Abstract
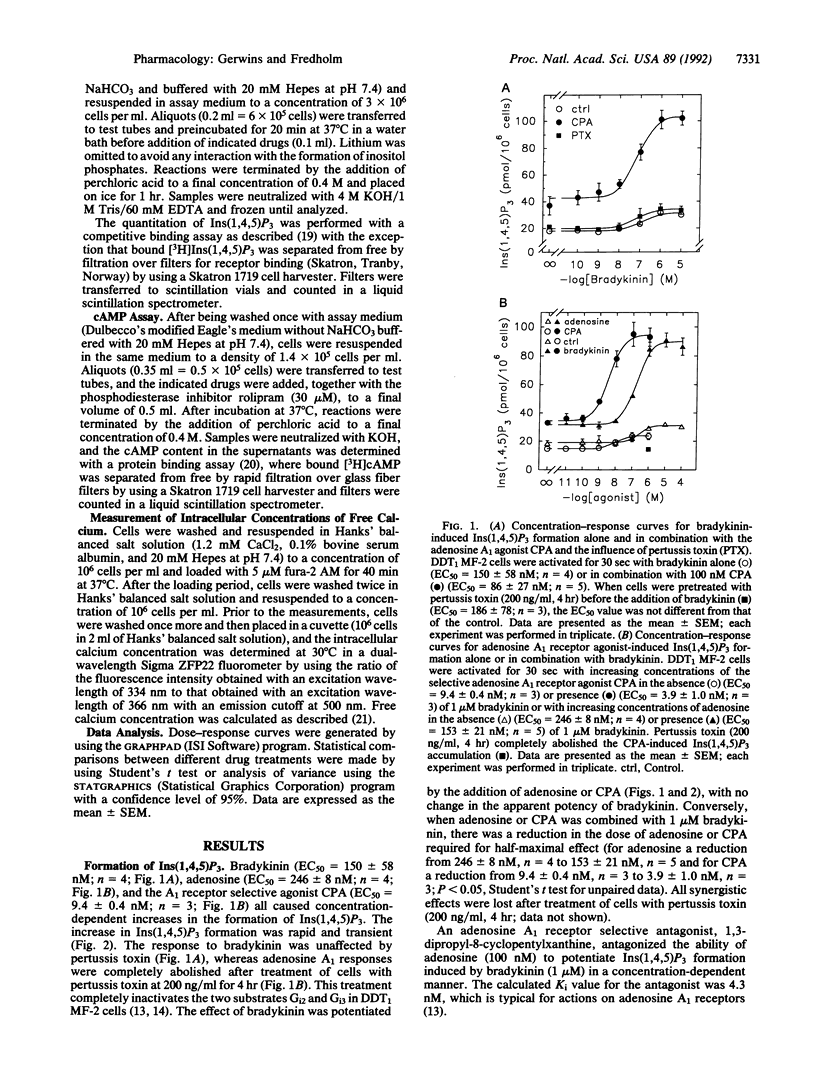
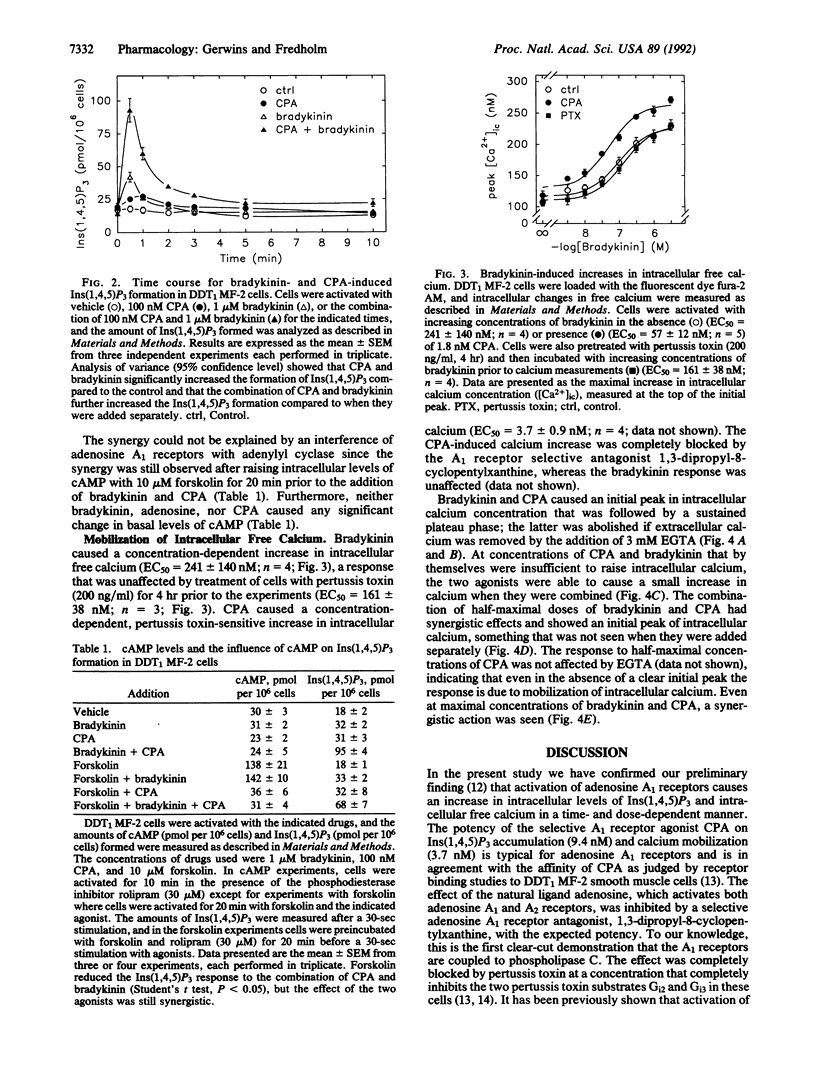
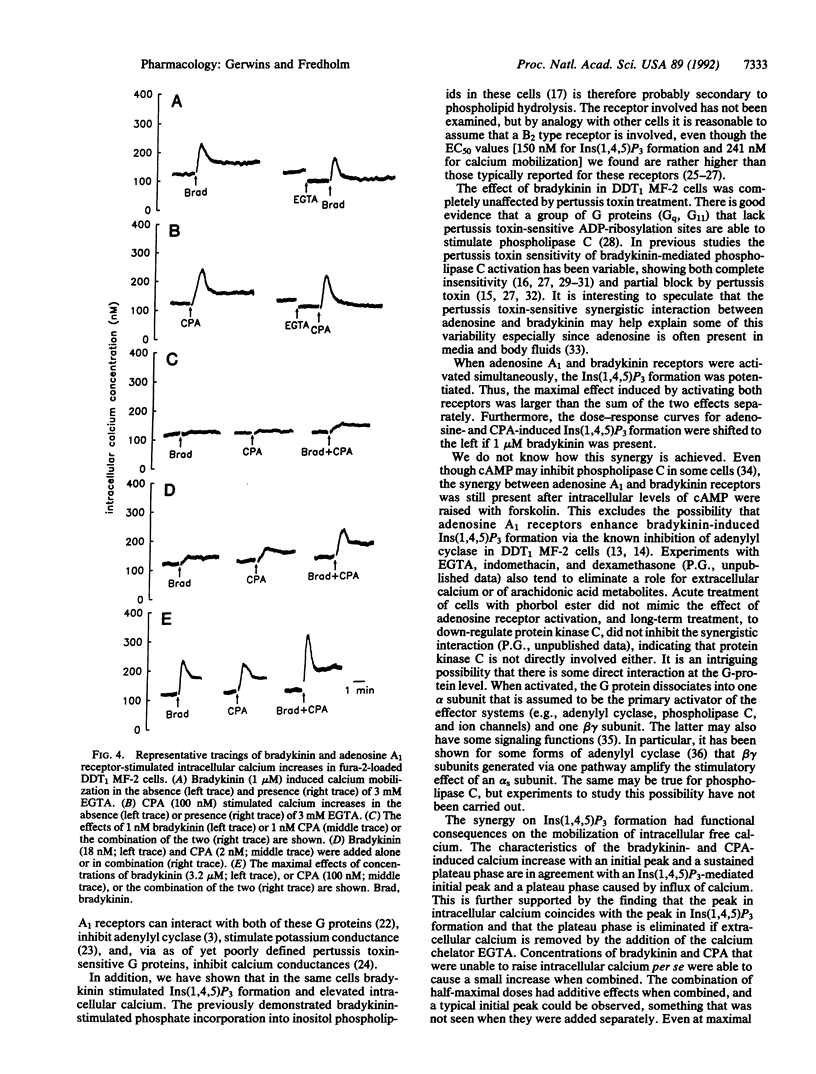
We have examined the cross talk between adenosine and bradykinin receptors in DDT1 MF-2 smooth muscle cells. Both adenosine and bradykinin mobilized intracellular free calcium via the formation of inositol 1,4,5-trisphosphate in a time- and dose-dependent manner. Adenosine exerted its actions via adenosine A1 receptors as demonstrated by the observations that N6-cyclopentyladenosine, a selective A1 receptor agonist, had an EC50 in the low nanomolar range and that a selective adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine, counteracted adenosine-mediated responses at concentrations typical for signaling via adenosine A1 receptors. Adenosine A1 receptors were coupled to phospholipase C via pertussis toxin-sensitive guanine nucleotide-binding regulatory protein(s) [G protein(s)], whereas bradykinin responses were unaffected by pertussis toxin. When adenosine or N6-cyclopentyladenosine was combined with bradykinin, the resulting formation of inositol 1,4,5-triphosphate was more than additive, and the EC50 value for adenosine and N6-cyclopentyladenosine was shifted to the left by bradykinin, the affinity of which was unaltered. Combining N6-cyclopentyladenosine and bradykinin also synergistically raised intracellular free calcium both at subthreshold levels and at maximal concentrations of the two agonists. The interaction was not dependent upon cAMP. In conclusion, stimulation of adenosine A1 receptors coupled to pertussis toxin-sensitive G protein(s) and bradykinin receptors coupled to pertussis toxin-insensitive G protein(s) synergistically mobilizes intracellular free calcium and inositol 1,4,5-trisphosphate formation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S. P., Kendall D. A., Hill S. J. Differences in the adenosine receptors modulating inositol phosphates and cyclic AMP accumulation in mammalian cerebral cortex. Br J Pharmacol. 1989 Dec;98(4):1241–1248. doi: 10.1111/j.1476-5381.1989.tb12670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascands J. L., Emond C., Pecher C., Regoli D., Girolami J. P. Bradykinin stimulates production of inositol (1,4,5) trisphosphate in cultured mesangial cells of the rat via a BK2-kinin receptor. Br J Pharmacol. 1991 Apr;102(4):962–966. doi: 10.1111/j.1476-5381.1991.tb12284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueb J. L., Mousli M., Bronner C., Rouot B., Landry Y. Activation of Gi-like proteins, a receptor-independent effect of kinins in mast cells. Mol Pharmacol. 1990 Dec;38(6):816–822. [PubMed] [Google Scholar]
- Burnatowska-Hledin M. A., Spielman W. S. Effects of adenosine on cAMP production and cytosolic Ca2+ in cultured rabbit medullary thick limb cells. Am J Physiol. 1991 Jan;260(1 Pt 1):C143–C150. doi: 10.1152/ajpcell.1991.260.1.C143. [DOI] [PubMed] [Google Scholar]
- Delahunty T. M., Cronin M. J., Linden J. Regulation of GH3-cell function via adenosine A1 receptors. Inhibition of prolactin release, cyclic AMP production and inositol phosphate generation. Biochem J. 1988 Oct 1;255(1):69–77. doi: 10.1042/bj2550069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M., Selzer E., Schütz W. Interactions of purified bovine brain A1-adenosine receptors with G-proteins. Reciprocal modulation of agonist and antagonist binding. Biochem J. 1991 May 1;275(Pt 3):651–656. doi: 10.1042/bj2750651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T., Okano Y., Nozawa Y. Bradykinin-induced generation of inositol 1,4,5-trisphosphate in fibroblasts and neuroblastoma cells: effect of pertussis toxin, extracellular calcium, and down-regulation of protein kinase C. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1429–1435. doi: 10.1016/s0006-291x(88)81035-0. [DOI] [PubMed] [Google Scholar]
- Gerwins P., Fredholm B. B. Glucocorticoid receptor activation leads to up-regulation of adenosine A1 receptors and down-regulation of adenosine A2 responses in DDT1 MF-2 smooth muscle cells. Mol Pharmacol. 1991 Aug;40(2):149–155. [PubMed] [Google Scholar]
- Gerwins P., Nordstedt C., Fredholm B. B. Characterization of adenosine A1 receptors in intact DDT1 MF-2 smooth muscle cells. Mol Pharmacol. 1990 Nov;38(5):660–666. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gustafsson L., Fredholm B. B., Hedqvist P. Theophylline interferes with the modulatory role of endogenous adenosine on cholinergic neurotransmission in guinea pig ileum. Acta Physiol Scand. 1981 Mar;111(3):269–280. doi: 10.1111/j.1748-1716.1981.tb06736.x. [DOI] [PubMed] [Google Scholar]
- Hedqvist P., Fredholm B. B. Effects of adenosine on adrenergic neurotransmission; prejunctional inhibition and postjunctional enhancement. Naunyn Schmiedebergs Arch Pharmacol. 1976 Jun;293(3):217–223. doi: 10.1007/BF00507344. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Nakahata N., Lovenberg T. W., DiGuiseppi J., Herman B., Earp H. S., Harden T. K. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J Biol Chem. 1987 Mar 5;262(7):2951–2956. [PubMed] [Google Scholar]
- Holck M. I., Marks B. H. Purine nucleoside and nucleotide interactions on normal and subsensitive alpha adrenoreceptor responsiveness in guinea-pig vas deferens. J Pharmacol Exp Ther. 1978 Apr;205(1):104–117. [PubMed] [Google Scholar]
- Hollingsworth E. B., De la Cruz R. A., Daly J. W. Accumulations of inositol phosphates and cyclic AMP in brain slices: synergistic interactions of histamine and 2-chloroadenosine. Eur J Pharmacol. 1986 Mar 11;122(1):45–50. doi: 10.1016/0014-2999(86)90156-1. [DOI] [PubMed] [Google Scholar]
- Häggblad J., Fredholm B. B. Adenosine and neuropeptide Y enhance alpha 1-adrenoceptor-induced accumulation of inositol phosphates and attenuate forskolin-induced accumulation of cyclic AMP in rat vas deferens. Neurosci Lett. 1987 Nov 23;82(2):211–216. doi: 10.1016/0304-3940(87)90132-7. [DOI] [PubMed] [Google Scholar]
- Jelsema C. L., Axelrod J. Stimulation of phospholipase A2 activity in bovine rod outer segments by the beta gamma subunits of transducin and its inhibition by the alpha subunit. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3623–3627. doi: 10.1073/pnas.84.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall D. A., Hill S. J. Adenosine inhibition of histamine-stimulated inositol phospholipid hydrolysis in mouse cerebral cortex. J Neurochem. 1988 Feb;50(2):497–502. doi: 10.1111/j.1471-4159.1988.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., DeBlasi A., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. I. Agonist-promoted desensitization and phosphorylation of alpha 1-adrenergic receptors coupled to inositol phospholipid metabolism in DDT1 MF-2 smooth muscle cells. J Biol Chem. 1987 Mar 5;262(7):3098–3105. [PubMed] [Google Scholar]
- Linden J. Structure and function of A1 adenosine receptors. FASEB J. 1991 Sep;5(12):2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- Mihara S., Shigeri Y., Fujimoto M. Neuropeptide Y-induced intracellular Ca2+ increases in vascular smooth muscle cells. FEBS Lett. 1989 Dec 18;259(1):79–82. doi: 10.1016/0014-5793(89)81499-1. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Williamson R. E., Rogulja I., Fluharty S. J., Williamson J. R. Angiotensin II effects on the cytosolic free Ca2+ concentration in N1E-115 neuroblastoma cells: kinetic properties of the Ca2+ transient measured in single fura-2-loaded cells. J Neurochem. 1990 Jan;54(1):278–287. doi: 10.1111/j.1471-4159.1990.tb13312.x. [DOI] [PubMed] [Google Scholar]
- Nazarea M., Okajima F., Kondo Y. P2-purinergic activation of phosphoinositide turnover is potentiated by A1-receptor stimulation in thyroid cells. Eur J Pharmacol. 1991 Jan 25;206(1):47–52. doi: 10.1016/0922-4106(91)90145-8. [DOI] [PubMed] [Google Scholar]
- Nordstedt C., Fredholm B. B. A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Anal Biochem. 1990 Sep;189(2):231–234. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- Norris J. S., Gorski J., Kohler P. O. Androgen receptors in a Syrian hamster ductus deferens tumour cell line. Nature. 1974 Mar 29;248(447):422–424. doi: 10.1038/248422a0. [DOI] [PubMed] [Google Scholar]
- Perney T. M., Miller R. J. Two different G-proteins mediate neuropeptide Y and bradykinin-stimulated phospholipid breakdown in cultured rat sensory neurons. J Biol Chem. 1989 May 5;264(13):7317–7327. [PubMed] [Google Scholar]
- Portilla D., Morrissey J., Morrison A. R. Bradykinin-activated membrane-associated phospholipase C in Madin-Darby canine kidney cells. J Clin Invest. 1988 Jun;81(6):1896–1902. doi: 10.1172/JCI113536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Minakuchi R., Kikkawa U., Sano K., Kaibuchi K., Yu B., Matsubara T., Nishizuka Y. Membrane phospholipid turnover, receptor function and protein phosphorylation. Prog Brain Res. 1982;56:287–301. doi: 10.1016/S0079-6123(08)63780-2. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991 Dec 6;254(5037):1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Voyno-Yasenetskaya T. A., Tkachuk V. A., Cheknyova E. G., Panchenko M. P., Grigorian G. Y., Vavrek R. J., Stewart J. M., Ryan U. S. Guanine nucleotide-dependent, pertussis toxin-insensitive regulation of phosphoinositide turnover by bradykinin in bovine pulmonary artery endothelial cells. FASEB J. 1989 Jan;3(1):44–51. doi: 10.1096/fasebj.3.1.2535990. [DOI] [PubMed] [Google Scholar]
- el-Etr M., Cordier J., Glowinski J., Premont J. A neuroglial cooperativity is required for the potentiation by 2-chloroadenosine of the muscarinic-sensitive phospholipase C in the striatum. J Neurosci. 1989 May;9(5):1473–1480. doi: 10.1523/JNEUROSCI.09-05-01473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



