Abstract
Tethered midbody remnants dancing across apical microvilli, encountering the centrosome, and beckoning forth a cilium—who would have guessed this is how polarized epithelial cells coordinate the end of mitosis and the beginning of ciliogenesis? New evidence from Bernabé-Rubio et al. (2016. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201601020) supports this emerging model.
In the final stages of mitosis, daughter cells are connected by a thin bridge packed with microtubules. Cytokinetic abscission severs the bridge to separate the daughters, but abscission is not a simple snip. Rather, the connection is resolved on either side or both sides of the dense midbody center. In this issue, Bernabé-Rubio et al. observed that during the growth of MDCK epithelial cells the midbody remnant remains associated with one of the daughter cells and that over time the remnant moves from the periphery toward the center of the apical surface. Using transmission electron microscopy of thin sections, Bernabé-Rubio et al. (2016) discovered that the midbody remnant remains connected to the apical plasma membrane by a thin tether. They also observed that many microvilli contact the midbody during cytokinesis and that the midbody remnant remains associated with microvilli on the apical cell surface.
The midbody remnant is not trash haplessly discarded by the cell after cytokinesis; instead the remains of the midbody are handled differently by different types of cells and can have a significant impact on cell fate and differentiation. Midbodies severed on both sides are released by some cancer cells and differentiating stem cells (Ettinger et al., 2011). Released midbodies can be endocytosed and degraded by autophagy (Crowell et al., 2014). Upon asymmetric severing, retention of the remnant can contribute to the identity of the daughter cell (Kuo et al., 2011). The discovery by Bernabé-Rubio et al. (2016) that MDCK midbody remnants are retained and move across the apical surface suggests that the remnant may have a biological role during polarization of epithelial cells.
Creation and resolution of the cytokinetic bridge involves many pathways and proteins that are also critical for formation of primary cilia. Primary cilia contain microtubules ensheathed by the ciliary plasma membrane, which is packed with receptors and coated with glycoproteins and glycolipids that transduce extracellular messages into the cell. Both cilia and midbodies contain acetylated microtubules, recruit Rab11- and Rab8-positive vesicles, and collect the Bardet-Biedle syndrome protein BBS6 (Kim et al., 2005; Yoshimura et al., 2007; Knödler et al., 2010; Guizetti et al., 2011; Kaplan and Reiner, 2011). In addition, many proteins in the midbody can also be found at the base of the cilium in the centrosome (Fabbro et al., 2005; Smith et al., 2011). IFT20 and IFT88 are important intraflagellar transport proteins that are required for ciliogenesis. Bernabé-Rubio et al. (2016) observed both IFT20 and IFT88 in the midbody and the midbody remnant that remained associated with the MDCK cells. Could the availability of shared midbody/cilia proteins be rate limiting for the formation of either structure?
Primary cilia can either grow from a centrosome docked at the cell surface or they can grow into a vesicle in the cytoplasm and emerge fully formed at the cell surface (Sorokin, 1968). In both cases, doublet microtubules grow out of the centriolar triplet microtubules to form the ciliary axoneme. Although similar in many aspects, generating cilia by these two pathways necessitates different regulatory steps. Molecular regulators of the intracellular biosynthesis pathway have been uncovered through studies of cilia from cell lines derived from deep tissue such as an immortalized retinal pigment epithelial cell line (Pugacheva et al., 2007; Liang et al., 2016). These cells form cilia when they enter Go and resorb the cilia upon entering the mitotic cycle. Primary cilia growth from centrosomes docked at the apical surface of cells that line tubules, such as bile ducts and kidney tubules (like MDCK cells), has also been investigated, and, in this case, cilia formation appears to be coupled to the process of polarization. Bernabé-Rubio et al. (2016) investigated whether there is a link between the cell cycle and ciliogenesis in cells that grow cilia from a docked centrosome, specifically whether the midbody remnant, containing IFT20 and many other cilia-related proteins, affects cilia formation.
Using four-dimensional imaging, Bernabé-Rubio et al. (2016) simultaneously followed the movement of the midbody remnant across the cell surface, the position of the centrosome, and the formation of the primary cilium. They observed that ciliogenesis occurred after the midbody remnant passed over the centrosome. Before cilia form, Bernabé-Rubio et al. (2016) observed a thin extension of fluorescent tubulin between the midbody remnant and the centrosome. They tested whether Rab8, a GTPase that is critical for intracellular ciliogenesis (Nachury et al., 2007), impacts ciliogenesis from docked centrosomes in MDCK cells and found that reduction of Rab8 by siRNA reduced the number of ciliated cells. Because Rab8 associates with intracellular vesicles, it was surprising that the midbody remnant failed to transit to the center of the apical surface in the absence of Rab8.
To test whether the midbody remnant is necessary for ciliogenesis, Bernabé-Rubio et al. (2016) developed a novel method to physically remove the midbody remnants from the periphery of cells. Using patch-clamp equipment they gently sucked the midbody remnant off of the cell surface. Instead of 80% of the cells forming primary cilia, only 20% of the cells were ciliated after removal of the midbody remnant. It is not clear whether the cilia that did form used a midbody remnant–independent pathway or if the midbody remnant removal was incomplete in some cases, but the results clearly indicate that midbody remnants can facilitate ciliogenesis.
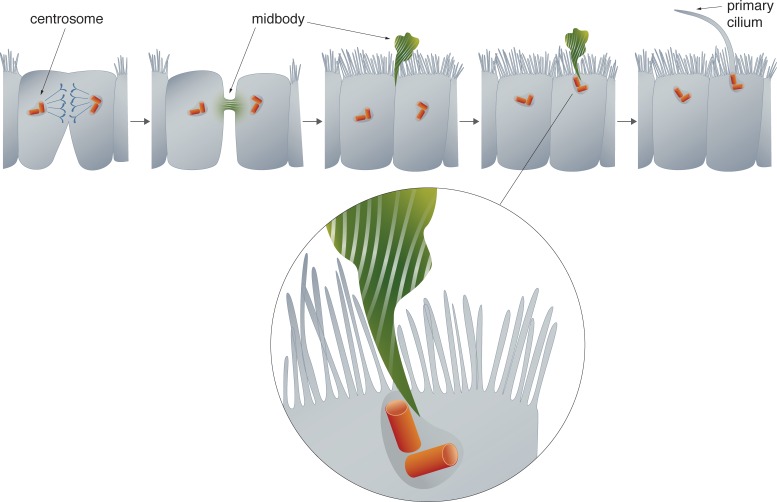
A fascinating model emerges that suggests that after abscission the midbody remnant remains tethered at the periphery of a daughter cell. The midbody remnant can transit from the periphery to the center of the apical surface and, when in close proximity to the centrosome, a bridge forms that somehow initiates a microtubule extension (Fig. 1). Fascinating questions emerge from consideration of this new paradigm. Are IFT20, IFT88, Rab8, or other proteins or lipids released from the midbody and transported to the centrosome? The presence of the microtubule bridge between the two structures makes this seem like an appealing possibility. A mechanism for direct transfer of proteins from the midbody to the centrosome would suggest that the overlapping protein palettes between these two structures is not simply a convenient coincidence, but that they function to regulate the maturation of one another.
Figure 1.
Primary cilia form after the midbody remnant passes over the centrosome. The midbody forms between daughter cells during the final stage of cytokinesis. In MDCK cells, the midbody is cleaved on only one side of the intracellular bridge and remains tethered to one of the daughter cells. The midbody remnant moves from the cell periphery to the center of the cell. At the center, microtubules extend between the midbody and the centrosome, and primary cilia formation ensues.
It is not clear how the tethered midbody travels across the cell surface. The remnant could be passed across the apical microvilli like a crowd surfer, or the association with microvilli could restrain movement and slow the arrival of the midbody remnant at the centrosome. Another interesting question is whether the centrosome must be docked at the plasma membrane when it encounters the midbody remnant or whether the association between the two structures promotes centrosome docking. Centrosome docking is not impaired in kidney epithelial cells lacking IFT20 (San Agustin et al., 2016). Future studies will hopefully also investigate whether IFT20 and IFT88 function during cytokinesis. Several previous studies have assessed IFT88 and IFT20 localization during mitosis (Follit et al., 2006; Patzke et al., 2010; Delaval et al., 2011) but Bernabé-Rubio et al. (2016) are the first to demonstrate that IFT88 and IFT20 localize to midbodies. This new observation could be a result of the specific antibodies used or of improved signal-to-noise imaging that facilitated the visualization of a small pool of these proteins in the midbody.
Bernabé-Rubio et al. (2016) tracked the progression of midbody remnant migration and ciliogenesis over time through multiple cell divisions. Midbody remnants accumulated in the population and they modeled the population transitions over generations as the midbody remnants accumulated at the center of the apical surface and cilia formed. They both modeled and observed that a reduction in the area of the cell footprint correlates with an increase of centralized midbody remnants and cilia formation. On cells grown on a patterned substrate, they observed that cells at the edge that had fewer neighbors were more likely to maintain the midbody remnant at the periphery, suggesting that junctions with neighbors are an important part of the process of polarized maturation. It is too early to conclude that changes in cell area cause the midbody to move. Midbody remnant relocation, changes in cell height, formation of junctions, and ciliogenesis are different faces of progressive cell polarization and it is not yet clear if one step facilitates the next or if all are consequences of a common regulatory mechanism.
The finding that the midbody remnant actively promotes ciliogenesis uncovers a new connection between cell division and ciliogenesis. In the 1960s, researchers reported a negative correlation between mitotic cells and cilia formation (Dingemans, 1969). Subsequent studies have elucidated several signaling pathways that stimulate cilia resorption before, or very early in, mitosis (Pugacheva et al., 2007). Serum starvation is commonly used to induce cells to exit the mitotic cycle and form primary cilia; however, epithelial cells can form cilia in the presence of serum and cell cycle exit occurs as a part of the epithelial polarization differentiation pathway. There has never before been evidence that a product from the final stage of cytokinesis could stimulate ciliogenesis, as described here by Bernabé-Rubio et al. (2016).
This mysterious new connection between the cytokinetic bridge and primary cilia will continue to unfold as we learn more about how each molecular component functions in each system. Future studies should probe whether the spatiotemporal regulation of common components controls cytokinetic abscission and ciliogenesis.
Acknowledgments
Thanks to Joe Brzostowski for helpful comments on the text. Julia Kuhl drew the figure in collaboration with C. Ott.
This work was supported by the Howard Hughes Medical Institute.
The author declares no competing financial interests.
References
- Bernabé-Rubio M., Andrés G., Casares-Arias J., Fernández-Barrera J., Rangel L., Reglero-Real N., Gershlick D.C., Fernández J.J., Millán J., Correas I., et al. 2016. Novel role for the midbody in primary ciliogenesis by polarized epithelial cells. J. Cell Biol. 10.1083/jcb.201601020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell E.F., Gaffuri A.-L., Gayraud-Morel B., Tajbakhsh S., and Echard A.. 2014. Engulfment of the midbody remnant after cytokinesis in mammalian cells. J. Cell Sci. 127:3840–3851. 10.1242/jcs.154732 [DOI] [PubMed] [Google Scholar]
- Delaval B., Bright A., Lawson N.D., and Doxsey S.. 2011. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat. Cell Biol. 13:461–468. 10.1038/ncb2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans K.P. 1969. The relation between cilia and mitoses in the mouse adenohypophysis. J. Cell Biol. 43:361–367. 10.1083/jcb.43.2.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger A.W., Wilsch-Bräuninger M., Marzesco A.-M., Bickle M., Lohmann A., Maliga Z., Karbanová J., Corbeil D., Hyman A.A., and Huttner W.B.. 2011. Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nat. Commun. 2:503 10.1038/ncomms1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro M., Zhou B.-B., Takahashi M., Sarcevic B., Lal P., Graham M.E., Gabrielli B.G., Robinson P.J., Nigg E.A., Ono Y., and Khanna K.K.. 2005. Cdk1/Erk2- and Plk1-dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis. Dev. Cell. 9:477–488. 10.1016/j.devcel.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Follit J.A., Tuft R.A., Fogarty K.E., and Pazour G.J.. 2006. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell. 17:3781–3792. 10.1091/mbc.E06-02-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizetti J., Schermelleh L., Mäntler J., Maar S., Poser I., Leonhardt H., Müller-Reichert T., and Gerlich D.W.. 2011. Cortical constriction during abscission involves helices of ESCRT-III–dependent filaments. Science. 331:1616–1620. 10.1126/science.1201847 [DOI] [PubMed] [Google Scholar]
- Kaplan A., and Reiner O.. 2011. Linking cytoplasmic dynein and transport of Rab8 vesicles to the midbody during cytokinesis by the doublecortin domain-containing 5 protein. J. Cell Sci. 124:3989–4000. 10.1242/jcs.085407 [DOI] [PubMed] [Google Scholar]
- Kim J.C., Ou Y.Y., Badano J.L., Esmail M.A., Leitch C.C., Fiedrich E., Beales P.L., Archibald J.M., Katsanis N., Rattner J.B., and Leroux M.R.. 2005. MKKS/BBS6, a divergent chaperonin-like protein linked to the obesity disorder Bardet-Biedl syndrome, is a novel centrosomal component required for cytokinesis. J. Cell Sci. 118:1007–1020. 10.1242/jcs.01676 [DOI] [PubMed] [Google Scholar]
- Knödler A., Feng S., Zhang J., Zhang X., Das A., Peränen J., and Guo W.. 2010. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl. Acad. Sci. USA. 107:6346–6351. 10.1073/pnas.1002401107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T.-C., Chen C.-T., Baron D., Onder T.T., Loewer S., Almeida S., Weismann C.M., Xu P., Houghton J.-M., Gao F.-B., et al. 2011. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat. Cell Biol. 13:1214–1223. 10.1038/ncb2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Meng D., Zhu B., and Pan J.. 2016. Mechanism of ciliary disassembly. Cell. Mol. Life Sci. 73:1787–1802. 10.1007/s00018-016-2148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury M.V., Loktev A.V., Zhang Q., Westlake C.J., Peränen J., Merdes A., Slusarski D.C., Scheller R.H., Bazan J.F., Sheffield V.C., and Jackson P.K.. 2007. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 129:1201–1213. 10.1016/j.cell.2007.03.053 [DOI] [PubMed] [Google Scholar]
- Patzke S., Redick S., Warsame A., Murga-Zamalloa C.A., Khanna H., Doxsey S., and Stokke T.. 2010. CSPP is a ciliary protein interacting with Nephrocystin 8 and required for cilia formation. Mol. Biol. Cell. 21:2555–2567. 10.1091/mbc.E09-06-0503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva E.N., Jablonski S.A., Hartman T.R., Henske E.P., and Golemis E.A.. 2007. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 129:1351–1363. 10.1016/j.cell.2007.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Agustin J.T., Klena N., Granath K., Panigrahy A., Stewart E., Devine W., Strittmatter L., Jonassen J.A., Liu X., Lo C.W., and Pazour G.J.. 2016. Genetic link between renal birth defects and congenital heart disease. Nat. Commun. 7:11103–11111. 10.1038/ncomms11103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.R., Kieserman E.K., Wang P.I., Basten S.G., Giles R.H., Marcotte E.M., and Wallingford J.B.. 2011. A role for central spindle proteins in cilia structure and function. Cytoskeleton (Hoboken). 68:112–124. 10.1002/cm.20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S.P. 1968. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3:207–230. [DOI] [PubMed] [Google Scholar]
- Yoshimura S., Egerer J., Fuchs E., Haas A.K., and Barr F.A.. 2007. Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 178:363–369. 10.1083/jcb.200703047 [DOI] [PMC free article] [PubMed] [Google Scholar]