Abstract
Background
Osteochondromas are benign bony protrusions that can be spontaneous or associated with radiation therapy (RT). Current treatment of high-risk neuroblastoma includes dose-intensive chemotherapy, local RT, anti-GD2 monoclonal antibody (MAb), and isotretinoin. Late effects are emerging.
Methods
We studied osteochondromas in 362 patients who were <10 years old when diagnosed with neuroblastoma; received MAb+isotretinoin since 2000; and survived ≥24 months from the first dose of MAb. The incidence rate of osteochondromas was determined using the competing risks approach where the primary event was osteochondroma calculated from the date of neuroblastoma diagnosis and the competing event was death without osteochondroma.
Results
Twenty-one osteochondromas were found in 14 patients who were 5.7-15.3 (median 10.4) years of age and 3.1-11.2 (median 8.2) years from neuroblastoma diagnosis. The cumulative incidence rate was 0.6% at five years and 4.9% at 10 years from neuroblastoma diagnosis. Nine osteochondromas were revealed incidentally during assessments of neuroblastoma disease status or bone age. Thirteen osteochondromas were outside RT portals and had characteristics of spontaneous forms. Complications were limited to pain necessitating resection in three patients, but follow-up is short at 0.3-7.7 (median 3.5) years.
Conclusions
Osteochondromas in long-term survivors of neuroblastoma should be expected because these benign growths can be RT-related and these patients undergo radiologic studies over years, are monitored for late toxicities through and beyond adolescence, and receive special attention (because of concern about relapse) if they develop a bony protuberance. A pathogenic role for chemotherapy, anti-GD2 MAb, or isotretinoin remains speculative.
Keywords: osteochondroma, bone tumors, radiotoxicity, long-term toxicity, neuroblastoma
Introduction
Osteocartilaginous exostosis or osteochondroma (OC) is a broad (sessile) or narrow (pedunculated) skeletal protrusion comprised of marrow and cortical bone, with an overlying cap of cartilage.1,2 OC is the most common benign skeletal tumor, with an estimated incidence of 1-2% in the general population; it accounts for 10-15% of all bone tumors, and shows a male predominance.1-4 Rather than a true neoplasm, OC can be classified as a developmental lesion that appears at a young age. It results from disturbance of enchondral (rather than membranous) ossification and therefore can arise from any bone that forms from cartilage. OCs can enlarge throughout childhood but usually not after skeletal maturity. Radiologic findings are pathognomonic: computed tomography and magnetic resonance imaging reveal an exophytic outgrowth with a thin layer of hyaline cartilage and cortical and medullary continuity between the abnormal mass and parent bone.1-4
Most OCs develop spontaneously without known cause and are discovered incidentally in children or adolescents between 10 and 15 years of age.2 These sporadic OCs can be solitary or multiple. A rare syndrome of multiple OCs is associated with two genes, EXT1 and EXT2 at 8q24 and 11p11-p12, respectively.5 Reports on late effects of oncologic therapy in children note OCs attributable to local radiation therapy (RT)6-12 or total body irradiation (TBI);13-24 a contributory role for growth hormone therapy has been suggested.18,22 A laboratory model shows radiation-related pathogenesis of OC.25 OCs associated with RT are indistinguishable pathologically and radiologically from sporadic forms.3
Early reports on OCs arising within RT fields included patients with what is now recognized as low-risk (i.e., readily curable) neuroblastoma (NB), namely, infants with localized NB.7-9,11 Such patients no longer receive RT,26,27 but irradiating the primary tumor bed is standard for high-risk NB.28-30 OCs in this patient population have been linked to TBI administered at a young age, with a 20-45% incidence in long-term survivors.17,19-22 In contrast, without TBI, 0/3019 and 1/5731 long-term NB survivors in multi-institutional studies developed an OC, and no OCs were noted in a North American study of long-term outcomes of 954 patients treated in 1970-1986 for all stages of NB.32 In recent single-institutional studies of late effects in high-risk NB patients, one OC (outside the RT field) was noted in 16 subjects in one report,33 whereas no OCs were described among 63 and 52 patients, respectively, in two other reports.34,35 A recent comprehensive review of late effects of childhood cancer therapy did not mention OC.36
RT and TBI together have been widely used in children with high-risk NB,20-22,30,37 setting these patients apart from those with other solid tumors (whose treatment can include local RT but not TBI) and from children with hematologic disorders (whose treatment can include TBI but not local RT). High-risk NB patients also stand out because their treatment now routinely includes differentiation therapy with isotretinoin37 and immunotherapy with anti-GD2 monoclonal antibody (MAb) ch14.1838 or 3F8.39 We now describe a series of long-term survivors of high-risk NB who developed OCs most of which were unrelated to local RT or TBI.
Methods
We studied OCs found in the 362 patients at Memorial Sloan Kettering Cancer Center who 1) were <10 years old at diagnosis of NB; 2) were enrolled since 2000 on clinical trials that used 3F8 plus granulocyte-macrophage colony-stimulating factor and isotretinoin (160 mg/m2/day, ×14 days/cycle, ×6 cycles), as described;39 and 3) survived ≥24 months from the first dose of anti-GD2 MAb. Every three months through five years from NB diagnosis, these patients underwent extent-of-disease evaluations that included computed tomography or magnetic resonance imaging of the primary site and 123I-meta-iodobenzylguanidine scan.40 Monitoring for late effects of therapy commenced ∼3 years from NB diagnosis.34,36 In accordance with rules of the institutional review board, informed written consents for evaluations and treatments were obtained from guardians, and, for this exploratory study via a retrospective review, a waiver was obtained for examination and analysis of patient records.
The incidence rate of OCs was determined using the competing risks approach where OC is the primary event. Time to event was calculated from the date of NB diagnosis until the date of OC, death, or last followup. Death without OC was considered as a competing event and patients alive at last follow-up were censored.
OCs outside the RT field versus RT-related OCs were compared, using the Wilcoxon rank sum test, as regards a) age at diagnosis of OC, and b) time from NB diagnosis to OC diagnosis.
Results
Neuroblastoma characteristics and treatment before discovery of OCs
Twenty-one OCs were identified in 14 patients (10 males, 4 females), including nine patients in first remission and five patients treated for relapse (Table 1). These patients were 0.8-4.3 (median 3.0) years old when diagnosed with stage 4 NB. Ten (71%) had MYCN-amplified NB. Treatments in all 14 patients included dose-intensive myelosuppressive chemotherapy; immunotherapy using anti-GD2 MAb; differentiation therapy with isotretinoin; and RT to the primary site (12 abdomen, 2 mediastinum) administered twice daily (hyperfractionated) in eight patients (2100 cGy),28 once daily in five patients (2160 cGy29 or 2400 cGy30), and as TBI (1200 cGy) and 1050 cGy in one patient (#5). Eleven of these 14 patients received myeloablative therapy with autologous stem-cell transplantation.
Table 1. Clinical characteristics of osteochondromas.
| Patient #/sex | Age at Dx of NB (yrs) | Age at Dx of OC (yrs) | Latency from Dx of NB (yrs) | Latency from RT (dose in cGy) (yrs) | Location | Growth hormone | Presentation | Follow-up (yrs) |
|---|---|---|---|---|---|---|---|---|
| Patients in continuous complete remission | ||||||||
| 1/F | 0.9 | 11.0 | 10.1 | n.a | 4th digit (L hand) | yes | incidental | 1.4 |
| and distal L fibula | painless bump | 1.4 | ||||||
| 2/M | 1.0 | 8.6 | 7.5 | n.a | distal R ulnaa | no | wrist swelling | 6.5 |
| 8.8 | 7.8 | n.a. | distal R femur | painless bump | 6.2 | |||
| 10.4 | 9.4 | n.a. | distal L radius | incidental | 4.6 | |||
| 3/M | 1.4 | 7.5 | 6.1 | 5.5 (5160)b | L tibia | no | painful bump | 0.3 |
| 4/M | 2.6 | 5.7 | 3.1 | 2.4 (2100) | posterior L iliac bonec | no | incidental | 7.7 |
| 5/M | 2.7 | 10.4 | 7.7 | 7.1 (1200, TBI) | distal L femur | no | painless bump | 4.4 |
| (2 sites)c | incidental | 4.4 | ||||||
| 6/M | 2.9 | 13.2 | 10.3 | n.a. | distal L radius | no | incidental | 4.2 |
| 7/M | 3.0 | 11.2 | 8.2 | n.a. | distal R femur | no | painless bump | 1.3 |
| 8/M | 3.1 | 12.1 | 9.1 | n.a. | distal R ulna | no | painless bump | 3.9 |
| 9/F | 3.2 | 14.4 | 11.2 | n.a. | proximal L ulna | yes | painful bump | 3.2 |
| Patients treated for neuroblastoma relapse | ||||||||
| 10/F | 1.9 | 10.7 | 8.8 | 8.0 (2160) | posterior R iliac bonec | no | incidental | 1.3 |
| 11/M | 1.4 | 7.5 | 6.2 | n.a. | proximal R humerusa | yes | painful bump | 4.4 |
| 12/M | 1.6 | 7.0 | 5.4 | 4.8 (2160) | R 4th ribc and | no | incidental | 1.3 |
| R scapulac | 1.3 | |||||||
| 13/M | 4.1 | 14.2 | 10.0 | n.a. | proximal R tibia: | |||
| anterior-mediala | no | painful bump | 2.6 | |||||
| poster ior-lateral | incidental | 2.6 | ||||||
| 15.3 | 11.1 | 8.7 (1500, lungs) | L scapulac | painless bump | 1.5 | |||
| 14/F | 4.4 | 8.7 | 4.4 | n.a. | anterior R iliac bone | no | incidental | 6.1 |
Abbreviations: Dx=diagnosis; n.a.=not applicable; NB=neuroblastoma; OC=osteochondroma; RT=radiotherapy; TBI=total body irradiation
Surgically removed
Initial dose was 2160 cGy, boost was 3000 cGy
Within or at edge of RT field
Osteochondroma characteristics
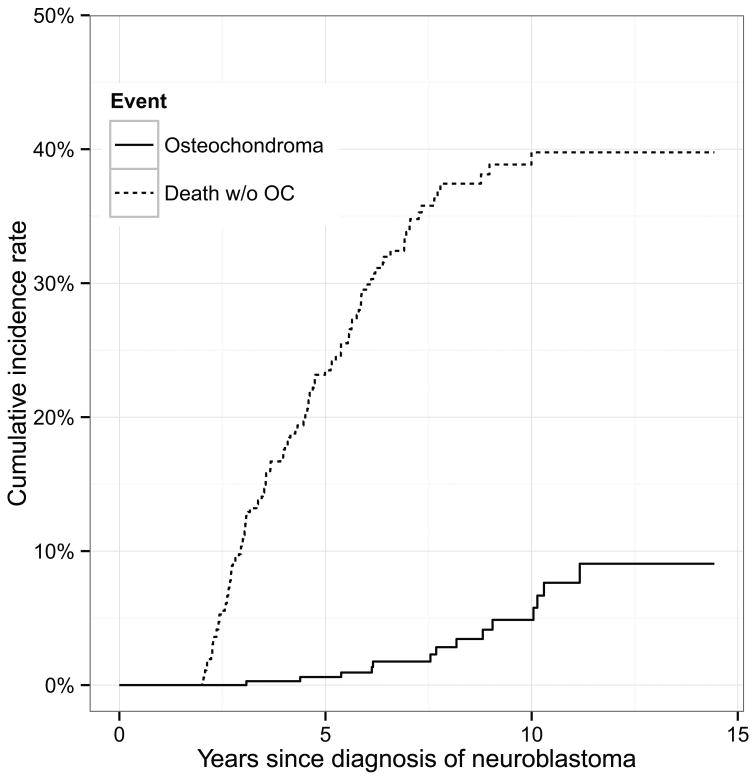
When the 21 OCs were found, the 14 patients were 3.1-11.2 (median 8.2) years from diagnosis of NB and 5.7-15.3 (median 10.4) years old (Table 1). The cumulative incidence rate of OC was 0.6% (95% CI 0%, 1.4%) at five years and 4.9% (95% CI 1.8%, 8.0%) at 10 years from NB diagnosis (Figure 1). Family histories were negative for OC. Nine patients had a single OC, three patients (#1, #5, and #12) had two OCs discovered simultaneously, one patient (#2) had three OCs detected sequentially, and one patient (#13) had three OCs, with two found simultaneously.
Figure 1.
The cumulative incidence rate of osteochondroma was 0.6% at five years and 4.9% at 10 years from the diagnosis of neuroblastoma.
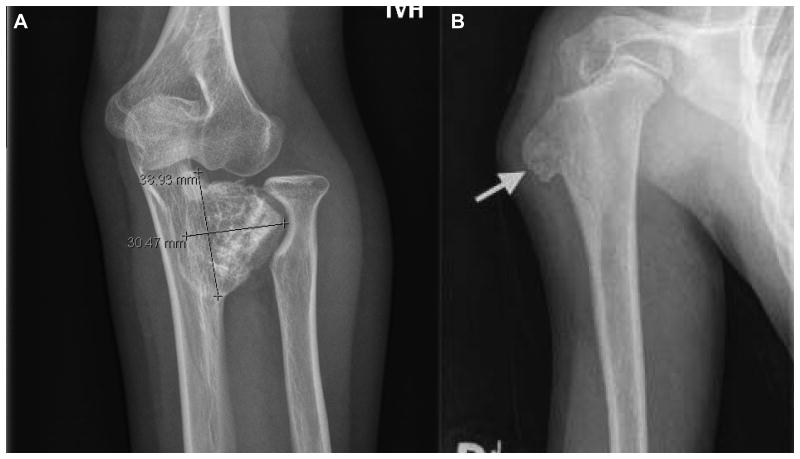
Twelve OCs presented as painless or painful bony protrusions (Figure 2). The other nine OCs were asymptomatic and were revealed incidentally by routine computed tomography or magnetic resonance imaging performed to assess NB status (patients #4, #10, #12, and #14); by x-rays performed to determine bone age in long-term survivors (patients #1, #2, and #6); and when scans to evaluate a new bony protuberance (which proved to be an OC) unexpectedly showed a second OC (patients #5 and #13).
Figure 2.
Osteochondroma unrelated to radiation therapy arising from (A) the proximal ulna in a 14.4 year old girl (patient #9), and (B) the proximal humerus in a 7.5 year old boy (patient #11). The humeral mass was resected to alleviate pain; pathology confirmed the diagnosis of osteochondroma.
The 13 OCs outside RT portals were found 4.4-11.2 (median 9.1) years from the diagnosis of NB, and the patients were 7.5-14.4 (median 11.0) years old. Eleven involved long bones, and one each arose from a digit and the iliac crest. These 13 OCs were identified at an older age (p=0.039) and at a longer time interval from NB diagnosis (p=0.083) compared to the eight RT-associated OCs. The latter were detected 2.4-8.7 (median 6.3) years post-RT and 3.1-11.1 (median 6.9) years from NB diagnosis; the patients were 5.7-15.3 (median 8.9) years old. The lesions included OCs in rib and scapula in a patient (#12) who had received 2160 cGy to a mediastinal primary site; a tibial OC in a patient (#3) who had received 2160 cGy followed by a boost of 3000 cGy to this site because of a persistent NB metastasis; and a scapular OC in a patient (#14) previously treated with 1500 cGy whole lung irradiation. Two OCs arising from iliac bone were at the edge of the RT field (patients #4 and #10). Finally, one patient (#5) treated with TBI developed two femoral OCs; no OCs were seen in the 12 other patients status-post TBI among the 362 study subjects.
Follow-up from OC diagnosis is 0.3-7.7 (median, 3.5) years. Three patients (#2, #11, and #13) had OCs resected because of pain; pathology revealed classic findings for this entity, and the patients had no post-operative complications or problems. Another patient (#9; Figure 2) had cosmetic concerns and limited mobility with an ulnar OC, but resection would have not have rectified the functional problem. No OC has undergone malignant transformation.
Three of the 14 patients were taking growth hormone, including one patient (#1) who had two OCs and two patients (#9 and #11) who each had a single OC (Figures 2 and 3). All three of these patients continued receiving growth hormone after OCs were diagnosed.
Discussion
This report is the first to describe OCs in a series of NB patients who received local RT, anti-GD2 MAb immunotherapy, and isotretinoin. These treatments are now standard for high-risk NB but were not routinely used in NB patients covered in recent reports on late effects of oncologic therapy.19-21,26,31-35 OCs were common with TBI,17,19-22 which is no longer in the NB therapeutic repertoire, but were virtually absent without TBI (see Introduction above).19,31-35 Improvements in therapy translate into increasing numbers of NB patients who achieve long-term survival - with a consequent evolution regarding the panoply and incidence of late events.
In past reports, the paucity of OCs among high-risk NB patients who never received TBI is possibly attributable to limited length of follow-up given that the incidence in our study patients is only 0.6% at five years but 4.9% at 10 years. This figure exceeds the estimated OC incidence of 1-2% in persons in the general population who undergo extensive radiographic evaluation.3 A majority of the OCs in our series were unrelated to RT and had hallmark characteristics of spontaneous forms of these benign developmental lesions1-4: single or multiple but no family history; predominance in long bones and males; discovered incidentally or presenting as a visible, sometimes painful, bony protuberance; pathognomonic radiologic features; and treatment (surgical resection) indicated in only a small minority.
Radiation has been implicated in the genesis of OCs through effects on the growth plate resulting in migration of undifferentiated cartilage tissue into the metaphysis.2,25 OC as a toxicity of local RT has been a concern for >50 years,6 and OCs as sequelae of TBI have been recognized since the 1990s.13 In our study, OCs outside RT portals included 12 in extremities and one in iliac bone, while the six OCs associated with local RT developed in scapula (n=2), iliac bone (n=2), tibia, and rib, and TBI preceded the detection of two femoral OCs in one patient. RT effects were among the possible causes for the younger age at OC diagnosis (p=0.039) and the shorter time interval from NB to OC diagnosis (p=0.083) with RT-associated OCs.
The absence of OCs in vertebrae might be considered unexpected since RT to the primary tumor bed encompasses spinal segments that are visualized repeatedly for years in radiologic studies performed to monitor disease status; hence, OCs would not be overlooked. Yet spinal OCs are in fact rare, which has raised the possibility that vertebral bodies and pedicles may be inherently less predisposed to radiation-related or spontaneous OCs.41,42 Spinal OCs were noted in two early reports on OCs and local RT,9,10 but not in others,6-8 and were not described in reports on toxicities of TBI13,16,17,21-23 or in other reports with details on sites of OCs.11,31,33
A dose-response relation between radiation and OC has not been established. OCs have been reported in sites that received 1050-5040 cGy.6-12 Early reports suggest OCs are more likely to develop with doses >2500 cGy.41,42 Lower doses, i.e., ∼2100 cGy,28,29 have become standard for RT to the primary site in NB patients, with less radiation absorbed by neighboring bony structures such as vertebrae and ilium, but NB treatment includes doxorubicin which is a radiation-sensitizer. Hyperfractionated RT, as received by eight of the 14 patients with OCs in this report, may have a less deleterious effect on normal tissues.43 Of note, linear growth is often compromised in survivors of high-risk NB,12,20,21,34 attributable to toxicity to vertebral bodies from local RT or TBI administered at a young age and/or to endocrine deficiencies from TBI. Growth hormone is used in these long-term survivors, including in patients who developed OCs. A causative link between growth hormone and OCs has been considered15,16,18,22 but never confirmed; recommendations are to continue hormonal supplementation despite emergence of an OC – as we did in all three of our patients with OCs who were taking growth hormone.
A pathogenic role for chemotherapy, anti-GD2 MAb, or isotretinoin in the emergence of OCs has never been reported and remains speculative. Chemotherapy may delay closure of epiphyseal plates and thereby increase the risk of disordered growth that eventuates in an OC. Anti-GD2 MAbs do not cross-react with bone but do cause transient neuropathic pain, albeit without long-term neuropathy,37,38 making it conceivable though unlikely that their interaction with nerve fibers has a pathologic effect on cartilage or skeletal structures. Excess retinol has a deleterious impact on bone, but the pathophysiology is not understood.44 The high-dose, short-term use of isotretinoin in NB patients37-39 has been associated with hypercalcemia, increased bone resorption and formation, and advanced bone age with premature closure of epiphyses.45-47
Complications from OCs in our subjects have been limited to pain, managed by surgical resection, in three patients (#2, #11, #13), and impaired mobility caused by proximity to an elbow, not amenable to surgical intervention, in one patient (#9; Figure 2). However, additional complications are foreseen since follow-up from OC diagnosis is short at 0.3-7.7 (median 3.1) years. To date, our patients have not had problems (e.g., vascular compromise or neurologic deficits) that can result from mass effect of OCs on adjacent tissues.1-4 Similarly, we have not seen problems arising from an OC itself, so-called intrinsic complications,4 which are rare and include fracture and malignant transformation. Malignancy is suspected if size increases rapidly or enlargement continues after the skeletal maturity concordant with puberty.1-5,48 OC-related malignancies are most often chondrosarcoma, occur in up to 5% or more of patients with hereditary OCs, are more likely with axially located OCs,1 display a thickened cartilage cap (>1 or 1.5 cm), and typically present in adulthood.1-5 Malignant neoplasms as sequelae of RT have latency periods >10-20 years; one must assume a greater risk with OCs arising in RT portals. Anticipatory monitoring is indicated.36
The detection of OCs in long-term survivors of high-risk NB should be expected not only because these benign skeletal lesions can be associated with local RT, which is standard of care, but also because these patients undergo comprehensive radiologic staging studies over years, are monitored for late effects of therapy through and beyond adolescence, and receive special medical attention (because of concern about relapse) if they develop a bony protuberance. OCs unrelated to RT have characteristics of spontaneous forms; hence, a pathogenic role for chemotherapy, anti-GD2 MAb, or isotretinoin remains uncertain and requires future analysis of large numbers of patients.
Acknowledgments
We thank Charles Sklar, M.D., for advice and support and Karima Yataghene, M.D., for help in data management.
Supported in part by grants from the National Institutes of Health (CA10450), Bethesda, MD; the Robert Steel Foundation, New York, NY; and Katie's Find A Cure Fund, New York, NY.
Footnotes
The authors have no financial disclosures to declare.
References
- 1.Giudici MA, Moser RP, Kransdorf MJ. Cartilaginous bone tumors. Radiol Clin N Amer. 1993;31:237–259. [PubMed] [Google Scholar]
- 2.Mavrogenis AF, Papagelopoulos PJ, Soucacos PN. Skeletal osteochondromas revisited. Orthopedics. 2008;31:1018–1028. [PubMed] [Google Scholar]
- 3.Murphey MD, Choi JJ, Kransdorf MJ, Flemming DJ, Gannon FH. Imaging of osteochondroma: variants and complications with radiologic-pathologic correlation. AFIP Archives. 2000;20:1407–1434. doi: 10.1148/radiographics.20.5.g00se171407. [DOI] [PubMed] [Google Scholar]
- 4.Lee KCY, Davies AM, Cassar-Pullicino VN. Imaging the complications of osteochondromas. Clin Radiol. 2002;57:18–28. doi: 10.1053/crad.2001.0719. [DOI] [PubMed] [Google Scholar]
- 5.Bovee JVMG. Multiple osteochondromas. Orphanet J Rare Dis. 2008;3:3. doi: 10.1186/1750-1172-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuhauser EB, Wittenborg MH, Berman CZ, Cohen J. Irradiation effect of roentgen therapy on the growing spine. Radiology. 1952;59:637–650. doi: 10.1148/59.5.637. [DOI] [PubMed] [Google Scholar]
- 7.Cole AR, Darte JM. Osteochondromata following irradiation in children. Pediatrics. 1963;32:285–288. [PubMed] [Google Scholar]
- 8.Katzman H, Waugh T, Berdon W. Skeletal changes following irradiation of childhood tumors. J Bone Joint Surg Am. 1969;51:825–842. [PubMed] [Google Scholar]
- 9.Libshitz HI, Cohen MA. Radiation-induced osteochondromas. Radiology. 1982;142:43–647. doi: 10.1148/radiology.142.3.6278535. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe N, Ried HL, Cohen M, McNeese MD, Sullivan MP. Radiation induced osteochondroma in long-term survivors of childhood cancer. Int J Radiat Oncol Biol Phys. 1983;9:665–670. doi: 10.1016/0360-3016(83)90232-8. [DOI] [PubMed] [Google Scholar]
- 11.Paulino AC, Fowler BZ. Secondary neoplasms after radiotherapy for a childhood solid tumor. Pediatr Hematol Oncol. 2005;22:89–101. doi: 10.1080/08880010590896459. [DOI] [PubMed] [Google Scholar]
- 12.Marcovici PA, Berdon WE, Liebling MS. Osteochondromas and growth retardation secondary to externally or internally administered radiation in childhood. Pediatr Radiol. 2007;37:301–304. doi: 10.1007/s00247-006-0382-0. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher BD, Crom DB, Krance RA, et al. Radiation-induced bone abnormalities after bone marrow transplantation for childhood leukemia. Radiology. 1994;191:231–235. doi: 10.1148/radiology.191.1.8134578. [DOI] [PubMed] [Google Scholar]
- 14.Maeda G, Yokoyama R, Ohtomo K, et al. Osteochondroma after total body irradiation in bone marrow transplant recipients: report of two cases. Jpn J Clin Oncol. 1996;26:480–483. doi: 10.1093/oxfordjournals.jjco.a023269. [DOI] [PubMed] [Google Scholar]
- 15.Harper GD, Dicks-Mireaux C, Leiper AD. Total body irradiation-induced osteochondromata. J Pediatr Orthop. 1998;18:356–358. [PubMed] [Google Scholar]
- 16.Bordigoni P, Turello R, Clement L, et al. Osteochondroma after pediatric hematopoietic stem cell transplantation: report of eight cases. Bone Marrow Transplant. 2002;29:611–614. doi: 10.1038/sj.bmt.1703424. [DOI] [PubMed] [Google Scholar]
- 17.Taitz J, Cohn RJ, White L, et al. Osteochondroma after total body irradiation: an age-related complication. Pediatr Blood Cancer. 2004;42:225–229. doi: 10.1002/pbc.10426. [DOI] [PubMed] [Google Scholar]
- 18.Sanders JE, Guthrie KA, Hoffmeister PA, Woolfrey AE, Carpenter PA, Appelbaum FR. Final adult height of patients who received hematopoietic cell transplantation in childhood. Blood. 2005;105:1348–1354. doi: 10.1182/blood-2004-07-2528. [DOI] [PubMed] [Google Scholar]
- 19.Flandin I, Hartmann O, Michon J, et al. Impact of TBI on late effects in children treated by megatherapy for stage IV neuroblastoma. A study of the French Society of Pediatric Oncology. Int J Radiat Oncol Biol Phys. 2006;64:1424–1431. doi: 10.1016/j.ijrobp.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Trahair TN, Vowels MR, Johnston K, et al. Long-term outcomes in children with high-risk neuroblastoma treated with autologous stem cell transplantation. Bone Marrow Transplant. 2007;40:741–746. doi: 10.1038/sj.bmt.1705809. [DOI] [PubMed] [Google Scholar]
- 21.Hobbie WL, Moshang T, Carlson CA, et al. Late effects in survivors of tandem peripheral blood stem cell transplant for high-risk neuroblastoma. Pediatr Blood Cancer. 2008;51:679–683. doi: 10.1002/pbc.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faraci M, Bagnasco F, Corti P, et al. Osteochondroma after hematopoietic stem cell transplantation in childhood. An Italian study on behalf of the AIEOP-HSCT group. Biol Blood Marrow Transplant. 2009;15:1271–1276. doi: 10.1016/j.bbmt.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Shido Y, Maeda N, Kato K, et al. Osteochondroma with metaphyseal abnormalities after total body irradiation followed by stem cell transplantation. J Pediatr Hematol Oncol. 2012;34:378–382. doi: 10.1097/MPH.0b013e3182332296. [DOI] [PubMed] [Google Scholar]
- 24.Mulcahy Levy JM, Tello T, Giller R, et al. Late effects of total body irradiation and hematopoietic stem cell transplant in children under 3 years of age. Pediatr Blood Cancer. 2013;60:700–704. doi: 10.1002/pbc.24252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langenskiold A, Edgren W. Initiation of chondrodysplasia by localized roentgen ray injury: an experimental study of bone growth. Acta Chir Scand. 1950;99:353–373. [Google Scholar]
- 26.Paulino AC, Mayr NS, Simon JH, Buatti JM. Locoregional control in infants with neuroblastoma: role of radiation therapy and late toxicity. Int J Radiat Onol Biol Phys. 2002;52:1025–1031. doi: 10.1016/s0360-3016(01)02713-4. [DOI] [PubMed] [Google Scholar]
- 27.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kushner BH, Wolden S, LaQuaglia MP, et al. Hyperfractionated low-dose (21 Gy) radiotherapy for high-risk neuroblastoma following intensive chemotherapy and surgery. J Clin Oncol. 2001;19:2821–2828. doi: 10.1200/JCO.2001.19.11.2821. [DOI] [PubMed] [Google Scholar]
- 29.Hans-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: a Children's Cancer Group study. Int J Radiat Oncol Biol Phys. 2003;56:28–39. doi: 10.1016/s0360-3016(02)04506-6. [DOI] [PubMed] [Google Scholar]
- 30.Zage PE, Kletzel M, Murray K, et al. Outcomes of the POG 9340/9341/9342 trials for children with high-risk neuroblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2008;51:747–753. doi: 10.1002/pbc.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno L, Vaidya SJ, Pinkerton CR, et al. Long-term follow-up of children with high-risk neuroblastoma: the ENSG5 trial experience. Pediatr Blood Cancer. 2013;60:1135–1140. doi: 10.1002/pbc.24452. [DOI] [PubMed] [Google Scholar]
- 32.Laverdiere C, Liu Q, Yasui Y, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:1131–1140. doi: 10.1093/jnci/djp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perwein T, Lackner H, Sovinz P, et al. Survival and late effects in children with stage 4 neuroblastoma. Pediatr Blood Cancer. 2011;57:629–635. doi: 10.1002/pbc.23036. [DOI] [PubMed] [Google Scholar]
- 34.Laverdiere C, Cheung NK, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45:324–332. doi: 10.1002/pbc.20331. [DOI] [PubMed] [Google Scholar]
- 35.Escobar MA, Grosfeld JL, Powell RL, et al. Long-term outcomes in patients with stage IV neuroblastoma. J Pediatr Surg. 2006;41:377–381. doi: 10.1016/j.jpedsurg.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 36.Dickerman JD. The late effects of childhood cancer therapy. Pediatrics. 2007;119:554–568. doi: 10.1542/peds.2006-2826. [DOI] [PubMed] [Google Scholar]
- 37.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children's Oncology Group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu A, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung N-KV, Cheung IY, Kushner BH, Ostrovnaya I, Kramer K, Modak S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kushner BH, Kramer K, Modak S, Cheung NK. Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol. 2009;27:1041–1046. doi: 10.1200/JCO.2008.17.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cree AK, Hadlow AT, Taylor TKF, Chapman GK. Radiation-induced osteochondroma in the lumbar spine. Spine. 1994;19:376–379. doi: 10.1097/00007632-199402000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Gorospe L, Madrid-Muniz C, Royo A, Garcia-Raya P, Alvarez-Ruiz F, Lopez-Barea F. Radiation-induced osteochondroma of the T4 vertebra causing spinal cord compression. Eur Radiol. 2002;12:844–848. doi: 10.1007/s003300101034. [DOI] [PubMed] [Google Scholar]
- 43.Thames HD, Withers HR, Peters LJ, et al. Changes in early and late radiation responses with altered fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 44.Michaelsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med. 2003;348:287–94. doi: 10.1056/NEJMoa021171. [DOI] [PubMed] [Google Scholar]
- 45.Marabelle A, Sapin V, Rousseau R, Periquet B, Francois D, Kanold J. Hypercalcemia and 13-cis-retinoic acid in post-consolidation therapy of neuroblastoma. Pediatr Blood Cancer. 2009;52:280–302. doi: 10.1002/pbc.21768. [DOI] [PubMed] [Google Scholar]
- 46.Cross SF, Pozza LD, Munns CF. Hypercalcemia and osteoblastic lesions induced by 13-cis-retinoic acid mimicking relapsed neuroblastoma. Pediatr Blood Cancer. 2009;53:666–668. doi: 10.1002/pbc.22052. [DOI] [PubMed] [Google Scholar]
- 47.Hobbie WL, Mostoufi-Moab S, Carlson CA, Gruccio D, Ginsberg JP. Prevalence of advanced bone age in a cohort of patients who received cis-retinoic acid for high-risk neuroblastoma. Pediatr Blood Cancer. 2011;56:474–476. doi: 10.1002/pbc.22839. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed AR, Tan T-S, Unni KK, Collins MS, Wenger DE, Sim FH. Secondary chondrosarcoma in osteochondroma: report of 107 patients. Clin Orthop Rel Res. 2003;411:193–206. doi: 10.1097/01.blo.0000069888.31220.2b. [DOI] [PubMed] [Google Scholar]