Abstract
Backgrounds and Aims Floral traits that attract pollinators may also attract seed predators, which, in turn, may generate conflicting natural selection on such traits. Although such selection trade-offs are expected to vary geographically, few studies have investigated selection mediated by pollinators and seed predators across a geographic mosaic of environments and floral variation.
Methods Floral traits were investigated in 14 populations of the bumble-bee-pollinated herb, Pedicularis rex, in which tubular flowers are subtended by cupular bracts holding rain water. To study potentially conflicting selection on floral traits generated by pollinators and florivores, stigmatic pollen loads, initial seed set, pre-dispersal seed predation and final viable seed production were measured in 12–14 populations in the field.
Key Results Generalized Linear Model (GLM) analyses indicated that the pollen load on stigmas was positively related to the exsertion of the corolla beyond the cupular bracts and size of the lower corolla lip, but so too was the rate of seed predation, creating conflicting selection on both floral traits. A geographic mosaic of selection mediated by seed predators, but not pollinators, was indicated by significant variation in levels of seed predation and the inclusion of two-, three- and four-way interaction terms between population and seed predation in the best model [lowest corrected Akaike Information Criterion (AICc)] explaining final seed production.
Conclusions These results indicate opposing selection in operation: pollinators generated selection for greater floral exsertion beyond the bracts, but seed predators generated selection for reduced exsertion above the protective pools of water, although the strength of the latter varied across populations.
Keywords: Corolla tube length, cupular bract, geographic selection mosaic, Pedicularis rex, pre-dispersal seed predation, phenotypic selection analysis, stigmatic pollen load, seed survival
INTRODUCTION
The evolution of floral traits has traditionally been thought to be moulded by the most frequent and effective pollinators (Stebbins, 1970). Floral traits attractive to pollinators, however, may also attract plant enemies such as herbivores that eat flower parts (florivores and seed predators). Although most early studies of plant–animal interactions investigated either attraction of pollinators or instead defence against herbivores, an increasing number of papers now suggest that most floral traits experience selection generated by both mutualists and antagonists (Strauss et al., 1996; Strauss and Armbruster, 1997; Galen, 1999; Mothershead and Marquis, 2000; Steffan-Dewenter et al., 2001; Irwin et al., 2003, 2004; McCall and Irwin, 2006; Rey et al., 2006; Strauss and Whittall, 2006; Parachnowitsch and Caruso, 2008; Bartkowska and Johnston, 2012; Theis and Adler, 2012; Kessler et al., 2013; Talluto and Benkman, 2014). For example, selection on floral traits generated by pre-dispersal seed predators might be in the opposite direction to that generated by pollinators, given that seed predators and pollinators may use the same floral traits (e.g. floral shape, colour and scent) to find flowers for oviposition or for mutualistically feeding on nectar, respectively (e.g. Ehrlén et al., 2002; de Waal et al., 2012; de Jager and Ellis, 2013; Pérez-Barrales et al., 2013). This effect might be especially strong if ovipositing seed predators can use floral traits that influence pollination success as a way to predict later host substrate quality for their offspring (e.g. Pérez-Barrales et al., 2013).
It is also increasingly apparent that geographic variation in the pollinator fauna, seed predator fauna and/or floral trait values often creates geographic mosaics of phenotypic selection (Thompson, 2005). Covariation between plant traits and pollinator and/or herbivore traits is an expected outcome of this situation, and such covariation observed in nature may, in turn, provide evidence for the existence of the selection mosaic (see Herrera et al., 2006). For example, corolla tube lengths in both Zaluzianskya microsiphon and Lapeirousia anceps covary geographically with tongue length of long-proboscid fly pollinators in South Africa. These were interpreted as a geographic selection mosaic because seed set and/or pollen deposition depended on the length of the flower tube relative to the length of the fly tongue (Anderson and Johnson, 2008; Pauw et al., 2009). Pollinators with longer tongues relative to floral tube length could ingest more nectar, and plants with longer tubes relative to pollinator tongue length benefited with higher pollen deposition. However, long-tubed flowers may experience selection in the opposite direction if seed predators (or nectar robbers) differentially exploit larger flowers. For example, conflicting selection was generated by pollinators and damage-inflicting ants, and was shown to influence the evolution of flower shape in bumble-bee-pollinated Polemonium viscosum. Plants bearing flowers with short, flared corollas were more attractive to bumble-bee pollinators but more vulnerable to ant predation (Galen and Cuba, 2001). While considerable research now suggests that selection mediated by seed predators (or nectar thieves) often runs counter to selection on the same traits mediated by pollinators (Herrera, 2000; Gómez, 2003, 2008; Irwin et al., 2003, 2010; Cariveau et al., 2004; Strauss and Whittall, 2006; Pérez-Barrales et al., 2013), geographic variation in selection generated by both pollinators and herbivores remains largely unexplored (but see Thompson and Pellmyr, 1992; Galen and Cuba, 2001; Siepielski and Benkman, 2010; Ågren et al., 2013; and reviews in Thompson 2005, 2013).
Here we investigate variation in floral traits over a large geographical area in a bumble-bee-pollinated sub-alpine herb, Pedicularis rex. We also assess variation in components of reproductive success as influenced by both pollinators and pre-dispersal seed predators. This study permits us to ask whether: (1) floral traits vary geographically; (2) there is conflicting selection generated by pollinators and seed predators within populations; and (3) there is variation among populations in these selective pressures. To examine the possible effects of selection by pollinators and herbivores on floral traits, we measured stigmatic pollen loads in flowers in 14 populations and seed production and seed predation in 12 of these populations. If flowers with wider corolla lobes or longer corolla tubes attract more pollinators and/or receive more pollen on stigmas, do they also attract more enemies, such as seed predators? We thus not only investigate possible conflicting selection by pollinators and florivores, but also attempt to compare patterns of selection on floral traits across multiple populations.
MATERIALS AND METHODS
Study species and sites
Pedicularis rex Franch. (Orobanchaceae) is a self-compatible, perennial herb endemic to south-west China (Yang et al., 1998; Tang et al., 2007). It flowers from late June to early August. Flowering individuals can grow up to 1·5 m and produce numerous vertical spike-like racemes with highly zygomorphic pink or yellow flowers. Flowers are arranged in whorls on each raceme (usually 3–5 flowers per whorl) and open in sequence from bottom to top. The pinnatisect to pinnatipartite leaves are borne in whorls, and the base of each whorl forms a cupular ‘bract’ (CB). Flowers are ‘approach’ herkogamous (Lloyd and Webb, 1992), with the receptive stigma exserted from the corolla and the anthers enclosed in the corolla. Very little self-pollination occurs in the absence of pollinators (Tang, 2011). The corolla comprises a tube, a trilobate lower lip, and a galeate upper lip enclosing the four introrse anthers (Fig. 1). The plants occur in various habitats including dry open slopes, forest edges or in shade, at elevations from 2500 to 4300 m across Sichuan and Yunnan Provinces. Our field survey revealed remarkable variation in plant size, flower number per whorl, and flower size across populations.
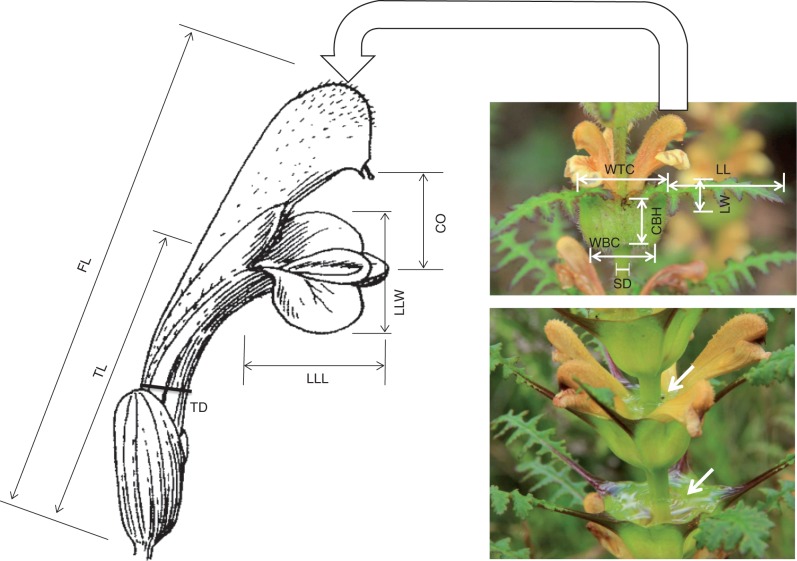
Fig. 1.
Measurements of six floral and six vegetative traits in Pedicularis rex. FL, flower length; TL, tube length; TD, tube diameter; LLL, lower lip length; LLW, lower lip width; CO, corolla opening; SD, stem diameter; LL, leaf length; LW, leaf width; WTC, width of top of cupular bract; WBC, width of bottom of cupular bract; CBH, cupular bract height. Arrows show water in the cupular bract.
The genus Pedicularis is extremely diverse in south-west China, with >300 species, most of which are pollinated almost entirely by bumble-bees (Yang et al., 1998; Tang et al., 2007; Eaton et al., 2012; Huang and Shi, 2013; Armbruster et al., 2014; Liu et al., 2016). Pedicularis rex is pollinated by several species of bumble-bees, including Bombus frieseanus and B. festivus.
The seeds of P. rex are fed upon by larvae of both fly (Diptera) and moth (Lepidoptera) pre-dispersal seed predators (Tang, 2011). The seed predators lay eggs on the ovaries after flowers are open but prior to the ovaries swelling, by piercing the sepals or corolla tubes from the outside of the flowers (cf. Thompson and Pellmyr, 1991). Because these larvae are not easily reared, we have identifications only to order.
Cupular bracts in P. rex (and its closest relatives) are usually full of rain water in which the capsules and the base of flowers are submerged (Fig. 1). We hypothesized, therefore, that CBs (containing water) might function to protect flowers from oviposition by pre-dispersal seed predators.
Traits measured
Twelve traits were measured on 16–36 (mean ± s.e. = 21·38 ± 1·04) individual plants in each field population. We used digital calipers precise to 0·1 mm to measure six vegetative traits (stem diameter, leaf length and width, width of top and bottom of CBs, and CB height). We also randomly chose two flowers from different whorls on each plant and used calipers to measure six floral traits [flower length, corolla tube length (the distance between the corolla base and the point where the lower lip extends out), corolla tube diameter, lower lip length and width, and corolla opening (the distance between the galea tip and lower lip); Fig. 1]. The mean of the two floral measurements from each plant was used in all subsequent analyses (Supplementary Data Appendix S1). We also calculated a composite trait to capture the proportion of the flower exserted above the water-bearing CB:
Geographical variation in traits
To investigate geographical variation in traits, we measured phenotypic variation in 14 populations in Sichuan and Yunnan, south-west China. Detailed information on sampled populations is provided in Supplementary Data Table S1. Based on specimen records from Flora of China, this species is mainly distributed in north-west Yunnan, and south-west and western Sichuan (Yang et al., 1998). These sampled populations are representative of its geographic variation and are at least 20 km apart from each other.
Measurement of fitness components
Lifetime reproductive fitness of individual plants was not easily assessed, nor could we measure the male component of fitness (dispersed pollen producing seeds on other plants). We instead measured three components that seemed likely to contribute to female fitness and would also be sensitive to the activities of pollinators and seed predators. Because seed production was almost certainly pollen limited (see the Results), the number of pollen grains on the stigma at the end of a flower’s period of receptivity should be a good indicator of potential seed production. Indeed, the relationship between initial seed set and number of pollen grains on the stigma was linear (results not shown). The proportion of ovules growing into seeds and those escaping seed predation also seemed to be important components of female fitness. Stigmatic pollen load was based on assessment of the same two flowers per plant measured above, and seed set and seed survival were based on a mean of 12·51 (s.d. = 5·60) capsules per plant. The erect raceme of one plant usually produces 5–10 whorls of flowers varying with plant size. To reduce confounding effects of plant size (possible resource limitation) on seed production, we sampled capsules from three whorls of each raceme at the upper, middle and bottom position. The plant mean of the above variables (Appendix S1) was used to characterize fitness components for each plant (see Cariveau et al., 2004).
Stigmatic pollen loads.
We measured pollen present on stigmas at the end of floral receptivity as an index of pollination success (i.e. pollen arrival) and a possible indicator of pollinator-mediated selection on floral traits (see Pauw et al., 2009). We counted the pollen on the stigmas of the same flowers that we measured morphologically. Specifically, we crushed onto slides the stigmas from flowers in late anthesis and counted under a microscope (see Fang and Huang, 2013) the spheroidal pollen grains of P. rex (diameter approx. 20 μm; Yu and Wang, 2008).
Estimation of initial seed set, seed predation and seed survival.
Seed set, seed predation and seed survival were estimated by counting viable seeds, damaged seeds and unfilled ovules (usually in six capsules each from 20 different individuals in each population, i.e., 120 fruits per population) 3 weeks after initial flowering. In seven populations (1, 3, 5, 8, 9, 10 and 11; see Supplementary Data Table S1), these were the same plants as used for measurement of floral traits and pollination success, although different flowers usually had to be selected. In five populations (2, 4, 6, 7 and 12; see Table S2), labels were lost during the intervening period, and we were unable to relate rates of seed production and predation to plant mean floral morphology. The data from these five populations were used only to assess variation in seed predation rates across populations (Fig. 4; Table S2).
Fig. 4.
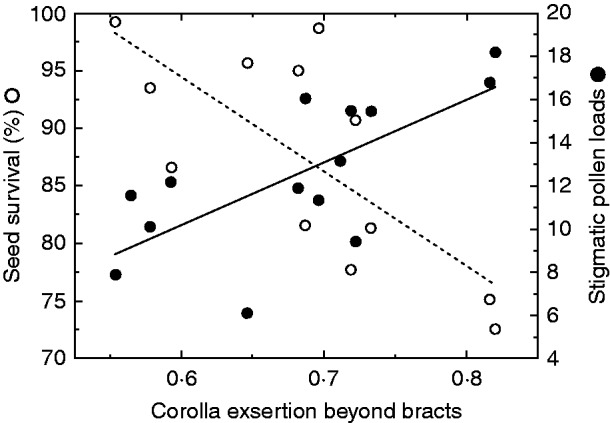
Relationship between population-mean values of the number of pollen grains on stigmas, proportion of seeds surviving predation and proportional exsertion of flower beyond water-bearing bracts.
Undamaged seeds, seeds damaged by larval seed predators and unfertilized ovules were identified in each capsule based on their morphological differences, and counted. Fully developed, viable seeds were obviously enlarged and undamaged. Unfertilized ovules were pale and much smaller. Seeds damaged by larvae were black and were usually missing part of the seed coat. The estimated total number of ovules (and maximum potential seed production per flower) is the sum of all three counts. We then calculated four parameters for each measured flower, the first two of which are proportions of maximum possible seed production for that flower (to correct for among-flower variation in ovule number; McKinney and Tomback, 2007; Xia et al., 2013). (1) Initial seed set is the sum of undamaged and damaged seeds divided by the total number of ovules. (2) Final seed production (intact seeds) is the number of undamaged seeds divided by the total number of ovules. (3) Proportional seed survival is the number of undamaged seeds divided by the sum of intact and damaged seeds. (4) Proportional seed predation is the number of damaged seeds divided by the sum of undamaged and damaged seeds (=1 – seed survival). Sometimes in heavily attacked populations capsule contents were completely consumed by larvae. In these cases (up to five fruits per population) seed number was impossible to ascertain, and these fruits could not be used in the analyses.
Data analysis
All statistical analyses were conducted using IBM SPSS 22 (IBM Corporation, 2014).
Variation among populations.
Multivariate analyses of variance (MANOVAs) were conducted separately on (1) vegetative traits and (2) floral traits to assess whether there was significant among-population variation in vegetative and floral traits. The difference between the initial seed set and the final seed production (= seed predation) was also compared across populations, and the variation was assessed using χ2 analysis.
Relating fitness to phenotype [Generalized Linear Models (GLMs)].
We conducted all analyses of fitness using individual plant means of traits and fitness components. In doing so, we assumed that most of the important floral variation affecting pollination and seed predation occurred at the among-plant rather than the among-flower level. We examined the effects of variation in the measured traits (flower length, corolla tube length, corolla tube diameter, lower lip length and width, corolla opening and corolla exsertion) on the four inter-related components of reproductive fitness described in the previous section. All proportional traits and proportional fitness components were arcsin-squareroot transformed in order to meet better the normality assumptions for analyses that follow. Of the seven floral traits measured or calculated, only corolla exsertion and lower corolla lip width were included in the final models, because only they explained enough additional variance to reduce the information criterion examined (Burnham and Anderson, 2002; see below).
To examine and compare across multiple populations the floral trait–fitness relationships at the individual plant level (within-population covariation), we used a GLM approach (with normal error distribution) similar to those described by Heisler and Damuth (1987), Scheiner et al., (2000), Okasha (2004) and Bolstad et al. (2010), these being extensions of the Lande–Arnold model of natural selection on quantitative traits (Lande and Arnold, 1983; see also Phillips and Arnold, 1989). By pooling population data and including terms for both population and population interaction, we were able to estimate overall mean population effects and test whether phenotypic selection on floral traits varied among populations (i.e. whether selection operated in a mosaic fashion).
We first assessed the number of pollen grains arriving on stigmas (mean = 12·28, s.d. = 5·30) in relation to floral traits, source population and trait × population interactions. We used a second model to evaluate controls over seed predation rates (proportion of seeds in a fruit damaged by seed predators; mean = 0·127, s.d. = 0·120) in relation to measured floral traits, amount of pollen on the stigmas, initial seed set, source population and the seed set × population interaction. We used a third model to examine initial seed set (proportion of the ovules in a fruit developing into seeds; mean = 10·53, s.d. = 2·42) in relation to floral traits, amount of pollen grains on the stigma, the source population, population × trait interactions and the population × pollen number interaction. We used a fourth model to evaluate controls over final production of viable seeds in relation to the amount of pollen on stigmas, initial seed set, seed predation rate, population identity and corresponding interactions.
The models were evaluated using model selection, where the lowest finite-sample-corrected Akaike Information Criterion (AICc) was assumed to indicate the best model, at least when the difference was substantial (e.g. ΔAICc > approx. 5, or a relative likelihood >20:1; see next section and Burnham and Anderson, 2002). In other words, the simpler model (fewer terms) was chosen over the more complex model when the explanatory powers were not significantly different (cf. Neter et al., 1985).
Relating fitness to phenotype (path analysis).
We used path analysis to visualize in one integrated causal model the relationships among components of reproductive success (see Scheiner et al., 2000), as well as effects of phenotypic traits on these components (i.e. operation of phenotypic selection), and the correlative relationships between the phenotypic traits present in the four best GLMs described above. This allowed the causal effects of variables and inter-related fitness parameters to be modelled using ‘biological common sense’ not necessarily captured in pure statistical models (Scheiner et al., 2000).
Because the path analysis that follows involves pooling the population data on the assumption of no significant interactions, we tested the best interaction models against the overall best model, following the procedure of Burnham and Anderson (2002), where the probability that the more complex model including the interaction minimizes information loss as well as the best model (which is also the relative likelihood) is calculated as:
The variables included in each portion of the path diagram were those shown by GLM selection to be important, in addition to those we expected to have potential direct effects on each component of reproductive success. (This approach does not create significant relationships, it just precludes impossible ones; Scheiner et al., 2000.) Note that inferences reported here include the caveat that true causality could be the result of effects of unmeasured correlates of the measured variables; only manipulative experiments can resolve this (see the Discussion). To remove the significant population effects identified by the GLM analyses above and to transform all fitness observations to within-population relative fitness (Endler, 1986), each observation was standardized to its populations mean by subtracting from each observation, i, its respective (jth) population mean: (xij – xj).
Ordinary least squares multiple regression was conducted on the population-mean-corrected observations to yield standardized regression coefficients, which are numerically identical to path coefficients calculated using simultaneous equations (Li, 1975; Shipley, 2000). The advantage of this approach is that the strength of standardized path coefficients can be compared numerically (all path coefficients vary theoretically between –1 and +1; Li, 1975; Scheiner et al., 2000). P-values reported for path coefficients in Fig. 2 come, however, from the more robust GLM analyses. The interactions were found to be ordinal, i.e. the result of differences in slope steepness but not direction. This meant that the main effects were biologically, as well as statistically, interpretable, despite several significant interactions (Pedzahur, 1997).
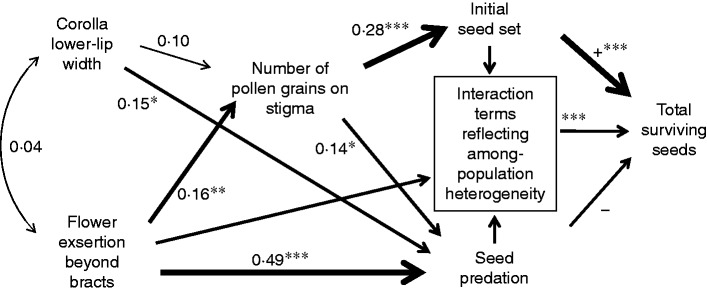
Fig. 2.
Path diagram, based in part on the four best GLMs obtained by AIC model selection of among-individual (within-population) relationships between floral morphology, pollination, initial seed set, seed predation and final seed production (after standardizing each observation to its respective population mean). Numbers are standardized regression coefficients (= path coefficients), which vary between 1 and –1 (0 = no effect, 1/–1 = complete determination). Coefficients of relationships affecting, or affected by, the interaction terms cannot be calculated in the same fashion as for the other paths and have not been included. (For details of the interactive effects on total seed set, see Table 1.) The main effects of seed predation and initial seed set on final seed production are interpretable despite interactions, because the interactions result largely from significant variation in slope steepness but not direction (i.e. interactions are ordinal), although the effect sizes (path coefficients) are not easily interpreted and have been omitted. Depicted significance levels come from the GLMs: *P < 0·05; **P < 0·01; ***P < 0·001.
RESULTS
Trait variation
The two MANOVAs indicated significant phenotypic variation among populations in both vegetative traits (F5, 280 = 3·835, P < 0·001) and floral traits (F5, 280 = 29·485, P < 0·001), with the effect size of the latter being much greater. Thus populations have differentiated phenotypically, with this being especially dramatic for floral traits.
Pollination and seed predation
The mean number of pollen grains deposited per stigma was 12·53 (s.d. = 5·36) (n = 299 plants, 598 flowers) across the 14 populations. Because each flower had on average 25·96 (s.d. = 6·33) ovules (n = 120), this result indicates that probably all populations experienced pollen limitation of seed production. Indeed, initial seed set in 12 populations varied from 31·10 to 48·53 %, consistent with the inference of pollen limitation. χ2 tests showed that final seed production differed significantly (P < 0·05) from initial seed set in six of 12 populations (Supplementary Data Table S2), indicating that these six populations probably experienced more seed predation than the others. Levels of seed predation ranged from 0·8 and 1·36 % in populations 11 and 3, respectively, to 18·5 and 27·42 % in populations 12 and 5, respectively (Table S2).
Multipopulation patterns of phenotypic selection within populations
Generalized Linear Models
The GLM analysis (with model selection) of the data from all 14 populations pooled indicated that pollen arrival on stigmas (Model 1) was affected positively by the exsertion of the corolla beyond the bracts, and positively, but more weakly, by the width of the lower corolla lobe (lower lip width). There was also a strong population effect, i.e. the mean amounts of pollen on stigmas varied significantly across populations (Table 1).
Table 1.
Generalized Linear Models of relationships between floral morphology, pollination, seed predation and seed production (all populations analysed jointly), as supported by the AICc model selection procedure (those with minimum AICc values were assumed to be best)
| 1. Determinants of pollination (number of pollen grains on stigmas) | |||
| a. | Full model | Pollen = constant + exsertion + lip width + population + population × exsertion + population × lip width | AICc = 1728·99 |
| b. | Best interaction model | Pollen = constant + exsertion + lip width* + population + population × exsertion | AICc = 1714·83 |
| c. | Best model | Pollen = constant + exsertion** + lip width + population*** | AICc = 1700·10 |
| 2. Determinants of seed predation | |||
| a. | Full model | Seed predation = constant + exsertion*** + lip width + pollen** + population + population × pollen + population × exsertion + population × pollen + population × lip | AICc = –128·48 |
| b. | Best interaction model | Seed predation = constant + exsertion*** + lip width* + pollen* + population + population × exsertion | AICc = –151·03 |
| c. | Best model | Seed predation = constant + exsertion*** + lip width* + pollen* + population*** | AICc = –156·11 |
| 3. Determinants of initial seed set | |||
| a. | Full model | Initial seed set = constant + exsertion* + lip + pollen*** + population*** + population × exsertion + population × lip* + population × pollen | AICc = –452·54 |
| b. | Best interaction model | Initial seed set = constant + pollen** + population** + population × pollen | AICc = –480·30 |
| c. | Best model | Initial seed set = constant + population*** + pollen*** | AICc = –493·44 |
| 4. Determinants of total surviving seeds | |||
| a. | Full model | Total seeds = constant + pollen + initial seed set*** – seed predation + exsertion + lip + population*** + population × initial seed set*** + population × seed predation*** + population × pollen + population×exsertion*** + population × lip + population × initial seed set × predation*** + population × initial seed set × predation × exsertion *** | AICc = –971·091 |
| b. | Best model | Total seeds = constant + initial seed set*** – seed predation + population*** + population×initial seed set*** + population × seed predation*** population × exsertion*** + population × initial seed set × predation*** + population × initial seed set × predation × exsertion *** | AICc = –1000·03 |
All proportions were arcsin-squareroot transformed to meet model assumptions of normality. (These transformations resulted in slightly lower AICs compared with untransformed data, but did not substantially change the results.) Models 1 and 3 are based on data from populations 1–14, whereas models 2 and 4 are based on data from populations 1, 3, 5 and 8–11. Note that Model 4 has only two models listed because the interaction model had a lower AIC than the no-interaction model.
Wald χ2 P-values for model-term effects:
*P < 0·05;
**P < 0·01;
***P < 0·001; no asterisk: P > 0·05.
The GLM analysis of seed predation rates from seven populations pooled (Model 2) indicated that the level of attack was affected positively by corolla exsertion, lip width and the amount of pollen on the stigmas. Attack rates also varied among populations (significant population term), consistent with the χ2 results above (Table 1).
Initial seed set (Model 3) was affected positively by the amount of pollen on the stigma and by the population term (Table 1).
Final viable seed production (Model 4) was affected positively by the initial seed set and negatively by the seed predation rate, as expected, although the steepness (but not direction) of the seed predation effects varied across populations (interactions were significant but ordinal). The population means of final viable seed also varied (the population effect was significant; Table 1).
Path analysis.
Testing the information content of the most biologically relevant interaction models against the best models suggested that population × trait interactions were statistically negligible in Models 1 and 3 and that pooling populations for the path-analytical model was appropriate. We found that for Model 1 (amount of pollen on stigmas), the model including the exsertion × population interaction was 0·0068 times as likely as the best model (see Table 1) to minimize the information loss, indicating no significant interaction. For Model 3 (initial seed set), the model including the pollen load × population interaction was 0·0120 times as likely as the best model to minimize the information loss, also indicating no significant interactions. For Model 2 (amount of seed predation), the likelihood analysis was less clear. The model including the exsertion × population interaction was 0·3450 times as likely as the best model (without interaction) to minimize the information loss. Despite weak support for the no-interaction model, we included it in the path diagram without interactions. In contrast, the best model for Model 4 (final seed production) included interaction terms (see Table 1), so interactions were indicated in the path diagram, and path coefficients were not calculated.
The path diagram (Fig. 2) shows the postulated causal, independent effects (i.e. holding other variable constant) of various floral characteristics on fitness components of P. rex plants after population effects have been removed (and assuming no interactions affecting pollen arrival, seed predation and initial seed set). The diagram also includes the detected direct and indirect causal influences on total viable seed production (plant means), from which interactions were not excluded. This integrated analysis indicates that flower exsertion strongly affected pollinator attraction as reflected in pollen arrival. In turn, pollen arrival strongly influenced initial seed set, which in turn had a strong effect on total number of surviving seeds. Floral exsertion had an even stronger effect on rates of attack by seed predators, which in turn had an effect on final seed production, mostly via population-interactive effects (i.e. populations varied significantly in the effect of seed predation on final seed production; Fig. 2; Table 1). In other words, there was a geographic mosaic in selection mediated by seed predators. In contrast, there was no evidence that selection mediated by pollinators varied among populations. Regardless, most populations experienced conflicting selection on flower exsertion beyond bracts, as mediated by pollinators vs. seed predators (Figs 2 and 3).
Fig. 3.
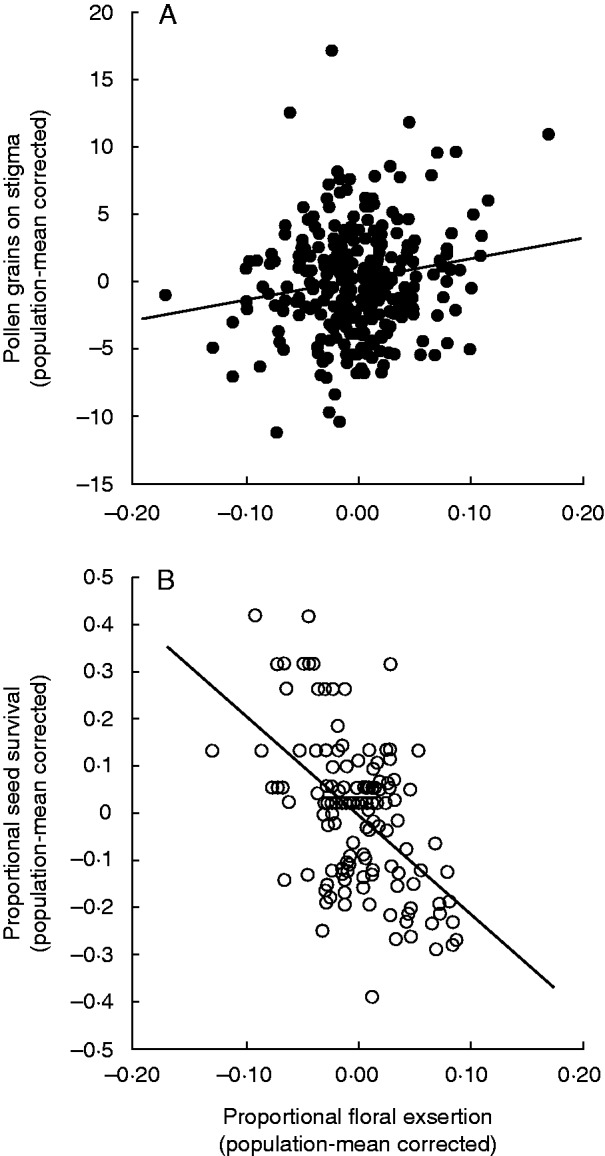
Conflicting selection detected by comparing variation in two components of female fitness – pollination and proportion of seeds escaping predation – in relation to flower exsertion beyond the water-bearing bracts. All variables were transformed by subtracting the mean of the population to which each observation belonged. This captures the pooled within-population relationships by removing the significant population effect detected in the GLM. Pooling of populations after standardization was statistically indicated because there were no detectable interactions. Note that other explanatory variables have not been partialled out as in the statistical models, hence large scatter of observations around the central tendencies. (A) Relationship between mean-corrected number of pollen grains on stigmas and the arcsin-squareroot-transformed proportional corolla exsertion (R2 = 0·024, P = 0·007). (B) Relationship between mean-corrected proportion of seeds surviving seed predation (arcsin-squareroot-transformed) and the arcsin-squareroot-transformed proportional corolla exsertion (R2 = 0·267, P < 0·001).
Among-population covariation between traits and mean fitness
An examination of the population means of initial seed set, seed survival and floral exsertion reveals a similar relationship to that seen within populations. Increasing floral exsertion (as a proportion of the corolla length) was associated with both increasing seed set and decreasing proportion of seeds surviving seed predation (Fig. 4).
DISCUSSION
Population differences
We found that the study populations of Pedicularis rex had diverged significantly in floral phenotype. Populations also differed significantly from one another in mean pollen loads on the stigma, mean seed set and mean rates of seed predation.
Pollination success and seed predation
We found that, as predicted, corollas that were more exserted from the bracts and those with wider lower lips attracted both more pollinators and more seed predators, indicating that direct positive and negative selection, respectively, acted on these traits. The apparent direct selection on floral exsertion (e.g. from the animal perspective) is actually correlational selection (from the plant perspective), because corolla exsertion is itself a relationship between one floral trait (corolla tube length) and one vegetative trait (bract height).
The apparent choice of flowers with broader lower corolla lips by both pollinators and seed predators suggests that both are responding to the same advertisement signals (see Pérez-Barrales et al., 2013). The same may be true of floral exsertion from the bracts (at least for pollinators), although in the case of the seed predators, the oviposition success rates may be the critical factor. Indeed, that less exserted flowers had lower rates of seed predation is consistent with the hypothesis that submergence of the base of the flower in water provides protection against seed predators. This interpretation has been confirmed experimentally in a companion study assessing the effects of draining the water from the cupular bracts (effectively increasing corolla exsertion from the water but not the bracts) on seed predation rates and pollination rates. The experimental study showed that flowers that were not submerged (i.e. high floral exsertion beyond water) had higher rates of seed predation (Sun and Huang, 2015). Greater exsertion beyond the water did not, however, increase pollination rates detectably (Sun and Huang, 2015), although the exsertion beyond the green bracts (more likely to influence showiness) remains to be manipulated and assessed. This prior study differs from the one reported here in that only six populations were studied and neither multivariate phenotypic selection nor its variation among populations were addressed.
Similar experimental results have been obtained in a water-calyx plant, Chrysothemis friedrichsthaliana (Gesneriaceae), in Costa Rica, where floral buds were frequently attacked by ovipositing moths. Experimental manipulation of water levels in the cup-like calyces showed that a liquid barrier over buds reduced per-flower egg deposition and subsequent herbivory by 50 %, suggesting that the water protected buds from florivores (Carlson and Harms, 2007).
We found, unexpectedly, that there were higher rates of seed predation on flowers that had more pollen on their stigmas (Table 1; Fig. 2). Although this makes intuitive sense, as well-pollinated flowers provide more seed resources for the adult seed predators’ larvae, the mechanisms generating this relationship are unclear. Oviposition occurs in the flowering stage rather than the fruiting stage, so direct assessment of seed resources by ovipositing adults is impossible. Adult seed predators appear instead to be making oviposition choices using floral cues (e.g. pollen odours or unmeasured floral traits influencing pollination) that predict which flowers are likely later to produce fruits with more seeds. This relationship seems unlikely to be simply the result of shared floral apparency to both pollinators and seed predators (as for the corolla lip), because most floral traits likely to involved in attraction were measured and no direct effects on seed predation rates could be found. If our interpretation is correct, this would be only the second or perhaps third time, to our knowledge, that such ‘predictive’ behaviour by seed predators has been detected using phenotypic selection analysis (Pérez-Barrales et al., 2013; see also Carlson and Holsinger, 2013). We suspect such predictive behaviour is actually common, however, given how adaptive it must be; it is just hard to distinguish from apparency. Indeed, use of predictive seed set signals may be the critical first step in the evolution of active pollination by seed predators (see Yoder et al., 2010).
Because the study presented here was based on standing variation without experimental manipulation of phenotype, it is possible that environmental correlations or other unknown correlates of the measured independent variables (e.g. microclimate, regional climate, soil conditions, activity of unknown mutualists or antagonists, etc.) could have contributed to the patterns we found. This could lead to misattributing causal significance to corolla exsertion and lower lip width, despite strong statistical support (see Rausher, 1992; Scheiner, 2002; Stinchcombe et al., 2002). However, the results of the companion study described above (Sun and Huang, 2015) confirm experimentally the operation of one mechanism invoked here, i.e. the protection of flowers from seed predators by deeper, water-bearing cupular bracts.
Conflicting selection on floral traits generated within populations by insect mutualists and antagonists
There is ample evidence of the influence of pollinator-mediated selection on floral evolution (see review in Fenster et al., 2004), and a growing number of studies have more recently shown the importance of florivore-mediated selection (e.g. Galen, 1999; Herrera, 2000; Cariveau et al., 2004; Irwin et al., 2004; Strauss and Whittall, 2006; Gómez, 2008; Carlson and Holsinger, 2010). Indeed it is increasingly apparent that floral traits are very commonly sculpted by conflicting selection generated by both mutualists and antagonists (Irwin et al., 2004; Theis and Adler, 2012; Kessler et al., 2013). For example, smaller (female) flowers experienced less attack by florivores than larger (hermaphrodite) flowers in a gynodioecious orchid (Huang et al., 2009), suggesting that an increase of flower size may increase attractiveness to pollinators but involve a higher risk of floral herbivory. In a recent study of Dalechampia scandens, blossoms with larger bracts received more pollen on their stigmas, but seed predators laid more eggs on these blossoms, indicating that selection for larger bract size exerted by pollinators was counteracted by the selection exerted by seed predators (Pérez-Barrales et al., 2013).
Previously studies have emphasized that, in Pedicularis, most of the diversity of flower morphology has been driven by selection generated by bumble-bee pollinators (Macior, 1982; Eaton et al., 2012). This emphasis is understandable, given the paucity of empirical studies of florivores on Pedicularis. Interestingly, there is one early study that reports intense pre-dispersal seed predation: lepidopteran larvae damaged 39 % of mature capsules of P. furbishiae in north-eastern America (Menges et al., 1986). To this observation we can add data presented here suggesting that evolution of floral traits in P. rex is influenced by antagonists as well as mutualists. Seed predators on P. rex generated selection conflicting with that mediated by pollinators for at least two floral traits: corolla exsertion from the cupular bracts and width of the lower lip. The conflicting selection on floral exsertion was particularly dramatic (Fig. 3).
Additive population effects
All four GLMs indicated that there were additive population effects influencing rates of pollination, seed predation, initial seed set and final viable seed production. This means that, for a given value of a phenotypic trait, e.g. floral exsertion, the average amount of pollen on stigmas, average rates of seed predation, etc. varied significantly among populations. This is consistent with the idea that some populations are pollination or seed predation ‘hot-spots’ and others relative ‘cold-spots’ because of different abundances of pollinators and seed predators. The lack of interactions for the first three models meant that populations could be safely combined to understand general trends in selection.
Among-population covariation between traits and mean fitness
The phenotypic variation among populations, if it has a genetic basis, might reflect a history of response to stronger selection by more abundant seed predators in some populations, leading to inconspicuous, less exserted flowers and lower pollination. In other populations, a history of less seed predation may have led to more conspicuous flowers and hence overall greater attractiveness to pollinators, relative to other plant species that generalist bumble-bee pollinators might otherwise visit in the same community. This interpretation is supported by analyses of population means of trait values and rates of pollinations, seed set and seed predation.
As was observed within populations, increasing mean floral exsertion was associated with increasing mean seed set and decreasing mean proportion of seeds surviving seed predation (Fig. 4). Thus, populations with large proportional corolla exsertion received more pollen on average (and set more seeds), but suffered higher average rates of seed predation. Populations with lower proportional corolla exsertion received less pollen on average, but experienced lower average seed predation. Although consistent with the within-population relationships, this result is somewhat surprising because natural selection is usually detected by comparing individuals within populations. It is possible that pollinators and seed predators are finding and choosing among nearby populations in the same fashion as they choose among flowers and/or plants within populations, although this seems a little unlikely. Nevertheless, populations can be spread out across the same governing adaptive surface as individuals within populations (e.g. Armbruster, 1990). If this is the case here, it is puzzling that the populations have not converged more closely onto a single adaptive peak. Perhaps the surface actually comprises multiple adaptive peaks. Further complicating our interpretation is the fact that populations may experience annual fluctuations in selection, so that the true selection history for any given populations is not reflected in any contemporary patterns.
Mosaic selection generated by seed predators but not pollinators
No population interaction term was retained in the best GLM of pollen arrival on stigmas, which suggests that pollinator-mediated selection on floral traits was similar across all populations. Indeed, the best interaction model (mosaic selection) was 0·0068 times as likely as the non-interaction model to minimize the information loss.
In contrast, the interaction model explaining seed predation was about one-third as likely as the non-interaction model to minimize the information loss, i.e. the two models were almost equally good, except for inclusion of an extra term and loss of a degree of freedom. This indicates that there may be geographic variation in selection mediated by seed predators, despite the interaction term not having been retained in the best model (perhaps because of relatively small sample sizes in each population). Even stronger evidence of a geographic mosaic in selection mediated by seed predators is the retention of the population × seed predation interaction term in the final seed production model. The retention of three- and four-way interactions involving seed predation also supports the geographic heterogeneity in selection generated by seed predators. The direct evidence of significant variation in intensity of seed predation across populations also supports the interpretation of a geographic mosaic in selection generated by seed predators. Taken together, these results are consistent with the expectation that selection commonly varies geographically, leading to the potential for geographic mosaics of evolution or coevolution (Thompson, 2005).
Concluding remarks
Populations of P. rex in south-western China exhibited large amounts of variation in morphology, levels of pollination and, most dramatically, levels of seed predation. Despite this variation among populations, there was also evidence of consistent conflicting selection within most populations on floral exsertion beyond the water-bearing bracts. Pollinators selected for greater exsertion, perhaps because such flowers are easier to see. Seed predators, in contrast, mediated selection against exsertion, as highly exserted flowers were less protected by water. There was also evidence of a geographic mosaic in selection generated by seed predators, but not in selection generated by pollinators. Many questions remain unanswered, however, especially with regard to among-population patterns of mean fitness in relation to floral trait variation. Future studies of this kind should focus on larger sample sizes (for each population) in order to increase parameter precision and improve the ability to detect geographic variation in selection generated by mutualists and antagonists.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: detailed information on location and altitude of 14 sampled populations of Pedicularis rex. Table S2: initial seed set (%), final seed set (%), seed predation (%) and comparison between initial and final seed set (χ2 analyses) shown for 12 populations. Appendix S1: means and standard errors for 12 phenotypic traits and pollination success in 14 populations of Pedicularis rex.
ACKNOWLEDGEMENTS
We thank Jin Ma, Xiao-Yue Wang, Min Yang and Jing-Ju Zhang for their help in the field study, and Sarah Corbet, Jeff Karron, Mark Rausher, Xiao-Xing Tang and three anonymous reviewers for valuable comments on this work. This work was supported by the National Science Foundation of China (NSFC no. 31030016, 31270281) to S.Q.H., and a UK Royal Society International Research grant to W.S.A.
LITERATURE CITED
- Ågren J, Hellström F, Toräng P, Ehrlén J. 2013. Mutualists and antagonists drive among-population variation in selection and evolution of floral display in a perennial herb. In: Proceedings of the National Academy of Sciences, USA 110: 18202–18207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Johnson SD. 2008. The geographical mosaic of coevolution in a plant–pollinator mutualism. Evolution 62: 220–225. [DOI] [PubMed] [Google Scholar]
- Armbruster WS. 1990. Estimating and testing the shapes of adaptive surfaces: the morphology and pollination of Dalechampia blossoms. American Naturalist 135: 14–31. [Google Scholar]
- Armbruster WS, Shi X-Q, Huang S-Q. 2014. Do specialised flowers promote reproductive isolation? Realised pollination accuracy of three sympatric Pedicularis species. Annals of Botany 113: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkowska MP, Johnston MO. 2012. Pollinators cause stronger selection than herbivores on floral traits in Lobelia cardinalis (Lobeliaceae). New Phytologist 193: 1039–1048. [DOI] [PubMed] [Google Scholar]
- Bolstad GH, Armbruster WS, Pélabon C, Pérez-Barrales R, Hansen TF. 2010. Direct selection at the blossom level on floral reward by pollinators in a natural population of Dalechampia schottii: full-disclosure honesty? New Phytologist 188: 370–384. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer-Verlag. [Google Scholar]
- Carlson JE, Harms KE. 2007. The benefits of bathing buds: water calyces protect flowers from a microlepidopteran herbivore. Biology Letters 3: 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JE, Holsinger KE. 2010. Natural selection on inflorescence color polymorphisms in wild Protea populations: the role of pollinators, seed predators, and intertrait correlations. American Journal of Botany 97: 934–944. [DOI] [PubMed] [Google Scholar]
- Carlson JE, Holsinger KE. 2013. Direct and indirect selection on floral pigmentation by pollinators and seed predators in a color polymorphic South African shrub. Oecologia 171: 905–919. [DOI] [PubMed] [Google Scholar]
- Cariveau D, Irwin RE, Brody AK, Garcia-mayeya LS, Von Der Ohe A. 2004. Direct and indirect effects of pollinators and seed predators to selection on plant and floral traits. Oikos 104: 15–26. [Google Scholar]
- Eaton DAR, Fenster CB, Hereford J, Huang S-Q, Ree RH. 2012. Floral diversity and community structure in Pedicularis (Orobanchaceae). Ecology 93: S182–S194. [Google Scholar]
- Ehrlén J, Käck S, Ǻgren J. 2002. Pollen limitation, seed predation and scape length in Primula farinosa. Oikos 97: 45–51. [Google Scholar]
- Endler JA. 1986. Natural selection in the wild.Princeton, NJ: Princeton University Press. [Google Scholar]
- Fang Q, Huang S-Q. 2013. A directed network analysis of heterospecific pollen transfer in a biodiverse community. Ecology 94: 1176–1185. [DOI] [PubMed] [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics 35: 375–403. [Google Scholar]
- Galen C. 1999. Why do flowers vary? BioScience 49: 631–640. [Google Scholar]
- Galen C, Cuba J. 2001. Down the tube: pollinators, predators, and the evolution of flower shape in the alpine skypilot, Polemonium viscosum. Evolution 55: 1963–1971. [DOI] [PubMed] [Google Scholar]
- Gómez JM. 2003. Herbivory reduces the strength of pollinator-mediated selection in the Mediterranean herb Erysimum mediohispanicum: consequences for plant specialization. American Naturalist 162: 242–256. [DOI] [PubMed] [Google Scholar]
- Gómez JM. 2008. Sequential conflicting selection due to multispecific interactions triggers evolutionary trade-offs in a monocarpic herb. Evolution 62: 668–679. [DOI] [PubMed] [Google Scholar]
- Hamrick JL, Godt MJW. 1996. Effects of life history traits on genetic diversity in plant species. Philosophical Transactions of the Royal Society B: Biological Sciences 351: 1291–1298. [Google Scholar]
- Heisler IL, Damuth J. 1987. A method for analyzing selection in hierarchically structured populations. American Naturalist 130: 582–602. [Google Scholar]
- Herrera CM. 2000. Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology 81: 2170–2176. [Google Scholar]
- Herrera CM, Castellanos MC, Medrano M. 2006. Geographical context of floral evolution: towards an improved research programme in floral diversification In: LD Harder, SCH Barrett, eds. The ecology and evolution of flowers. Oxford: Oxford University Press, 278–294. [Google Scholar]
- Huang S-Q, Shi X-Q. 2013. Floral isolation in Pedicularis: how do congeners with shared pollinators minimize reproductive interference? New Phytologist 199: 858–865. [DOI] [PubMed] [Google Scholar]
- Huang S-Q, Lu Y, Chen Y-Z, Luo Y-B, Delph LF. 2009. Parthenogenesis maintains male sterility in a gynodioecious orchid. American Naturalist 174: 578–584. [DOI] [PubMed] [Google Scholar]
- IBM Corporation. 2014. IBM SPSS Statistics 20. Armonk, NY: IBM Corporation. [Google Scholar]
- Irwin RE, Strauss SY, Storz S, Emerson A, Guibert G. 2003. The role of herbivores in the maintenance of a flower color polymorphism in wild radish. Ecology 84: 1733–1743. [Google Scholar]
- Irwin RE, Adler LS, Brody AK. 2004. The dual role of floral traits: pollinator attraction and plant defense. Ecology 85: 1503–1511. [Google Scholar]
- Irwin RE, Bronstein JL, Manson JS, Richardson L. 2010. Nectar robbing: ecological and evolutionary perspectives. Annual Review of Ecology, Evolution and Systematics 41: 271–292. [Google Scholar]
- de Jager ML, Ellis AG. 2013. Floral polymorphism and the fitness implications of attracting pollinating and florivorous insects. Annals of Botany 113: 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D, Diezel C, Clark DG, Colquhoun TA, Baldwin IT. 2013. Petunia flowers solve the defence/apparency dilemma of pollinator attraction by deploying complex floral blends. Ecology Letters 16: 299–306. [DOI] [PubMed] [Google Scholar]
- Li CC. 1975. Path analysis – a primer. Pacific Grove, CA: Boxwood Press. [Google Scholar]
- Liu YN, Li Y, Yang FS, Wang XQ. 2016. Floral nectary, nectar production dynamics, and floral reproductive isolation among closely related species of Pedicularis. Journal of Integrative Plant Biology 58: 178–187. [DOI] [PubMed] [Google Scholar]
- Lloyd DG, Webb CJ. 1992. The evolution of heterostyly In: Barrett SCH, ed. Evolution and function of heterostyly. Berlin: Springer-Verlag, 151–178. [Google Scholar]
- Macior LW. 1982. Plant community and pollinator dynamics in the evolution of pollination mechanisms in Pedicularis (Scrophulariaceae). In: JA Armstrong, JM Powell, AJ Richards, eds. Pollination and evolution. Sydney, Australia: Royal Botanic Gardens, 29–45. [Google Scholar]
- McCall AC, Irwin RE. 2006. Florivory: the intersection of pollination and herbivory. Ecology Letters 9: 1351–1365. [DOI] [PubMed] [Google Scholar]
- McKinney ST, Tomback DF. 2007. The influence of white pine blister rust on seed dispersal in whitebark pine. Canadian Journal of Forestry Research 37: 1044–1057. [Google Scholar]
- Menges ES, Waller DM, Gawler SC. 1986. Seed set and seed predation in Pedicularis furbishiae, a rare endemic of the St. John River, Maine. American Journal of Botany 73: 1168–1177. [Google Scholar]
- Mothershead K, Marquis RJ. 2000. Fitness impacts of herbivory through indirect effects on plant–pollinator interactions in Oenothera macrocarpa. Ecology 81: 30–40. [Google Scholar]
- Neter J, Wasserman W, Kutner MH. 1985. Applied linear statistical models, 2nd edn. Homewood, IL: Irwin. [Google Scholar]
- Okasha S. 2004. Multi-level selection, covariance and contextual analysis. British Journal of Philosophical Science 55: 481–504. [Google Scholar]
- Parachnowitsch AL, Caruso CM. 2008. Predispersal seed herbivores, not pollinators, exert selection on floral traits via female fitness. Ecology 89: 1802–1810. [DOI] [PubMed] [Google Scholar]
- Pauw A, Stofberg J, Waterman RJ. 2009. Flies and flowers in Darwin’s race. Evolution 63: 268–279. [DOI] [PubMed] [Google Scholar]
- Pedhazur EJ. 1997. Multiple regression in behavioural research, 3rd edn. Orlando, FL: Harcourt Brace. [Google Scholar]
- Pérez-Barrales R, Bolstad GH, Pélabon C, Hansen TF, Armbruster WS. 2013. Pollinators and seed predators generate conflicting selection on Dalechampia blossoms. Oikos 122: 1411–1428. [Google Scholar]
- Phillips PC, Arnold SJ. 1989. Visualizing multivariate selection. Evolution 43: 1209–1222. [DOI] [PubMed] [Google Scholar]
- Rausher MD. 1992. The measurement of selection on quantitative traits: biases due to environmental covariances between traits and fitness. Evolution 46: 616–626. [DOI] [PubMed] [Google Scholar]
- Rey PJ, Herrera CM, Guitián J, et al. 2006. The geographic mosaic in predispersal interactions and selection on Helleborus foetidus (Ranunculaceae). Journal of Evolutionary Biology 19: 21–34. [DOI] [PubMed] [Google Scholar]
- Scheiner SM. 2002. Reducing environmental bias when measuring natural selection. Evolution 56: 2156–2167. [DOI] [PubMed] [Google Scholar]
- Scheiner SM, Mitchell RJ, Callahan HS. 2000. Using path analysis to measure natural selection. Journal of Evolutionary Biology 13: 423–433. [Google Scholar]
- Shipley B. 2000. Cause and correlation in biology. Cambridge: Cambridge University Press [Google Scholar]
- Siepielski AM, Benkman CW. 2010. Conflicting selection from an antagonist and a mutualist enhances phenotypic variation in a plant. Evolution 64: 1120–1128 [DOI] [PubMed] [Google Scholar]
- Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics 1: 307–326. [Google Scholar]
- Steffan-Dewenter I, Münzenberg U, Tscharntke T. 2001. Pollination, seed set and seed predation on a landscape scale. In: Proceedings of the Royal Society B: Biological Sciences 268: 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JR, Rutter MT, Burdick DS, Tiffin P, Rausher MD, Mauricio R. 2002. Testing for environmentally induced bias in phenotypic estimates of natural selection: theory and practice. American Naturalist 160: 511–523. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Armbruster WS. 1997. Linking herbivory and pollination: new perspectives on plant and animal ecology and evolution. Ecology 78: 1617–1618. [Google Scholar]
- Strauss SY, Whittall JB. 2006. Non-pollinator agents of selection on floral traits In: Harder LD, Barrett SCH, eds. The ecology and evolution of flowers. Oxford: Oxford University Press, 120–135. [Google Scholar]
- Strauss SY, Conner JK, Rush SL. 1996. Foliar herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. American Naturalist 147: 1098–1107. [Google Scholar]
- Sun S-G, Huang S-Q. 2015. Rainwater in cupulate bracts repels seed herbivores in a bumblebee-pollinated subalpine flower. AoB Plants 7: plv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talluto MV, Benkman CW. 2014. Conflicting selection from fire and seed predation drives fine-scaled phenotypic variation in a widespread North American conifer. In: Proceedings of the National Academy of Sciences, USA 111: 9543–9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X-X. 2011. A comparative study of phenotypic selection on Pedicularis corolla. PhD thesis, Wuhan University, Wuhan, China.
- Tang Y, Xie J, Sun H. 2007. The pollination ecology of Pedicularis rex subsp. lipskyana and P. rex subsp. rex (Orobanchaceae) from Sichuan, southwestern China. Flora 202: 209–217. [Google Scholar]
- Theis N, Adler LS. 2012. Advertising to the enemy: enhanced floral fragrance increases beetle attraction and reduces plant reproduction. Ecology 93: 430–435. [DOI] [PubMed] [Google Scholar]
- Thompson JN. 2005. The geographic mosaic of coevolution. Chicago IL: University of Chicago Press. [Google Scholar]
- Thompson JN. 2013. Relentless evolution. Chicago IL: University of Chicago Press. [Google Scholar]
- Thompson JN, Pellmyr O. 1991. Evolution of oviposition behavior and host preference in Lepidoptera. Annual Review of Entomology 36: 65–89. [Google Scholar]
- Thompson JN, Pellmyr O. 1992. Mutualism with pollinating seed parasites amid co-pollinators: constraints on specialization. Ecology 73: 1780–1791. [Google Scholar]
- de Waal C, Barrett SCH, Anderson B. 2012. The effect of mammalian herbivory on inflorescence architecture in ornithophilous Babiana (Iridaceae): implications for the evolution of a bird perch. American Journal of Botany 99: 1096–1103. [DOI] [PubMed] [Google Scholar]
- Xia J, Sun S-G, Liu G-H. 2013. Evidence of a component Allee effect driven by predispersal seed predation in a plant (Pedicularis rex, Orobanchaceae). Biology Letters 9: 20130387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Holmgren NH, Mill RR. 1998. Pedicularis L In: Wu Z-Y, Raven PH, eds. Flora of China, Vol. 18 Beijing, China: Science Press, 97–209. [Google Scholar]
- Yoder JB, Smith CI, Pellmyr O. 2010. How to become a yucca moth: minimal trait evolution needed to establish the obligate pollination mutualism. Biological Journal of the Linnean Society 100: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W-B, Wang H. 2008. Pollen morphology of Pedicularis sect. Cyathophora, a group endemic to the eastern Himalaya–Hengduan Mountains Region. Journal of Integrative Plant Biology 50: 244–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.