Abstract
Background and Aims The mechanisms involved in breaking seed dormancy in species with woody endocarps are poorly understood. In a landmark study examining the role of endocarps in regulating germination, our aim was to investigate the effects of the natural sequence of environmental conditions on dormancy break of a species with a woody endocarp (Persoonia longifolia).
Methods The role of the endocarp in germination was investigated through imbibition and endocarp removal germination tests. The use of burial to break dormancy was examined and results from these experiments were used to guide laboratory investigations into the use of wet/dry cycling and stratification to break dormancy.
Key Results Endocarps were water-permeable. Germination increased from 0 to 92·5 % when endocarps were removed. During burial in the field and nursery, 41·6 and 63·7 % of the endocarps germinated, respectively, after 36 months. Ex situ post-burial germination was cyclical and highest after 30 months of burial (45·4 % nursery and 31·8 % field). Highest germination occurred in wet/dry trials when the dry summer was long (20 weeks), had fluctuating temperatures (30/50 °C) and two long (7 d) wet cycles and was followed by moist winters at 10/20 °C. A stratification trial found that highest germination occurred following incubation for 12 weeks at 30 °C (including 2 weeks moist) + 6 weeks moist at 8 °C then placement at 20/10 °C for germination.
Conclusions Summer conditions break physiological dormancy of the embryo and promote opening of the endocarp, allowing seeds to germinate during winter conditions. By closely monitoring the environment that endocarps are exposed to in nature, dormancy breaking mechanisms can be identified and used to improve germination. These results outline for the first time how dormancy and germination are regulated in a species with a hard woody endocarp, insights which will significantly improve our understanding of other species with similar reproductive features.
Keywords: Persoonia longifolia, woody endocarp, dormancy break, burial, physiological dormancy, stratification, wet/dry cycles, seed germination
INTRODUCTION
The germination unit of species in various plant families is a seed enclosed by a hard woody indehiscent endocarp (see table 3·10 in Baskin and Baskin, 2014), and species with woody endocarps can be found in vegetation zones from the tropics to the boreal/subalpine. The challenge in understanding the germination ecology of seeds covered by a hard endocarp is that characteristics of both the endocarp and the embryo may be involved in delaying germination. That is, the endocarp could be water-impermeable (Li et al., 1999), or it could be water-permeable but exert strong mechanical resistance to expansion of the embryo (Nikolaeva, 1969). Also, the embryo may have some level of physiological dormancy (PD) and warm and/or cold stratification may be required for dormancy break to occur (Chien et al., 2002; Baskin et al., 2005; Persson et al., 2006; Chen et al., 2007; Imani et al., 2011). However, after PD is broken, it is still uncertain whether the embryo has enough growth potential to overcome the mechanical resistance of the water-permeable endocarp (Nikolaeva, 1969) as the covering structure may require some form of weakening. In some species the endocarp splits into two parts during seed germination, but in others the endocarp opens via a lid-like structure (Hill, 1933, 1937).
For most species with woody indehiscent endocarps, little is known about how the timing of germination is controlled in the natural habitat. Also, the effect that the sequence of environmental conditions between the time of dispersal and germination has on the embryo and the endocarp is largely unknown. To explore these questions, we investigated dormancy break and germination of Persoonia longifolia (Proteaceae, Persoonioideae) in its Western Australian Mediterranean habitat.
Persoonia longifolia is one of 98 species of Persoonia, all of which are Australian endemics with a long Gondwanan heritage stretching back to the Cretaceous (Dettmann and Jarzen, 1998). It is commonly found in the jarrah forests (Eucalyptus marginata) of south-western Western Australia, an area that experiences a Mediterranean climate with hot dry summers and cool wet winters. The species is a distinctive resprouting, mid-storey tree that is difficult to propagate either by cuttings or seeds. The shape and size of the tree makes it desirable for use in the native horticultural industry, and due to its distinctive leaves it is currently used as a filler species in the cut flower trade (Weston, 2003). The natural dispersal unit of Persoonia species is a drupe, with a fleshy mesocarp and a hard indehiscent endocarp. The embryo inside the endocarp is surrounded by a thin papery testa and, unlike other species of Persoonia, the embryo of P. longifolia is recurved (rather than linear) making endocarp removal problematic (Weston, 1994; Norman and Koch, 2008).
Although a significant amount of research has been conducted on the germination biology and alleviation of dormancy in P. longifolia over the past 13 years (Dixon et al., 2002; Mullins et al., 2002; Preston et al., 2002; Norman and Koch, 2005, 2006, 2008), the dormancy breaking and germination requirements remain essentially unknown for this species. Germination studies also have been undertaken on other species of Persoonia from New South Wales and Queensland. In particular, P. virgata and P. sericea, two obligate seeder species, have been studied in some detail (Ketelhohn et al., 1998; Bauer et al., 1999, 2001, 2004; Bauer and Johnston, 1999), with germination increasing from 0 to 64·6 % in P. sericea and to 87·5 % in P. virgata after at least half of the woody endocarp was removed. Studies on P. pinifolia also revealed that removal of the woody endocarp and application of gibberellic acid (GA3) promoted germination (McIntyre, 1969). However, seed extraction of P. pauciflora, an endangered species from New South Wales, resulted in only 2 % germination (Frith and Offord, 2010). Whilst chipping of P. longifolia endocarps resulted in moderate (36·7 %) germination (Norman and Koch, 2006), no studies have as yet investigated the effect of complete endocarp removal.
Drupes of P. longifolia mature and are dispersed in winter but do not germinate until the second (or later) winter (Chia et al., 2015), and during this time they may become buried in the soil. Mullins et al. (2002) found that sowing endocarps of P. longifolia on the soil surface resulted in higher germination than burial at a depth of 1 cm under nursery conditions (10 and 0 %, respectively) after 7 months. Norman and Koch (2008) found that burial of the fruits at a depth of 2 cm in the jarrah forest for 21 months resulted in the highest germination of P. longifolia (36·7 %) after retrieval, chipping the endocarp, treating with GA3 and surface sowing in the nursery. In addition to possibly becoming buried, endocarps of P. longifolia would be subjected to wetting and drying in the natural habitat. However, the effects of wetting and drying on opening the endocarps of this species have not been tested. Wetting and then rapid drying of Santalum spicatum endocarps resulted in 98 % of them cracking (Woodall, 2004), with germination increasing from 10 % in non-cracked endocarps to 100 % in cracked endocarps. Similarly, heating and chilling of teak (Tectona grandis) drupes resulted in improved germination (Rajput and Tiwari, 2001), and this was later attributed to weakening of a predefined, regular section (valve) on the woody endocarp (Slator et al., 2013).
The broad objective of our studies was to determine the dormancy breaking and germination requirements of P. longifolia dispersal units under natural conditions and to replicate them under laboratory and nursery conditions to facilitate the development of reliable propagation approaches. We hypothesized that exposure of the natural germination units, i.e. seed enclosed by endocarp, to the natural sequence of temperature and soil moisture conditions characteristic of the Mediterranean climate in Western Australia would have effects on both the embryo and the endocarp, and thereby result in germination. Thus, to validate (or not) our hypothesis, the specific aims of our research were to: (1) characterize the endocarp and seed structure, (2) determine the role of the endocarp in the regulation of dormancy, (3) investigate the impacts of natural field burial on endocarp opening and (4) examine the optimal germination requirements for P. longifolia seeds after dormancy was broken.
METHODS
Fruit collection and processing
Fruits (drupes) of Persoonia longifolia R.Br. were collected annually (2010–2014) between June and September from Moodiarrup West Road, Cordering, Western Australia (33·55°S, 116·60°E), for all trials except the burial trial. Fruits for the burial trial were collected in September 2010 from the Sotico and Saddleback areas near Boddington (32·95°S, 116·49°E), Western Australia. For trials in which clean endocarps were required, the mesocarp was removed and clean endocarps were stored in the seed drying room (maintained at 15 % relative humidity and 15 ± 2 °C) at Kings Park and Botanic Garden, Perth, Western Australia, until used in experiments.
Before use in any experiment, a sample of endocarps from each seed collection was examined for seed fill using an X-ray machine (Faxitron X-Ray Specimen Radiography System, Faxitron Bioptics, LLC, Tucson, AZ, USA). To assess whether the seeds in filled endocarps were viable, three replicates of 20 cleaned endocarps were examined for seed fill and then tested for viability using 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) (Lakon, 1949). A portion of the endocarp (the lid) was removed along a natural facture line using diagonal cutting pliers, and the remaining endocarp and seed were placed in Petri dishes on germination paper moistened with 1 % (w/v) TTC and incubated at 30 °C in the dark for 48 h. Embryos that stained red were considered to be viable, and those that did not stain were assumed to be non-viable.
Endocarp and seed characteristics
Eighteen replicates of 20 freshly collected fruits (including the mesocarp and exocarp) were weighed. Also, 15 intact seed-filled endocarps were each individually weighed. The seed was then extracted by gently squeezing longitudinally in an industrial vice to crack open the endocarp. Each individual seed was weighed separately after extraction from the endocarp. The contribution of the seed to the mass of the germination unit was calculated as a percentage of the combined endocarp and seed mass.
The morphological characteristics of endocarps were examined by scanning electron microscopy (SEM) (JEOL 6000 with an accelerating voltage of 15 kV). Each endocarp was placed on double sided carbon conductive adhesive tape on 13 mm SEM specimen stubs and sputter coated with a layer of 200 nm of gold for examination by SEM.
Seed imbibition and moisture content
An imbibition test was conducted at room temperature (22–24 °C) on three replicates of 20 whole fruits, intact endocarps, endocarps with lid removed plus seeds and extracted seeds. Each replicate was weighed, submerged in water for 10 min in Petri dishes, then blotted dry and reweighed (time 0), then returned to the dishes. Replicates were then removed from the Petri dishes, blotted dry and reweighed after 1, 2, 4, 8, 24, 48 and 72 h. The percentage increase in mass was determined according to Turner et al. (2009): % increase in mass = [(Wi – Wd)]/Wd] × 100, where Wi and Wd are the mass of imbibed and dry (pre-imbibed) seeds (or endocarps), respectively.
Imbibition studies in relation to burial were also conducted, as there was some evidence that burial in soil in a jarrah forest for 18 months promoted germination (S. R. Turner, unpubl. res.). Whole fruits and intact endocarps were placed in mesh bags (20-mm porous nylon mesh), buried in clean washed river sand in large deep tubs in spring 2011 (i.e. late September) and placed in a nursery under 70 % shade cloth where they received natural rainfall. Fruits and endocarps were retrieved from the soil after 18 months of burial, i.e. second summer (March 2013). Imbibition tests, as described above, were conducted on fruits, intact endocarps, endocarps from which the lid had been removed, and seeds that were extracted from the endocarps. Some germination occurred during the period of burial and some seeds were damaged (and discarded) during the cracking process. Thus, the number of seeds, endocarps or fruits used in the imbibition trial varied from eight to 20 per replicate. Non-buried endocarps (controls) were stored in the seed drying room at Kings Park until required.
To further evaluate effects of the endocarp on imbibition of the seed, moisture content (MC) was determined for intact air-dried endocarps, hydrated endocarps, air-dried extracted seeds and hydrated extracted seeds. Hydrated endocarps and seeds were soaked for 96 h prior to determining MC, then all seeds and endocarps were weighed and dried for 17 h at 103 °C and reweighed (International Seed Testing Association, 1999). In addition, MC was determined for seeds extracted from endocarps hydrated for 96 h and also 8 weeks. For each treatment three replicates of 20 seeds were used. Seed MC was determined gravimetrically on an oven dry mass (DW) basis as follows: % Seed MC = [(Wi – Wd)/Wd] × 100.
Endocarp removal germination tests
To determine the effect of the endocarp on germination, four treatments were used: intact endocarp (control), endocarp with the lid removed, endocarp with 50–80 % (hereafter 50 %) of the endocarp removed and extracted seeds. There were four replicates of 20 endocarps (or seeds) for each treatment. Endocarps were surface sterilized in a 1 % (w/v) calcium hypochlorite (Ca(ClO)2) solution for 30 min. Extracted seeds were surface sterilized for only 15 min due to concern that the sterilization process might damage them. Seeds and endocarps were then rinsed three times in sterile water (Turner et al., 2009) and then placed in 90-mm Petri dishes on germination paper (Advantec, Dublin, CA, USA) moistened with either 100 p.p.m. GA3 (Sigma-Aldrich, NSW, Australia), 100 p.p.b. karrikinolide (KAR1) (Flematti et al., 2005) or water. However, due to embryo breakage during the extraction process there were only enough seeds with 50 % endocarp removal and extracted seeds to test them with water and one of the germination stimulants (KAR1) in each treatment. Seeds and endocarps were incubated in light (12 h light/12 h dark) at 15 °C and checked weekly for germination (extension of the radicle) for 8 weeks. After this time, mould became an issue, and the trial was terminated.
Endocarp burial and retrieval
Fifty intact endocarps were placed in each of 88 nylon mesh bags, and 22 bags were buried at each of four locations in the jarrah forest at Haddleton Nature Reserve, Cordering (33°34·687′S, 116°37·274′E), Western Australia, in spring 2010 (early October). Each burial area (1 × 0·7 m) was cleared of litter and then excavated to a depth of 2–3 cm. Bags were buried using the soil previously excavated, and leaf litter was re-spread over the area before it was covered by wire mesh. Twelve bags of 50 endocarps also were buried in each of four plastic tubs (38 × 30 × 18 cm) filled with clean washed silica sand and placed under 70 % shade cloth in nursery conditions (Capercup, 32°55·610′S, 116°26·427′E) and exposed to natural rainfall (i.e. not watered). Soil moisture and temperature probes (Hobo®) were buried at a depth of 2–3 cm at each of the field burial sites and in two of the nursery tubs to record soil moisture and temperature at 1-h intervals. In addition, 32 bags of 50 endocarps each were stored at Kings Park and Botanic Garden in constant temperature and relative humidity (RH) (20 °C and 50 % RH) conditions. These endocarps were used as a control.
Two bags of 50 endocarps each were retrieved from each of the field and nursery burial sites and the Kings Park incubator at approx. 6-month intervals (6, 18, 24, 30 and 36 months), which correspond to autumn (March) and spring (September). Additional retrievals of individual bags were made (throughout winter) from the field site after 9, 16, 17, 19, 20, 21 and 22 months. At each retrieval time, endocarps in all bags were scored for in situ germination (emergence of the radicle from the endocarp). Empty endocarps without the lid attached were assumed to have germinated as there was evidence of old, shrivelled P. longifolia seedlings in the bags. Therefore, a seed was defined as having germinated if the lid had broken away from the endocarp, even if no seedling was attached. Germinated endocarps were removed, and the remaining endocarps were placed in a sealed envelope and stored at room temperature for 24 h prior to further experimentation.
All endocarps that had not germinated were examined by X-ray analysis for seed fill (as previously described), and those not containing a seed were discarded. In addition, four endocarps from each replicate retrieved at 6-month intervals were X-rayed to determine if any structural changes had occurred. One bag from each 6-month retrieval was used for examination of endocarps with SEM and for viability testing of seeds using TTC, as previously described. The SEM examination of non-cracked endocarps focused on the surface area around the lid, micropyle and hilum, while the internal structure of the micropyle and other channels and cavities were examined using cracked endocarps.
Filled endocarps from the second bag retrieved at 6-month intervals and the additional single bag retrievals from the field were used for ex situ germination. After in situ germinants were removed the number of endocarps remaining ranged from four to 50. Endocarps were surface sterilized, placed in Petri dishes on sterilized silica sand and half were irrigated with 1 μm KAR1 and the other half with distilled water. Petri dishes were sealed with plastic film and incubated in the light at 15 °C and checked weekly for germination (emergence of the radicle from the endocarp) for 3 months.
Optimal winter temperature for germination
Nursery burial and summer watering previously have been linked to dormancy break (Chia and Turner, 2013). Thus, to prepare material for this experiment, 20 cleaned intact endocarps were placed in each of three bags and buried at a depth of 2–3 cm in the nursery tubs filled with clean washed silica sand for 6 months over the summer period (2012/2013). Tubs did not receive natural rainfall (they were kept in a nursery under a cover that allowed sunlight and natural air flow) and instead were artificially watered four times with the equivalent of 25 mm rainfall during the summer months to ensure dormancy break and thus promote germination during the subsequent laboratory germination trials. Endocarps were retrieved and cracked to remove 50 % of the endocarp. The seeds with partial endocarps were sterilized in an acidified 1 % (w/v) Ca(ClO)2 solution plus Tween® 20, after which they were placed under vacuum for 10 min, released from vacuum for 10 min and vacuumed again for 10 min, adapted from Bunn et al. (2007). To help minimize fungal contamination, each seed with a partial endocarp was placed in an individual 55-mm Petri dish, and 30 dishes, each containing one seed, were incubated in light at each of the following temperatures: 10, 15, 20, 25, 30, 10/20 and 15/25 °C. Temperatures are reflective of the range of winter soil temperatures experienced in southern Western Australia. Germination was defined as a change in colour of cotyledons from white to green, which was assumed to be an indication that metabolic activity associated with germination was occurring. Due to the length of time required for radicle extension and potential for mould with P. longifolia seeds, embryo greening was considered to be the best means of defining germination for this trial.
Simulation of summer wet/dry cycles to promote germination
Two different wet/dry experiments were undertaken from 2010 to 2014 with each running for up to 2 years. All endocarps were surface sterilized as previously described and placed on dry sterilized white silica sand in Petri dishes. Four replicates of 20 seeds were used for each treatment. In experiment 1, endocarps were subjected to wet periods (i.e. simulated summer rainfall events) and to decreased temperatures that may be associated with summer rains. These summer treatments were: (1) a summer heat burst for 4 weeks; (2) long (7 d) or short (24 h) wet cycles; (3) two or four wet cycles throughout the summer; and (4) a decrease in the temperature during the wet cycles. The experimental design included 18 treatments/controls (Table 1). During the summer cycles, all endocarps were on dry sand in Petri dishes at 30 °C, unless otherwise indicated.
Table 1.
The experimental design for wet/dry experiment 1
| Treatment | Incubator week no. |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 1 (Control) | 30 | 30 | 30 | 30 | 50 | 50 | 50 | 50 | 30 | 30 | 30 | 30 |
| 2 (Control) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 3 | 30 | 30 | 30 | 10/20 | 50 | 50 | 50 | 50 | 30 | 30 | 10/20 | 30 |
| 4 | 30 | 30 | 30 | 10/20 | 30 | 30 | 30 | 30 | 30 | 30 | 10/20 | 30 |
| 5 | 30 | 30 | 30 | 30 | 50 | 50 | 50 | 50 | 30 | 30 | 30 | 30 |
| 6 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 7 | 30 | 30 | 30 | 10/20 | 50 | 50 | 50 | 50 | 30 | 30 | 10/20 | 30 |
| 8 | 30 | 30 | 30 | 10/20 | 30 | 30 | 30 | 30 | 30 | 30 | 10/20 | 30 |
| 9 | 30 | 30 | 30 | 30 | 50 | 50 | 50 | 50 | 30 | 30 | 30 | 30 |
| 10 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 11 | 30 | 10/20 | 30 | 10/20 | 50 | 50 | 50 | 50 | 10/20 | 30 | 10/20 | 30 |
| 12 | 30 | 10/20 | 30 | 10/20 | 30 | 30 | 30 | 30 | 10/20 | 30 | 10/20 | 30 |
| 13 | 30 | 30 | 30 | 30 | 50 | 50 | 50 | 50 | 30 | 30 | 30 | 30 |
| 14 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 15 | 30 | 10/20 | 30 | 10/20 | 50 | 50 | 50 | 50 | 10/20 | 30 | 10/20 | 30 |
| 16 | 30 | 10/20 | 30 | 10/20 | 30 | 30 | 30 | 30 | 10/20 | 30 | 10/20 | 30 |
| 17 | 30 | 30 | 30 | 30 | 50 | 50 | 50 | 50 | 30 | 30 | 30 | 30 |
| 18 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
Number(s) in each box indicate the temperature that endocarps were exposed to for that week. Temperature decreases to 10/20 °C were for 24 h and then endocarps were returned to 30 °C for the remainder of the week. Light shading indicates short (24 h) and dark shading long (7 d) wet cycles.
Endocarps subjected to a summer heat burst were alternately incubated in light at 30 °C for 4 weeks, in darkness at 50 °C for 4 weeks and then back to 30 °C for 4 weeks. When endocarps were subjected to wet cycles, they were removed from the dry sand and placed on wet germination paper in Petri dishes for either 24 h or 7 d, after which time the endocarps were placed on dry sand and returned to the relevant temperature regime. When endocarps were subjected to temperature decreases similar to those that occur in the habitat following summer rainfall events, they were placed at 10/20 °C for 24 h during the wet cycle and then returned to 30 °C (either moist for long wet cycles or dry for short wet cycles); the controls were kept at 30 °C during the wet cycle.
At the end of the 12 weeks of summer, all Petri dishes were moved to light at 15 °C (winter) for 3 months and scored weekly for germination (emergence of the radicle from the endocarp). All treatments were cycled through four summers and four winters, over a period of 2 years. Generally, each cycle was a 12-week period; however, in winters three and four germinants were still emerging at the end of the 12-week period so these two winters were extended (by up to 3 weeks) until germination had ceased.
Experiment 2 was conducted with the aim of examining the impacts of variations in winter temperatures on germination following the application of summer dormancy breaking techniques. Based on the results from the first wet/dry experiment and those from a previous laboratory germination experiment (S. R. Turner, unpubl. res.), the following treatments were used: (1) summer heat burst as per experiment 1; (2) two or four wet cycles (as per experiment 1); (3) variations on the length of the summer period (i.e. 12 weeks, 12 weeks + 4 weeks autumn, 16 weeks or 20 weeks); and (4) variations in winter temperatures (15, 20 and 10/20 °C). Four replicates of 20 endocarps were cycled through three summer and three winters as described above.
Stratification experiment
The purpose of this experiment was to determine the effects of warm (≥15 °C) and/or cold (≤10 °C) stratification on dormancy break. Furthermore, if both warm and cold stratification are required, the order in which they occur may be important (i.e. cold and then warm stratification or warm and then cold stratification) to obtain high germination during the winter cycle. Four replicates of 20 endocarps were used in each of the following treatments: (1) constant temperature of either 8, 15 or 20 °C and constant moisture for the duration of the experiment; (2) cold moist stratification only (either 4, 6 or 8 weeks at 8 °C) then into winter temperatures (10/20 °C); (3) warm incubation only (either 6 or 12 weeks at the dry summer temperature of 30 °C with two 7-d wet cycles within the period) then into winter temperatures; (4) cold (either 4, 6 or 8 weeks wet at 8 °C) + warm incubation (12 weeks at 30 °C with two, 7-d wet cycles) and then to winter temperatures; and (5) warm incubation (either 6 or 12 weeks with two 7-d wet cycles) + 6 weeks of cold stratification at 8 °C and then into winter temperatures. Once endocarps were placed at winter germination temperatures (10/20 °C), they were checked weekly for germination (emergence of the radicle from the endocarp). Treatments at constant temperatures of 8, 15 or 20 °C also were checked weekly for germination for the duration of the experiment.
Statistical analysis
Imbibition and MC data were analysed using a linear regression model. The data were inspected graphically using plots of residuals and quantile-probability (qq) plots to assess model assumptions (Enright et al., 2011). MC data were loge transformed to satisfy assumptions of normality. The full model with seed state, burial treatment and interactions was examined. Seed viability, seed fill and germination data were analysed using a binomial generalized linear mixed model (GLMM) with a logit link function to determine the effects of the different treatment factors. Initial exploration of the data was through a simple model including treatment only. Data were analysed for zero inflation.
For the removal of endocarp germination experiment, the full model including seed state (i.e. seed, 50 % of endocarp removed, lid removed or intact endocarp), treatment (GA3, KAR1 or water) and interactions was examined. Analysis of in situ germination from the burial trial included burial location (either in the nursery, field or Kings Park laboratory storage), time of retrieval and interactions in the full model. The model examining ex situ germination data also included the ex situ treatments (KAR1 or water), the month of retrieval, year of retrieval and all relevant interactions.
For the laboratory wet/dry experiments, full models for each experiment were analysed individually (including length of wet/dry cycle, number of wet dry cycles, temperature during wet/dry cycle, summer temperatures, winter temperatures, length of the summer cycles and all relevant two and three-way interactions). A full model combining all the germination data after the third winter from the two wet/dry experiments was analysed with a further ‘Trial’ factor. Factors included in the binomial GLMM for the stratification trial were the number of weeks the seeds were warm-treated, number of weeks cold stratified, warm and cold treatment order and all relevant interactions.
All models were then reduced by omitting all non-significant interactions (5 % significance level) through stepwise variable selection. Comparisons between the different treatments were made using Tukey’s honest significant difference test. Soil temperature and moisture data were converted to mean daily maximum and minimum temperatures and moisture contents and nursery and field data for the same dates were analysed with a paired t-test. Non-transformed data appear in all figures and tables. All analyses were undertaken in the statistical program R (R Core Team, 2013) using the mgcv and lsmeans packages (Wood, 2011; Lenth, 2014).
RESULTS
Endocarp and seed characteristics
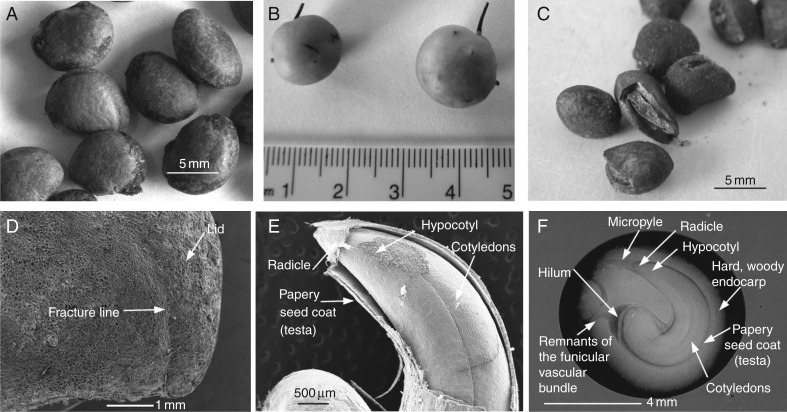
Cleaned endocarps (Fig. 1A) had a seed fill of 98·3 ± 1·7 % and TTC testing indicated that 94·7 ± 3·0 % of the seeds were viable. The mean mass of 20 fresh fruits (Fig. 1B) was 20·4 ± 0·3 g, and mean mass of single intact cleaned endocarps was 150·4 ± 5·3 mg. Based on mass, the seed component was 11·6 ± 0·2 % of the intact germination unit. The endocarp has a predefined fracture line at the radicle end (Fig. 1C, D), and the micropyle and remnants of the funicular vascular bundle remain as open channels in the wall of the endocarp (Fig. 1F).
Fig. 1.
External and internal structure of Persoonia longifolia endocarps and seeds. (A) Clean endocarps, (B) mature fresh fruit, (C) aged endocarps with lid detached, (D) SEM image of the endocarp fracture line, (E) SEM of P. longifolia seed, (F) X-ray image of the internal structure of P. longifolia endocarp and seed.
Seed imbibition and moisture content
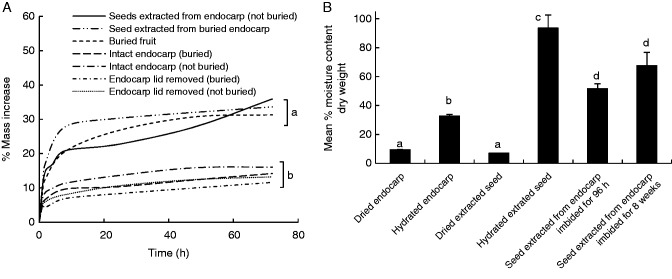
All seeds and endocarps increased in mass (Fig. 2A) over the course of the imbibition trial. There was no significant effect of burial on the ability of seeds or endocarps to imbibe water, although removal of the seed from the endocarp resulted in a significant increase in water uptake (Table 2, Fig. 2A). Endocarps with the lid removed did not imbibe significantly more water than those from which the lid was not removed (Table 2), and this did not vary significantly with either buried or non-buried endocarps (Fig. 2A). Fruits that had been buried and retrieved increased in mass by 31·3 ± 1·2 %, and this increase largely occurred over a period of 48 h.
Fig. 2.
(A) Imbibition curves (mean percentage increase in mass) for three replicates of 20 fresh and buried P. longifolia endocarps and seeds immersed in water. Standard error range from 0 to 7·4 has been omitted for clarity (B) Mean moisture content (± s.e.) of three replicates of 20 P. longifolia endocarps and seeds after being air dried, soaked in water for 96 h or soaked for 8 weeks. Treatments with the same letter do not differ significantly.
Table 2.
Percentage increase in mass after 72 h of imbibition
| Treatment | Level | Percentage increase in mass (g) (mean ± s.e.) | P |
|---|---|---|---|
| Burial | Buried | 22·7 ± 3·2 | NS |
| Not buried | 21·8 ± 3·6 | ||
| Reproductive unit | Fruit + endocarp + seed | 31·3 ± 1·2a | <0·001 |
| Endocarp + seed | 15·2 ± 0·1b | ||
| Endocarp with latch removed + seed | 12·4 ± 0·3b | ||
| Extracted seed | 34·8 ± 2·6a |
Levels within each treatment with the same letter do not differ significantly.
The MC of hydrated, extracted seeds (93·5 ± 9·0 %) was significantly higher than that of all other treatments (Fig. 2B, P < 0·001). Air dried endocarps and excised seeds had significantly lower MC than those in all other treatments (9·5 ± 0·04 and 7·1 ± 0·1 %, respectively, Fig. 2B). There was no significant difference in MC between seeds extracted from endocarps imbibed for either 8 weeks (67·6 ± 9·2 %) or 96 h (51·7 ± 3·3 %, Fig. 2B).
Endocarp removal germination tests
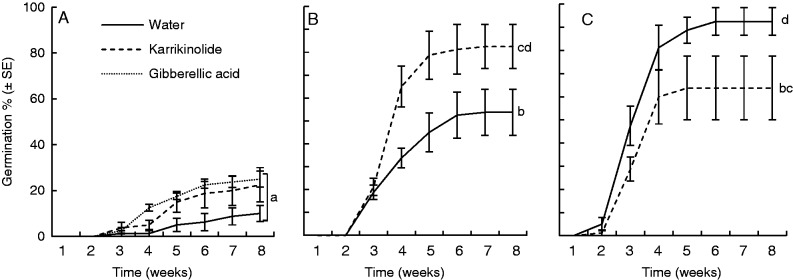
Endocarp treatment, type of germination stimulant and the interactions had significant effects on final germination percentage of P. longifolia endocarps and seeds (P < 0·001 in all three cases) (Fig. 3). Removal of both the entire endocarp and 50 % of the endocarp resulted in significantly higher germination percentages (78·1 ± 8·8 and 68·1 ± 8·4 %, respectively) than either removing the lid (19·2 ± 3·4 %) or leaving the endocarp intact (0·0 %, P < 0·001). GA3 resulted in significantly less germination (12·5 ± 5·0 %) than either KAR1 (42·2 ± 9·4 %) or water (39·1 ± 9·9 %, P < 0·001 in both cases); however, this treatment was only used on seeds with intact endocarps or with the lid removed. There was no significant difference between water and KAR1 (P = 0·700) in promoting germination.
Fig. 3.
Mean cumulative germination percentage (± s.e.) of four replicates of 20 Persoonia longifolia seeds with varying degrees of endocarp removal. (A) Lid removed, (B) 50 % endocarp removed, (C) extracted seed. No germination occurred in intact endocarps, and therefore the results are not presented here. Treatments with the same letter did not differ significantly in final germination percentage.
Contamination by mould was a significant problem particularly for seeds treated with KAR1. However despite the presence of mould, almost 100 % of viable seeds germinated in the water treatment (seed viability was assessed as 94·7 % using TTC test), and this level of germination was significantly higher than that in all other treatments except 50 % endocarp removal + KAR1 where 82·5 ± 9·7 % of the viable seeds germinated (P < 0·001, Fig. 3).
Endocarp burial and retrieval
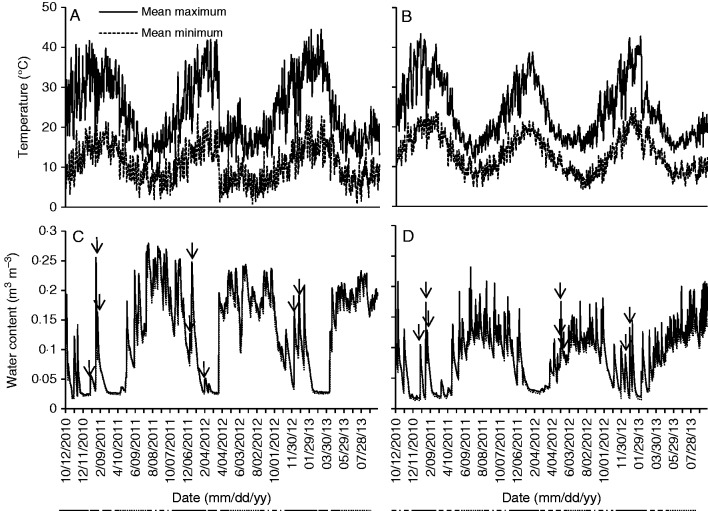
Generally, the nursery-buried endocarps experienced a greater variation in temperatures than the field-buried endocarps (Fig. 4). Maximum temperatures in summer months of the nursery soils were 2–3 °C higher than those in field soils (although this difference was not significant), and winter temperatures were significantly lower by 2–3 °C (P < 0·001). Both mean maximum and mean minimum soil MC were significantly higher in the nursery than in the field (Fig. 4, P < 0·001). Both field and nursery soils were exposed to the same rainfall, yet water penetration in the nursery was generally greater than that of field soils, with the nursery soils having a greater water content following rainfall than the field soils. In addition, nursery soils tended to stay moist longer than field soils. Rainfall events greater than 10 mm occurred in all three summers: three in 2010/11, three in 2011/12 and two in 2012/13 (Fig. 4).
Fig. 4.
Mean minimum and maximum daily temperatures and soil moisture levels of field and nursery soils in which endocarps were buried. (A) Nursery soil temperatures, (B) field soil temperatures, (C) nursery soil moisture levels, (D) field soil moisture levels. Lines underneath dates indicate seasons – solid line = summer, dashed line = autumn, dotted line = winter and dash/dot line = spring. Arrows indicate when summer thunderstorms greater than 10 mm occurred.
Seed fill of intact endocarps in both the field and the nursery treatments declined over time from 93·5 ± 1·9 to 83·1 ± 6·4 % for field-buried endocarps and to 68·5 ± 5·4 % for nursery-buried endocarps after 3 years. Seed fill of endocarps in the control treatment at Kings Park did not change significantly over the 3 years. After 3 years of burial, viability of field-buried endocarps had decreased significantly from 90·4 ± 0·5 to 57·5 ± 11·9 % (P < 0·001), while that of nursery-buried endocarps declined but not significantly. Viability of control endocarps did not decline significantly over the same time frame.
X-ray analysis of endocarps at each retrieval showed that control endocarps at Kings Park did not develop the fracture line to the same extent as those that had been buried either in the field or in the nursery (Fig. 5). The fracture line became visible after 12 months of burial and was very evident after 3 years of burial. The lid became more defined and the micropyle opening more evident on buried compared with control endocarps. There did not appear to be any change in the various channels within the endocarp itself. SEM images also revealed that the buried endocarps acquired more cracks and fissures over time than those of the controls (data not shown).
Fig. 5.
X-ray analysis of Persoonia longifolia endocarps. The development of the fracture line and lid in the endocarp is indicated with the black arrow. (A) Field-buried for 6 months, (B) field-buried for 36 months, (C) nursery-buried for 6 months, (D) nursery-buried for 36 months, (E) stored at Kings Park (control) for 6 months, (F) stored at Kings Park (control) for 36 months.
In situ germination increased with time and was higher in the endocarps retrieved from the nursery than the field at every sampling time (Fig. 6A, P < 0·001). Germination reached 63·7 ± 6·7 % in the nursery-buried endocarps and 41·6 ± 8·1 % in field-buried endocarps after 3 years of burial; no in situ germination occurred in the control endocarps. Germination was first recorded at 9 months for field-buried endocarps and 12 months for nursery-buried endocarps (note that a retrieval was not conducted at 9 months for nursery-buried endocarps), indicating that germination commenced in the first winter that endocarps were buried.
Fig. 6.
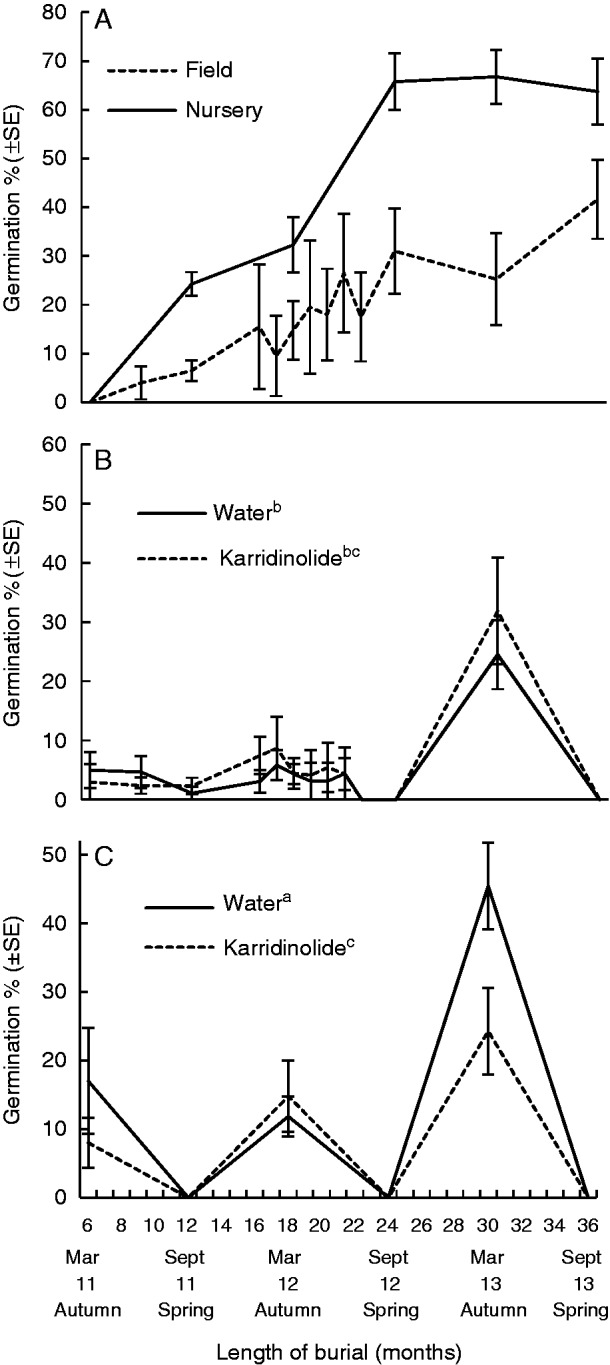
Germination percentage (mean ± s.e.) of nursery- and field-buried endocarps. (A) In situ germination in four replicate bags of 50 endocarps after retrieval, (B) ex situ germination of field-buried endocarps, (C) ex situ germination of nursery-buried endocarps. Treatments with the same letter are not significantly different.
Ex situ germination of endocarps commenced after only 6 months burial (March retrieval/autumn) in both the field and the nursery (Fig. 6B, C). Germination was cyclical with little or no germination occurring when endocarps were retrieved at the end of winter/beginning of spring (i.e. 12, 24 and 36 months). Ex situ germination peaked in the autumn retrievals (i.e. 6, 18 and 30 months), with burial after 30 months resulting in higher germination than at any other retrieval time. Overall, germination was significantly higher for endocarps buried in the nursery and then treated with water than in any of the other treatments (P = 0·040). When ex situ germination was examined in combination with the month of the year, there was no significant difference for any month between use of KAR1 or water.
Optimal winter temperatures for germination
Germination occurred at all the laboratory temperatures tested in endocarps that had been buried and artificially watered four times throughout the summer months to break dormancy (Fig. 7). Final germination percentage was significantly higher at 20 °C (76·7 ± 3·3 %) than at all other temperatures, and the second best temperature regime was 10/20 °C (66·7 ± 8·8 %, P < 0·001).
Fig. 7.
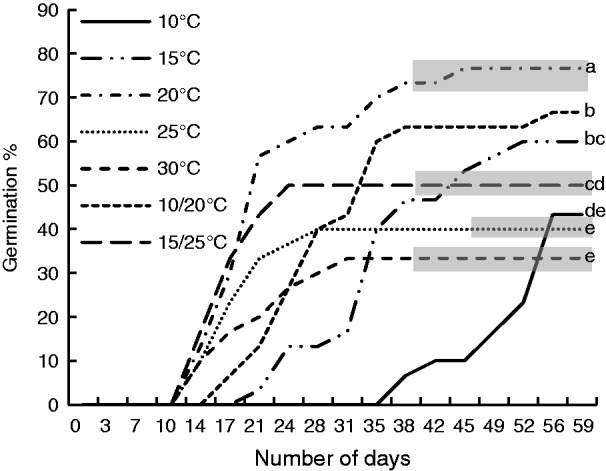
Mean cumulative germination percentage of 30 individual seeds at various winter temperatures of Persoonia longifolia seeds with 50 % of the endocarp removed following 6 months of burial in the nursery plus four artificial wet cycles during the summer months. Grey bars indicate where mould became an issue in each of the different treatments. Treatments with the same letter did not differ significantly.
Simulation of summer wet/dry cycles to promote germination
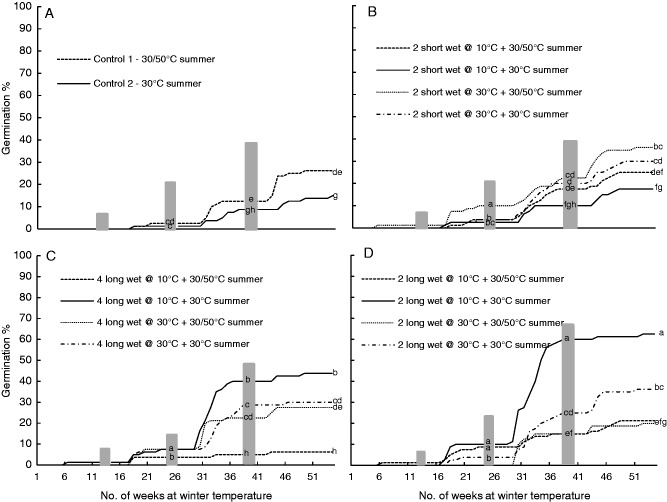
In experiment 1, < 5 % germination occurred in any treatment after the first winter. After the second winter, germination was still low but had commenced in most treatments (i.e. 13 out of 18 treatments). The majority of germination occurred after the third winter phase (Fig. 8). The best germination (62·5 ± 10·1 %) occurred following two long wet cycles at 10/20 °C and a constant 30 °C summer (Fig. 8D) and this was significantly greater than in all other treatments. No germination occurred in treatments with four short wet cycles. Long wet cycles during the summer months resulted in significantly higher germination than either short wet cycles or no wet cycles, and both two and four wet cycles resulted in significantly more endocarps germinating than those that experienced dry summers (control/no wet cycles) (Table 3). Whilst more endocarps germinated after being exposed to four wet cycles compared with two wet cycles, this difference was not statistically significant at the completion of the trial (Table 3). The temperature of the endocarps during the summer wet cycles (either 10/20 °C for 24 h or 30 °C) had no significant effect on germination (Table 3). However, the use of a heat burst (50 °C) (during dry periods in summer) reduced germination (Table 3). Further investigation into the interactions between summer temperatures and other factors indicated that when there were no wet cycles (i.e. dry summers) then a heat burst of 50 °C improved germination but not significantly (P = 0·973). However, if the wet cycles were long, then a heat burst significantly reduced germination (P < 0·001).
Fig. 8.
Mean cumulative germination percentages of four replicates of 20 endocarps in response to simulated summer wet/dry cycles (Experiment 1) under laboratory conditions. (A) Control treatments with no wet/dry cycles during the summer months, (B) two short (24 h) wet cycles, (C) four long (7 d) wet cycles, (D) two long wet cycles. Grey shading on all graphs indicates the final three of four summers with the first summer preceding the data shown. Letters within the summer bars indicate significant differences in germination percentages between treatments on all four graphs at the completion of the preceding winter. No germination occurred in treatments with four short wet cycles so these results are not presented.
Table 3.
Germination percentages (mean ± s.e.) for P. longifolia endocarps in the different simulations of summer wet/dry cycles (experiment 1); very little germination occurred in the first winter and therefore data have not been included here
| Factor | Treatment | Germ. % after 2nd winter | P | Germ. % after 3rd winter | P | Germ. % after 4th/final winter | P |
|---|---|---|---|---|---|---|---|
| Length of wet cycles | No wet cycle | 7·5 ± 2·5a | <0·001 | 12·1 ± 3·1 | NS | 20·6 ± 5·1b | 0·03 |
| Short | 8·9 ± 1·6b | 21·5 ± 3·5 | 27·2 ± 3·3b | ||||
| Long | 10·8 ±1·4c | 30·2 ± 4·1 | 35·4 ± 4·0a | ||||
| Number of wet cycles | No wet cycle | 7·5 ± 2·5a | <0·001 | 12·1 ± 3·1 | NS | 20·6 ± 5·1b | <0·001 |
| 2 wet cycles | 8·5 ± 1·0b | 25·5 ± 3·6 | 31·1 ± 3·2a | ||||
| 4 wet cycles | 15·0 ± 2·2c | 32·1 ± 5·8 | 35·8 ± 6·3a | ||||
| Temp. during wet cycles | 10/20 °C | 4·5 ± 1·1a | 0·004 | 32·8 ± 5·7 | NS | 35·2 ± 5·3 | NS |
| 30° | 4·1 ± 1·1b | 23·3 ± 3·0 | 30·0 ± 2·8 | ||||
| Summer temp. | 30°/50 °C | 4·03 ± 1·0 | NS | 18·3 ± 1·7a | <0·001 | 26·0 ± 2·2a | <0·001 |
| 30 °C only | 4·03 ± 1·0 | 32·1 ± 4·9b | 34·8 ± 4·5b |
Treatments with the same superscript letter are not significantly different from other treatments within the same factor and season. NS, not significant.
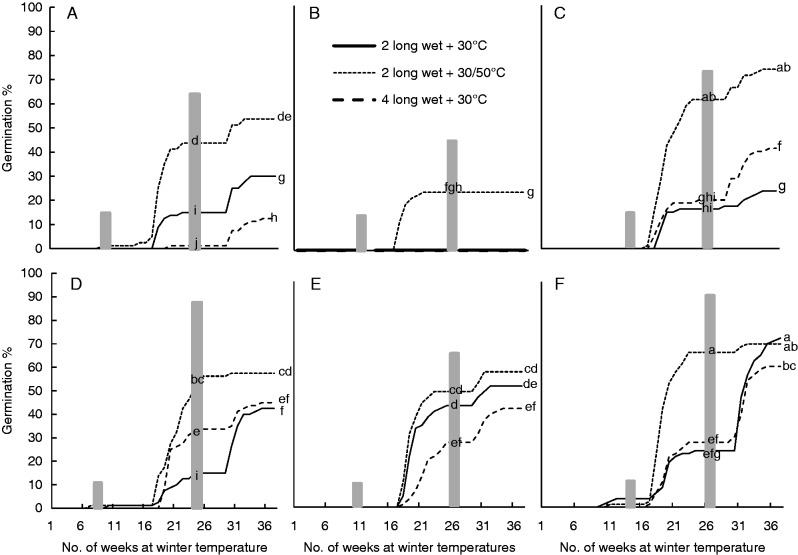
Germination in experiment 2 was low in the first winter (< 5 %), with most of the germination occurring during the second winter (Fig. 9). The highest germination (75·0 ± 6·1 %) was achieved when endocarps were exposed to a 20-week summer period with a fluctuating summer temperature (30/50 °C), two long wet cycles and winter temperatures at 10/20 °C (Fig. 9F). Unlike the optimal winter temperature trial, treatments at the winter temperature of 20 °C resulted in very low germination in comparison to winter temperatures of 15 or 10/20 °C (Fig. 9B). The longer the summer period, the higher was the germination, with the best germination occurring in treatments with a 20-week summer (Table 4). Germination after the longer summer period was up to 85 % in one replicate after three winters. If the summer period is only 12 weeks long, then a heat burst in summer (i.e. 30/50 °C) improves germination significantly (P < 0·001). This trend (increased germination after a 4-week heat burst of 50 °C) also was apparent for a 16-week summer and 12-week summer + 4-week autumn but was not statistically significant. Germination was highest after two wet cycles compared with four and this was opposite of the results obtained from experiment 1. A heat burst improved germination significantly (Table 4) and further analysis revealed that this heat burst was only significant if the summer is short. In addition, earlier in the experiment (i.e. in the second winter), the fluctuation in summer temperatures (30/50 °C) resulted in significantly greater germination (P < 0·001) than constant summer temperatures (30 °C) but this effect diminished (but was still significant) after the third summer.
Fig. 9.
Cumulative mean germination percentage of four replicates of 20 endocarps in response to simulated variations in winter temperatures and length of summer (Experiment 2) under laboratory conditions. (A) Winter temperatures of 15 °C, (B) winter temperatures of 20 °C, (C) winter temperatures of 10/20 °C, (D) summer of 16 weeks + winter temperatures of 10/20 °C, (E) summer of 16 weeks + autumn of 4 weeks + winter temperatures of 10/20 °C, (F) summer of 20 weeks + winter temperatures of 10/20 °C. Grey shading on all graphs indicates the final two of three summers with the first summer preceding the data shown. Letters within the grey summer bars indicate significant differences percentages between treatments on all six graphs at the completion of the preceding winter.
Table 4.
Germination percentages (mean ± s.e.) for P. longifolia endocarps in experiment 2 (simulation of variations in winter temperatures); very little germination occurred in the first winter and therefore data have not been included here
| Factor | Treatment | Germ. % after 2nd winter | P | Germ. % after 3rd winter | P |
|---|---|---|---|---|---|
| Number of wet cycles | 2 wet cycles | 39·2 ± 3·6a | <0·003 | 52·7 ± 3·4a | <0·001 |
| 4 wet cycles | 26·2 ± 4·7b | 41·5 ± 5·9b | |||
| Summer temperatures | 30°/50 °C | 50·8 ± 4·2 | NS | 56·9 ± 4·9a | 0·007 |
| 30 °C only | 25·3 ± 3·1 | 44·3 ± 3·7b | |||
| Temperature during winter | 15° | 26·7 ± 7·6a | <0·001 | 32·1 ± 7·4a | <0·001 |
| 20° | 22·9 ± 3·6a | 27·1 ± 4·3b | |||
| 10/20° | 39·3 ± 3·5b | 57·3 ± 3·1c | |||
| Summer length | 12 weeks | 30·0 ± 4·6a | <0·001 | 38·3 ± 5·0a | <0·001 |
| 16 weeks | 35·0 ± 6·7ab | 48·3 ± 6·3b | |||
| 16 weeks + 4 weeks autumn | 41·6 ± 5·9b | 52·5 ± 4·9b | |||
| 20 weeks | 40·8 ± 7·6b | 70·8 ± 3·1c |
Treatments with the same superscript letter are not significantly different from other treatments within the same factor and season.
Stratification experiment
No germination occurred in endocarps kept constantly wet at 8, 15 or 20 °C (data not shown). Minimal germination (<5 %) occurred in those endocarps exposed to warm incubation only, cold only or cold plus warm. The only treatment with >5 % germination was 12 weeks warm + 6 weeks cold stratification, in which germination reached 13·8 ± 4·3 %.
DISCUSSION
The dormancy and germination biology of species with hard woody endocarps such as P. longifolia has been poorly understood for many years even though this type of plant structure is remarkably common, occurring in many well-known and economically important species such as plums (Prunus domestica), peaches (Prunus persica), dates (Phoenix dactylifera) and olives (Olea europaea) (Merritt et al., 2007; Janick and Paull, 2008). Prior to this study there was no clear picture as to how germination was regulated in species with these types of reproductive structures, with various hypotheses focusing on the presence of an impermeable barrier preventing water uptake, mechanical dormancy restricting the capacity of the embryo to grow and expand, and physiological dormancy due to the presence of a physiological inhibiting mechanism within the embryonic tissues proposed as the dominant factor/s regulating germination (Baskin and Baskin, 2014). Indeed, it appears based on this study that two principal processes are responsible for controlling germination: (1) mechanical dormancy imposed by the hard woody endocarp restricting embryo growth and (2) physiological dormancy residing within the embryo, which is overcome by cycles of warm and cold stratification. Overall, these results add to our current understanding of the nature of hard indehiscent endocarps in Persoonia species and provide some fresh insight into the dormancy and germination requirements of other taxa with similar indehiscent endocarps.
Role of the endocarp and embryo in seed dormancy
Extracted seeds, intact endocarps and fruits of P. longifolia imbibed water, indicating that they were permeable and that delayed germination was not due to a water-impermeable barrier, i.e. physical dormancy. However, seeds extracted from intact endocarps after hydration for 96 h or 8 weeks had significantly lower MC than those hydrated after extraction. Thus, while the endocarp is permeable, it does restrict water uptake to some degree. Endocarps of P. longifolia showed no increase in water uptake when they were buried or when the lid was removed. The amount of water imbibed by buried endocarps of P. longifolia was similar to that reported by Norman and Koch (2008) (10–16 %) and is similar to that observed in Astroloma xerophyllum (a Western Australian species also with a hard woody endocarp) in which mass increase was 38 % for extracted seeds but only 16 % for those obtained from intact endocarps (Turner et al., 2009). African olive (Olea europaea subsp. cuspidata), which has an endocarp structure similar to that of P. longifolia, also did not show any appreciable increase in water uptake when scarified on one end (Cuneo et al., 2010).
Despite issues associated with contamination in germination trials, which is a common problem with Persoonia species (McIntyre, 1969; Bauer and Johnston, 1999; Frith and Offord, 2010), germination percentages of P. longifolia increased when the endocarp was removed, indicating that it exerts a mechanical constraint to germination. Germination of other species of Persoonia also has been shown to increase when the endocarp is removed, for example P. sericea and P. virgata (Bauer and Johnston, 1999) and in P. pinifolia, but only when GA was applied (McIntyre, 1969; Rintoul and McIntyre, 1975). However, simply filing the ends or scarifying the endocarp does not seem to have the same effect as complete removal of the endocarp or even removal of the endocarp lid in either P. longifolia (Norman and Koch, 2006) or P. elliptica (Abbott and van Heurck, 1988).
Based on the results from our study, P. longifolia can therefore be classified as having PD seeds. That is, when dormant, the embryo has limited growth potential and cannot overcome the mechanical constraints of the endocarp. Furthermore, removal of the lid from fresh endocarps was mostly ineffective in promoting germination, and exposure of fresh endocarps with the lid removed to GA3 resulted in only 25 % germination (Fig. 3A). Thus, GA3 did not stimulate embryo growth potential sufficiently to promote mass germination from endocarps in which the lid was removed. After the endocarps were buried under natural temperature and soil moisture conditions in both the field and nursery for 30 months, the embryo had gained enough growth potential to germinate to 24·5 and 45·4 %, respectively, in water (Fig. 6B, C). These observations demonstrate that exposure to the natural sequence of seasonal environmental changes had broken the physiological dormancy in this proportion of the embryos. Marked physical changes to the surrounding endocarp were also observed over the period of time (Fig. 5).
Effect of burial on germination
Many species with hard woody endocarps in Australia have been shown to germinate well both in situ and ex situ after soil burial for a period of time (Tieu et al., 2001; Merritt et al., 2007; Ooi et al., 2007; Turner et al., 2009), particularly when treated with smoke water after burial (Dixon et al., 1995; Roche et al., 1997). There have been contrasting reports on the effects of burial on germination of P. longifolia endocarps and fruits, with surface sowing improving germination in one report (Mullins et al., 2002) and burial improving germination in several others (Norman and Koch, 2008; Chia and Turner, 2013). Exposure to the varying temperatures and moisture conditions when buried in the field may result in either weakening of the endocarp and/or physiological changes within the embryo that increase its growth potential enough for it to push open the lid of the endocarp and germinate. When buried endocarps were retrieved from the field, the lid became more evident over time (Fig. 5), and it also became much easier to dislodge. Prior to burial it was not possible to remove the lid without some mechanical intervention such as excision with diagonal cutting pliers. Also, after exposure to the natural wetting and drying and temperature fluctuations associated with burial in the soil, cracks and fissures were observed to develop in the endocarp especially around the lid region (as seen in our SEM examinations on soil aged endocarps). These cracks and fissures would no doubt help weaken the endocarp and break down the suberin that fuses the lid to the rest of the endocarp, making it easier for the embryo to push open the lid and germinate when empowered to do so.
Our results suggest that it is not necessarily the length of time that endocarps are buried but rather the environmental conditions to which they are exposed during burial that are important in dormancy-break. First observations of in situ germination during burial occurred at 9 months (first winter), after endocarps had been buried for one summer. In previous burial trials undertaken at Kings Park, in situ germination in P. longfolia was not observed until 18 months after burial and, even then, germination was low with only 11·5 % of seeds germinating (S. R. Turner, unpubl. res.) compared with 24 % germination after only 12 months burial in the nursery in the present study (Fig. 6a).
A close examination of rainfall and temperature data recorded in our study and those from the previous Kings Park burial trials (S. R. Turner, unpubl. res.) reveals that the environmental conditions experienced by the endocarps during the summer months were quite different in each trial. The first summer that endocarps were buried at Kings Park was exceptionally dry, and four out of five rainfall events were less than 7 mm and the fifth was only 12·5 mm (Bureau of Meteorology, 2015). By comparison, during the first summer of burial in the present study, endocarps experienced eight rainfall events, two of which were greater than 20 mm (41·8 and 27·8 mm) and two were greater than 10 mm (11·8 and 14·4 mm). The second summer of the Kings Park trial experienced one large thunderstorm of 43 mm (Bureau of Meteorology, 2015) and the following winter was when in situ germination was first recorded. Thus, endocarp germination in winter appears to be positively correlated with summer rainfall.
The difference between in situ germination that occurred in our field-buried endocarps compared with nursery-buried endocarps is no doubt related to the fact that the endocarps in the nursery experienced a greater range of temperature and moisture variations than those in the field. After summer thunderstorms, soil moisture levels and temperatures in the nursery and field took about 1 week and 2 d, respectively, to return to pre-storm conditions (Fig. 4). Therefore, the variations in the summer temperature and moisture regimes experienced in the nursery environment were more conducive to germination in winter than those in the field.
Seasonal simulations to induce germination
In the optimal winter temperature trial, 20 °C proved to be the best temperature for germination of P. longifolia, whilst in the wet/dry laboratory experiments 10/20 °C resulted in best germination. This difference could be explained by the removal of most of the endocarp in the optimal winter temperature trial and the use of an unconventional method of identifying germination (i.e. greening of the embryo) in an attempt to deal with the mould problem. The fluctuating winter temperature of 10/20 °C reflects the winter temperatures in the natural environment of this species.
A study of 28 jarrah forest species from southern Western Australia found that the highest germination percentages generally occurred between 10 and 15 °C (Bell and Bellairs, 1992). Gradual dormancy alleviation over summer ensures that seeds will germinate during the cooler and wetter winter months when conditions are most favourable for seedling establishment and survival. A return to a dormant state during the spring months would ensure that seeds do not germinate over the summer months when survival is likely to be poor. Finch-Savage and Leubner (2006) describe dormancy as a moving target continuously reacting to the environment and adjusting the conditions required for germination and this appears to be the case for P. longifolia where cyclical germination was observed.
Germination in P. longifolia was significantly improved through the manipulation of temperatures and moisture regimes during the summer months. Most importantly, the seeds required brief wet periods during the summer months. The environment in which these plants are found is subjected to between one and four summer thunderstorms annually (Bureau of Meteorology, 2015). The results from the wet/dry trials and the nursery trials indicated that these thunderstorms are one of the key drivers in breaking dormancy in the endocarp and seed. The length of the wet cycles that the endocarps are exposed to (rather than the number of wet cycles) appears to be a critical factor in breaking dormancy. A small summer rainfall event, when endocarps are moist for only 24 h, is not sufficient to promote dormancy break. Instead, the endocarps require a significant rainfall event (i.e. of > 10 mm) for dormancy to be broken, as previously observed by Hoyle et al. (2008) and Hidayati et al. (2012) for several other Australian native species.
PD in seeds can be broken by cold, warm, or warm + cold stratification (Baskin and Baskin, 2014). An investigation of the role of stratification in dormancy break of P. longifolia showed that warm stratification followed by cold stratification is required but only if the endocarps were exposed to a long warm period, i.e. 12 weeks compared with 6 weeks, including a total of 14 d of warm, moist conditions. Although germination was low (<15 %) in the stratification trial, it was still greater than germination after only one winter cycle in almost all other laboratory trials undertaken to date, and in this context these results are highly encouraging. Norman and Koch (2006) found that a period of warm incubation (including a wet cycle every 3–4 d) at 15/30 °C for 2 weeks, prior to sowing the endocarps in the greenhouse, resulted in significantly better germination than all other treatments. However, this treatment still only produced 13 % germination over two winter cycles. The role of warm (moist) stratification and cold (moist) stratification in promoting germination through breaking of PD within the embryo or by weakening of the endocarp in P. longifolia is not clearly defined here. Warm (moist) stratification has been linked to breaking of PD in Empetrum hermaphroditum, another species with a stony endocarp (Baskin et al., 2002), and Persson et al. (2006) linked warm stratification with weakening of the surrounding endocarp and cold stratification with breaking of PD within the embryo in Crataegus monogyna.
The length of time that endocarps were exposed to summer conditions also was important in breaking dormancy. A short summer period of 12 weeks promoted less germination than a long summer period of 20 weeks. Summer temperatures in the jarrah forest soils of ≥ 30 °C can occur from mid-October to late March (Fig. 4), i.e. for a period of between 19 and 24 weeks. This concept of a longer summer period has not been investigated in great detail for Western Australian species and may be of importance for other native species, particularly for those considered to be deeply dormant (Merritt et al., 2007). The stratification experiment with P. longifolia endocarps included a 12-week summer, and only 2 weeks during this period were wet. Thus, germination potentially could be improved with either longer wet periods, more wet periods and/or a longer summer period, and these conditions should be the focus of future investigations.
The series of experiments undertaken here are the culmination of over 5 years of research and indicate just how complex natural germination systems can be for some species. In the case of P. longifolia, environmental conditions are critical to opening the hard woody endocarp and breaking physiological dormancy of the embryo. Closely monitoring these environmental conditions provides direction for laboratory germination studies that can be used to clearly identify the factors that break seed dormancy. One set of conditions may be required to open the endocarp and a second set may be required to break dormancy of the embryo. Clearly, temperature, moisture level and the timing of these conditions are important for regulating seed dormancy-break for P. longifolia during the summer months (endocarp weakening) and perhaps during the cold of winter as well (removal of embryo PD). Overall, the timing of endocarp opening and the breaking of embryo dormancy result in most seeds germinating at a time that is favourable for seedling establishment and these results provide a useful framework for understanding the germination requirements of P. longifolia and related species. Indeed, the insights provided by this study will not only improve our ability to propagate this species in future but will also aid the development of effective propagation approaches for other similar taxa as well for conservation, restoration and horticultural purposes.
DISCLOSURE
The authors declare that there is no conflict of interests regarding the publication of this paper.
ACKNOWLEDGEMENTS
We would like to thank Alcoa of Australia Limited and South 32 Worsley Alumina for both financial and practical assistance that made this work possible. We also acknowledge the valued comments of two anonymous reviewers whose suggestions greatly improved this paper. This work was supported by the Australian Government through an Australian Postgraduate Award, and a University of Western Australia top up scholarship provided to K.A. Chia. It was also supported by Alcoa of Australia Limited, South 32 Worsley Alumina and the Mining Research Institute of Western Australia.
LITERATURE CITED
- Abbott I, van Heurck P. 1988. Widespread regeneration failure of Persoonia elliptica (Proteaceae) in the northern jarrah forest of Western Australia. Journal of the Royal Society of Western Australia 71: 15–22. [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination, 2nd edn Amsterdam: Elsevier. [Google Scholar]
- Baskin CC, Zackrisson O, Baskin JM. 2002. Role of warm stratification in promoting germination of seeds of Empetrum hermaphroditum (Empetraceae), a circumboreal species with a stony endocarp. American Journal of Botany 89: 486–493. [DOI] [PubMed] [Google Scholar]
- Baskin CC, Baskin JM, Yoshinaga A, Thompson K. 2005. Germination of drupelets in multi-seeded drupes of the shrub Leptecophylla tameiameiae (Ericaceae) from Hawaii: a case for deep physiological dormancy broken by high temperatures. Seed Science Research 15: 349–356. [Google Scholar]
- Bauer LM, Johnston ME. 1999. Propagation of Persoonia virgarta for the development of a new floricultural export Crop. School of Land and Food, The University of Queensland, Gatton College. [Google Scholar]
- Bauer LM, Johnson ML, Williams R. 1999. Persoonias − potential for domestication. In Slater A, ed. Proceedings of the Fifth Australian Wildflower Conference. ‘New Flowers, Products and Technologies’. PR conference consultants, Carlton Crest Hotel, Melbourne, 119–121.
- Bauer LM, Johnston ME, Williams RR. 2001. Rate and timing of vegetative growth, flowering and fruit development of Persoonia virgata (Proteaceae). Australian Journal of Botany 49: 245–251. [Google Scholar]
- Bauer LM, Johnston ME, Williams RR. 2004. Fruit processing, seed viability and dormancy mechanisms of Persoonia sericea A. Cunn. ex R. Br. and P. virgata R. Br.(Proteaceae). Seed Science and Technology 32: 663–670. [Google Scholar]
- Bell DT, Bellairs SM. 1992. Effect of temperature on the germination of selected Australian native species used in rehabilitation of bauxite mining disturbance in Western Australia. Seed Science and Technology 20: 47–55. [Google Scholar]
- Bunn E, Turner S, Panaia M, Dixon KW. 2007. The contribution of in vitro technology and cryogenic storage to conservation of indigenous plants. Australian Journal of Botany 55: 345–355. [Google Scholar]
- Bureau of Meteorology. 2015. Climate data online. Available at www.bom.gov.au/climate/data/index.shtml (last accessed 14 December 2015).
- Chen S-Y, Chien C-T, Chung J-D, Yang Y-S, Kuo S-R. 2007. Dormancy-break and germination in seeds of Prunus campanulata (Rosaceae): role of covering layers and changes in concentration of abscisic acid and gibberellins. Seed Science Research 17: 21–32. [Google Scholar]
- Chia KA, Turner SR. 2013. Manipulation of summer watering regimes during seed burial breaks dormancy in seeds of Persoonialongifolia (R. Br. Proteaceae). In: Seed Ecology IV Conference. Shenyang: China Meteorological Press. [Google Scholar]
- Chia KA, Koch JM, Sadler R, Turner SR. 2015. Developmental phenology of Persoonia longifolia (Proteaceae) and the impact of fire on these events. Australian Journal of Botany 63: 415–425. [Google Scholar]
- Chien CT, Chen SY, Yang JC. 2002. Effect of stratification and drying on the germination and storage of Prunus campanulata seeds. Taiwan Journal of Forest Science 17: 413–420. [Google Scholar]
- Cuneo P, Offord C, Lieshman M. 2010. Seed ecology of the invasive woody plant African olive (Olea europaea subsp. cuspidata): implications for management and restoration. Australian Journal of Botany 58: 342–348. [Google Scholar]
- Dettmann ME, Jarzen DM. 1998. The early history of the Proteaceae in Australia: the pollen record. Australian Systematic Botany 11: 401–438. [Google Scholar]
- Dixon KW, Roche S, Pate JS. 1995. The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants. Oecologia 101: 185–192. [DOI] [PubMed] [Google Scholar]
- Dixon KW, Tieu A, Jefferson L. et al. 2002. Dormancy mechanisms of Australian native plant species. Perth: Kings Park and Botanic Gardens. [Google Scholar]
- Enright NJ, Fontaine JB, Westcott VC, Lade JC, Miller BP. 2011. Fire interval effects on persistence of resprouter species in Mediterranean-type shrublands. Plant Ecology 212: 2071–2083. [Google Scholar]
- Finch-Savage WE, Leubner G. 2006. Tansley review − seed dormancy and the control of germination. New Phytologist 171: 501–523. [DOI] [PubMed] [Google Scholar]
- Flematti G, Ghisalberti E, Dixon K, Trengove R. 2005. Synthesis of the seed germination stimulant 3-methyl-2H-furo[2,3-c] pyran-2-one. Tetrahedron Letters 46: 5719–5721. [Google Scholar]
- Frith A, Offord C. 2010. Investigation into the germination and propagation of Persoonia pauciflora P.H. Weston. In: Australian Network for Plant Conservation 8th National Conference. Perth: Australian Network for Plant Conservation, 42.
- Hidayati SN, Walck JL, Merritt DJ, Turner SR, Turner DW, Dixon KW. 2012. Sympatric species of Hibbertia (Dilleniaceae) vary in dormancy break and germination requirements: implications for classifying morphophysiological dormancy in Mediterranean biomes. Annals of Botany 109: 1111–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AW. 1933. The method of germination of seeds enclosed in a stony endocarp. Annals of Botany 47: 873–887. [Google Scholar]
- Hill AW. 1937. The method of germination of seeds enclosed in a stony endocarp. II. Annals of Botany 1: 239–256. [Google Scholar]
- Hoyle GL, Daws MI, Steadman KJ, Adkins SW. 2008. Mimicking a semi-arid tropical environment achieves dormancy alleviation for seeds of Australian native Goodeniaceae and Asteraceae. Annals of Botany 101: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imani A, Rasouli M, Tavakoli R. et al. 2011. Optimization of seed germination in Prunus species combining hydrogen peroxide or gibberellic acid pre-treatment with stratification. Seed Science and Technology 39: 204–207. [Google Scholar]
- International Seed Testing Association. 1999. International rules for seed testing. Seed Science and Technology 27: 271–335. [Google Scholar]
- Janick J, Paull RE. 2008. The encyclopedia of fruit and nuts. Wallingford, UK: CABI Publishing. [Google Scholar]
- Ketelhohn LM, Johnston ME, Williams RE. 1998. Propagation of Persooniavirgata for commercial development In JA Considine, J Gibbs, eds. Third International Symposium on New Floricultural Crops. Perth: International Society for Horticultural Science, 157–163. [Google Scholar]
- Lakon G. 1949. The topographical tetrazolium method for determining the germinating capacity of seeds. Plant Physiology 24: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV. 2014. Least-Squares Means, Version 2.11. In R Package. Available at http://CRAN.R-project.org/package=lsmeans (last accessed 1 September 2014).
- Li X, Baskin JM, Baskin CC. 1999. Anatomy of two mechanisms of breaking physical dormancy by experimental treatments in seeds of two North American Rhus species (Anacardiaceae). American Journal of Botany 86: 1505–1511. [PubMed] [Google Scholar]
- McIntyre DK. 1969. The germination of dormant Persoonia pinifolia R.Br. seeds by the use of gibberellic acid. Canberra: Canberra Botanic Gardens. [Google Scholar]
- Merritt DJ, Turner SR, Clarke S, Dixon KW. 2007. Seed dormancy and germination stimulation syndromes for Australian temperate species. Australian Journal of Botany 55: 336–344. [Google Scholar]
- Mullins RG, Koch JM, Ward SC. 2002. Practical method of germination for a key jarrah forest species: snottygobble (Persoonia longifolia). Ecological Management and Restoration 3: 97–103. [Google Scholar]
- Nikolaeva MG. 1969. Physiology of deep dormancy in seeds. Leningrad: Izdatel’stvo ‘Nauka’. [Google Scholar]
- Norman MA, Koch JM. 2005. Differences in species abundance between sites rehabilitated with direct return and stockpiled soil. Perth: Aloca World Alumina Australia. [Google Scholar]
- Norman MA, Koch JM. 2006. The investigation of seed coat chipping, seed coat ageing and warm temperature stratificiation for snottygobble (Persoonia longifolia). Pinjarra, WA: Aloca of Australia, Pty Ltd. [Google Scholar]
- Norman MA, Koch JM. 2008. The effect of in situ seed burial on dormancy break in three woody-fruited species (Ericaceae and Proteaceae) endemic to Western Australia. Australian Journal of Botany 56: 493–500. [Google Scholar]
- Ooi MKJ, Auld TD, Whelan RJ. 2007. Distinguishing between persistence and dormancy in soil seed banks of three shrub species from fire-prone southeastern Australia. Journal of Vegetation Science 18: 405–412. [Google Scholar]
- Persson L, Jensen M, Eriksen EN, Mortensen LC. 2006. The effect of endocarp and endocarp splitting resistance on warm stratification requirement of hawthorn seeds (Crataegus monogyna). Seed Science and Technology 34: 573–584. [Google Scholar]
- Preston C, Adkins SW, Bellairs SM. et al. 2002. Dormancy mechanisms of Australian native plant species. School of Land and Food Science and Center for Mine Land rehabilitation, University of Queensland. [Google Scholar]
- R Core Team. 2013. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; Available at www.R-project.org/ (last accessed 4 November 2013). [Google Scholar]
- Rajput A, Tiwari KP. 2001. Effects of alternate chilling/heating on germination of fresh teak (Tectona grandis L.F.) drupes, without scarification of felty mesocarp. Seed Science and Technology 29: 57–64. [Google Scholar]
- Rintoul I, McIntyre DK. 1975. Investigation into Seed Dormancy in Persoonia pinifolia. Canberra: Canberra Botanic Gardens. [Google Scholar]
- Roche S, Dixon KW, Pate JS. 1997. Seed ageing and smoke: partner cues in the amelioration of seed dormancy in selected Australian native species. Australian Journal of Botany 45: 783–815. [Google Scholar]
- Slator NJ, Callister AN, Doland Nichols J. 2013. Mechanical but not physical dormancy is a cause of poor germination in teak (Tectona grandis L.f.). New Forests 44: 39–49. [Google Scholar]
- Tieu A, Dixon KW, Meney KA, Sivasithamparam K. 2001. Interaction of soil burial and smoke on germination patterns in seeds of selected Australian native plants. Seed Science Research 11: 69–76. [Google Scholar]
- Turner S, Commander LE, Baskin JM, Baskin CC, Dixon KW. 2009. Germination behaviour of Astroloma xerophyllum (Ericaceae), a species with woody indehiscent endocarps. Botanical Journal of the Linnean Society 160: 299–311. [Google Scholar]
- Weston PH. 1994. The Western Australian species of subtribe Persooniinae (Proteaceae; Persoonioideae: Persoonieae). Telopea 6: 51–165. [Google Scholar]
- Weston PH. 2003. Proteaceae subfamily Persoonioideae. Australian Plants 22: 62–91. [Google Scholar]
- Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society 73: 3–36. [Google Scholar]
- Woodall GS. 2004. Cracking the woody endocarp of Santalum spicatum nuts by wetting and rapid drying improves germination. Australian Journal of Botany 52: 163–169. [Google Scholar]