Abstract
Background and Aims The stomata of Equisetum – the sole extant representative of an ancient group of land plants – are unique with respect to both structure and development, yet little is known about details of ultrastructure and patterning, and existing accounts of key developmental stages are conflicting.
Methods We used light and electron microscopy to examine mature stomata and stomatal development in Equisetum myriochaetum, and compared them with other land plants, including another putative fern relative, Psilotum. We reviewed published reports of stomatal development to provide a comprehensive discussion of stomata in more distantly related taxa.
Key Results Stomatal development in Equisetum is basipetal and sequential in strict linear cell files, in contrast with Psilotum, in which stomatal development occurs acropetally. In Equisetum, cell asymmetry occurs in the axial stomatal cell file, resulting in a meristemoidal mother cell that subsequently undergoes two successive asymmetric mitoses. Each stomatal cell complex is formed from a single precursor meristemoid, and consists of four cells: two guard cells and two mesogene subsidiary cells. Late periclinal divisions occur in the developing intervening cells.
Conclusions In addition to the unique mature structure, several highly unusual developmental features include a well-defined series of asymmetric and symmetric mitoses in Equisetum, which differs markedly from Psilotum and other land plants. The results contribute to our understanding of the diverse patterns of stomatal development in land plants, including contrasting pathways to paracytic stomata. They add to a considerable catalogue of highly unusual traits of horsetails – one of the most evolutionarily isolated land-plant taxa.
Keywords: Equisetum, meristemoids, Psilotum, sphenophytes, stomatal development
INTRODUCTION
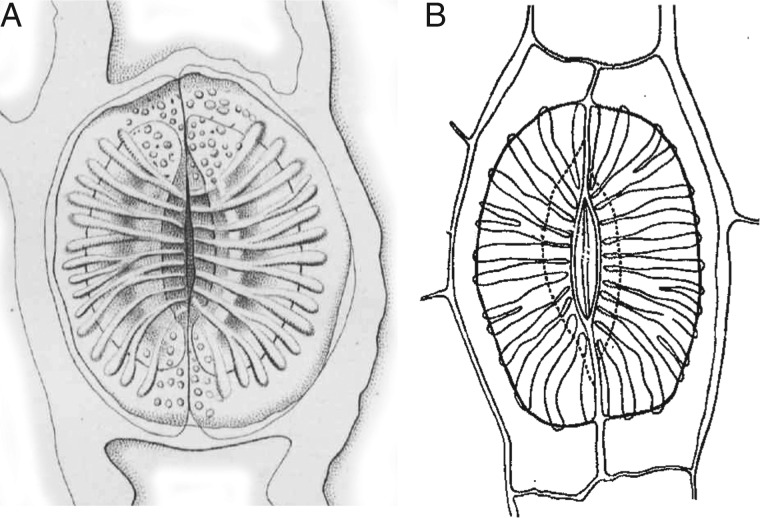
The stomata of horsetails (Equisetum L.) are unique in both their mature structure and their development. In mature stomata, two neighbouring (subsidiary) epidermal cells are superimposed immediately above the guard cells and together form the pore (e.g. Dayanandan and Kaufman, 1973). This condition contrasts with all other extant plants, in which the paired guard cells alone form the pore. Furthermore, in the pore-forming subsidiary cells of Equisetum, the lower wall possesses highly characteristic silicified thickenings radiating outwards from the pore (Fig. 1). These unusual features of Equisetum stomata also characterize well-preserved fossil material of this genus (e.g. Thomas, 1912; Thomasson, 1980; Channing et al., 2011), and hence readily allow identification of well-preserved fossils of extinct members of this ancient clade, such as the coal-swamp calamites that proliferated in the Carboniferous (Husby, 2013) and their likely progenitors, the archaeocalamites (Rowe, 1986; Bateman, 1991). Although anatomical details of the stomata are frequently poorly preserved in such ancient fossils, stomata of some extinct Carboniferous groups, such as calamites and sphenophylls, also possess characteristic radiating ribs, as in Equisetum (Thomas, 1912; Good, 1973).
Fig. 1.
Drawings of Equisetum stomata. (A) E. palustre; fig. 10 from Duval-Jouve (1864). (B) E. fluviatile; fig. 12 from Riebner (1925).
Despite a series of comparative studies spanning >150 years, the stomata of Equisetum have not hitherto been examined in detail ultrastructurally and only hand-drawn diagrams exist of contrasting developmental stages (Duval-Jouve, 1863; Strasburger, 1867; Riebner, 1925; Hauke, 1957; Chatterjee, 1964; Pant and Mehra, 1964; Pant and Kidwai, 1968; Dayanandan and Kaufman, 1973). Indeed early developmental stages are unknown in Equisetum, and debate continues regarding the sequence and homologies of the different cell types, and whether the meristemoidal division is periclinal or anticlinal. This paper represents one of a series investigating stomatal patterning and development in a broad range of land plants (cf. Rudall et al., 2012; Rudall and Knowles, 2013), the ultimate goal being to identify potential ‘fossil fingerprints’ of developmental regulation of stomata (Rudall et al., 2013).
The tracheophyte genus Equisetum is the only extant representative of an ancient plant group – the equisetophytes or sphenophytes for which the broader phylogenetic relationships remain ambiguous. Molecular phylogenies of land plants are data-rich but species-poor due to the inevitable absence of data for large numbers of extinct higher taxa. They invariably place both Equisetum and Psilotum (whisk ferns) together with ‘true’ ferns in a broad ‘fern’ clade that is itself usually resolved as sister to seed plants (Pryer et al., 2001; Ruhfel et al., 2014). However, there is some conflict in the detailed placements of these taxa within the broad ‘fern’ clade. Some molecular analyses place Equisetum as sister to a small subclade consisting of Psilotum plus Ophioglossum (e.g. Karol et al., 2010; Grewe et al., 2013), whereas others place it as sister to all other extant ferns, including Psilotum plus Ophioglossum (Rai and Graham, 2010; Rothfels et al., 2015). Conversely, morphological phylogenetic analyses are relatively data-poor but species-rich due to inclusion of extinct taxa known only from fossils. Morphological analyses place these groups in a stepwise series of clades on the spine of the land-plant tree (e.g. Rothwell and Nixon, 2006). Specifically, these stepwise clades are: the Psilotum lineage, the ‘true’ fern lineage and the Equisetum lineage, respectively. This distinction between molecular and morphological analyses is crucial, because it has considerable influence on interpretations of morphological character evolution across the tracheophytes.
In addition to documenting in detail the remarkable stomata of Equisetum (specifically those of the giant horsetail, Equisetum myriochaetum), we examine stomatal development to determine the relative roles of asymmetric divisions in the development of the stomatal complex and epidermis. Equisetum is also highly unusual among extant non-angiosperms in possessing paracytic stomata (a typological term that describes mature stomata with a distinct pair of lateral subsidiary cells oriented parallel to the guard cells; for a glossary of the complex terminology of stomata, see Rudall et al., 2013). However, paracytic stomata can be achieved via different developmental pathways, so comparative developmental studies are necessary to provide insights regarding the evolution of stomatal patterning.
MATERIALS AND METHODS
Material
Material of Equisetum myriochaetum Schlecht & Cham. was obtained from the living collections at the Royal Botanic Gardens (RBG), Kew (accession number: 1994-3391). For comparison, we also examined material of Psilotum nudum (L.) P.Beauv. from both RBG Kew (no accession number) and RBG Edinburgh (1969-7569), and Psilotum × intermedium W.H.Wagner from RBG Edinburgh (2006-0724A; this is the hybrid of P. complanatum Sw. × P. nudum). Material for light microscopy (LM) and scanning electron microscopy (SEM) was fixed in FAA and stored in 70 % ethanol. Material for transmission electron microscopy (TEM) was dissected into small pieces and fixed in 3 % phosphate-buffered glutaraldehyde followed by 1 % osmium tetroxide.
Methods
For permanent slides, samples were embedded in JB4 resin using a Leica TP1020 tissue processor. Resin blocks were cut to the required size and sectioned using either a Leica RM2155 rotary microtome with a tungsten-carbide knife or a Reichert-Jung Ultracut ultramicrotome with a glass knife. Samples were stained using toluidine blue, which stains nucleic acids and polysaccharides. Thick sections for temporary slides were prepared using a Reichert MICROM freezing-stage microtome at approx. –30ºC. Two microscopes were used for LM imaging: a Leica DMLB microscope fitted with a Zeiss RM2155 camera, and a Leica DM6000 fitted with a Leica DFC295 camera. Stomatal measurements were taken using the built-in AxioVision rel. 4·8 software on the Leica DMLB microscope.
For TEM examination, material was embedded in LR White resin after osmium staining and then sectioned using a Reichert–Jung Ultracut ultramicrotome. Ultrathin sections (50–100 nm) were collected on formvar-coated and non-coated copper grids and imaged using a Hitachi H-7650 transmission electron microsope. For SEM examination, fixed material was dried using a Supercritical Autosamdri 815B critical point drier (CPD). Dried specimens were mounted onto Cambridge stubs and coated with platinum using a Quorum Sputter Coater Q150TES. Samples were imaged at 2 kV using a Hitachi S-4700 scanning electron microscope.
RESULTS
Stomatal distribution and surface features
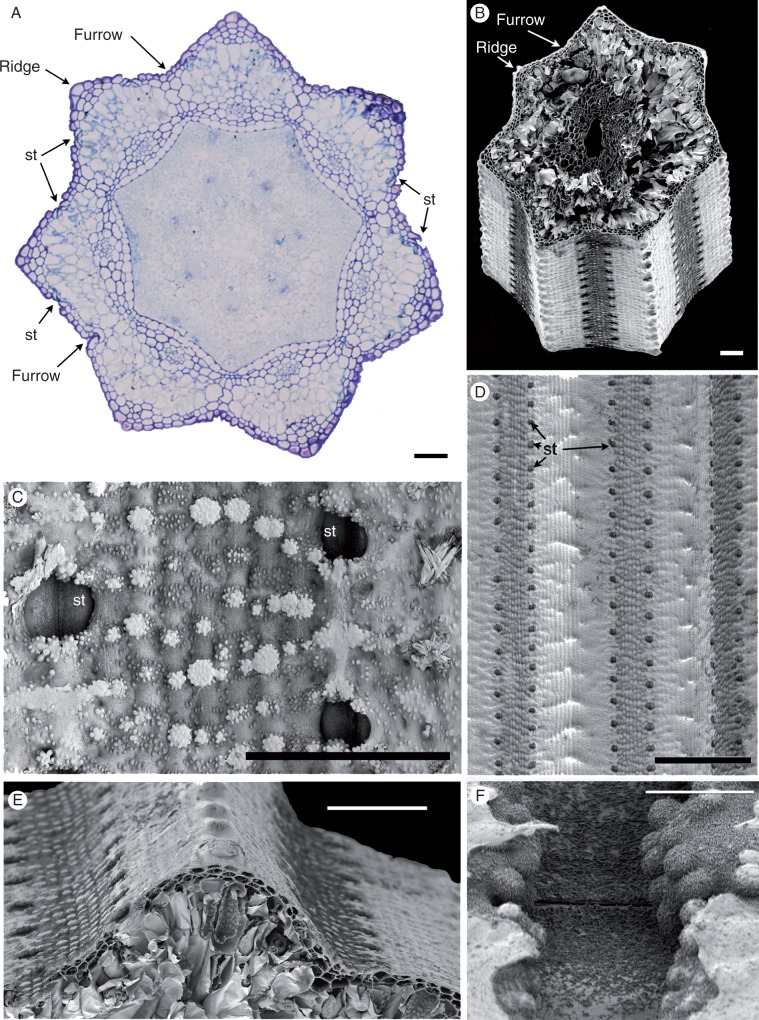
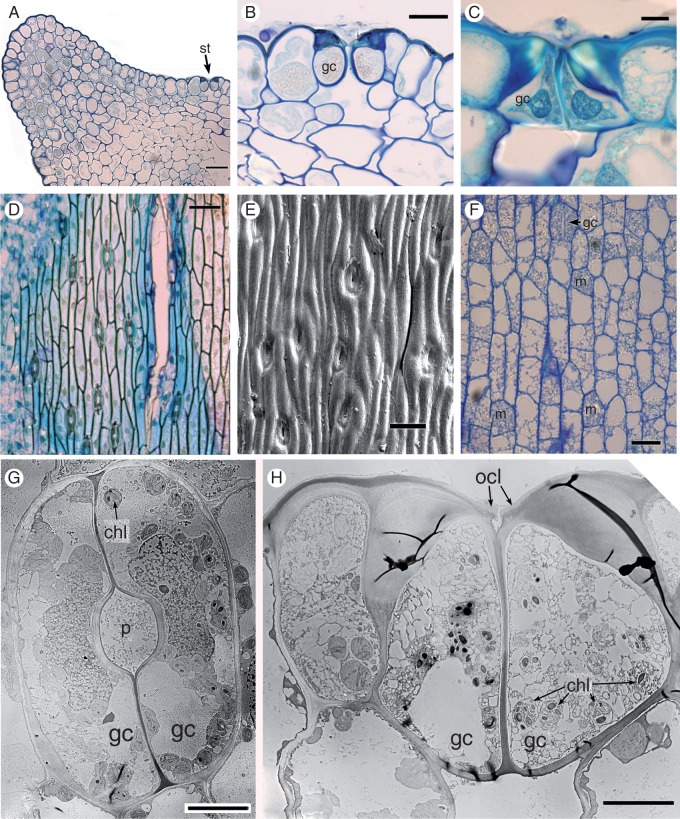
Stem transverse sections (Fig. 2A, B) have a characteristic outline with about seven ridges and intervening furrows. At the outermost point of each ridge, one or two prominent cells have relatively thickened walls. Stomata occur in furrows in two distinct axial cell files (Fig. 2A–E) mid-way between the ridges. On the leaf sheaths (not shown), stomata are present in linear rows on the abaxial epidermis; some stomata are also present on the adaxial surface, where they are restricted to the apical region.
Fig. 2.
Equisetum myriochaetum (A, LM; B–F, SEM). (A) Transverse section of the stem just above a node, showing encircling fused leaf sheaths around the stem. (B). Transverse section of the stem at the internode. (C) Details of the stem surface with sunken stomata and silica. (D, E) Stem surface showing rows of stomata midway between ridges and furrows. (F) View of a sunken (closed) stomatal pore with fine epicuticular wax flakes. St, stomata. Scale bars = 100 μm in (A), (D), (E), 10 μm in (B), (F), 50 μm in (C).
Clumps of silica particles are distributed across the plant surface (Fig. 2C). Very fine epicuticular wax flakes are also widely distributed on the plant surface (Fig. 2F); clumps of larger coiled wax rodlets are more localized.
Mature stomatal structure
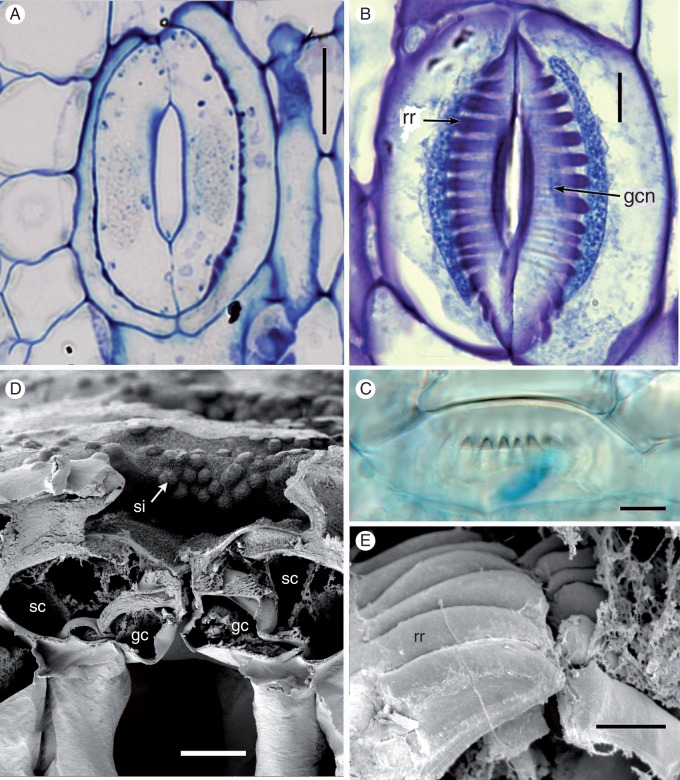
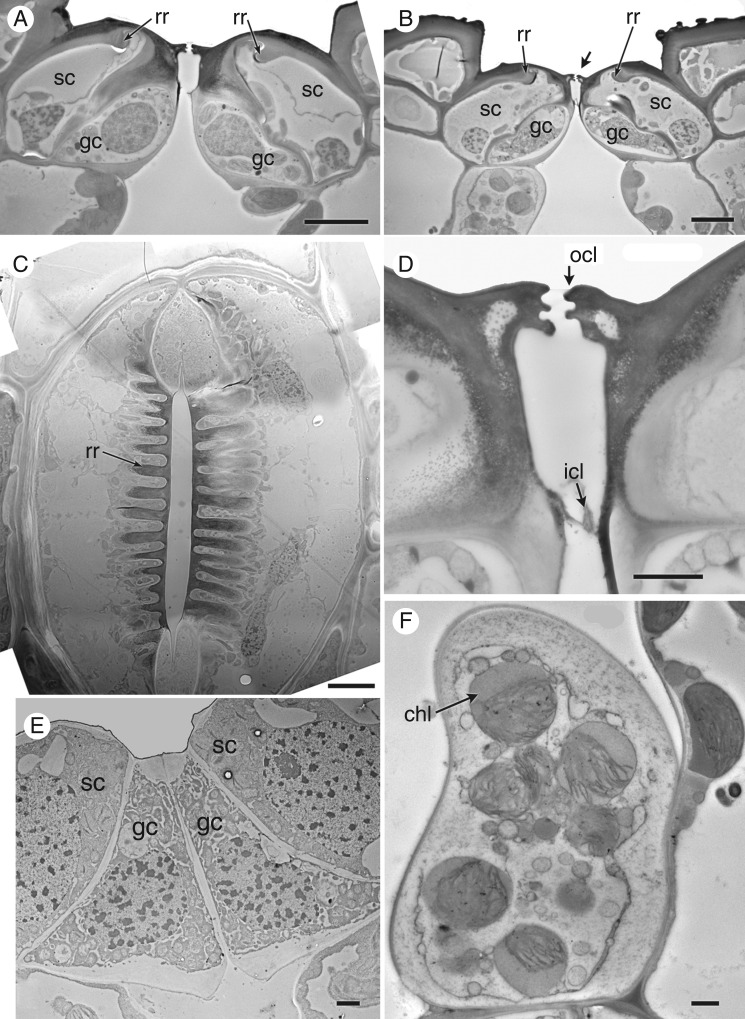
Stem stomata are described below, except where stated. All mature stomata (Figs 3 and 4) are approximately the same size (subsidiary pore-forming cells approx. 70 μm long × 60 μm wide). The stomatal complex consists of a pair of guard cells flanked by a pair of lateral subsidiary cells that over-arch the guard cells. At later stages of development, characteristic ‘ribs’ are present on the lower walls of the subsidiary cells, radiating outward from the central pore (Figs 3A–C, E and 4C). Starch is present in both the guard cells and the subsidiary cells; the subsidiary cells are also lipid-rich, particularly around the radiating ribs (Fig. 4C). The guard cells contain starch granules, many vacuoles and an elongated nucleus (Figs 3B and 4C). Chloroplasts are present in the sub-epidermal mesophyll cells (Fig. 4F), but apparently absent from the guard cells. The apertural opening is narrow (Fig. 4C, D). The subsidiary cells have an interlocking outer ledge that delimits the pore; the two guard cells also produce an inner ledge and thickenings on their lower walls (Fig. 4A, B, D).
Fig. 3.
Equisetum myriochaetum (A, B, LM; C, DIC; E, F, SEM). (A) Thin paradermal section of a mature stoma showing radiating ribs on subsidiary cells. (B) Thick paradermal section of a mature stoma with radiating ribs. Both guard cells and superadjacent subsidiary cells are visible. (C) Oblique view of a mature stoma showing radiating ribs. (D) Transverse section of a mature sunken stoma, showing silica on the surface of subsidiary cells. (E) Macerated stoma showing radiating ribs. gc, guard cell; gcn, guard cell nucleus; rr, radiating ribs; sc, subsidiary cell; sc, silica. Scale bars: 10 μm in (A−D), 5 μm in (E).
Fig. 4.
Equisetum myriochaetum (TEM). (A, B) Transverse sections of mature stomata. (C) Paradermal view of a mature stomatal complex with radiating ribs; parts of both guard cells and superadjacent subsidiary cells are visible in this plane of the section, which lies below the outer ledges that delimit the pore. (D) Detail of interlocking outer cuticular ledges on subsidiary cells, and thinner ledges on guard cells below. (E) Transverse section of a young stoma. (F) Transverse section of a mesophyll cell below the stoma. chl, chloroplast; gc, guard cell; icl, inner cuticular ledge (on guard cells); ocl, outer cuticular ledge (on subsidiary cells); rr, radiating ribs; sc, subsidiary cell. Scale bars: 10 μm in (A–C), 2 μm in (D−F).
It was difficult to judge from our material whether the apertures are open only in young stomata that lack radiating ribs, or remain almost closed; in surface view, they appear to lock shut in mature stomata (Fig. 2F).
Stomatal development
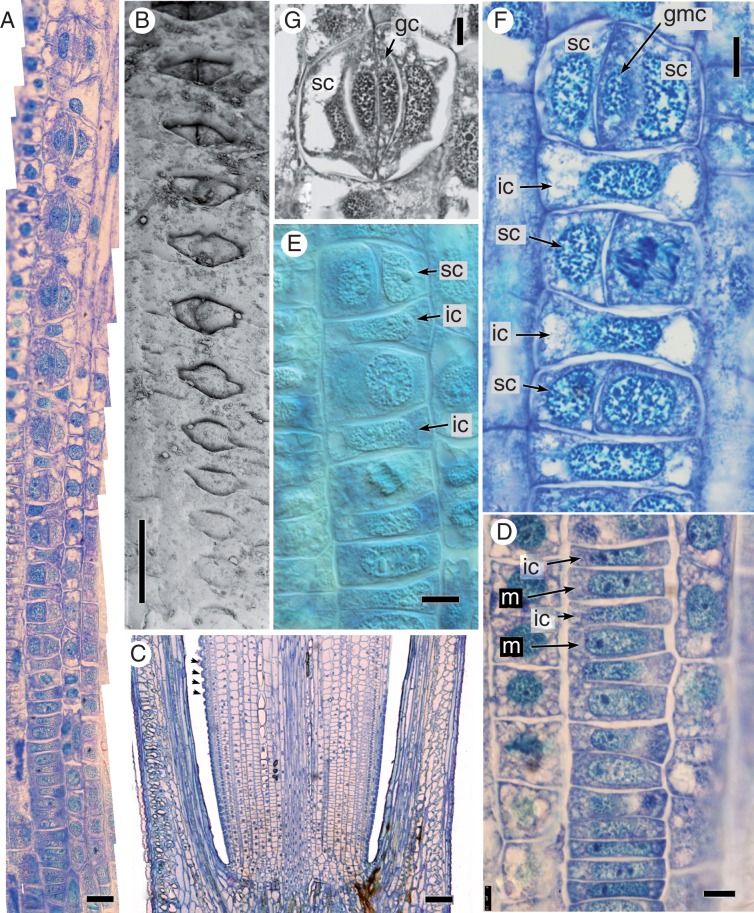
Development is sequential within axial cell files (Figs 5 and 6) and basipetal, as in grasses (i.e. within each internode, stomata closer to the apex are more differentiated and more sunken relative to those closer to the node below).
Fig. 5.
Equisetum myriochaetum, stomatal development (A, C−F, LM; B, SEM; all images oriented with plant apex uppermost). (A) Composite image showing the series of developmental stages along a single axial stomatal cell file. (B) Series of developmental stages in surface view, increasingly sunken towards the apex. (C) Longitudinal section of a stem with fully differentiated stomata arrowed; less well-developed stomata are closer to the internode. (D) Undifferentiated cells in a stomatal cell file, close to the internode; meristemoids are slightly larger than intervening cells. (E) Later stages of development, showing initial asymmetric cell division and the resulting pair of cells. (F) Later stages of development, showing the second asymmetric cell division and resulting triad. (G) Differentiated stomatal complex. gc, guard cell; gmc, guard mother cell; ic, intervening cell; m, meristemoid; sc, subsidiary cell, st, stoma. Scale bars = 20 μm in (A), 100 μm in (B), (C), 7.5 μm in (D), (F), (G).
Fig. 6.
Equisetum myriochaetum, transverse sections showing stomatal development (all LM). (A) Part of a young stem showing rib and meristemoids. (B) Detail of a meristemoid. (C) Meristemoids undergoing the first asymmetric division. (D) Detail showing recent periclinal divisions of epidermal cells. (E) An intervening cell undergoing periclinal division. (F). Stomatal triad. (G) Detail of a differentiated stoma not yet showing silicified radiating ribs. gc, guard cell, m, meristemoid; ocl, outer cuticular ledge; p, pore; sc, subsidiary cell; st, stoma. Scale bars = 50 μm in (A), 10 μm in (B), (D), (G), 20 μm in (C), 5 μm in (E), (F).
In paradermal view (Fig. 5A), at the start of a stomatal cell file (i.e. close to the internode), all cells appear undifferentiated (Fig. 5D). The meristemoids become slightly more rounded than the intervening cells. An asymmetric division takes place in the meristemoid and the smaller daughter cell forms one of the subsidiary cells (Fig. 5E). The larger daughter cell also divides asymmetrically to form both the second subsidiary cell and the central guard mother cell (GMC), resulting in a triad of cells (Fig. 5F). The direction of the first asymmetric division appears to be random. The GMC divides symmetrically to form two equal guard cells (Fig. 5G). Stomatal size increases distally along the cell file, and the stomatal cell triad becomes increasingly sunken during development (Figs 5B and 6G). After the first asymmetric division of the meristemoid, the intervening epidermal cells begin to grow over the stomatal complex and silica deposition begins. A sub-stomatal cavity forms by cell separation (i.e. schizogenously). Periclinal divisions occur in the intervening cells in the axial stomatal cell files (Fig. 6E).
In transverse sections of developmental stages (Fig. 6), the meristemoid appears much larger than adjacent cells, which divide periclinally. The first meristemoidal division sometimes occurs at a slight angle (Fig. 6C). The young stomata are clearly visible at early stages, though the silicaceous radiating ribs develop relatively late in stomatal ontogeny (Fig. 6E, F).
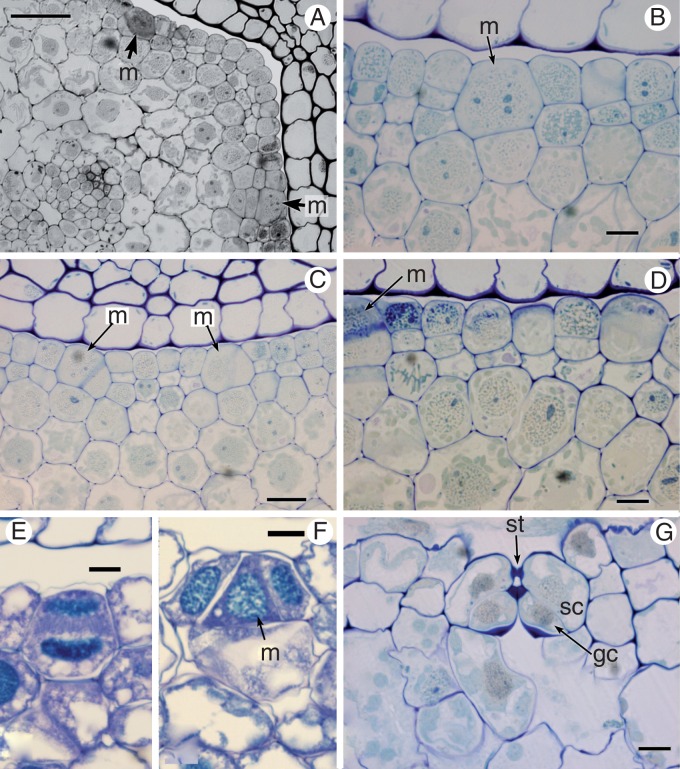
Psilotum stomata (Fig. 7)
Fig. 7.
Stomata of Psilotum (A, B, E, F, G, H, P. nudum; C, D, P. intermedium). (A, B) P. nudum, transverse section of a mature stem with detail of a stoma in (B) (C) P. intermedium, transverse section of a mature stoma. (D) P. intermedium, LM stem surface. (E) P. nudum, SEM stem surface. (F) P. nudum, paradermal section of the epidermis with guard mother cells and a recently divided stoma. (G) P. nudum, TEM paradermal section of a mature stoma. (G) P. nudum, TEM transverse section of a mature stoma (slightly off-centre, since most stomata are not quite parallel with the axis). chl, chloroplast; gc, guard cell; m, meristemoid; st, stoma. Scale bars = 50 μm in (A), 10 μm in (B), (C), (G), (H); 100 μm in (D), (E); 25 μm in (F).
In Psilotum, there is no intercalary meristem at the base of each internode, in contrast with Equisetum. Mature stomata in Psilotum are oriented parallel to the axis but do not invariably occur in discrete linear cell files, as in Equisetum (Fig. 7D–F). Asymmetric divisions in epidermal cells distort existing cell files and create new ones. Neighbouring cells to guard cells are not morphologically distinct from other epidermal cells; thus, subsidiary cells are absent (Fig. 7D, E). A range of different stomatal stages can occupy cell files in the same region, in an apparently haphazard pattern (Fig. 7F). Thus, stages of meristemoid initiation were not readily observed. Meristemoids divide symmetrically to form a pair of guard cells (Fig. 7F). In contrast with Equisetum, stomata of Psilotum lack radiating ribs, and the guard cells form the pore (Fig. 7G, H). Mature stomata in Psilotum are relatively round in surface view compared with the more elongated stomata of Equisetum. They possess a pair of guard cells with heavily thickened outer walls and an outer stomatal ledge (Fig. 7B, C, H). Small chloroplasts are present in the guard cells (Fig. 7G, H).
DISCUSSION
Mature structure and function of Equisetum stomata
Perhaps the most distinctive feature of mature Equisetum stomata is the vault-like radiating ribs in the subsidiary cells, which allow comparison of this isolated ‘living fossil’ with extinct sphenophytes known only from fossils. Other studies of extant Equisetum stomata have shown that these characteristic ribs are composed of cellulose impregnated with silica; indeed, they are primarily silicaceous in regions close to the stomatal pore (Kaufman et al., 1971; Dayanandan and Kaufman, 1973). Many studies have suggested that the thickenings are located in the guard cells, an erroneous conclusion that is unfortunately readily drawn from observations that are made entirely in surface view. In particular, studies of fossil cuticles typically report thickenings in the guard cells, because it is difficult to distinguish between guard cells and subsidiary cells in poorly preserved material; for example, Thomas (1912) reported this feature in the leaves of fossil calamites. However, our investigation clearly shows that the thickenings in Equisetum are located in the overlying subsidiary cells that together form the pore (Figs 3 and 4).
Our investigation highlights ultrastructural details of the mature stomata of Equisetum. To our knowledge, the only previously published TEM image of Equisetum stomata depicts E. hyemale (Sack, 1987), in which the silicified radiating ribs appear relatively large. Our study shows that the radiating ribs are formed late in stem development, consistent with the relatively late deposition of silica (compare relatively young stages in Figs 5G and 6G with older stages in Fig. 4A–C). Within Equisetum, the stomatal apparatus is taxonomically significant in delimiting the two subgenera (e.g. Channing et al., 2011), though one subgenus is paraphyletic in molecular trees (Des Marais et al., 2003; Guillon, 2004, 2007). Stomatal cell size and the number of rib thickenings in the subsidiary cells also differ among species.
With regard to stomatal function in Equisetum, the function of the radiating ribs is obscure and clearly merits research. However, it appears highly unlikely that mature silicified stomata are functional in terms of pore opening or closure, though injection experiments have suggested limited stomatal movement in younger stomata (Barber, 1961; Kaufman et al., 1973). Equisetum is probably unique among extant tracheophyte taxa in that the subsidiary cells form the pore. The subsidiary cells appear to lock shut in mature stomata (Fig. 2F; see also Dayanandan and Kaufman, 1973). The two closely interlocking pairs of ledges on subsidiary cell and guard cell walls effectively create a chamber that presumably acts as a barrier to excessive water loss and could also prevent water from entering the sub-stomatal cavities or reduce gas diffusion, as in stomata on moss sporophytes (Merced and Renzaglia, 2013). However, they apparently do not prevent wilting of cut horsetail stems placed in water (J. Duckworth, pers. comm.; and pers. obs.). Relatively thin-walled stomata are common on the adaxial surface of the leaf sheath (i.e. facing the axis), where they are often reported as hydathodes (e.g. Dayanandan and Kaufmann, 1973). We found starch in both the guard cells and the lipid-rich subsidiary cells in Equisetum, but chloroplasts were absent from both cell types, suggesting relatively low concentrations of the energy-bearing molecule ATP, a feature that is sometimes correlated with reduced active stomatal movement (Wang et al., 2014). Since cell wall-bound silica reinforces mechanical properties, it seems likely that the silicaceous thickenings provide rigidity that effectively blocks all movement of the cell walls in older stomata (Riebner, 1925; Dayanandan and Kaufmann, 1973).
In other land plants, the stomata of ferns and lycophytes do not respond to the phytohormone abscisic acid (ABA) and lack active stomatal control (McAdam and Brodribb, 2012a, b); stomatal movement is also absent from hornworts (Pressel et al., 2014). Ziegler (1987) considered the guard-cell movements of Psilotum to be similar to that of the clubmoss Huperzia; both conform to the passive hydraulic model. Our results on Psilotum found that small chloroplasts are present in the guard cells, but the outer walls are greatly thickened in mature stomata (Fig. 7), suggesting that pore movements are at best limited at this stage. In contrast, studies report an active ABA response in stomata of the mosses Physcomitrella and Funaria (Chater et al., 2011, 2013) and the lycophyte Selaginella (Ruszala et al., 2011), leading these authors to conclude that active stomatal control occurs throughout land plants, with possible loss of this feature in the fern lineage. These contrasting observations indicate that stomatal function requires further investigation in land plants, and may well differ among major groups. In particular, Equisetum and Psilotum, both among the few extant representatives of ancient land-plant lineages, could represent phylogenetically critical taxa in this ongoing debate.
Development
Stomatal development in Equisetum is basipetal from an intercalary meristem; it is also sequential in strict linear cell files, so that the youngest stages are closest to the internode. In contrast, stomatal development in Psilotum occurs acropetally, so that the youngest stages are closest to the apical meristem. In this respect, the growth pattern in Equisetum is analogous with the intercalary growth of distantly related taxa such as grasses; both groups achieve relatively rapid axial extension through intercalary meristems located above each node (e.g. Golub and Wetmore, 1948; Husby, 2013).
Some authors (e.g. Duval-Jouve, 1864; Chatterjee, 1964) have controversially suggested that a periclinal division of the meristemoid occurs during stomatal development in Equisetum. Chatterjee (1964) reported that the resulting inner cell forms a substomatal mesophyll cell, and successive anticlinal divisions of the outer cell form the stomatal complex. In agreement with other studies (e.g. Strasburger, 1867; Hauke, 1957; Pant and Mehra, 1964; Pant and Kidwai, 1968), our study found no evidence for a periclinal division in stomatal meristemoids of Equisetum, though the first asymmetric division of the meristemoid is oriented at a slight angle to the surface. However, we note that a periclinal division does occur in the intervening cells between meristemoid mother cells in axial cell files. Of the two daughter cells resulting from this periclinal division, the outer one forms an epidermal cell and the inner one forms part of the underlying tissue. We also note that periclinal epidermal divisions do occur at early stages in stem formation in Equisetum. In contrast, periclinal divisions in seed plants are confined to the shoot apex, and cease as soon as a distinct epidermis has differentiated.
The developmental pathway of the guard cells in Equisetum is markedly different from that of Psilotum (and indeed from those of all other extant taxa). Equisetum has mesogenous stomata derived from a series of asymmetric mitoses, whereas development in Psilotum is probably entirely perigenous, though this aspect requires more detailed investigation. Our observations of Psilotum axes were inconclusive in this respect, in common with earlier studies of Psilotales that concluded that development is most probably perigenous (Pant and Mehra, 1963; Maroti, 1966; Pant and Khare, 1971; Payne, 1979; Mickle et al., 2012). In Equisetum, our study and some previous studies (e.g. Pant and Kidwai, 1968) have demonstrated two successive asymmetric mitoses that form the two lateral subsidiary cells in the stomatal complex, followed by a symmetric division of the central cell to form the guard cells. Hence, Equisetum is apparently unique among taxa with stomata that develop in axial cell files, in that both lateral subsidiary cells are mesogenous (i.e. share a common initial cell with the same meristemoid). Our study also explores earlier stages, and uncovers further cell asymmetry in the earlier formed axial stomatal cell file, resulting in a meristemoidal mother cell that subsequently undergoes two successive asymmetric mitoses, as described above.
Paracytic stomata are widely considered to represent a defining trait for angiosperms, in common with the extinct seed-plant order Bennettitales and some extant Gnetales (e.g. Doyle and Donoghue, 1992; reviewed by Rudall et al., 2013), though debate continues regarding the ancestral state for this developmental character. Thus, the presence of paracytic stomata in Equisetum – a taxon that is phylogenetically remote from these groups – is noteworthy. The fact that development of the stomatal complex differs radically between Equisetum and angiosperms with paracytic stomata strongly supports the conclusion that they represent character convergence. For example, grasses possess paracytic stomata with distinct lateral subsidiary cells, but, in grasses, the meristemoid that is formed by asymmetric division in the axial cell file divides only once (symmetrically) to form a pair of guard cells. Thus, in contrast to Equisetum, the lateral subsidiary cells of grasses are perigenous cells derived from asymmetric mitoses in the neighbouring cell lineages. In maize, the PANGLOSS1 (PAN1) gene controls the formation of the lateral subsidiary cells (Peterson et al., 2010).
In the archetypal model angiosperm Arabidopsis stomatal development is at least partly controlled by a group of basic helix–loop–helix (bHLH) transcription factors (e.g. Pillitteri et al., 2011): SPEECHLESS (SPCH), which initiates asymmetric divisions; MUTE, which controls acquisition of GMC identity; FAMA, which initiates guard-cell differentiation; and two partially redundant bHLH genes, SCRM and SCRM2. Given the highly divergent mode of stomatal development shown by Equisetum, it would be interesting to determine whether these group IA bHLH genes are functionally conserved in this isolated taxon. Although both groups are tracheophytes, the phylogenetic relationship between sphenopsids and angiosperms is very distant. Earlier phylogenies of these genes based on a restricted taxon sampling indicate that early land plants lacked SPCH-like activity and that FAMA is closest to the ancestral form within this gene family (MacAlister and Bergmann, 2011).
Conclusions
It is interesting to compare horsetails with the grass family (Poaceae), because they possess several morphological similarities that are either unique or highly unusual, but ultimately highly divergent in many respects, consistent with the vast phylogenetic distance between these two groups. Stomatal development in Equisetum is basipetal and sequential in strict linear cell files, as in grasses. Both taxa possess intercalary meristems that facilitate rapid regrowth (Golub and Wetmore, 1948). Another highly unusual feature, a mixed-linkage hemicellulose (MLG), characterizes cell walls of both grasses and Equisetum (Fry et al., 2008a; Sørensen et al., 2008). Since both horsetails and grasses also accumulate high concentrations of silica in their cell walls, Fry et al. (2008a) suggested that MLG has a role in cell-wall silicification. However, the occurrence of a unique enzyme (MXE) in walls of horsetails (but not in grasses), which binds MLG to xyloglucan, indicates different mechanisms of cell-wall modification between the two groups (Fry et al., 2008b). Furthermore, both grasses and horsetails possess perforations in the cell walls linking the two guard cells, large enough for plastids to pass through (Dayanandan and Kaufman, 1973; Sack, 1987; this study), though this feature is poorly known and could be relatively widespread. Finally, stomata are clearly paracytic in both grasses and horsetails, both groups possessing lateral subsidiary cells that differ greatly in morphology from adjacent cells.
However, these similarities are relatively superficial and mostly relate to mature patterning rather than to development or function. Our study highlights that stomatal development differs greatly between Equisetum and grasses, as outlined above. Furthermore, stomatal function probably also differs greatly between Equisetum and grasses. In grasses, stomatal opening is facilitated by the lateral displacement of turgor via osmotic ‘see-sawing’ between the guard cells and subsidiary cells, whereas stomata of Equisetum are entirely closed in mature stems.
In conclusion, our investigation underlines the divergence of stomata of Equisetum from those of all other extant taxa. Stomata of ferns, Psilotum and horsetails differ radically from each other (reviewed by Payne, 1979; Rudall et al., 2013). In addition to features that were already known (though relatively poorly documented) in Equisetum, such as the pore-forming subsidiary cells with vault-like silicified radiating ribs and interlocking ledges, several developmental features are unique or highly unusual. These features include basipetal development and a well-defined series of asymmetric and symmetric mitoses. They add to a considerable catalogue of highly unusual traits of horsetails – evidently one of the most divergent and phylogenetically isolated land-plant taxa.
ACKNOWLEDGEMENTS
We thank Richard Bateman for critically reading the manuscript, and Louis Ronse De Craene for supplying material of Psilotum from the Royal Botanic Garden Edinburgh. Thomas Gregory provided lab support and training for one of us (E.C.) during her research year based at Kew.
LITERATURE CITED
- Barber DA. 1961. Gas exchange between Equisetum limosum and its environment. Journal of Experimental Botany 12: 243–251. [Google Scholar]
- Bateman RM. 1991. Palaeobiological and phylogenetic implications of anatomically-preserved archaeocalamites from the Dinantian of Oxroad Bay and Loch Humphrey Burn, southern Scotland. Palaeontographica B 223: 1–59 + 8 plates. [Google Scholar]
- Channing A, Zamuner A, Edwards D, Guido D. 2011. Equisetum thermale sp. nov. (Equisetales) from the Jurassic San Agustin hot spring deposit, Patagonia: anatomy, paleoecology, and inferred paleoecophysiology. American Journal of Botany 98: 680–697. [DOI] [PubMed] [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, et al. 2011. Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Current Biology 21: 1025–1029. [DOI] [PubMed] [Google Scholar]
- Chater C, Gray JE, Beerling DJ. 2013. Early evolutionary acquisition of stomatal control and development gene signalling networks. Current Opinion in Plant Biology 16: 638–646. [DOI] [PubMed] [Google Scholar]
- Chatterjee J. 1964. Stomata in Equisetum ramosissimum Desf. subsp. ramosissimum. Phytomorphology 14: 451–457. [Google Scholar]
- Dayanandan P, Kaufman PB. 1973. Stomata in Equisetum. Canadian Journal of Botany 51: 1555–1564. [Google Scholar]
- Des Marais DL, Smith AR, Britton DM. 2003. Phylogenetic relationships and evolution of extant horsetails, Equisetum, based on chloroplast DNA sequence data (rbcL and trnF). International Journal of Plant Sciences 164: 737–751. [Google Scholar]
- Doyle JA, Donoghue MJ. 1992. Fossils and seed plant phylogeny reanalyzed. Brittonia 44: 89–106. [Google Scholar]
- Duval-Jouve J. 1863. Histoire naturelle des Equisetum de France. Paris: J. B. Bailiere. [Google Scholar]
- Fry SC, Nesselrode BHWA, Miller JG, Mewburn BR. 2008a. Mixed-linkage (1→3,1→4)-β-d-glucan is a major hemicellulose of Equisetum (horsetail) cell walls. New Phytologist 179: 104–115. [DOI] [PubMed] [Google Scholar]
- Fry SC, Mohler KE, Nesselrode BHWA, Franková L. 2008b. Mixed-linkage β-glucan:xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. The Plant Journal 55: 240–252. [DOI] [PubMed] [Google Scholar]
- Golub SJ, Wetmore RH. 1948. Studies of development in the vegetative shoot of Equisetum arvense L. II. The mature shoot. American Journal of Botany 35: 767–781. [Google Scholar]
- Good CW. 1973. Studies of Sphenophyllum shoots: species delimitation within the taxon Sphenophyllum. American Journal of Botany 60: 929–939. [Google Scholar]
- Grewe F, Guo W, Gubbels EA, Hansen AK, Mower JP. 2013. Complete plastid genomes from Ophioglossum californicum, Psilotum nudum, and Equisetum hyemale reveal an ancestral land plant genome structure and resolve the position of Equisetales among monilophytes. BMC Evolutionary Biology 13: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon JM. 2004. Phylogeny of horsetails (Equisetum) based on the chloroplast rps4 gene and adjacent noncoding sequences. Systematic Botany 29: 251−259. [Google Scholar]
- Guillon JM. 2007. Molecular phylogeny of horsetails (Equisetum) including chloroplast atpB sequences. Journal of Plant Research 120: 569–574. [DOI] [PubMed] [Google Scholar]
- Hauke RL. 1957. The stomatal apparatus of Equisetum. Bulletin of the Torrey Botanical Club 84: 178–181. [Google Scholar]
- Husby C. 2013. Biology and functional ecology of Equisetum with emphasis on the giant horsetails. Botanical Review 79: 147–177. [Google Scholar]
- Karol KG, Arumuganathan K, Boore JL, et al. 2010. Complete plastome sequences of Equisetum arvense and Isoetes flaccida: implications for phylogeny and plastid genome evolution of early land plant lineages. BMC Evolutionary Biology 10: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PB, Bigelow WC, Schmid R, Ghosheh NS. 1971. Electron microprobe analysis of silica in epidermal cells of Equisetum. American Journal of Botany 58: 309–316. [Google Scholar]
- Kaufman PB, Lacroix JD, Dayanandan P, Lawrence F, Rosen JJ, Bicelow CW. 1973. Silicification of developing internodes in the perennial scouring rush (Equisetum hyemale var. affine). Developmental Biology 31: 124–135. [DOI] [PubMed] [Google Scholar]
- MacAlister AC, Bergmann DC. 2011. Sequence and function of basic helix–loop–helix proteins required for stomatal development in Arabidopsis are deeply conserved in land plants. Evolution and Development 13: 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroti I. 1966. Development of Tmesopsida and Pteropsida leaves and histogenesis of the epidermis. Acta Biologica 12: 37–60. [Google Scholar]
- McAdam AM, Brodribb TJ. 2012a. Fern and lycophyte guard cells do not respond to endogenous abscisic acid. The Plant Cell 24: 1510–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam AM, Brodribb TJ. 2012b. Stomatal innovation and the rise of seed plants. Ecology Letters 15: 1–8. [DOI] [PubMed] [Google Scholar]
- Merced A, Renzaglia KS. 2013. Moss stomata in highly elaborated Oedipodium (Oedipodiaceae) and highly reduced Ephemerum (Pottiaceae) sporophytes are remarkably similar. American Journal of Botany 100: 2318–2327. [DOI] [PubMed] [Google Scholar]
- Mickle JE, Barone Lumaga MR, De Luca P. 2012. Stomatal development in aerial axes of Psilotum nudum (Psilotaceae). Journal of the North Carolina Academy of Science 128: 95–99. [Google Scholar]
- Pant DD, Khare PK. 1971. Epidermal structure of Psilotales and stomatal ontogeny of Tmesipteris tannensis Bernh. Annals of Botany 35: 151–157. [Google Scholar]
- Pant DD, Kidwai PF. 1968. Development of stomata in Equisetum. Annals of Botany 32: 601–608. [Google Scholar]
- Pant DD, Mehra B. 1963. Development of stomata in Psilotum nudum (L.) Beauv. Current Science 32: 420–422. [Google Scholar]
- Pant DD, Mehra B. 1964. Development of stomata in some fern allies. Proceedings of the National Institute of Sciences, India 30: 92–98. [Google Scholar]
- Payne WW. 1979. Stomatal patterns in embryophytes: their evolution, ontogeny and interpretation. Taxon 28: 117–132. [Google Scholar]
- Peterson KM, Rychel AL, Torii KU. 2010. Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. The Plant Cell 22: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Peterson KM, Horst RJ, Torii KU. 2011. Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. The Plant Cell 23: 3260–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressel S, Goral T, Duckett JG. 2014. Stomatal differentiation and abnormal stomata in hornworts. Journal of Bryology 36: 87–103. [Google Scholar]
- Pryer KM, Schneider H, Smith AR, et al. 2001. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature 409: 618–622. [DOI] [PubMed] [Google Scholar]
- Rai HS, Graham SW. 2010. Utility of a large, multigene plastid data set in inferring higher-order relationships in ferns and relatives (Monilophytes). American Journal of Botany 97: 1444–1456. [DOI] [PubMed] [Google Scholar]
- Riebner F. 1925. Ũber bau und funktion der spaltoffnungsapparate bei den Equisetinae und Lycopodiinae. Planta 1: 260–300. [Google Scholar]
- Rothfels CJ, Li FW, Sigel EM, et al. 2015. The evolutionary history of ferns inferred from 25 low-copy nuclear genes. American Journal of Botany 102: 1089–1107. [DOI] [PubMed] [Google Scholar]
- Rothwell GW, Nixon KC. 2006. How does the inclusion of fossil data change our conclusions about the phylogenetic history of euphyllophytes? International Journal of Plant Sciences 167: 737–749. [Google Scholar]
- Rowe NP. 1986. The fossil flora of the Drybrook Sandstone (Lower Carboniferous) of the Forest of Dean, Gloucestershire. PhD thesis, University of Bristol, UK.
- Rudall PJ, Knowles EVW. 2013. Ultrastructure of stomatal development in early-divergent angiosperms reveals contrasting patterning and pre-patterning. Annals of Botany 112: 1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudall PJ, Rowland AV, Bateman RM. 2012. Ultrastructure of stomatal development in Ginkgo biloba. International Journal of Plant Sciences 173: 849–860. [Google Scholar]
- Rudall PJ, Hilton J, Bateman RM. 2013. Several developmental and morphogenetic factors govern the diversity of stomatal development in land plants. New Phytologist 200: 598–614. [DOI] [PubMed] [Google Scholar]
- Ruhfel BD, Gitzendanner MA, Soltis PS, Soltis DE, Burleigh JG. 2014. From algae to angiosperms – inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evolutionary Biology 14: 23. doi:10.1186/1471-2148-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, et al. 2011. Land plants acquired active stomatal control early in their evolutionary history. Current Biology 21: 1030–1035. [DOI] [PubMed] [Google Scholar]
- Sack FD. 1987. The development and structure of stomata In: Zeiger E, Farquhar GD, Cowan IR, eds. Stomatal function. Stanford, CA: Stanford University Press, 59–89. [Google Scholar]
- Sørensen I, Pettolino FA, Wilson SM, et al. 2008. Mixed-linkage (1→3), (1→4)-β-d-glucan is not unique to the Poales and is an abundant component of Equisetum arvense cell walls. The Plant Journal 54: 510–521. [DOI] [PubMed] [Google Scholar]
- Strasburger E. 1867. Ein Beitrag zur Entwicklungsgeschichte der Spaltöffnungen. Jahrbücher fur Wissenschaftliche Botanik 5: 297–342. [Google Scholar]
- Thomas HH. 1912. On the leaves of calamites (Calamocladus section). Philosophical Transactions of the Royal Society B: Biologial Sciences 202: 51–92. [Google Scholar]
- Thomasson JR. 1980. A fossil Equisetum sp. (family Equisetaceae, subgenus Hippochaetae) from the Late Tertiary Ash Hollow Formation of Nebraska. American Journal of Botany 67: 125–127. [Google Scholar]
- Wang SW, Ying L, Zhang XL, et al. 2014. Lacking chloroplasts in guard cells of crumpled leaf attenuates stomatal opening: both guard cell chloroplasts and mesophyll contribute to guard cell ATP levels. Plant, Cell and Environment 37: 2201–2210. [DOI] [PubMed] [Google Scholar]
- Ziegler H. 1987. The evolution of stomata In: Zeiger E, Farquhar GD, Cowan IR, eds. Stomatal function. Stanford, CA: Stanford University Press, 29–57. [Google Scholar]