Abstract
Background and aims Leaf nitrogen distribution in the plant canopy is an important determinant for canopy photosynthesis. Although the gradient of leaf nitrogen is formed along light gradients in the canopy, its quantitative variations among species and environmental responses remain unknown. Here, we conducted a global meta-analysis of leaf nitrogen distribution in plant canopies.
Methods We collected data on the nitrogen distribution and environmental variables from 393 plant canopies (100, 241 and 52 canopies for wheat, other herbaceous and woody species, respectively).
Key Results The trends were clearly different between wheat and other species; the photosynthetic nitrogen distribution coefficient (Kb) was mainly determined by leaf area index (LAI) in wheat, whereas it was correlated with the light extinction coefficient (KL) and LAI in other species. Some other variables were also found to influence Kb. We present the best equations for Kb as a function of environmental variables and canopy characteristics. As a more simple function, Kb = 0·5KL can be used for canopies of species other than wheat. Sensitivity analyses using a terrestrial carbon flux model showed that gross primary production tended to be more sensitive to the Kb value especially when nitrogen content of the uppermost leaf was fixed.
Conclusion Our results reveal that nitrogen distribution is mainly driven by the vertical light gradient but other factors such as LAI also have significant effects. Our equations contribute to an improvement in the projection of plant productivity and cycling of carbon and nitrogen in terrestrial ecosystems.
Keywords: Canopy photosynthesis, functional group, leaf area index, light extinction coefficient, light distribution, model, nitrogen allocation, nitrogen use, optimization
INTRODUCTION
In a leaf canopy, leaf nitrogen content per unit leaf area (Narea) is highest at the top of the canopy and decreases with depth (Mooney et al., 1981; DeJong and Doyle, 1985; Charles-Edwards et al., 1987; Hirose and Werger, 1987b). Such a gradient of Narea contributes to an efficient use of nitrogen at the whole-plant level, as photosynthetic carbon gain of a leaf increases with increasing Narea at high irradiance but is less dependent at low irradiance (Mooney and Gulmon, 1979; Hirose and Werger, 1987a; Hikosaka and Terashima, 1995). Given that nitrogen is one of the most limiting resources for plant growth (Aerts and Chapin, 2000), the distribution of Narea within a leaf canopy is one of the most important determinants of canopy carbon gain.
To date, many studies have discussed optimal Narea distribution that maximizes canopy carbon gain. Field (1983) indicated that canopy photosynthesis is maximized when Narea is distributed such that the marginal gain of nitrogen investment is identical among leaves. Assuming linear relationships between Amax (light-saturated rate of CO2 assimilation) and Narea, Farquhar (1989) suggested that canopy photosynthesis is maximized when Amax is proportional to relative light intensity. Anten et al. (1995) derived a mathematical solution of the optimal Narea distribution. When the gradient of light availability and Narea are described with an exponential function as
| (1) |
| (2) |
optimal Kb that maximizes canopy carbon gain is given as follows:
| (3) |
where F is the cumulative leaf area index (LAI) from the canopy top, I and Io are photosynthetically active photon flux density (PPFD) at F and the canopy top, respectively, No is Narea of the top leaves, Nb is the x-intercept of the Amax–Narea relationship, which can be regarded as representing non-photosynthetic nitrogen content, and KL and Kb are the light extinction coefficient and photosynthetic nitrogen distribution coefficient, respectively (see Anten et al., 1995, for how this solution is derived). Several simulation studies have reported that the Narea distribution observed in actual canopies significantly improves canopy photosynthesis compared with the uniform Narea distribution (for a review, see Hirose, 2005). This optimality theory has been used to describe canopy Narea distribution in various models of plant functioning because of its simplicity (Sellers et al., 1992; Anten, 2002; Franklin and Ågren, 2002; Hikosaka, 2003; Hikosaka and Anten, 2012).
Nevertheless, the Narea gradients in actual canopies have been shown to be less steep than the optimal gradients (e.g. Hirose and Werger, 1987b; Yin et al., 2003; Niinemets, 2012; Buckley et al., 2013). Furthermore, Hikosaka (2014) demonstrated that the optimal Narea distribution is steeper under direct and diffuse light than under diffuse light only. The difference between actual and optimal Narea distribution is therefore even larger than considered in previous studies that assumed only diffuse light in their calculation of optimal Narea distributions. Many studies have intensively discussed the physiological and physical constraints explaining discrepancies between theoretical and actual Narea distribution (Pons et al., 1989; Stockhoff, 1994; Hollinger, 1996; Schieving and Poorter, 1999; Kull, 2002; Buckley et al., 2002, 2013; Lloyd et al., 2010; Dewar et al., 2012; Peltoniemi et al., 2012; Niinemets, 2012; Tarvainen et al., 2013; Coble and Cavaleri, 2015).
Given that actual Narea distribution is suboptimal, several questions arise: how steep is the Narea gradient in actual canopies? Is the gradient different between species or between functional groups? Can we predict Narea gradients in plant canopies located in different environmental conditions? As Narea distribution is one of the critical elements for determining canopy photosynthesis (i.e. equivalent to gross primary productivity, GPP) in crop growth models (Yin and Struik, 2010) and vegetation–climate models (Sitch et al., 2003; Krinner et al., 2005; Ito et al., 2005; Bonan et al., 2011), this information is required to improve the accuracy of the model predictions. Anten et al. (2000) demonstrated that Kb was significantly correlated with KL across various plant stands, implying that Narea distribution is regulated depending on the light gradient. However, they used a limited number of plant stands and did not investigate stands of woody species (i.e. forest). In contrast, by examining Narea distribution in canopies of many wheat cultivars, Moreau et al. (2012) reported that the Narea distribution depended on LAI in the canopy rather than KL. Recently, Niinemets et al. (2015) conducted a meta-analysis of leaf trait gradient within a canopy, in which they focused on the relationship between traits and light availability of leaves. However, their results are not necessarily useful for canopy photosynthesis modelling because they did not study how Narea distribution is related to KL and LAI in the canopies. Here, we conducted a meta-analysis of nitrogen distribution coefficients in plant canopies. We collected data of Narea distribution from many plant stands and analysed interspecific variation and environmental dependence of the nitrogen distribution coefficient. We further analysed the sensitivity in canopy carbon gain in vegetation types to the value of nitrogen distribution coefficient.
MATERIALS AND METHODS
Data collection
We collected data for canopy traits of 393 stands from 41 studies including published (37 studies) and unpublished results (four studies) (Supplementary Data Table S1). The published papers were sourced from the Web of Science, Google Scholar and citations within other studies. The quality of data in unpublished studies was considered to be equivalent to that in published studies. We chose data that had total LAI of the canopy and Narea values for at least two positions in the canopy in terms of cumulative LAI from the top. The locations of the stands ranged from 43°S to 58°N in latitude and from –0·6 to 1025 m in altitude (Table 1). Ten stands were multispecies forests consisting of species belonging to the same functional group, whereas the other stands consisted of or were dominated by single species (43 species). The dataset included natural (44 canopies) and artificial stands (349 canopies) that were established in the field (290 canopies) or in greenhouses (103 canopies), and included both wild and domesticated (crop) species. We did not collect data from stands grown only under artificial light sources.
Table 1.
Basic statistics of the data
| Mean | SD | Min. | Max. | |
|---|---|---|---|---|
| Latitude (°, absolute value) | 39.2 | 9.3 | 3.0 | 58.0 |
| Altitude (m) | 172.1 | 173.1 | –0.6 | 1025 |
| [CO2] (μmol mol–1) | 398.0 | 72.2 | 350 | 705.6 |
| Air temperature (°C) | 21.6 | 5.0 | 5.7 | 30.6 |
| Leaf area index (m2 m–2) | 4.18 | 2.64 | 0.19 | 14.2 |
| KL | 0.58 | 0.18 | 0.29 | 1.31 |
| KN | 0.26 | 0.21 | –0.14 | 1.71 |
| Kb | 0.41 | 0.35 | 0.01 | 2.55 |
The number of stands was 264, 393 and 236 for KL (light extinction coefficient), KN (coefficient of leaf nitrogen distribution) and Kb (coefficient of photosynthetic nitrogen distribution), respectively.
We studied two nitrogen distribution coefficients, Kb and KN. Kb is the slope of photosynthetic nitrogen content (Narea–Nb) given by eqn (2). Nb was obtained as the x-intercept of the relationship between photosynthetic capacity and Narea (Anten et al., 1995). KN is the slope of Narea in the canopy given as follows:
| (4) |
KN values were available for all the studied stands, whereas Kb values were available for 236 stands because of a lack of photosynthetic data. KL values, which were given in the literature or calculated according to eqn (1) based on direct measurements, were available for 264 stands.
We obtained latitude (Lat) and altitude (Alt) of the site, date of measurement, mean daily or monthly air temperature (Temp) at the date or month of measurement and growth CO2 concentration (CO2), if available. If not, monthly air temperature data near the site were collected from other sources such as websites and growth CO2 concentration was obtained from the global mean (http://ds.data.jma.go.jp/gmd/wdcgg/pub/global/globalmean.html; Japan Meteorological Agency). We calculated noon solar angle (SA) at the measurement date or month. Precipitation was not considered because some crop stands were irrigated. The effects of field/greenhouse and that of natural/artificial canopies were not assessed because they were not significant in a preliminary test.
Species were categorized according to growth form (annual herb, A; perennial herb, P; deciduous woody species, D; and evergreen woody species, E), phylogeny (Phyl: gymnosperm, G; monocot, M; and dicot, D) and photosynthetic metabolism (C3 or C4). Growth phase was divided into vegetative or reproductive based on the presence of reproductive organs in the studied plants. Additionally, we conducted a separate analysis using data only for Triticum aestivum (wheat) and for other species, because wheat showed different trends from the other species (see Results).
Statistics
We applied multiple regression analyses to detect factors responsible for nitrogen coefficients (Kb and KN) and the light extinction coefficient (KL) using a generalized linear model (GLM). As explanatory variables, we used KL (only for nitrogen coefficients), LAI, Temp, SA, Lat, Alt, CO2, Phyl, C3/C4 and growth phase in the full model of the GLM. The absolute value of latitude was used. We selected the best model that provided the lowest values of Akaike’s information criterion (AIC) by a backward stepwise process. In the present study, we did not incorporate the random effects for the studies in the GLM. This is because the reported species in each study were highly dependent on the literature (i.e. some papers only reported Oryza sativa). Therefore, we assumed that every study had a common true effect size in this study. Multicollinearity was assessed using the variance inflation factor (VIF). When the VIF value was higher than 10, the model was rejected and the best model was selected eliminating a parameter that was related to others. The analyses were performed using the R statistical software version 3.2.0 with packages mgcv and car (R Core Team, 2015).
Sensitivity analysis
To examine quantitative influences of Kb values on ecosystem-scale carbon flux, we conducted a sensitivity analysis using a process-based terrestrial carbon cycle model at three contrastive sites. We used the Vegetation Integrative SImulator for Trace gases (VISIT; Ito, 2010) to simulate GPP through the year. In the model, a sun/shade canopy scheme by de Pury and Farquhar (1997) was incorporated. The analysis was conducted using data from three sites: (1) a cool-temperate deciduous broadleaved forest in Takayama, Japan, (2) an evergreen tropical forest in Lambir, Malaysia, and (3) a cool grass/shrubland at Chokurdakh, Russia. These sites are occupied by C3 plants.
We conducted seven simulations for each site: Sim1, a baseline simulation using the Kb value obtained from our meta-analysis; Sim2 and Sim5, the value of Kb was changed by + 10 % from Sim1; Sim3 and Sim6, the value of Kb was changed by –10 % from Sim1; and Sim4 and Sim7, a reference simulation according to the model of de Pury and Farquhar (1997), where nitrogen distribution in the canopy is described using another nitrogen distribution coefficient, Ka:
| (5) |
where Ft is the total LAI in the canopy. The value of Ka was fixed at 0·713 in Sim4 and Sim7 (de Pury and Farquhar, 1997); note that Kb values change with LAI when Ka is fixed. For the baseline simulation (i.e. Sim1), ecophysiological parameters such as canopy-top nitrogen concentration were calibrated, so that the estimated GPP came close to observations (Saigusa et al., 2005 for Takayama; Katayama et al., 2013 for Lambir; Iwahana et al., 2014 for Chokurdakh). In Sim2–4, total leaf nitrogen in the canopy was identical to that in Sim1. In Sim5–7, Narea of top leaves (No) was identical to that in Sim1. Sim5–7 were applied because No values are available rather than canopy nitrogen in most experimental sites and thus many simulation models use No values for estimating GPP. Because the simulated carbon flux was stabilized through a sufficient length of initialization (i.e. spin-up), for clarity we focused on annual GPP in this sensitivity analysis.
RESULTS
Nitrogen distribution coefficients
The global means of KN and Kb values in the database were 0·26 and 0·41, respectively (Table 1). The mean value of LAI was 4·18, which was very close to our previous meta-analysis of LAI for forests (4·21; Iio et al., 2014). The mean value of KL was 0·58.
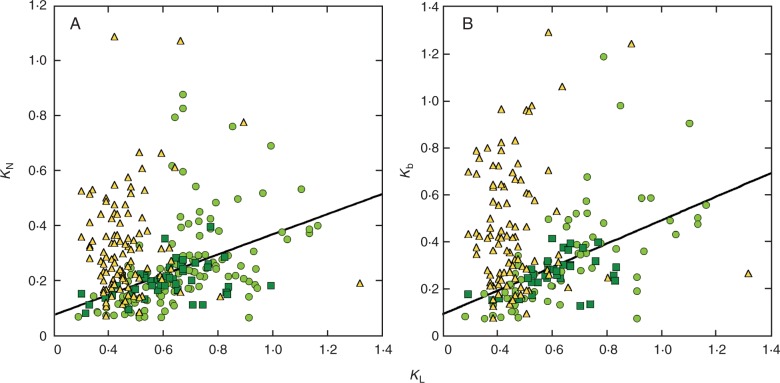
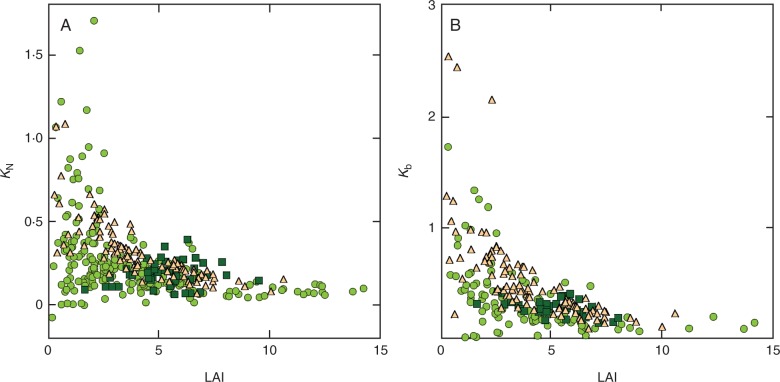
When all the data were pooled, KL, LAI, SA and growth form were selected in the best model for the variation in KN (Table 2). KL, LAI, latitude and solar angle were selected for the variation in Kb (Table 2). Because the relationship between the nitrogen distribution coefficients and KL was very different between wheat and the other species (Fig. 1), we analysed these groups separately. In wheat, KN and Kb were not related to KL (Fig. 1) but rather to LAI (Fig. 2). Air temperature at harvest was also selected as a significant factor for the variation in Kb in wheat (Table 2). In contrast, in the species other than wheat, KN was related to KL, LAI, growth form and growth phase (Table 2) and Kb was related to KL, LAI, temperature, Lat and Alt (Table 2). KN and Kb were nearly proportional to KL; the regression line was Kb =0·5KL (Fig. 1). KN and Kb were negatively correlated with LAI not only in wheat but also in other species (Fig. 2). We further separated species other than wheat into herbaceous and woody species. In all cases, KL was a significant factor for the variation in the nitrogen distribution coefficients (Table 2). LAI was also selected in every case, although it was not significant in the analysis for Kb of herbs. Some of the geographical and environmental factors were selected in the best models; however, the selected factors were different depending on whether KN or Kb was considered. Air temperature at harvest was suggested to significantly influence both KN and Kb in woody species; an increase by 1 °C in temperature decreased nitrogen coefficients by 0·006–0·015, whereas it was positively related to Kb in herbs (Table 2). Altitude was found to be negatively related to Kb in herbs. Perennial herbs were found to have lower KN than annual herbs. Evergreen woody species were found to have lower KN than deciduous woody species. Coefficients of other factors selected in the model were not significant.
Table 2.
Results of multiple regression analysis for nitrogen distribution coefficients
| Full model | AIC | Selected model | d.f. | AIC | Coefficient | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KN | All | KL + LAI + Temp + Lat + SA + CO2 + Alt + GF + Phyl + Phase + C3/C4 | –133·6 | KL + LAI + SA + GF | 183 | –196·1 | Intercept | KL | LAI | SA | GF C | GF D | GF E | GF P |
| 0·455 | 0·136 | –0·0323 | –0·00210 | –0·06270 | –0·190 | –0·0679 | –0·103 | |||||||
| *** | * | *** | ** | *** | ||||||||||
| VIF | 1·313 | 1·410 | 1·164 | 1·199 | ||||||||||
| Wheat | KL + LAI + Temp + Lat + SA + CO2 + Alt + Phase | 10·2 | LAI + Lat | 98 | –110·7 | Intercept | LAI | Lat | ||||||
| 0·563 | –0·0573 | –0·00009 | ||||||||||||
| * | *** | |||||||||||||
| VIF | 1·034 | 1·034 | ||||||||||||
| Non-wheat | KL + LAI + Temp + Lat + SA + CO2 + Alt + GF + Phyl + Phase + C3/C4 | –158·5 | KL + LAI + GF + Phase | 129 | –176·9 | Intercept | KL | LAI | GF D | GF E | GF P | Veg | ||
| 0·231 | 0·242 | –0·0252 | 0·00862 | –0·1370 | –0·0766 | –0·0361 | ||||||||
| *** | *** | *** | * | *** | ||||||||||
| VIF | 1·486 | 1·513 | 1·506 | 1·319 | ||||||||||
| Herbs (except wheat) | KL + LAI + Temp + Lat + SA + CO2 + Alt + GF + Phyl + Phase + C3/C4 | –101·4 | KL + LAI + GF + Phase | 104 | –120·7 | Intercept | KL | LAI | GF P | Veg | ||||
| 0·239 | 0·240 | –0·0255 | –0·0700 | –0·0494 | ||||||||||
| ** | ** | *** | * | |||||||||||
| VIF | 1·460 | 1·420 | 1·350 | 1·330 | ||||||||||
| Woody species | KL + LAI + Temp + Lat + SA + CO2 + Alt + GF + Phase | –114·8 | KL + LAI + Temp + GF | 42 | –159·4 | Intercept | KL | LAI | Temp | GF E | ||||
| 0·337 | 0·176 | –0·0195 | –0·00599 | –0·09950 | ||||||||||
| *** | *** | *** | *** | *** | ||||||||||
| VIF | 1·228 | 1·130 | 1·246 | 1·399 | ||||||||||
| Kb | All | KL + LAI + Temp + Lat + SA + CO2 + Alt + GF + Phyl + Phase + C3/C4 | 43·6 | KL + LAI + Lat + SA | 124 | 20·7 | Intercept | KL | LAI | Lat | SA | |||
| 1·319 | 0·251 | –0·0381 | –0·0042 | –0·0121 | ||||||||||
| *** | + | ** | *** | |||||||||||
| VIF | 1·529 | 1·781 | 2·187 | 2·912 | ||||||||||
| Wheat | KL + LAI + Temp + Lat + SA + CO2 + Alt + Phase | 62·9 | LAI + Temp | 97 | 53·5 | Intercept | LAI | Temp | ||||||
| 1·651 | –0·1070 | –0·0424 | ||||||||||||
| *** | *** | * | ||||||||||||
| VIF | 1·159 | 1·159 | ||||||||||||
| Non-wheat | KL + LAI + Temp + Lat + SA + CO2 + Alt + GF + Phyl + Phase + C3/C4 | –82·7 | KL + LAI + Temp + Lat + Alt | 95 | –114·4 | Intercept | KL | LAI | Temp | Lat | Alt | |||
| –0·181 | 0·454 | –0·0208 | 0·00834 | 0·00347 | –0·00014 | |||||||||
| *** | *** | + | * | * | ||||||||||
| VIF | 1·877 | 1·683 | 2·050 | 1·945 | 1·114 | |||||||||
| Herbs (except wheat) | KL + LAI + Temp + Lat + SA + CO2 + Alt + GF + Phyl + Phase + C3/C4 | –36·9 | KL + LAI + Temp + Lat + Alt | 61 | –52·2 | Intercept | KL | LAI | Temp | Lat | Alt | |||
| –0·276 | 0·538 | –0·0149 | 0·0100 | 0·00333 | –0·00019 | |||||||||
| *** | * | |||||||||||||
| VIF | 2·492 | 1·909 | 2·438 | 1·913 | 1·193 | |||||||||
| Woody species | KL + LAI + Temp + Lat + SA + CO2 + Alt + GF + Phase | –76·5 | KL + LAI + Temp + CO2 | 29 | –99·1 | Intercept | KL | LAI | Temp | CO2 | ||||
| 0·459 | 0·251 | –0·0205 | –0·01500 | 0·00226 | ||||||||||
| *** | ** | * | *** | |||||||||||
| VIF | 1·399 | 1·568 | 1·164 | 1·310 | ||||||||||
A generalized linear model with stepwise selection was applied to obtain the best model based on Akaike’s information criterion (AIC). Coefficient values (not standardized) with significance are shown (***P < 0·001, **P < 0·01, *P < 0·05, +P < 0·1). Coefficients for the categorical variable are the value relative to annual plant (growth form), dicot species (phylogeny) and reproductive phase (phase). All the variables were treated as a fixed factor. Alt, altitude (m); CO2, atmospheric CO2 concentration (μmol mol–1); GF, growth form (C, conifer; D, deciduous tree; E, evergreen tree; P, perennial herb); Kb, coefficient of photosynthetic nitrogen distribution; KL, light extinction coefficient; KN, coefficient of leaf nitrogen distribution; LAI, leaf area index; Lat, latitude (degrees); Phase, vegetative or reproductive phase; Phyl, phylogeny (gymnosperm, monocot or dicot); SA, solar angle at harvest (degrees); Temp, air temperature at harvest (°C); Veg, vegetative phase; VIF, variance inflation factor.
Fig. 1.
Relationship between nitrogen distribution coefficients and light extinction coefficient (KL). (A) The coefficient of leaf nitrogen distribution (KN) and (B) the coefficient of photosynthetic nitrogen distribution (Kb). Circles, herbaceous species except wheat; triangles, wheat; and squares, woody species. Regression lines are KN = 0·368KL (r2 = 0·22) and Kb = 0·499KL (r2 = 0·37), with data for wheat not included. Note that three data points that had Kb values higher than 1·5 were omitted from B (see Supplementary Data Fig. S1 for the whole data set).
Fig. 2.
Relationship between nitrogen distribution coefficients and leaf area index (LAI). (A) The coefficient of leaf nitrogen distribution (KN) and (B) the coefficient of photosynthetic nitrogen distribution (Kb). Circles, herbaceous species except wheat; triangles, wheat; and squares, woody species.
Variation in KL among species was analysed. When all the species were pooled, KL was significantly related to LAI, temperature, SA, growth form and phylogeny (Supplementary Data Table S2). Annual plants had a higher KL than deciduous woody species. When separated into different growth forms, KL in wheat was related to LAI and SA. In herbs other than wheat, KL was related to LAI, temperature, growth form, phylogeny (gymnosperm, monocot or dicot) and growth phase. In woody species, KL was related to SA and growth form.
Sensitivity analysis
Here, we assess the effect of incorrect values of nitrogen distribution on GPP estimation. At the three sites, the baseline simulation by the VISIT model retrieved intersite differences in annual GPP (Table 3): high productivity of Lambir tropical forest, low productivity of cool grass/shrubland, and intermediate productivity in the Takayama temperate forest. In Sim2–4, Kb values were altered with the same value of canopy nitrogen as for Sim1. An increase in Kb increased GPP, indicating that the predicted Kb is less steep than optimal. However, the deviations in GPP from Sim1 were small except for Sim4, suggesting that the effects of small changes in Kb on GPP are generally small. On the other hand, in Sim5–7, where Kb values were altered with the same No value as for Sim1, the deviations were larger than those in Sim2–4. Whereas optimality models fix canopy nitrogen as in Sim2–4, many simulations of ecosystem carbon cycling use leaf properties of top leaves rather than canopy nitrogen as in Sim5–7. Therefore, correct Kb values are necessary for accurate projection of carbon cycling.
Table 3.
Sensitivity analysis for effects of photosynthetic nitrogen distribution coefficient (Kb) on gross primary production (GPP) in three ecosystems, Takayama (deciduous broadleaved forest), Lambir (evergreen tropical forest) and Chokurdakh (grass/shrubland)
| Site | Mean Kb | GPP (g C m−2 year−1) |
||||||
|---|---|---|---|---|---|---|---|---|
| Same canopy nitrogen |
Same No |
|||||||
| Sim1 | Sim2 | Sim3 | Sim4 | Sim5 | Sim6 | Sim7 | ||
| Takayama | 0·371 | 1006 | 1011 | 1000 | 1125 | 973 | 1042 | 1162 |
| +0·5 % | –0·6 % | +11·9 % | –3·4 % | +4·0 % | +17·6 % | |||
| Lambir | 0·180 | 3442 | 3460 | 3423 | 3430 | 3365 | 3417 | 3377 |
| +0·5 % | –0·5 % | –0·3 % | –0·8 % | +0·8 % | +0·4 % | |||
| Chokurdakh | 0·222 | 382 | 382 | 382 | 372 | 287 | 325 | 216 |
| +0·05 % | –0·05 % | –2·7 % | –5·7 % | +6·6 % | +29·1 % | |||
Sim1, a baseline simulation using Kb values obtained from the meta-analysis (the equation of ‘woody species’ for Takayama and Lambir and that of ‘non-wheat’ for Chokurdakh; Table 2); Sim2 and Sim5, the Kb value was changed by + 10 % from Sim1; Sim3 and Sim6, the Kb value was changed by –10 % from Sim1; Sim4 and Sim7, a reference simulation using Ka value (0·713) assumed in de Pury and Farquhar (1997) (see text for details). For Sim2–4, canopy nitrogen content per ground area was the same as that for Sim1, whereas for Sim5–7, nitrogen content of top leaves (No) was the same as that for Sim1. Note that the Kb value in Sim1–3 changes for each site depending on other variables such as temperature and the mean value is shown. For Takayama, the mean Kb value was calculated for seasons with LAI > 1.
DISCUSSION
Our meta-analysis clearly demonstrated that the nitrogen distribution coefficients are sensitive to species traits and environmental factors. In particular, wheat has a different dependence of nitrogen coefficients on the traits and factors from other species. We presented the best models to explain the variation in nitrogen partitioning coefficients in Table 2. These models contribute to prediction of nitrogen partitioning in canopies of species belonging to different functional groups under various environmental conditions.
For species other than wheat, KN and Kb were mostly explained by KL. Kb was nearly proportional to KL. Therefore, we can use a simple model, Kb = 0·5KL, to predict nitrogen partitioning in plant canopies when information on other factors is lacking. Given that Kb = KL is optimal under diffuse light (Anten et al., 1995; Hikosaka, 2014; see Introduction), the Kb to KL ratio <1 indicates that nitrogen distribution in actual canopies is suboptimal. However, a strong correlation between Kb and KL suggests that light distribution in the canopy is an important driver of nitrogen distribution within the canopy across a wide variety of species, as has been indicated by many researchers (Hirose and Werger, 1987b; Hirose et al., 1988, 1989; Ackerly, 1992; Ackerly and Bazzaz, 1995; Pons et al., 1993; Hikosaka et al., 1994; Anten et al., 1995; Niinemets et al., 2015).
It was found that KN and Kb were negatively related to LAI. This was partly explained by the fact that greater KL values were found only with lower LAI, especially in herbaceous species. A canopy with both greater KL and LAI values may be unrealistic because carbon gain in leaves at lower positions becomes negative due to very low light availability (Monsi and Saeki, 1953). Higher KN and Kb at low LAI may be related to nitrogen availability. When plants are exposed to low nitrogen availability, senescence of old leaves and thus retranslocation of nitrogen from old to new leaves is accelerated (Thomas and Staddart, 1980). Moreau et al. (2012) showed that wheat stands in low nutrient availability had higher Kb.
Several environmental and geographical factors were found to influence nitrogen distribution coefficients. Air temperature at harvest was negatively related to KN and Kb in woody species. This is consistent with a study showing that an experimentally warmed Scots pine stand had a less steep gradient of leaf nitrogen content (Kellmäki and Wang, 1997). Although effects of other factors such as latitude and altitude were found to be significant in some cases, their effects were not consistent between KN and Kb. For example, effects of altitude were significant for Kb in herbaceous species but not for KN, although KN and Kb are closely related to each other (see eqns 2 and 4). In our dataset, KN values were available in all stands, whereas Kb values were available for 60 % of the studied stands. This implies that the results for KN are more reliable than those for Kb and that the environmental and geographical factors may influence a limited number of species.
Many researchers have discussed why nitrogen distributions in leaf canopies are generally more uniform than the optimal distribution (see Introduction). Although the present study did not directly analyse the underlying mechanisms, comparisons between different functional groups may provide some insights. It has been indicated that leaf ageing may contribute to forming the nitrogen gradient because Narea generally decreases with leaf age (Mooney et al., 1981). Hikosaka et al. (1994) raised a vine plant (Ipomoea tricolor) horizontally to avoid shading between leaves and found that nitrogen gradient was formed along leaf age when the plants were grown at low nutrient availability. As a vertical leaf age gradient is formed in herbaceous dicot stands but not in most woody species stands, one may expect that herbaceous stands have a steeper gradient of nitrogen content than stands of woody species. In the present study, annual herbs had a higher KN than evergreen woody species (Table 2). However, such a difference was not found for Kb. Furthermore, deciduous woody species had higher KN values than annual herbs, although this was not significant (Table 2). Therefore, we can reject a hypothesis that leaf age gradient plays an important role for forming the nitrogen gradient in field stands. Peltoniemi et al. (2012) demonstrated theoretically that suboptimal distribution of hydraulic conductance between upper and lower leaves may explain suboptimal nitrogen distribution. However, a similar nitrogen gradient between annual herbs and deciduous trees may suggest that hydraulic constraints are not an important factor as hydraulic limitations are less important in herbs than in taller trees. Further studies may be necessary for understanding the ecological significance of suboptimal nitrogen distribution in the canopy.
In wheat canopies, in contrast to other species, nitrogen distribution was not related to KL but to LAI, as has been indicated by Moreau et al. (2012). In addition, their KN and Kb values were generally greater than that of other species even though they have lower KL values. Intriguingly, the results for wheat were contrary to those for other Poaceae species included in the present study. For example, rice had a similar Kb to KL ratio to other species, 0·34 ± 0·12. Berthloot et al. (2008) reported that there was no gradient of Narea within flag leaves of wheat although there was a large gradient in light. They considered that light signal is integrated at the whole leaf lamina level and that the nitrogen gradient is mainly due to differences between leaves. If this is true, the vertical gradient of Narea may be steeper in a canopy consisting of smaller individual leaves where LAI is lower. Another explanation may originate from the fact that the wheat ear has a high photosynthetic ability and its contribution to grain filling is greater than that of flag leaves (e.g. Sanchez-Bragado et al., 2014). Previous optimization models assume that the slope of the relationship between photosynthetic ability and Narea is constant across leaves. However, if the slope is very different between ear and flag leaves, this assumption may not hold true, and a more complex model would need to be used to determine optimal N distributions. Although the mechanisms remain unclear, our meta-analysis clearly showed that the relationship between canopy structure and the nitrogen distribution differs from that of the other species in the present study. This effect will need to be considered when wheat productivity is modelled.
The equations to predict KN and Kb values (Table 2) include information on geography, abiotic environment and canopy characteristics. Whereas the geographical and environmental variables can be estimated using geographical information systems (GIS), canopy characteristics, LAI and KL may need to be determined with field investigation. Recently, Iio et al. (2014) conducted a meta-analysis of forest LAI where LAI was expressed as a function of mean annual temperature, wetness index (the ratio of annual precipitation to potential evapotranspiration) and plant functional type, which may be useful to predict LAI in a given ecosystem. KL varies considerably among plant stands (see Table 1) mainly depending on leaf angle (Monsi and Saeki, 1953). The equations shown in Table S2 may be useful to predict KL values in plant stands.
How KL values theoretically influence canopy photosynthesis varies depending on model assumptions. In previous optimality studies, the sensitivities of canopy photosynthesis to KL values were studied keeping a constant canopy nitrogen per ground area, because nitrogen is regarded as a cost for photosynthesis (Field, 1983; Hirose and Werger, 1987b). In Sim2–4, canopy nitrogen was the same as Sim1 and an increase in the Kb value increased GPP (Table 3) because the Kb value was lower than the optimum. On the other hand, in vegetation models that consider canopy photosynthesis, Narea of the top leaves (No) is given (e.g. de Pury and Farquhar, 1997). In these models, a decrease in the Kb value resulted in a decrease in canopy nitrogen per ground area (see eqn 2), which leads to a decrease in canopy photosynthesis. In Sim5–7, No was the same as that in Sim1 and an increase in the Kb value decreased canopy nitrogen and thus GPP (Table 2).
Our sensitivity analysis clearly revealed that GPP is sensitive to the value of Kb especially when No is given. Therefore, accuracy of the Kb value is indispensable for accurate projection of canopy gas exchange rates, plant production and ecosystem carbon budget. Furthermore, the Kb value also contributes to estimation of leaf nitrogen content in the canopy, which is an important variable for ecosystem nitrogen cycling. This information may contribute to a functional coupling of models for carbon and nitrogen cycling in terrestrial ecosystems.
CONCLUSION
The present study has demonstrated that the nitrogen distribution coefficients of a plant canopy are influenced by various factors. It is surprising that wheat has a different dependence of nitrogen distribution coefficients on the factors from other species. In other species, the nitrogen distribution coefficients were significantly correlated with the light extinction coefficient, suggesting that the vertical light gradient is a main driver of nitrogen distribution in the canopy. Other factors such as LAI were also found to influence nitrogen distribution coefficients. The best models shown in the present study contribute to an accurate estimation of nitrogen distribution coefficients in a given vegetation and to an improvement of projection of plant productivity and cycling of carbon and nitrogen in terrestrial ecosystems.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: relationship between the coefficient of photosynthetic nitrogen distribution and light extinction coefficient. Table S1: dataset of leaf area index, light extinction coefficient, coefficient of leaf nitrogen distribution and coefficient of photosynthetic nitrogen distribution. Table S2: result of multiple regression analysis for light extinction coefficient.
ACKNOWLEDGMENTS
We thank Prof. Tadaki Hirose for providing data and comments. The study was supported by KAKENHI (2114009, 25660113, 25440230, 25291095) and CREST, JST, Japan.
LITERATURE CITED
- Ackerly DD. 1992. Light, leaf age, and leaf nitrogen concentration in a tropical vine. Oecologia 89: 596–600. [DOI] [PubMed] [Google Scholar]
- Ackerly DD, Bazzaz FA. 1995. Leaf dynamics, self-shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia 101: 289–298. [DOI] [PubMed] [Google Scholar]
- Aerts R, Chapin FS., III 2000. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Advances in Ecological Research 30: 1–67. [Google Scholar]
- Anten NPR. 2002. Evolutionarily stable leaf area production in plant populations. Journal of Theoretical Biology 217: 15–32. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Schieving F, Werger MJA. 1995. Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C3 and C4 mono- and dicotyledonoous species. Oecologia 101: 504–513. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Hikosaka K, Hirose T. 2000. Nitrogen utilization and the photosynthetic system In Marshal B, Robe J, eds. Leaf development and canopy growth. Sheffield: Sheffield Academic, 171–203. [Google Scholar]
- Berthloot J, Martre P, Andrieu B. 2008. Dynamics of light and nitrogen distribution during grain filling within wheat canopy. Plant Physiology 148: 1707–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonan GB, Lawrence PJ, Oleson KW, et al. 2011. Improving canopy processes in the Community Land Model version 4 (CLM4) using global flux fields empirically inferred from FLUXNET data. Journal of Geophysical Research 116: G02014 [Google Scholar]
- Buckley TN, Miller JM, Farquhar GD. 2002. The mathematics of linked optimisation for nitrogen and water use in a canopy. Silva Fennica 36: 639–669. [Google Scholar]
- Buckley TN, Cescatti A, Farquhar GD. 2013. What does optimization theory actually predict about crown profiles of photosynthetic capacity when models incorporate greater realism? Plant, Cell and Environment 36: 1547–1563. [DOI] [PubMed] [Google Scholar]
- Charles-Edwards DA, Stutzel H, Ferraris R, Beech DF. 1987. An analysis of spatial variation in the nitrogen content of leaves from different horizons within a canopy. Annals of Botany 60: 421–426. [Google Scholar]
- Coble AP, Cavaleri MA. 2015. Light acclimation optimizes leaf functional traits despite height‐related constraints in a canopy shading experiment. Oecologia 177: 1131–1143. [DOI] [PubMed] [Google Scholar]
- DeJong TM, Doyle JF. 1985. Seasonal relationships between leaf nitrogen content (photosynthetic capacity) and leaf canopy light exposure in peach (Prunus persica) Plant, Cell and Environment 8: 701–706. [Google Scholar]
- de Pury DGG, Farquhar GD. 1997. Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant, Cell and Environment 20: 537–557 [Google Scholar]
- Dewar RC, Tarvainen L, Parker K, Wallin G, McMurtrie RE. 2012. Why does leaf nitrogen decline within tree canopies less rapidly than light? An explanation from optimization subject to a lower bound on leaf mass per area. Tree Physiology 32: 520–534. [DOI] [PubMed] [Google Scholar]
- Farquhar GD. 1989. Models of integrated photosynthesis of cells and leaves. Philosophical Transactions of the Royal Society of London, Series B 323: 357–367. [Google Scholar]
- Field C. 1983. Allocating leaf nitrogen for the maximization of carbon gain: leaf age as a control on the allocation program. Oecologia 56: 341–347. [DOI] [PubMed] [Google Scholar]
- Franklin O, Ågren GI. 2002. Leaf senescence and resorption as mechanisms of maximizing photosynthetic production during canopy development at N limitation. Functional Ecology 16: 727–733. [Google Scholar]
- Hikosaka K. 2003. A model of dynamics of leaves and nitrogen in a canopy: an integration of canopy photosynthesis, leaf life-span, and nitrogen-use efficiency. American Naturalist 162: 149–164. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. 2014. Optimal nitrogen distribution within a leaf canopy under direct and diffuse light. Plant, Cell and Environment 9: 2077–2085. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Anten NPR. 2012. An evolutionary game of leaf dynamics and its consequences on canopy structure. Functional Ecology 26: 1024–1032. [Google Scholar]
- Hikosaka K, Terashima I. 1995. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant, Cell and Environment 18: 605–618. [Google Scholar]
- Hikosaka K, Terashima I, Katoh S. 1994. Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading. Oecologia 97: 451–457. [DOI] [PubMed] [Google Scholar]
- Hirose T. 2005. Development of the Monsi–Saeki theory on canopy structure and function. Annals of Botany 95: 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Werger MJA. 1987a. Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of a Solidago altissima stand. Physiologia Plantarum 70: 215–222. [Google Scholar]
- Hirose T, Werger MJA. 1987b. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72: 520–526. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA, Pons TL, Van Rheenen JWA. 1988. Canopy structure and leaf nitrogen distribution in a stand of Lysimachia vulgaris L. as influenced by stand density. Oecologia 77: 145–150. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA, Van Rheenen WA. 1989. Canopy development and leaf nitrogen distribution in a stand of Carex acutiformis. Ecology 70: 1610–1618. [Google Scholar]
- Hollinger DY. 1996. Optimality and nitrogen allocation in a tree canopy. Tree Physiology 16: 627–634. [DOI] [PubMed] [Google Scholar]
- Iio A, Hikosaka K, Anten NPR, Nakagawa Y, Ito A. 2014. Global dependence of field-observed leaf area index on climate in woody species: a systematic review. Global Ecology and Biogeography 23: 274–285. [Google Scholar]
- Ito A. 2010. Changing ecophysiological processes and carbon budget in East Asian ecosystems under near-future changes in climate: implications for long-term monitoring from a process-based model. Journal of Plant Research 123: 577–588. [DOI] [PubMed] [Google Scholar]
- Ito A, Saigusa N, Murayama N, Yamamoto S. 2005. Modeling of gross and net carbon dioxide exchange over a cool-temperate deciduous broad-leaved forest in Japan: analysis of seasonal and interannual change. Agriculture and Forest Meteorology 134: 122–134. [Google Scholar]
- Iwahana G, Takano S, Petrov RE, et al. 2014. Geocryological characteristics of the upper permafrost in a tundra-forest transition of the Indigirka River Valley, Russia. Polar Science 8: 96–113. [Google Scholar]
- Katayama A, Kume T, Komatsu H, et al. 2013. Carbon allocation in a Bornean tropical rainforest without dry seasons. Journal of Plant Research 126: 505–515. [DOI] [PubMed] [Google Scholar]
- Kellmäki S, Wang KY. 1997. Effects of long-term CO2 and temperature elevation on crown nitrogen distribution and daily photosynthetic performance of Scats pine. Forest Ecology and Management 99: 309-326. [Google Scholar]
- Krinner G, Viovy N, de Noblet-Ducoudré N, et al. 2005. A dynamic global vegetation model for studies of the coupled atmosphere-biosphere system. Global Biogeochemcal Cycles 19: GB1015. [Google Scholar]
- Kull O. 2002. Acclimation of photosynthesis in canopies: models and limitations. Oecologia 133: 267–279. [DOI] [PubMed] [Google Scholar]
- Lloyd J, Patino S, Paiva RQ, et al. 2010. Optimisation of photosynthetic carbon gain and within-canopy gradients of associated foliar traits for Amazon forest trees. Biogeosciences 7:1833–1859. [Google Scholar]
- Mooney HA, Gulmon SL. 1979. Environmental and evolutionary constraints on the photosynthetic characteristics of higher plants In Solbrig OT, Jain S, Johnson GB, Raven PH, eds. Topics in plant population biology. New York: Columbia University Press, 316–337. [Google Scholar]
- Mooney HA, Field C, Gulmon SL, Bazzaz FA. 1981. Photosynthetic capacities in relation to leaf position in desert versus old field annuals. Oecologia 50: 109–112. [DOI] [PubMed] [Google Scholar]
- Monsi M, Saeki T. 1953. Über den Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung für die Stoffproduktion. Japanese Journal of Botany 14: 22–52. [Google Scholar]
- Moreau D, Allard V, Gaju O, Le Gouis J, Foulkes MJ, Martre P. 2012. Acclimation of leaf nitrogen to vertical light gradient at anthesis in wheat is a whole-plant process that scales with the size of the canopy. Plant Physiology 160: 1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü. 2012. Optimization of foliage photosynthetic capacity in tree canopies: towards identifying missing constraints. Tree Physiology 32: 505–509. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Keenan TF, Hallik L. 2015. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytologist 205: 973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltoniemi MS, Duursma RA, Medlyn BE. 2012. Co-optimal distribution of leaf nitrogen and hydraulic conductance in plant canopies. Tree Physiology 32: 510–519. [DOI] [PubMed] [Google Scholar]
- Pons TL, Schieving F, Hirose T, Werger MJA. 1989. Optimization of leaf nitrogen allocation for canopy photosynthesis in Lysimachia vulgaris In Lambers H, Cambridge ML, Konings H, Pons TL, eds. Causes and consequence of variation in growth rate and productivity of higher plants. The Hague: SPB Academic Publishing, 175–186. [Google Scholar]
- Pons TL, Van Rijnberk H, Scheurwater I, Van der Werf A. 1993. Importance of the gradient in photosynthetically active radiation in a vegetation stand for leaf nitrogen allocation in two monocotyledons. Oecologia 95: 416–424. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2015. R: A language and environment for statistical computing [Internet] Vienna, Austria: R Foundation for Statistical Computing, 171–203. [Google Scholar]
- Saigusa N, Yamamoto S, Murayama S, Kondo H. 2005. Inter-annual variability of carbon budget components in an AsiaFlux forest site estimated by long-term flux measurements. Agricultural and Forest Meteorology 134: 4–16. [Google Scholar]
- Sanchez-Bragado R, Molero G, Reynolds MP, Araus JL. 2014. Relative contribution of shoot and ear photosynthesis to grain filling in wheat under good agronomical conditions assessed by differential organ δ13C. Journal of Experimental Botany 65: 5401–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieving F, Poorter H. 1999. Carbon gain in a multispecies canopy: the role of specific leaf area and photosynthetic nitrogen-use efficiency in the tragedy of the commons. New Phytologist 143: 201–211. [Google Scholar]
- Sellers PJ, Berry JA, Collatz GJ, Field CB, Hall FG. 1992. Canopy reflectance, photosynthesis and transpiration. III: a reanalysis using improved leaf models and a new canopy integration scheme. Remote Sensing of the Environment 42: 187–216. [Google Scholar]
- Sitch S, Smith B, Prentice IC, et al. 2003. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Global Change Biology 9: 161–185. [Google Scholar]
- Stockhoff BA. 1994. Maximisation of daily canopy photosynthesis: effects of herbivory on optimal nitrogen distribution. Journal of Theoretical Biology 169: 209–220. [Google Scholar]
- Tarvainen L, Wallin G, Räntfors M, Uddling J. 2013. Weak vertical canopy gradients of photosynthetic capacities and stomatal responses in a fertile Norway spruce stand. Oecologia 173: 1179–1189. [DOI] [PubMed] [Google Scholar]
- Thomas H, Staddart JL. 1980. Leaf senescence. Annual Review of Plant Physiology 31: 83–111. [Google Scholar]
- Yin X, Struik PC. 2010. Modelling the crop: from system dynamics to systems biology. Journal of Experimental Botany 61: 2171–2183. [DOI] [PubMed] [Google Scholar]
- Yin X, Lantinga EA, Schapendonk AHCM, Zhong X. 2003. Some quantitative relationships between leaf area index and canopy nitrogen content and distribution. Annals of Botany 91: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.