Abstract
Despite tremendous advancement in the characterization of nasal enzyme expression, knowledge of the role of the nasal mucosa in the metabolism of xenobiotics is still inadequate, primarily due to the limited availability of in vitro models for nasal metabolism screening studies. An extensive knowledge of the oxidative and conjugative metabolizing capacity of the cattle (Bos taurus) olfactory and respiratory mucosa can aid in efficient use of these tissues for pre-clinical investigations of the biotransformation and toxicity of therapeutic agents following nasal administration or inhalation. Cows are also exposed to a variety of airborne pollutants and pesticides during their lifetime, the metabolism of which can have profound toxicological and ecological consequences. The aim of the present study was to characterize cytochrome P450 (CYP) enzyme expression in the bovine nasal mucosa. Amplification of the specific genes through real time RT-PCR confirmed expression of several CYP enzymes in bovine hepatic and nasal tissues. The results demonstrate that bovine nasal olfactory and respiratory mucosal and liver tissues express similar populations, families, and distributions of CYP enzymes, as has been previously reported with other species, including humans. Bovine ex vivo tissues can serve as a readily available reference tissue to elucidate preclinical toxicokinetic effects resulting from exposure to substances in the environment or following drug administration.
Keywords: Nasal Mucosa, Metabolism, Cytochrome P450, Gene Expression, Immunohistochemistry, Real Time-PCR
1. Introduction
Pre-systemic elimination by local enzymatic degradation is one of the key barriers to the transport of xenobiotics across the nasal mucosa. The nasal olfactory and respiratory mucosae have been well documented to host a diverse array of phase I and II drug metabolizing enzymes (Gu et al., 2000; Heydel et al., 2013; Legendre et al., 2014; Thiebaud et al., 2013, 2010; Thornton-Manning, 1997; Watelet et al., 2009; Wong and Zuo, 2010; Zhang et al., 2005), some of which have been implicated in tissue-specific toxicological reactions. An increasing number of reports of the induction of nasal tumors or lesions in laboratory animals following inhalation of compounds undergoing metabolic activation to toxic compounds fueled interest in the area of enzymatic barrier properties of the nasal mucosa (Brittebo, 1997; Dahl and Hadley, 1991; Ding and Kaminsky, 2003; Reed, 1993; Thornton-Manning, 1997). The activity of nasal enzymes in metabolizing intranasally administered therapeutic agents during their transfer across the nasal mucosal layers, and the toxicological consequences of those metabolic reactions on the structural organization and integrity of the nasal mucosa, have not yet been carefully studied. The nasal olfactory and respiratory mucosae of rodents and humans have been relatively well characterized for enzyme expression (Chen, 2003; Gu et al., 2000; Ling et al., 2004; Marini et al., 2001; Minn et al., 2005; Su et al., 1996; Zhang et al., 2005). In contrast, however, the nasal mucosa of cattle has received relatively less attention.
Cows are inexpensive and easily accessible sources of ex-vivo tissues in the United States. The use of bovine tissues for studying the enzymatic activity in various organs, such as the liver, lungs, intestinal mucosa, kidneys, coronary arteries, and tongue, has become increasingly popular in recent years (Darwish et al., 2010(a), (b); Maté et al., 2015; Virkel et al., 2010; Zancanella et al., 2010). The proteolytic capacity of the bovine nasal mucosa has been used to investigate peptide transport and metabolism across the nasal mucosa (Lang et al., 1998, 1996; Schmidt et al., 2000). However, research reports characterizing the CYP-dependent monooxygenases in cattle are still scarce. In rodents and laboratory animals, the small size of the nasal cavity and the larger surface area coverage of the olfactory mucosa make it virtually impossible to effectively distinguish between the olfactory and respiratory mucosa. However, in cattle, the size of the nasal cavity is large and the organization of the nasal mucosa resembles that in humans, facilitating distinction and easy preparation of the olfactory and respiratory mucosa (Smith and Bhatnagar, 2004).
Evaluation of the metabolic capacity of the bovine nasal mucosa is critical for several reasons. An extensive knowledge of the expression and activity of the oxidative and conjugative drug metabolizing capacity of the bovine olfactory and respiratory mucosa can aid in efficient use of these tissues for high-throughput in vitro screening of the biotransformation of therapeutic agents during transport across the nasal mucosa. An improved understanding of cattle nasal biotransformative capacity is also essential to evaluate the risk of contamination of dairy or meat products with potentially toxic metabolites from chronic inhalation of pesticide or other airborne chemicals. In addition, knowledge of the distribution and activity of the nasal enzymes can be used to evaluate the effects of enzyme inhibitors and inducers on overall biotransformative capabilities of the nasal mucosa.
The aim of the present study was to investigate the metabolic capability of the bovine nasal mucosa through a screening of CYP gene expression and tissue-specific protein localization. Using quantitative RT-PCR, the relative expression levels of major CYP enzymes in the bovine olfactory and respiratory mucosae were determined, and the gene expression of the enzymes in the bovine liver was also examined and compared with that in the nasal tissues to better estimate the metabolic barrier presented by the nasal tissues. The nomenclature used for bovine CYP enzymes studied using RT-PCR in this study is given in Table I along with their NCBI accession numbers (Edgar et al., 2002). Localization of the major CYP enzymes in the bovine olfactory and respiratory explants was examined by immunofluorescence staining to confirm the translation of gene sequences into the encoded proteins and to determine the distribution of those enzymes within the nasal tissues.
Table I.
Primers used for quantitative real time RT-PCR (Edgar et al., 2002)
| Enzyme | Primers | Sequence (5′-3′) | Product size (bp) | Annealing temp (°C) | Position (5′) | Accession number |
|---|---|---|---|---|---|---|
| Bovine CYP1A1 | Sense | GACCTGAATCAGAGGTTCTACGTCT | 81 | 60 | 861 | XM_588298.7 |
| Antisense | CCGGATGTGACCCTTCTCAA | 941 | ||||
| Bovine CYP1A2 | Sense | ACCATGACCCGAAGCTGTG | 78 | 60 | 1301 | NM_001099364.1 |
| Antisense | CAATGGTGGTGCCATCAGAC | 1378 | ||||
| Bovine CYP2A6 | Sense | CCAGAATGGAGCTCTTCCTCT | 195 | 58 | 26 | DQ114539.1 |
| Antisense | CTATTTGCTCCTCCCACCAG | 220 | ||||
| Bovine CYP2C42 | Sense | TCCCTGGACATGAACAACCC | 71 | 60 | 760 | XM_612374.5 |
| Antisense | TTGTGCTTTTCCTGTTCCATCTT | 830 | ||||
| Bovine CYP2C31 | Sense | TCCCAAGGGCACAACCATA | 56 | 60 | 1146 | XM_600421.5 |
| Antisense | CCTTGCCATCGTGCAGG | 1201 | ||||
| Bovine CYP3A5 | Sense | GCCAGAGCCCGAGGAGTT | 77 | 60 | 1309 | NM_174531.2 |
| Antisense | GCAGGTAGACGTAAGGATTTATGCT | 1385 | ||||
| Bovine CYP3A4 | Sense | GGAAACCTGGGTTCTCCTGGCT | 308 | 58 | 114 | NM_001099367.1 |
| Antisense | CCGATGGACCAAAAACCCTCCG | 421 | ||||
| Bovine GAPDH | Sense | ATGGAGAAGGCTGGGGCTCACT | 209 | 58 | 379 | NM_001034034.2 |
| Antisense | AGTCCCTCCACGATGCCAAAGT | 587 |
2. Materials and Methods
2.1 Materials
For PCR reactions, TRIzol® reagent for RNA extraction and SuperScript® III reverse transcriptase for cDNA synthesis were purchased from Invitrogen (Life Technologies™, Carlsbad, CA). Oligonucleotide primers were obtained from Integrated DNA Technologies Inc. (Coralville, IA). Real-time qPCR Master Mix SYBR® Advantage® was purchased from Clontech Laboratories, Inc. (Mountain View, CA). MicroAmp® Fast optical 48-well reaction plates were obtained from Applied Biosystems® (Life Technologies™, Carlsbad, CA), and the thermal adhesive sealing film to cover the PCR plates was purchased from Research Products International Corp. (Mount Prospect, IL). QIAquick® gel extraction kit was purchased from Qiagen Inc. (Valencia, CA).
Bovine CYP specific monoclonal antibodies are not commercially available. Hence antibodies cross-reactive to bovine proteins were obtained. Primary antibodies, including mouse monoclonal anti-human CYP2A6 (AB56069) and mouse monoclonal anti-human CYP1A2 (AB22717), were obtained from Abcam Inc. (Cambridge, MA). Rabbit polyclonal anti-human antibody to CYP 2C8, 2C9, 2C19 (CR 3280), and rabbit monoclonal anti-human antibody to CYP3A4 (CR 3340) were obtained from Biomol International LP (Plymouth Meeting, PA). Alexa fluor 488 conjugated secondary antibodies, goat anti-mouse immunoglobulin G (IgG) and goat anti-rabbit IgG as well as horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG, the nuclear stain ToPro3, and Dulbecco’s phosphate buffered saline (PBS) were obtained from Molecular Probes® (Life Technologies™, Carlsbad, CA). All other reagents were obtained from Sigma Chemical (Sigma-Aldrich, St. Louis, MO).
2.2 Preparation of animal tissues
Bovine (Bos taurus) (six animals, approximately two years old) nasal explants and liver specimens were obtained from and dissected at Bud’s Custom Meats Co. (Riverside, IA). The nasal respiratory and olfactory mucosae were collected by making incisions along the lateral walls of the nasal cavity and a horizontal incision along the ocular plane. The ethmoturbinates, covered by the olfactory mucosa, and the maxilloturbinates, covered by the respiratory mucosa, as well as liver tissues, were excised immediately after death. For histological and immunofluorescence examinations, the excised olfactory and respiratory mucosal sections were fixed immediately in zinc formalin (1% zinc sulfate in 3.7% unbuffered formalin) for 36 hours. For RNA extraction, the nasal and liver tissues were frozen in liquid nitrogen immediately after dissection and were kept frozen at −80 °C until use.
2.3 RNA extraction
RNA extraction and real-time RT PCR were carried out at the College of Pharmacy, University of Iowa (Iowa City, IA). Total RNA was isolated from frozen tissues using TRIzol® reagent according to the manufacturer’s instructions. Briefly, 1 mL of TRIzol® was added to a small piece of frozen tissue and homogenized at 4 °C to release the nucleic acids. The RNA was isolated using a phenol-chloroform extraction method provided in the TRIzol® kit. The concentration and purity of RNA was checked by absorbance spectroscopy using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE). The integrity of the RNA samples was assessed by denaturing electrophoretic analysis on 1% agarose gels stained with ethidium bromide using FisherBiotech electrophoresis system (Fisher Scientific, Pittsburgh, PA).
2.4 Reverse transcription
Complementary DNA (cDNA) was synthesized from RNA samples using a SuperScript® III reverse transcriptase kit in a Bio-Rad MyCycler™ thermal cycler (Bio-Rad Laboratories Inc., Hercules, CA). The reaction was performed at 50°C for 90 minutes with the use of 2.5 μg of total RNA, 200 units of SuperScript® III reverse transcriptase enzyme, 2.5 μM oligo(dT) primer, and 5 mM MgCl2 in the presence of 50 mM Tris-HCl buffer (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol in a total volume of 20 μl. The reverse transcriptase was inactivated by heating to 70 °C for 15 minutes. The quality of the synthesized cDNA was confirmed by denaturing agarose gel electrophoresis on 1% agarose gels stained with ethidium bromide using FisherBiotech electrophoresis system (Fisher Scientific, Pittsburgh, PA).
2.5 Real time RT-PCR
The Bos taurus primer pairs used for quantitative real time PCR are given in Table I. Primer pairs for CYP1A1, 1A2, 2C9, 2C19 and 3A5 genes have been described previously(Giantin et al., 2008). The primers for the remaining genes and for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were designed according to the sequences in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) with the help of Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Specificity of the primers used was confirmed by BLAST analysis of the GenBank database from NCBI (Edgar et al., 2002).
One microliter of the reverse transcription product (cDNA) was added to the PCR mixture containing 5 μl of SYBR® Advantage® premix, and 1 μM each of the sense and antisense primers (Table I) in a final volume of 10 μl prepared with with RNase free water in the wells of the MicroAmp® Fast optical 48-well reaction plate maintaind on ice. The wells were covered with the thermal seal adhesive film, and the plate was centrifuged at 250 × g for 30 seconds at 4 °C using an Eppendorf 5804R centrifuge (Eppendorf, Hauppauge, NY). The real time PCR reactions were performed in a StepOne PCR machine (Version 1.0, Applied Biosystems®, Life Technologies™, Carlsbad, CA). The PCR run was initiated with a 5-minute cycle at 95 °C. A three step cycling program was used to monitor amplification for 40 cycles with a 30-second denaturating step at 95 °C, a 30-second annealing step at a primer-specific annealing temperature (Table I) and a 30-second elongation step at 72 °C. At the end of the PCR cycle, a melting (dissociation) curve analysis was performed to verify the amplification of a single amplicon. Negative control reactions with no added reverse transcriptase product were performed for each PCR run to monitor potential contamination of reagents. Electrophoretic analysis of the PCR products was performed by separating the products on ethidium bromide-stained 2 % agarose gels and visualizing by transillumination with ultraviolet light. The real time PCR products were purified using the QIAquick® gel extraction kit and sequenced by the DNA Sequencing Facility of the University of Iowa (Iowa City, IA).
2.6 Calculation of gene expression levels and statistical analysis
For real time PCR, the relative expression level (L) for each gene was calculated from the threshold cycle value (CT(Schmittgen and Livak, 2008)) using the equation L = 2−CT. Normalization of the expression level of the CYP genes to the endogenous control (GAPDH) was performed by dividing the expression levels of each gene of interest by the expression level of GAPDH. The tissue expression of mRNA for each CYP enzyme was expressed as mean ± standard deviation of six samples from different animals. Statistically significant differences between expression levels of CYP enzymes among the nasal olfactory and respiratory mucosae and the liver were evaluated using one-way ANOVA and pairwise comparisons were performed using Student’s t-test with a Bonferroni correction. A p-value of less than 0.05 was considered to be significant.
2.7 Histological examination of bovine nasal mucosa
Fixed nasal mucosal explants were dehydrated and embedded in low temperature melting paraffin using an RMC paraffin tissue processor (Model 1530, Kroslak Enterprises Inc., Midland, ON, Canada). The slides for histological examination were prepared at the Central Microscopy Research Facility at the University of Iowa (Iowa City, IA). For morphologic examination, 5 μm thick sections were cut using a Leica UC6 ultramicrotome (Leica Microsystems Inc., Bannockburn, IL) and mounted onto Superfrost® Plus microscope slides (Fisher Scientific Inc., Pittsburgh, PA). The mounted sections were dehydrated overnight and deparaffinized by placing them in an oven maintained at 60 °C to melt the paraffin followed by rinsing the slides in xylene. Staining of the sections with hematoxylin and eosin was carried out using a DRS-601 Sakura Diversified Stainer (GMI Inc., Ramsey, MN). Finally, the sections were coverslipped and examined using an Olympus BX-51 light microscope (Olympus America Inc., Center Valley, PA) equipped with a DP-71 digital camera.
2.8 Immunohistochemistry
The slides for immunohistochemical examination were prepared at the Central Microscopy Research Facility at the University of Iowa (Iowa City, IA). For CYP immunofluorescence imaging, 5 μm sections of respiratory and olfactory mucosa were deparaffinized and dehydrated using a DRS-601 Sakura Diversified Stainer (GMI Inc, Ramsey, MN). Heat-induced epitope retrieval of the sections immersed in citrate buffer was carried out in a microwave (Pelco-Biowave, Ted-Pella Inc, Redding, CA) set at 650 watts with a target temperature of 95 °C (5 min warm, 5 min rest, 5 min warm). Non-specific binding was minimized by incubating the sections in blocking buffer (10% normal goat serum, 1% bovine serum albumin in phosphate buffered saline (PBS), pH 7.4) for 20 minutes at room temperature. The sections were incubated with the following dilutions of primary antibody solutions for 90 minutes at room temperature: mouse anti-human CYP1A2 (1:100), mouse anti-human CYP2A6 (1:1000), rabbit anti-human CYP2C (1:1000) and rabbit anti-human CYP3A4 (1:1000). The primary antibody aliquots were diluted to the desired concentrations in PBS. Negative controls were incubated with species-matched, non-specific immunoglobulins at concentrations identical to those of the primary antibodies.
After rinsing in PBS, the tissues were exposed to secondary antibody solutions [goat anti-mouse Alexa 488 (1:500) for CYP1A2 and CYP2A6 or goat anti-rabbit Alexa 488 (1:500) for CYP2C and CYP3A4] for 30 minutes at room temperature. Following washing, the sections were incubated with the nuclear stain, To-Pro3, and mounted in Vectashield (Vector Laboratories, Burlingame, CA). A BioRad 1024 confocal laser-scanning microscope (BioRad, Carl Zeiss, Germany) equipped with a krypton/argon laser at excitation wavelengths of 488 nm and 647 nm was used to examine the section. Digital image processing was performed using ImageJ software (Abramoff et al., 2004; Schneider et al., 2012) (U.S. National Institutes of Health, Bethesda, MD). For comparison of the signal intensity of each CYP enzyme between the olfactory and respiratory mucosae, the nasal explants were matched for animal donor, antibody concentration and the staining protocol, and the incubations were all performed at the same time.
3. Results
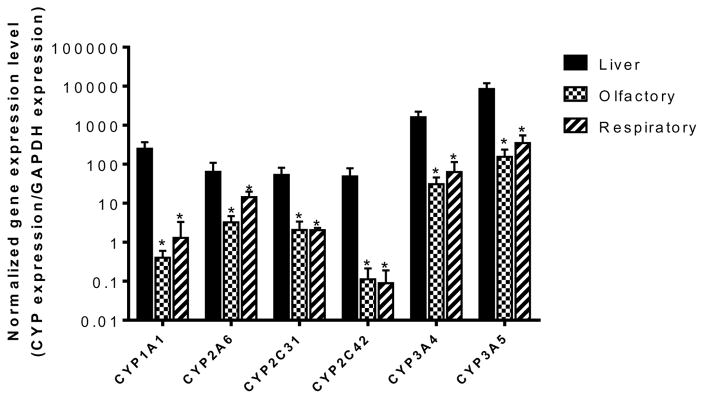
The data obtained by RT-PCR confirmed the expression of several CYP genes in the bovine olfactory and respiratory mucosae. As seen in gel electrophoresis images (Figure 1), transcripts of each of the enzymes selected were detected as real time PCR products. Figure 2 shows the gene expression levels of the selected CYP enzymes normalized with respect to GAPDH. Bovine hepatic tissue exhibited significantly higher gene expression levels for all the CYP enzymes examined (CYP1A1, CYP2A6, CYP2C31, CYP2C42, CYP3A4, and CYP3A5), than the corresponding mRNA levels in the olfactory and respiratory mucosa. No statistically significant differences were observed between the mRNA expression levels obtained from the olfactory and the respiratory mucosa for any of the genes studied. Both of the nasal tissues exhibited abundant expression of three genes: CYP2A6, CYP3A4 and CYP3A5. The remaining enzymes, including CYP1A1, CYP2C31, and CYP2C42 were expressed in both bovine olfactory and respiratory mucosa, but exhibited low expression levels compared to those obtained in bovine hepatic tissues (Table II). The RT-PCR results from the current studies showed that the expression of the CYP2C42 mRNA was mainly confined to bovine hepatic tissues. The expression levels of CYP2C31 mRNA obtained in both bovine olfactory and respiratory tissues were significantly higher than those obtained for CYP2C42 mRNA in the bovine olfactory and respiratory mucosa.
Figure 1.
Gel electrophoretic analysis of RT-PCR products obtained during amplification of the selected CYP genes from bovine hepatic, olfactory, and respiratory tissues extracted from six different cows.
Figure 2.
Gene expression levels of the selected CYP enzymes normalized with respect to GAPDH expression in bovine hepatic, olfactory, and respiratory tissues analyzed using real time RT-PCR. Results are presented as mean ± standard deviation for 6 animals. Error bars represent standard deviations. * represents statistically significant difference in the enzyme expression levels for liver vs. the nasal (olfactory or respiratory) mucosa at p<0.05. No statistically significant differences between the mRNA expression levels were observed between the olfactory and the respiratory mucosa for any of the enzymes studied.
Table II.
Comparison of reported CYP expression in human, rat, and bovine hepatic and nasal tissues
| Enzyme | Human liver | Human nasal mucosa | Rat liver | Rat nasal mucosa | Bovine liver | Bovine nasal (olfactory and respiratory) mucosa |
|---|---|---|---|---|---|---|
| CYP1A1 | mRNA: Low Protein: Low and variable (Martignoni et al., 2006; Schweikl et al., 1993) |
mRNA: moderate (Ling et al., 2004) | mRNA: not detected Protein: low (Martignoni et al., 2006) |
mRNA: moderate (Minn et al., 2005) | mRNA: moderate | mRNA: moderate |
| CYP1A2 | mRNA: variable Protein: variable (Gunes and Dahl, 2008; Schweikl et al., 1993; Zhou et al., 2010) |
mRNA: moderate and variable (Ling et al., 2004; Zhou et al., 2010) | mRNA: moderate-to-high (Lu and Li, 200) | mRNA: moderate (Minn et al., 2005) | mRNA: high | mRNA: variable Protein: weak |
| CYP2A Subfamily | mRNA: high (Martignoni et al., 2006; Koskela et al., 1999) | mRNA: low in adults, high CYP 2A mRNA in fetal olfactory mucosa (Gu et al., 2000; Koskela et al., 1999; Su et al., 1996) | mRNA: low (Martignoni et al., 2006) | mRNA: moderate (Robottom-Ferreira et al., 2003; Su et al., 1996) | mRNA: moderate (CYP2A6) | mRNA: moderate Protein: high (CYP2A6) |
| CYP2C Subfamily | mRNA: high Protein: high (Shimada et al., 1994) |
Protein: moderate (Yokose et al., 1999) | mRNA: high Protein: high (Nedelcheva and Gut, 1994; Martignoni et al., 2006) |
mRNA: low (Minn et al., 2005) | mRNA: high | mRNA: low Protein: high |
| CYP3A Subfamily | mRNA: high Protein: high (Watanabe et al., 2004; Shimada et al., 1994) |
Protein: moderate (Yokose et al., 1999) | mRNA: high and variable Protein: high and variable (Martignoni et al., 2006; Meredith et al., 2003; Nishimuta et al., 2013) |
mRNA: moderate Protein: moderate (Minn et al., 2005; Thornton-Manning, 1997) |
mRNA: high (Maté et al., 2015) | mRNA: high Protein: high |
Scoring of enzyme expression is based on our RT-PCR and immunohistochemistry results for bovine nasal and hepatic mucosa and an extensive qualitative review of available literature for nasal cytochrome P450 gene and protein expression in humans and rats.. For bovine hepatic RT-PCR expression, a normalized expression of greater than 1000 units (expression level in hepatic tissues/GAPDH expression in hepatic tissues) was assigned a “high” score, and a normalized expression of 40–1000 units was assigned a “moderate” score. For bovine nasal RT-PCR expression, a normalized expression of greater than 150 units (expression level in nasal tissues/GAPDH expression in nasal tissues) was assigned a “high score”; a normalized expression of 5–15 units was assigned a “moderate score”; a normalized expression of <5 units was assigned a “low” score.
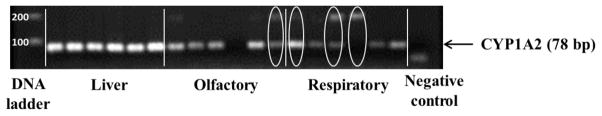
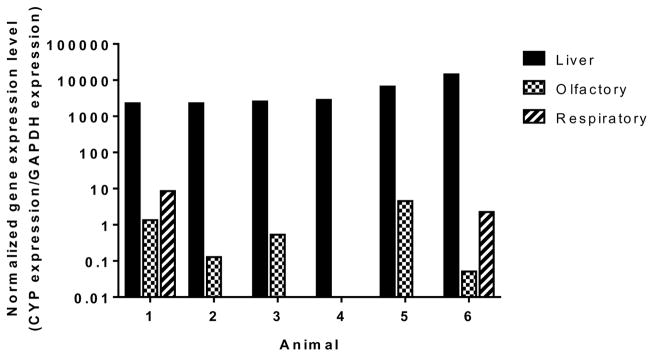
Amplification of the CYP1A2 gene from mRNA extracted from bovine respiratory mucosal tissues resulted in multiple PCR products, yielding multiple bands on electrophoretic analysis as shown in Figure 3. Figure 4 demonstrates the observed gene expression levels of CYP1A2 in bovine hepatic, olfactory and respiratory tissues. Real time PCR monitoring of one out of six olfactory tissues and four out of six respiratory tissues resulted in an indeterminate CT value for the desired transcript of CYP1A2. To identify the sequences of the fragments amplified during real time RT-PCR, the individual DNA fragments were extracted from the agarose gel and sequenced to identify possible sequence homology with the bovine CYP1A2 genes (NCBI accession number NM_001099364.1). The sequence of the amplified products partially aligned with the nucleotide sequence of bovine CYP1A2 mRNA, indicating the possible expression of a spliced variant of CYP1A2 genes in the bovine olfactory and respiratory mucosae.
Figure 3.
Gel electrophoretic analysis of RT-PCR products from amplification of CYP1A2 gene in bovine hepatic, olfactory, and respiratory tissues. Amplification of the CYP1A2 gene from mRNA extracted from several bovine respiratory and olfactory mucosal tissues resulted in multiple PCR products. Lanes showing multiple bands following amplification are highlighted with ovals.
Figure 4.
Gene expression levels of the CYP1A2-like gene normalized with respect to GAPDH expression in bovine hepatic, olfactory, and respiratory tissues analyzed using real time RT-PCR. Real time PCR monitoring of one of six olfactory tissues and four of six respiratory tissues resulted in an indeterminate CT value for the desired transcript of CYP1A2.
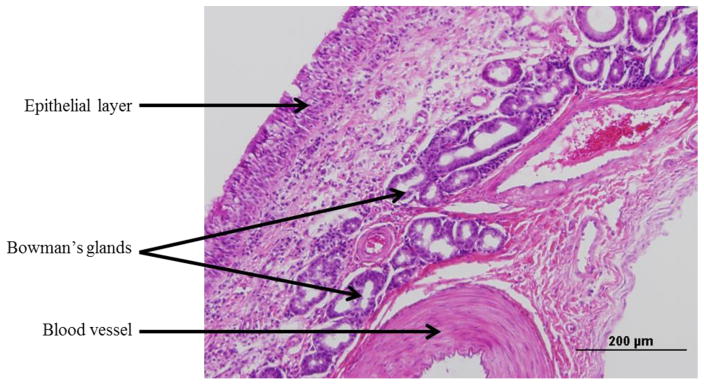
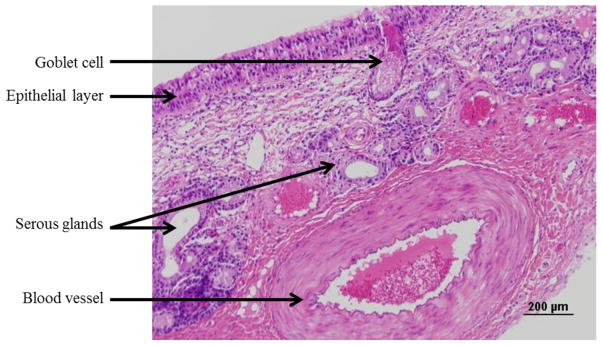
The histological organization of the bovine olfactory mucosa, obtained from the ethmoid turbinate is shown in Figure 5. The olfactory mucosa consists of a pseudostratified columnar epithelial layer and an underlying highly-cellular lamina propria. The submucosal region contains a significant population of the cells of Bowman’s glands and blood vessels. The respiratory mucosa, shown in Figure 6, has a similar cellular organization, where the pseudostratified columnar epithelial layer is interspersed with mucus producing goblet cells, and the lamina propria contains respiratory serous glands, blood vessels and connective tissue.
Figure 5.
Brightfield image of a formalin-fixed, paraffin-embedded, and hematoxylin and eosin stained section (5 μm) of bovine olfactory mucosa. Scale bar: 200 μm.
Figure 6.
Brightfield image of a formalin-fixed, paraffin-embedded, and hematoxylin and eosin stained section (5 μm) of bovine respiratory mucosa. Scale bar: 200 μm.
Due to the possibility of undesirable cross-reactivity among different bovine CYP epitopes with commercially-available primary antibodies, only one enzyme from each of the CYP subfamilies (1A, 2A, 2C, and 3A) was selected for the investigation of representative protein localization in bovine nasal mucosa. Cross-reactivity between different enzymes of the CYP subfamily could not be ruled out during these studies, however, the overall expression patterns obtained for the enzymes studies were identical, confirming CYP distribution in specific tissues of the bovine nasal olfactory and respiratory mucosa.
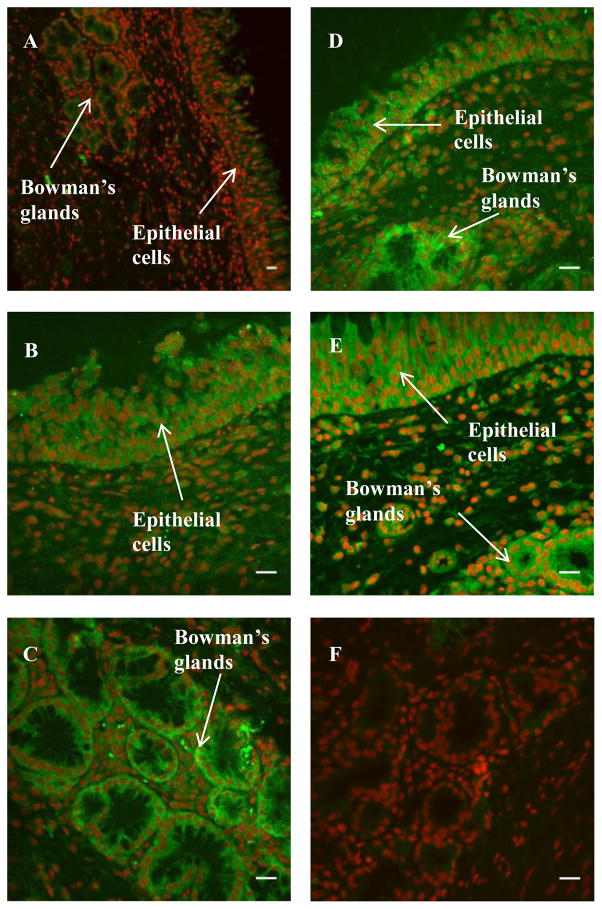
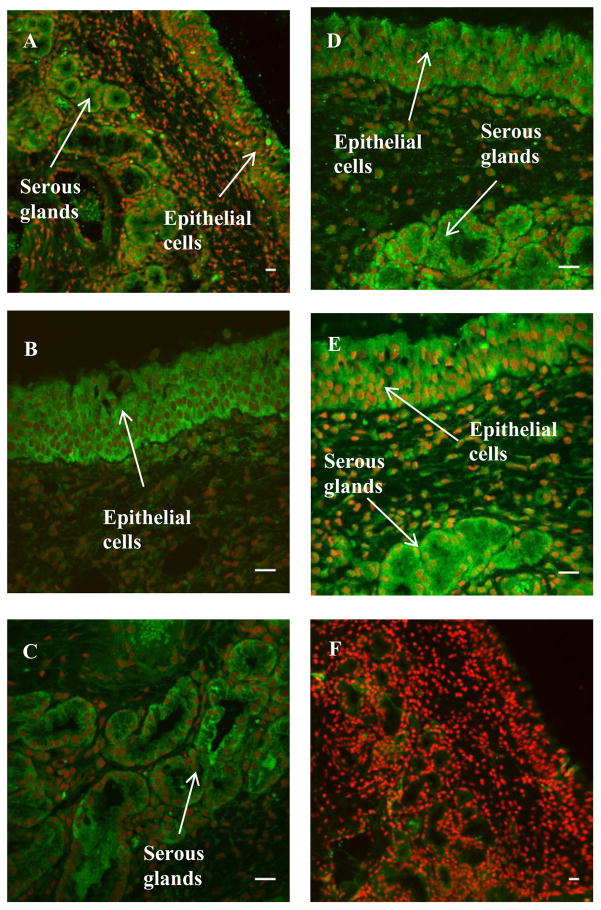
Immunohistochemical analyses (Figures 7 and 8) of the localization of the CYP enzymes showed that the enzymes belonging to the subfamilies 1A, 2A, 2C, and 3A were expressed in the bovine nasal mucosa where positive immunoreactivity is indicated by green fluorescence. ToPro3 was used to stain the nuclei red, which helped distinguish the cytoplasmic green fluorescence signal obtained from the cellular immunostaining from that of the autofluorescent red blood cells. In the olfactory mucosal sections, CYP 3A4, 2C8/9/19, and 2A6 protein immunoreactivity was observed in the pseudostratified columnar epithelial layer and the acinar cells of Bowman’s glands in the submucosal layer (Table II). In the immunopositive tissues, immunofluorescence was mainly confined to the cytoplasm. Submucosal endothelial cells did not exhibit significant immunoreactivity. Replacement of the primary antibody with species-matched immunoglobulins (negative control) in the staining protocol resulted in a markedly lower level of staining in the tissue sections, including the endothelial cells which are a common site for nonspecific IgG staining. For the respiratory mucosal sections, strong immunoreactivity was observed in the pseudostratified columnar epithelium and the serous glands for all four CYP subfamilies studied, including 3A, 2C, 2A, and 1A. This, as previsouly explained, could be due to cross-reactivity of primary antibodies towards multiple CYP enzymes. While the CYP1A2 fluorescent signal obtained in the respiratory sections was stronger than that obtained for the olfactory sections (Figures 7 and 8), the signal obtained in the olfactory sections treated for CYP1A2 immunoflurescence was not visibly different from the signal obtained in the negative control.
Figure 7.
Immunohistochemical staining of CYP1A2 (A), CYP2A6 (B–C), CYP2C (D), and CYP3A4 (E) in bovine olfactory mucosa. A representative negative control image is shown in F. Green fluorescence indicates positive immunoreactivity; red coloration indicates nuclei (To-Pro3). Scale bar: 20 μm.
Figure 8.
Immunohistochemical staining of CYP1A2 (A), CYP2A6 (B–C), CYP2C (D), and CYP3A4 (E) in bovine respiratory mucosa. A representative negative control image is shown in F. Green fluorescence indicates positive immunoreactivity; red coloration indicates nuclei (To-Pro3). Scale bar: 20 μm.
4. Discussion
Gene expression levels examined by RT-PCR illustrated that CYP2A6, CYP3A4, and CYP3A5 were the dominant CYP enzymes in the bovine olfactory and respiratory mucosal tissues, and a summary of the comparison of reported CYP expression in rats, human and bovine hepatic and nasal tissues is shown in Table II. The prominent expression of CYP2A6 in the bovine nasal mucosa was anticipated, as the enzymes of the CYP2A subfamily have been shown to be mainly extra-hepatic, and their nasal mucosal expression and activity has been extensively studied in humans and laboratory animals (Chen, 2003; Gu et al., 2000; Su and Ding, 2004; Su et al., 2000, 1996). Su et al. demonstrated that the CYP2A subfamily was present in human and rat nasal mucosa, but the relative mRNA expression levels in humans were much lower than the expression of the CYP2A genes in the rodent nasal mucosa when normalized with respect to each species’ liver CYP2A content (Su et al., 1996).
The enzymes of the CYP3A subfamily play key roles in catalyzing hepatic first-pass metabolism of orally administered therapeutic agents. CYP3A enzyme expression and activity has been extensively studied in the hepatic tissues of humans and rodents (Meredith et al., 2003; Nishimuta et al., 2013; Watanabe et al., 2004), and studies on cattle liver expression have also been reported (Maté et al., 2015). However, information available about the presence and activity of CYP3A enzymes in the nasal mucosa is scarce. CYP3A proteins have been previously identified in the respiratory and olfactory mucosa of rats, rabbits and humans by immunohistochemistry (Ding and Kaminsky, 2003; Thornton-Manning, 1997; Yokose et al., 1999), and CYP3A4-like gene expression was reported by Darwish et al. in mRNA samples extracted from bovine lungs and kidneys (Darwish et al., 2010(b)). Quantitative analysis of nasal mucosal CYP3A expression has not been previously reported, however. Real time RT-PCR with bovine primer pairs targeted towards two CYP3A enzymes showed that both bovine CYP3A4 and CYP3A5 mRNAs were expressed in the bovine olfactory and respiratory mucosae of the 6 animals studied. The expression levels were approximately 1–12% of the corresponding gene expression levels in the liver. The expression of several members of the CYP3A subfamily in bovine olfactory and respiratory tissues suggests that the bovine nasal mucosa possesses significant levels of CYP3A4-like catalytic activity.
CYP1A1 was expressed in both the bovine olfactory and respiratory mucosa, but exhibited low expression levels (< 1%) compared to bovine hepatic tissues. CYP1A1 is primarily an extra-hepatic enzyme, as its hepatic expression is reported to be very low in rats and humans (Hadley, 1982; Ling et al., 2004; Martignoni et al., 2006; Schweikl et al., 1993; Voigt et al., 1993). However, our results demonstrating the expression of CYP1A1 in bovine liver are in agreement with previous reports where CYP1A1 has been shown to be highly expressed in the hepatic tissues in cows (Darwish et al., 2010 (a); Sivapathasundaram et al., 2001). CYP1A is also the most inducible CYP subfamily, and many chemicals present in dietary plant components, in addition to environmental pollutants like polycyclic aromatic hydrocarbons have been reported to enhance its expression (Ioannides, 1999; Ling et al., 2004). Given the typical pasture characteristics for domestic livestock in the midwestern United States, dietary or environmental CYP1A1 inducers may have played a major role in influencing the apparent expression of CYP1A1 mRNA in the bovine liver samples that were used in these studies.
For the case of CYP1A2 mRNA amplification by RT-PCR, the liver and the nasal tissues presented notable differences in mRNA expression patterns among the six animals studied. The mRNA extracted from the livers of all six animals exhibited nearly identical expression of the CYP1A2. However, amplification of the CYP1A2 transcript from mRNA extracted from some of the bovine olfactory and respiratory mucosal tissues yielded multiple products. Real time PCR monitoring of one of six olfactory tissues and four of six respiratory tissues resulted in indeterminate CT values for the desired transcript of CYP1A2 (Figure 4). The variability in bovine CYP1A2 nasal expression is not entirely surprising, as pronounced inter-individual differences (40–130 fold) have been reported in CYP1A2 activity in humans (Gunes and Dahl, 2008; Zhou et al., 2010). A significant amount of work has been directed towards studying the genetic, epigenetic and environmental factors affecting CYP1A2 expression and catalytic activity in humans and rodents (Eaton et al., 1995; Gunes and Dahl, 2008; Ikeya et al., 1989; Rajkumar et al., 2013; Zhou et al., 2009).
The CYP2C subfamily primarily consists of 2C8, 2C9, 2C18, and 2C19 enzymes in humans. The enzymes of this subfamily constitute approximately 20% of total human hepatic CYP contents (Shimada et al., 1994), and the expression of CYP2C mRNAs and proteins has been reported in the small intestine and other extra-hepatic tissues in humans (Klose et al., 1999). In rats, the CYP2C subfamily is abundantly expressed in the hepatic tissues and is involved in the oxidation of chemicals like aflatoxin B1 and steroids (Martignoni et al., 2006; Nedelcheva and Gut, 1994).
The enzyme distribution patterns observed from the immunohistochemical analyses indicate inter-species similarity in nasal CYP enzyme distribution, and the epithelial and submucosal enzyme distribution patterns are consistent with the reported cellular localization of CYP in the human nasal mucosa (Getchell et al., 1993) as well as the distribution of CYP2A related proteins in fetal and adult human nasal mucosa (Chen, 2003) and with the staining patterns obtained for CYP2S1 in human nasal mucosa by in situ hybridization and immunohistochemistry (Saarikoski et al., 2005). Similar observations were also reported by Voigt et al. who observed CYP1A1 immunoreactivity localized in the cells of Bowman’s glands in rat olfactory mucosa with minimal staining of the epithelium (Voigt et al., 1993). Our findings are also consistent with the observed cytochrome P450 localization in epithelia, sustentacular cells and Bowman’s glands in rat olfactory mucosa (Chen et al., 1992).
5. Conclusions
Bovine nasal tissues possess a multi-cellular organization comparable to the adult human nasal mucosa, and results from the RT-PCR and immunohostochemical studies demonstrate that bovine nasal olfactory and respiratory mucosae express similar populations and distributions of CYP enzymes as have been previously reported in other species, including humans and rats. The expression and localization patterns of cytochrome P450 enzymes in the bovine nasal mucosa makes these tissues a promising ex vivo tool for pharmaco-toxicological studies in the investigation of enzyme-induced transformations of chemicals by the nasal mucosa, including potential toxicities from environmental exposures or diminished effectiveness of therapeutic agents targeted to or absorbed via the nasal mucosa.
Acknowledgments
This work was funded in part by National Institutes of Health; NIH R01DC08374.
Abbreviations
- cDNA
complementary DNA
- DNA
Deoxyribonucleic acid
- CYP
Cytochrome P450
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HCl
Hydrochloric acid or hydrochloride
- IgG
Immunoglobulin G
- KCl
Potassium chloride
- MgCl2
Magnesium chloride
- mRNA
Messenger ribonucleic acid
- PBS
Phosphate buffered saline
- PCR
Polymerase chain reaction
- RNA
Ribonucleic acid
- RT-PCR
Reverse transcriptase polymerase chain reaction
Footnotes
Declaration of Interest
The authors report no conflict of interest in the work described in this report.
References
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Brittebo EB. Metabolism-dependent activation and toxicity of chemicals in nasal glands. Mutat Res. 1997;380:61–75. doi: 10.1016/s0027-5107(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Chen Y. Immunoblot analysis and immunohistochemical characterization of CYP2A expression in human olfactory mucosa. Biochem Pharmacol. 2003;66:1245–1251. doi: 10.1016/s0006-2952(03)00476-3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Getchell M, Ding X, Getchell T. Immunolocalization of two cytochrome P450 isozymes in rat nasal chemosensory tissue. Neuroreport. 1992;3:749–752. doi: 10.1097/00001756-199209000-00007. [DOI] [PubMed] [Google Scholar]
- Dahl AR, Hadley WM. Nasal cavity enzymes involved in xenobiotic metabolism: effects on the toxicity of inhalants. Crit Rev Toxicol. 1991;21:345–372. doi: 10.3109/10408449109019571. [DOI] [PubMed] [Google Scholar]
- Darwish WS, Ikenaka Y, Eldaly EA, Ohno M, Sakamoto KQ, Fujita S, Ishizuka M. Cytochrome P450 1A-dependent activities in deer, cattle and horses. J Vet Med Sci. 2010a;72:561–566. doi: 10.1292/jvms.09-0318. [DOI] [PubMed] [Google Scholar]
- Darwish WS, Ikenaka Y, El-Ghareeb WR, Ishizuka M. High expression of the mRNA of cytochrome P450 and phase II enzymes in the lung and kidney tissues of cattle. Animal. 2010b;4(12):2023–2029. doi: 10.1017/S1751731110001394. [DOI] [PubMed] [Google Scholar]
- Ding X, Kaminsky LS. Humans extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol. 2003;43:149–173. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Gallagher EP, Bammler TK, Kunze KL. Role of cytochrome P4501A2 in chemical carcinogenesis: implications for human variability in expression and enzyme activity. Pharmacogenetics. 1995;5:259–274. doi: 10.1097/00008571-199510000-00001. [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell M, Chen Y, Xinxin D, Sparks D, Getchell T. Immunohistochemical localization of a cytochrome P-450 isozyme in human nasal mucosa: age-related trends. Ann Otol Rhinol Laryngol. 1993;102:368–374. doi: 10.1177/000348949310200509. [DOI] [PubMed] [Google Scholar]
- Giantin M, Carletti M, Capolongo F, Pegolo S. Effect of breed upon cytochromes P450 and phase II enzyme expression in cattle liver. Drug Metab Dispos. 2008;36:885–893. doi: 10.1124/dmd.107.019042. [DOI] [PubMed] [Google Scholar]
- Gu J, Su T, Chen Y, Zhang QY, Ding X. Expression of biotransformation enzymes in human fetal olfactory mucosa: potential roles in developmental toxicity. Toxicol Appl Pharmacol. 2000;165:158–162. doi: 10.1006/taap.2000.8923. [DOI] [PubMed] [Google Scholar]
- Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9:625–37. doi: 10.2217/14622416.9.5.625. [DOI] [PubMed] [Google Scholar]
- Hadley W. Cytochrome P-450 dependent monooxygenase activity in rat nasal epithelial membranes. Toxicol Lett. 1982;10:417–422. doi: 10.1016/0378-4274(82)90240-5. [DOI] [PubMed] [Google Scholar]
- Heydel JM, Coelho A, Thiebaud N, Legendre A, Le Bon AM, Faure P, Neiers F, Artur Y, Golebiowski J, Briand L. Odorant-binding proteins and xenobiotic metabolizing enzymes: Implications in olfactory perireceptor events. Anat Rec. 2013;296:1333–1345. doi: 10.1002/ar.22735. [DOI] [PubMed] [Google Scholar]
- Ikeya K, Jaiswal AK, Owens RA, Jones JE, Nebert DW, Kimura S. Human CYP1A2: sequence, gene structure, comparison with the mouse and rat orthologous gene, and differences in liver 1A2 mRNA expression. Mol Endocrinol. 1989;3:1399–1408. doi: 10.1210/mend-3-9-1399. [DOI] [PubMed] [Google Scholar]
- Ioannides C. Effect of diet and nutrition on the expression of cytochromes P450. Xenobiotica. 1999;29:109–154. doi: 10.1080/004982599238704. [DOI] [PubMed] [Google Scholar]
- Klose TS, Blaisdell JA, Goldstein JA. Gene structure of CYP2C8 and extrahepatic distribution of the human CYP2Cs. J Biochem Mol Toxicol. 1999;13:289–295. doi: 10.1002/(sici)1099-0461(1999)13:6<289::aid-jbt1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Koskela S, Hakkola J, Hukkanen J, Pelkonen O, Sorri M, Saranen A, Anttila S, Fernandez-Salguero P, Gonzalez F, Raunio H. Expression of CYP2A genes in human liver and extrahepatic tissues. Biochem Pharmacol. 1999;57:1407–1413. doi: 10.1016/s0006-2952(99)00015-5. [DOI] [PubMed] [Google Scholar]
- Lang S, Rothen-Rutishauser B, Perriard JC, Schmidt MC, Merkle HP. Permeation and pathways of human calcitonin (hCT) across excised bovine nasal mucosa. Peptides. 1998;19:599–607. doi: 10.1016/s0196-9781(97)00470-1. [DOI] [PubMed] [Google Scholar]
- Lang S, Staudenmann W, James P, Manz HJ, Kessler R, Galli B, Moser HP, Rummelt A, Merkle HP. Proteolysis of Human Calcitonin in Excised Bovine Nasal Mucosa: Elucidation of the Metabolic Pathway by Liquid Secondary lonization Mass Spectrometry (LSIMS) and Matrix Assisted Laser Desorption Ionization Mass Spectrometry (MALDI) Pharm Res. 1996;13:1679–1685. doi: 10.1023/a:1016492723930. [DOI] [PubMed] [Google Scholar]
- Legendre A, Faure P, Tiesset H, Potin C, Jakob I, Sicard G, Schaal B, Artur Y, Coureaud G, Heydel JM. When the nose must remain responsive: Glutathione conjugation of the mammary pheromone in the newborn rabbit. Chem Senses. 2014;39:425–437. doi: 10.1093/chemse/bju013. [DOI] [PubMed] [Google Scholar]
- Ling G, Gu J, Genter MB, Zhuo X, Ding X. Regulation of cytochrome P450 gene expression in the olfactory mucosa. Chem Biol Interact. 2004;147:247–258. doi: 10.1016/j.cbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Lu C, Li AP. Species comparison in P450 induction: Effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague-Dawley rat, minipig, and beagle dog. Chem Biol Interact. 2001;134:271–281. doi: 10.1016/s0009-2797(01)00162-4. [DOI] [PubMed] [Google Scholar]
- Marini S, Longo V, Zaccaro C, De Matteis F, Gervasi PG. Selective inactivation of rat and bovine olfactory cytochrome P450 by three haloethanes. Toxicol Lett. 2001;124:83–90. doi: 10.1016/s0378-4274(01)00323-x. [DOI] [PubMed] [Google Scholar]
- Martignoni M, Groothuis GMM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- Maté ML, Ballent M, Larsen K, Lifschitz A, Lanusse C, Virkel G. Gene expression and enzyme function of two cytochrome P450 3A isoenzymes in rat and cattle precision cut liver slices. Xenobiotica. 2015:1–8. doi: 10.3109/00498254.2014.1002122. [DOI] [PubMed] [Google Scholar]
- Meredith C, Scott MP, Renwick AB, Price RJ, Lake BG. Studies on the induction of rat hepatic CYP1A, CYP2B, CYP3A and CYP4A subfamily form mRNAs in vivo and in vitro using precision-cut rat liver slices. Xenobiotica. 2003;33:511–527. doi: 10.1080/0049825031000085960. [DOI] [PubMed] [Google Scholar]
- Minn A, Pelczar H, Denizot C, Martinet M, Heydel JJM, Walther B, Goudonnet H, Artur Y, Poincare H. Characterization of microsomal cytochrome P450-dependent monooxygenases in the rat olfactory mucosa. Drug Metab Dispos. 2005;33:1229–1237. doi: 10.1124/dmd.105.004085. [DOI] [PubMed] [Google Scholar]
- Nedelcheva V, Gut I. P450 in the rat and man: methods of investigation, substrate specificities and relevance to cancer. Xenobiotica. 1994;24:1151–1175. doi: 10.3109/00498259409038673. [DOI] [PubMed] [Google Scholar]
- Nishimuta H, Nakagawa T, Nomura N, Yabuki M. Species differences in hepatic and intestinal metabolic activities for 43 human cytochrome P450 substrates between humans and rats or dogs. Xenobiotica. 2013;43:948–955. doi: 10.3109/00498254.2013.787155. [DOI] [PubMed] [Google Scholar]
- Rajkumar AP, Poonkuzhali B, Kuruvilla A, Srivastava A, Jacob M, Jacob KS. Association between CYP1A2 gene single nucleotide polymorphisms and clinical responses to clozapine in patients with treatment-resistant schizophrenia. Acta Neuropsychiatr. 2013;25:2–11. doi: 10.1111/j.1601-5215.2012.00638.x. [DOI] [PubMed] [Google Scholar]
- Reed CJ. Drug metabolism in the nasal cavity: relevance to toxicology. Drug Metab Rev. 1993;25:173–205. doi: 10.3109/03602539308993975. [DOI] [PubMed] [Google Scholar]
- Robottom-Ferreira AB, Aquino SR, Queiroga R, Albano RM, Ribeiro Pinto LF. Expression of CYP2A3 mRNA and its regulation by 3-methylcholanthrene, pyrazole, and beta-ionone in rat tissues. Brazilian J Med Biol Res. 2003;36:839–844. doi: 10.1590/s0100-879x2003000700003. [DOI] [PubMed] [Google Scholar]
- Saarikoski ST, Wikman Ha-L, Smith G, Wolff CHJ, Husgafvel-Pursiainen K. Localization of cytochrome P450 CYP2S1 expression in human tissues by in situ hybridization and immunohistochemistry. J Histochem Cytochem. 2005;53:549–556. doi: 10.1369/jhc.4C6576.2005. [DOI] [PubMed] [Google Scholar]
- Schmidt MC, Simmen D, Hilbe M, Boderke P, Ditzinger G, Sandow J, Lang S, Rubas W, Merkle HP. Validation of excised bovine nasal mucosa as in vitro model to study drug transport and metabolic pathways in nasal epithelium. J Pharm Sci. 2000;89:396–407. doi: 10.1002/(SICI)1520-6017(200003)89:3<396::AID-JPS10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweikl H, Taylor JA, Kitareewan S, Linko P, Nagorney D, Goldstein JA. Expression of CYP1A1 and CYP1A2 genes in human liver. Pharmacogenetics. 1993;3:239–49. doi: 10.1097/00008571-199310000-00003. [DOI] [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- Sivapathasundaram S, Magnisali P, Coldham NG, Howells LC, Sauer MJ, Ioannides C. A study of the expression of the xenobiotic-metabolising cytochrome P450 proteins and of testosterone metabolism in bovine liver. Biochem Pharmacol. 2001;62:635–645. doi: 10.1016/s0006-2952(01)00710-9. [DOI] [PubMed] [Google Scholar]
- Smith TD, Bhatnagar KP. Microsmatic primates: reconsidering how and when size matters. Anat Rec Part B New Anat. 2004;279:24–31. doi: 10.1002/ar.b.20026. [DOI] [PubMed] [Google Scholar]
- Su T, Bao Z, Zhang Q, Smith T, Hong J, Ding X. Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000;60:5074–5079. [PubMed] [Google Scholar]
- Su T, Ding X. Regulation of the cytochrome P450 2A genes. Toxicol Appl Pharmacol. 2004;199:285–294. doi: 10.1016/j.taap.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Su T, Sheng JJ, Lipinskas TW, Ding X. Expression of CYP2A genes in rodent and human nasal mucosa. Drug Metab Dispos. 1996;24:884–890. [PubMed] [Google Scholar]
- Thiebaud N, Sigoillot M, Chevalier J, Artur Y, Heydel JM, Le Bon AM. Effects of typical inducers on olfactory xenobiotic-metabolizing enzyme, transporter, and transcription factor expression in rats. Drug Metab Dispos. 2010;38:1865–1875. doi: 10.1124/dmd.110.035014. [DOI] [PubMed] [Google Scholar]
- Thiebaud N, Veloso Da Silva S, Jakob I, Sicard G, Chevalier J, Ménétrier F, Berdeaux O, Artur Y, Heydel JM, Le Bon AM. Odorant Metabolism Catalyzed by Olfactory Mucosal Enzymes Influences Peripheral Olfactory Responses in Rats. PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0059547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton-Manning J. Metabolic capacity of nasal tissue:: interspecies comparisons of xenobiotic-metabolizing enzymes. Mutat Res. 1997;380:43–59. doi: 10.1016/s0027-5107(97)00126-7. [DOI] [PubMed] [Google Scholar]
- Virkel G, Carletti M, Cantiello M, Della Donna L, Gardini G, Girolami F, Nebbia C. Characterization of xenobiotic metabolizing enzymes in bovine small intestinal mucosa. J Vet Pharmacol Ther. 2010;33:295–303. doi: 10.1111/j.1365-2885.2009.01137.x. [DOI] [PubMed] [Google Scholar]
- Voigt JM, Guengerich FP, Baron J. Localization and induction of cytochrome P450 1A1 and aryl hydrocarbon hydroxylase activity in rat nasal mucosa. J Histochem Cytochem. 1993;41:877–885. doi: 10.1177/41.6.8315279. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kumai T, Matsumoto N, Tanaka M, Suzuki S, Satoh T, Kobayashi S. Expression of CYP3A4 mRNA is correlated with CYP3A4 protein level and metabolic activity in human liver. J Pharmacol Sci. 2004;94:459–62. doi: 10.1254/jphs.94.459. [DOI] [PubMed] [Google Scholar]
- Watelet JB, Strolin-Benedetti M, Whomsley R. Defence mechanisms of olfactory neuro-epithelium: mucosa regeneration, metabolising enzymes and transporters. B-ENT. 2009;5(Suppl 13):21–37. [PubMed] [Google Scholar]
- Wong YC, Zuo Z. Intranasal delivery-modification of drug metabolism and brain disposition. Pharm Res. 2010;27:1208–1223. doi: 10.1007/s11095-010-0127-5. [DOI] [PubMed] [Google Scholar]
- Yokose T, Doy M, Taniguchi T, Shimada T, Kakiki M, Horie T, Matsuzaki Y, Mukai K. Immunohistochemical study of cytochrome P450 2C and 3A in human non-neoplastic and neoplastic tissues. Virchows Arch. 1999;434:401–411. doi: 10.1007/s004280050359. [DOI] [PubMed] [Google Scholar]
- Zancanella V, Giantin M, Lopparelli RM, Patarnello T, Dacasto M, Negrisolo E. Proposed new nomenclature for Bos taurus cytochromes P450 involved in xenobiotic drug metabolism. J Vet Pharmacol Ther. 2010;33:528–536. doi: 10.1111/j.1365-2885.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang QY, Liu D, Su T, Weng Y, Ling G, Chen Y, Gu J, Schilling B, Ding X. Expression of cytochrome p450 and other biotransformation genes in fetal and adult human nasal mucosa. Drug Metab Dispos. 2005;33:1423–1428. doi: 10.1124/dmd.105.005769. [DOI] [PubMed] [Google Scholar]
- Zhou SF, Wang B, Yang LP, Liu JP. Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metab Rev. 2010;42:268–354. doi: 10.3109/03602530903286476. [DOI] [PubMed] [Google Scholar]
- Zhou SF, Yang LP, Zhou ZW, Liu YH, Chan E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11:481–494. doi: 10.1208/s12248-009-9127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]