The presence of Px on RBCs demonstrated in the in vitro assays was also indicated by similar AChE activity profiles in vivo following a s.c. injection of Px (Fig. 4, lower line) and in vitro mixing experiments (Fig. 4, middle line) in contrast to the lack of suppression observed when the RBC were pre-incubated with OPH prior to assay (Fig. 4, upper line). Although the early inhibition observed following administration of Px both by s.c. injection or by aerosol is not well understood, it is clear that the lipophilicity of Px and its transfer from RBC to AChE is partly responsible for this observation. Similar results were observed in studies using BChE (not shown).
Fig. 4.
Inhibition of control (PB) RBC-AChE activity in in vitro assays by the addition of lysed or intact Px-exposed-RBC collected at different times after Px exposure of macaques (middle line) is very similar to the RBC-AChE inhibition profiles observed in vivo in macaques injected with Px (lower line). Preincubation with OPH eliminates RBC-AChE inhibition (upper line).
4. Discussion
The results of this study demonstrate that a PEG-conjugated form of rMaBChE can protect against one or two s.c. injections of Px when the pretreatment is delivered parenterally by i.v. injection; similar to the protection afforded by the aer-rBChE form previously reported [17]. Thus, except for the transient inhibition of RBC-AChE and plasma BChE observed immediately after Px injection, monkeys were totally protected (no AChE inhibition) by both 5 and 7 mg/kg of PEG-rMaBChE against a 12 µg/kg dose of Px given one hr later, that without pretreatment, would normally lead to a ~75% inhibition of RBC-AChE. Following a second Px dose at 72 hr, macaques showed no signs of toxicity with only a 10–20% decrease in pre-exposure RBC-AChE activity.
Px is an active metabolite of the inactive pesticide parathion, which has been responsible for many accidental poisonings and deaths and was recently phased out of use in the US. It was chosen for these studies because it inhibits AChE, BChE and carboxylesterase [22], has a relatively low LD50, is stabile in aqueous solution and has proven to be a useful surrogate for the modeling of nerve agent exposure. Although the LD50 of Px administered s.c. in macaques is not established, the inhibition of AChE observed with a 12 µg/kg Px dose used in these studies is consistent with previous findings in rodents and primates. For example in rats, a s.c. dose of 190 µg/kg Px (0.83 × LD50) produced no signs of cholinergic hyperactivity and < 20% inactivation of AChE in the brain or diaphragm [23]; 1 × LD50 of Px was reported in the range of 230–430 µg/kg in rats following s.c. injection [24]. By comparison, 15 µg/kg of aer-Px resulted in 63% inhibition of RBC-AChE and plasma BChE activity in macaques [17]. In other primate studies, exposure of baboons to 13.4 µg/kg soman and 30 µg/kg sarin vapors (= 2 × LD50) resulted in 93% and 91% decreases in peak RBC-AChE activity, respectively [25], while ~4 ug/kg of inhaled sarin caused a 50% inhibition of RBC-AChE in humans [26]. Overall, these findings confirm those of Maxwell et al. [27] who showed that a ~10-fold lower dose of OP elicited the same inhibition in primates as compared to rodents; the resistance in the latter being due to the presence of carboxylesterase.
The transfer of the highly lipophilic Px on RBC from macaques, exposed by either s.c. or pulmonary routes, appears to play a role in the early and transient inhibition of AChE observed in vivo, even in protected animals [17], which is supported by the inhibition of RBC-AChE observed in the in vitro mixing experiments. Thus, it should be noted that, the observed transient inhibition of AChE and BChE activity appears to be an artifact of the ChE assay and may not occur in vivo. Since the inhibition of AChE and BChE was demonstrated in the in vitro mixing studies using intact Px-exposed-RBC, which had been washed three times, it is unlikely that the Px was transferred from serum proteins such as albumin.
The proportion of OP-inhibited cholinesterases (ChEs) in peripheral circulation is always a result of the balance between the amount of available OP in circulation, the inhibition rate constant (ki) for the specific OP involved, the spontaneous reactivation rate of OP-inhibited ChE and the final “aging” of OP-inhibited ChE. This is all predicated on the inherent level of ChE in circulation. In the current study, PEG-rMaBChE afforded protection of RBC-AChE by providing additional esterase binding sites to absorb circulating OP, conserving available circulating background AChE levels on RBC and the critical target organs of the AChE enzyme.
In spite of this straightforward concept of protection of AChE by a PEG-rMaBChE bioscavenger, the results raised interesting questions. Following the first injection of Px, 25–30 U/ml of rMaBChE were consumed by 12 µg/kg Px (~0.8 × LD50); a decrease of 30 U/ml equivalent to 0.51 nmol. This is remarkably similar to that observed in minipigs where 200 U/ml (3.43 nmol) of native HuBChE was required to neutralize 5–5.5 × LD50 of soman (equivalent to 0.54 nmol / 0.8 × LD50)[8]. However, based on the BChE levels of ~17 U/ml at 72 hr, comprising ~11 U/ml of residual exogenous BChE plus ~6 U/ml of the endogenous BChE, there appeared to be sufficient BChE to neutralize most of the second dose of Px administered at that time. This is consistent with MaBChE functioning as a stoichiometric bioscavenger of Px, since the amount of rMaBChE used was 5 mg/kg, which is equivalent to 77 nmol/kg (MaBChE: 900U = 1 mg = 15.3 nmol) and the dose of Px of 12 µg/kg corresponds to 41 nmol/kg. It was therefore surprising that AChE levels did not return closer to 100% activity and circulating BChE levels were not more depleted. In the assumption of complete mixing of OP within the peripheral circulation upon absorption into this compartment, AChE and BChE would have equal access to being inhibited by the OP. The comparative percentage of inhibition of each ChE will then be initially driven by the respective ki for each enzyme. In this context, the ki for Px is 2–3 times higher for rHuBChE than for rHuAChE [28, 29], suggesting that BChE would be more efficient than AChE at removing OP from the general circulation.
The post-exposure “rebounds” of AChE and BChE activity shown in Fig. 2 were previously observed in blood of minipigs and mice as well as guinea pig bronchoalveolar fluid (BAL) fluid [30–32]. For example, in a study of VX-induced pulmonary edema in swine, rapid increases (10–15 min post-exposure) in plasma BChE activity were observed following each VX i.v. infusion [30] while a rapid recovery of BChE by 24 hr reaching 130% by 72 hr was observed in aer-PEG-rMaBChE-pretreated macaques following aer-Px (Y.R. unpub. obs). In the present study, AChE levels were observed to rebound above the 100% activity baseline in two aer-PEG-rMaBChE-pretreated monkeys post Px (#4947, #4955 in Fig.1). In mice, AChE levels reached 160% of pre-exposure levels 3 days after administration of aer-Px (Y.R., unpub. obs), whilst Jiang et. al. have shown a >200% increase in AChE activity by 4 days in spleens and lungs of mice treated with 1,500 mg/kg of the OP tri-o-cresyl phosphate; the rebound thought to correlate with cell apoptosis [31]. Whatever the mechanism, these findings indicate that while spontaneous reactivation may return some of the ChE activity, a second process appears to occur that causes the rebound to exceed the initial pre-exposure levels. Since new synthesis can be ruled out in many cases, some possibilities include migration of RBC from organs into the blood in the case of RBC-bound AChE in primates (which lack soluble AChE), release of AChE from damaged cells and the presence of a “sink” or tissue reservoir of BChE. Although speculative, rapid antibody-independent clearance (MRT ~50 hr) in 2/4 macaques following a i.v. injection of a low dose (3,000U =~1.3 mg/kg) of native MaBChE [14] is compatible with the replenishment of a reservoir from the exogenous enzyme injected into blood.
Recombinant HuBChE bioscavenger produced in CHO cells
First demonstration of protection by an i.v. rBChE pretreatment against the OP pesticide paraoxon
Protection measured by lack of inhibition of RBC-AChE and plasma BChE in macaques
Transfer of Px on RBC from monkeys may cause inhibition RBC-AChE and plasma BChE in in vitro assays
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak d, Shafferman A, Silman I, Sussman JL. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999;38:7032–7039. doi: 10.1021/bi982678l. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberry TL, Mallender WD, Thomas PJ, Szegletes T. A steric blockade model for inhibition of acetylcholinesterase by peripheral site ligands and substrate. Chem Biol Interact. 1999;119–120:85–97. doi: 10.1016/s0009-2797(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 3.Taylor P. Anti cholinesterase Agents. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics / Edition 12. New York: McGraw-Hill Publishing Company; 2010. pp. 239–254. [Google Scholar]
- 4.Guven M, Sungur M, Eser B, Sari I, Altuntas F. The effects of fresh frozen plasma on cholinesterase levels and outcomes in patients with organophosphate poisoning. J Toxicol Clin Toxicol. 2004;42:617–623. doi: 10.1081/clt-200026967. [DOI] [PubMed] [Google Scholar]
- 5.Ashani Y. Prospective of human butyrylcholinesterase as a detoxifying antidote and potential regulator of controlled-release drugs. Drug Development Research. 2000;50:298–308. [Google Scholar]
- 6.Lenz DE, Maxwell DM, Koplovitz I, Clark CR, Capacio BR, Cerasoli DM, Federko JM, Luo C, Saxena A, Doctor BP, Olson C. Protection against soman or VX poisoning by human butyrylcholinesterase in guinea pigs and cynomolgus monkeys. Chem Biol Interact. 2005;157:205–210. doi: 10.1016/j.cbi.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Saxena A, Sun W, Fedorko JM, Koplovitz I, Doctor BP. Prophylaxis with human serum butyrylcholinesterase protects guinea pigs exposed to multiple lethal doses of soman or VX. Biochem Pharmacol. 2011;81:164–169. doi: 10.1016/j.bcp.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Saxena A, Sun W, Dabisch PA, Hulet SW, Hastings NB, Jakubowski EM, Mioduszewski RJ, Doctor BP. Pretreatment with human serum butyrylcholinesterase alone prevents cardiac abnormalities, seizures, and death in Gottingen minipigs exposed to sarin vapor. Biochem Pharmacol. 2011;82:1984–1993. doi: 10.1016/j.bcp.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg YJ, Saxena A, Sun W, Jiang XM, Chilukuri N, Luo C, Doctor BP, Lee KD. Demonstration of in vivo stability and lack of immunogenicity of a polyethyleneglycol-conjugated recombinant CHO-derived butyrylcholinesterase bioscavenger using a homologous macaque model. Chem Biol Interact. 2010;187:279–286. doi: 10.1016/j.cbi.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Huang YJ, Huang Y, Baldassarre H, Wang B, Lazaris A, Leduc M, Bilodeau AS, Bellemare A, Cote M, Herskovits P, Touati M, Turcotte C, Valeanu L, Lemee N, Wilgus H, Begin I, Bhatia B, Rao K, Neveu N, Brochu E, Pierson J, Hockley DK, Cerasoli DM, Lenz DE, Karatzas CN, Langermann S. Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc Natl Acad Sci USA. 2007;104:13603–13608. doi: 10.1073/pnas.0702756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer BC, Kannan L, Garnaud PE, Broomfield CA, Cadieux CL, Cherni I, Hodgins SM, Kasten SA, Kelley K, Kilbourne J, Oliver ZP, Otto TC, Puffenberger I, Reeves TE, Robbins N, 2nd, Woods RR, Soreq H, Lenz DE, Cerasoli DM, Mor TS. Plant-derived human butyrylcholinesterase, but not an organophosphorous-compound hydrolyzing variant thereof, protects rodents against nerve agents. Proc Natl Acad Sci USA. 2010;107:20251–20256. doi: 10.1073/pnas.1009021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg YJ, Jiang XM, Mao LJ, Hernandez-Abanto S, Lee KD. Development of a prophylactic butyrylcholinesterase bioscavenger to protect against insecticide toxicity using a homologous macaque model. Insecticides Basic and Others Applications: InTech Press. 2012:79–100. [Google Scholar]
- 13.Chilukuri N, Parikh K, Sun W, Naik R, Tipparaju P, Doctor BP, Saxena A. Polyethylene glycosylation prolongs the circulatory stability of recombinant human butyrylcholinesterase. Chem Biol Interact. 2005;157–158:115–121. doi: 10.1016/j.cbi.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg YJ, Luo C, Ashani Y, Doctor BP, Fischer R, Wolfe G, Saxena A. Pharmacokinetics and immunologic consequences of exposing macaques to purified homologous butyrylcholinesterase. Life Sciences. 2002;72:125–134. doi: 10.1016/s0024-3205(02)02203-8. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins T, Balinsky D, Patient DW. Cholinesterase in plasma: first reported absence in the Bantu; half-life determination. Science. 1967;156:1748–1749. doi: 10.1126/science.156.3783.1748. [DOI] [PubMed] [Google Scholar]
- 16.Cascio C, Comite C, Ghiara M, Lanza G, Ponchione A. Use of serum cholinesterases in severe organophosphorus poisoning. Our experience. Minerva Anestesiol. 1988;54:337–338. [PubMed] [Google Scholar]
- 17.Rosenberg YJ, Laube B, Mao LJ, Jiang XM, Hernandez-Abanto S, Lee KD, Adams R. Pulmonary delivery of an aerosolized recombinant human butyrylcholinesterase pretreatment protects against aerosolized paraoxon in macaques. Chem Biol Interact. 2013;203:167–171. doi: 10.1016/j.cbi.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gearhart JM, Jepson GW, Clewell HJ, Andersen ME, Conolly RB. Physiologically based pharmacokinetic model for the inhibition of acetylcholinesterase by organophosphate esters. Environmental health perspectives. 1994;102(Suppl. 11):51–60. doi: 10.1289/ehp.94102s1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poet TS, Kousba AA, Dennison SL, Timchalk C. Physiologically based pharmacokinetic/ pharmacodynamic model for the organophosphorus pesticide diazinon. Neurotoxicology. 2004;25:1013–1030. doi: 10.1016/j.neuro.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 21.Wales ME, Reeves TE. Organophosphorus hydrolase as an in vivo catalytic nerve agent bioscavenger. Drug Test Anal. 2012;4(3–4):271–281. doi: 10.1002/dta.381. [DOI] [PubMed] [Google Scholar]
- 22.Levine ES. Nerve Agent Simulants: Can they be used as substitutes for nerve agents in biomedical research? Prepared for the U.S. Army Medical Research Institute of Chemical Defense under. 2006 Contract No. GS-23F-8006H. [Google Scholar]
- 23.Milatovic D, Dettbarn WD. Modification of acetylcholinesterase during adaptation to chronic, subacute paraoxon application in rat. Toxicol Appl Pharmacol. 1996;136:20–28. doi: 10.1006/taap.1996.0003. [DOI] [PubMed] [Google Scholar]
- 24.Villa AF, Houze P, Monier C, Risede P, Sarhan H, Borron SW, Megarbane B, Garnier R, Baud FJ. Toxic doses of paraoxon alter the respiratory pattern without causing respiratory failure in rats. Toxicology. 2007;232:37–49. doi: 10.1016/j.tox.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Anzueto A, deLemos RA, Seidenfeld J, Moore G, Hamil H, Johnson D, Jenkinson SG. Acute inhalation toxicity of soman and sarin in baboons. Fundam Appl Toxicol. 1990;14:676–687. doi: 10.1016/0272-0590(90)90293-s. [DOI] [PubMed] [Google Scholar]
- 26.Oberst FW, Crook JW, Christensen MK, Cresthull P, Koon WS, Freeman G. Inhaled GB retention studies in man at rest and during activity. Army Chemical Center Md.: Chemical Corps Research and Development Command, DTICAD226805. 1959 [Google Scholar]
- 27.Maxwell DM, Brecht KM, Koplovitz I, Sweeney RE. Acetylcholinesterase inhibition: does it explain the toxicity of organophosphorus compounds? Arch Toxicol. 2006;80:756–760. doi: 10.1007/s00204-006-0120-2. [DOI] [PubMed] [Google Scholar]
- 28.Worek F, Diepold C, Eyer P. Dimethylphosphoryl-inhibited human cholinesterases: inhibition, reactivation, and aging kinetics. Arch Toxicol. 1999;73(1):7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]
- 29.Amitai G, Moorad D, Adani R, Doctor BP. Inhibition of acetylcholinesterase and butyryl-cholinesterase by chlorpyrifos-oxon. Biochem Pharmacol. 1998;56(3):293–299. doi: 10.1016/s0006-2952(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 30.Dorandeu F, Foquin A, Briot R, Delacour C, Denis J, Alonso A, Froment MT, Renault F, Lallement G, Masson P. An unexpected plasma cholinesterase activity rebound after challenge with a high dose of the nerve agent VX. Toxicology. 2008;248:151–157. doi: 10.1016/j.tox.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, Duysen EG, Lockridge O. Induction of plasma acetylcholinesterase activity and apoptosis in mice treated with the organophosphate toxicant, tri-o-cresyl phosphate. Toxicol Res. 2012;1:55–61. [Google Scholar]
- 32.Graham JR, Wright BS, Rezk PE, Gordon RK, Sciuto AM, Nambiar MP. Butyrcholinesterase in guinea pig lung lavage: a novel biomarker to assess lung injury following inhalation exposure to nerve agent VX. Inhalation Toxicology. 2006;18:493–500. doi: 10.1080/08958370600602116. [DOI] [PubMed] [Google Scholar]