Abstract
Background and Aims Adaptations allowing plants to cope with drying are particularly relevant in the light of predicted climate change. Dehydration tolerance (DhT, also dehydration-tolerant) is one such adaptation enabling tissue to survive substantial drying. A great deal of work has been conducted on highly DhT species. However, bryophytes showing less intense and variable DhT are understudied, despite the potential for these species to provide an informative link between highly tolerant and sensitive species. In this study, we tested the degree to which DhT varies across populations and the sexes of a species expected to exhibit a moderate DhT phenotype.
Methods To test predicted patterns of tolerance we assessed DhT in males and females of Marchantia inflexa from two distinct habitat types that differ in water availability. Both common garden and field-collected tissue was subjected to drying assays at multiple intensities and recovery was monitored by chlorophyll florescence. Verification studies were conducted to confirm the level of dehydration, the rate of drying and the associated changes in photosynthetic physiology.
Key Results We confirmed our expectation that M. inflexa is able to tolerate moderate dehydration. We also found that females exhibited higher DhT than males, but populations did not differ in DhT when cultured in a common garden. However, field-collected samples exhibited differences in DhT corresponding to environmental dryness, suggesting plasticity in DhT.
Conclusions By studying a less extreme DhT phenotype we gained insight into how more sensitive (yet still tolerant) organisms cope with dehydration. Additionally, the identified sex-specific variation in DhT may explain ecological patterns such as female-biased sex ratios. Furthermore, plasticity in DhT has the potential to inform management practices aimed at increasing tolerance to drought conditions.
Keywords: Marchantia inflexa, sex difference, acclimation, chlorophyll fluorescence, desiccation tolerance, dehydration, plasticity
INTRODUCTION
In a warming and increasingly variable climate, adaptations to cope with water scarcity are particularly relevant. Knowledge of such adaptive strategies can inform both natural and agricultural applications. For some plant species, unique adaptations provide a means for tissues to resurrect from near complete dehydration and apparent death. This capacity is known as desiccation tolerance, an extreme case of dehydration tolerance (DhT, also dehydration-tolerant). A variety of research has been conducted on the natural history (Alpert, 2000), physiology (Bewley, 1979), mechanisms (Le and McQueen-Mason, 2006; Gechev et al., 2012) and prevalence (Wood, 2007) of DhT with particular focus on highly desiccation-tolerant species.
Dehydration tolerance is not common across the Tree of Life and most plant and animal tissue cannot withstand cellular water potentials below −10 MPa (Gaff, 1997; Walters et al., 2002). However, DhT tissues consistently recover from cellular water potentials below this level (Oliver et al., 2010), and desiccation-tolerant tissues survive cellular water potentials below −100 MPa (Gaff, 1971). Dehydration-tolerant organisms have a unique ability to leverage both protection and repair systems to combat the myriad drying stresses, such as membrane rupture (Hoekstra and Golovina, 1999), protein aggregation and oxidative damage (Leprince et al., 1990; Oliver et al., 2005). To date, considerable effort has been devoted to understanding the mechanism of DhT. Soluble sugar accumulation, antioxidant production, cell wall flexibility and late embryogenesis abundant (LEA) protein accumulation have been associated with tolerance in multiple species (see Black and Pritchard, 2002; Lüttge et al., 2011, and citations within). Much of this work has focused on driving positive economic impact in the biotech and agricultural industries (Alpert, 2005; Toldi et al., 2009), and although progress has been made many unanswered questions remain.
Despite being uncommon, DhT is found across many life forms, from bacteria (Billi and Potts, 2002) to animals (Clegg, 2005; Gusev et al., 2014) and plants. In plants DhT likely evolved as a necessary adaptation for the transition from water to land by early plants (Oliver et al., 2000). Through evolutionary time most vascular plants lost DhT in their vegetative tissues, perhaps as a trade-off for more complex water storage and transport systems supporting larger growth forms (Alpert, 2006; Gaff and Oliver, 2013). However, species from multiple lineages of vascular plants re-evolved vegetative DhT independently (Farrant and Moore, 2011). Interestingly, most dehydration-sensitive plants retain DhT in their pollen, seeds or spores, suggesting that a wider array of plants may have the genetic potential for DhT, but that it is constrained to specific tissue types and developmental stages as a product of taxon-specific life history (Walters et al., 2002; Grene et al., 2011). Although there are DhT representatives across taxonomic groups of land plants, DhT is most prominent among bryophytes, lichens and green algae (Proctor et al., 2007; Wood, 2007).
To date, a great deal of vegetative DhT research has focused on the extremely DhT (or desiccation-tolerant) species, which has contributed valuable insight into the process and mechanisms of DhT. However, such extreme phenotypes are not applicable to many plant forms and may obscure nuanced components of the process. There has been a relative paucity of research on bryophytes that show lower levels of tolerance, although some recent studies address this (Koster et al., 2010; Cruz de Carvalho et al., 2011, 2012, 2014, 2015; Pardow and Lakatos, 2013; Bader et al., 2013; Stark et al., 2013), and numerous studies have investigated moderate levels of dehydration in the drought response of vascular plants (Hsiao, 1973; Osakabe et al., 2014). Bryophytes showing moderate DhT likely occupy a large ecological niche, and understanding their response to abiotic stress is important for predicting species range shifts due to environmental changes. Additionally, studying more moderate DhT in a relatively simple tissue could increase fundamental understanding of the spectrum of dehydration responses.
Intraspecific variability in DhT has also been relatively underexplored, though its existence has long been acknowledged (Schonbeck and Bewley, 1981). Evidence indicates that DhT is not a fixed trait within a species, and examples suggesting plasticity in dehydration responses (Dilks and Proctor, 1976; Schonbeck and Bewley, 1981; Robinson et al., 2000), seasonal variation (Beckett and Hoddinott, 1997; Farrant et al., 2009), differences between developmental stages (Koster and Leopold, 1988; Stark et al., 2004, 2007) and sex-specific variation (Newton, 1972; Stieha et al., 2014) exist. Plasticity in DhT may be particularly informative for understanding parameters that modulate DhT. Additionally, sex differences in DhT provide insight into the genetic underpinnings of DhT and may inform the existence of biased population sex ratios in nature.
In this study we tested for intraspecific variation in DhT in the tropical dioecious liverwort Marchantia inflexa. We hypothesized that M. inflexa would show moderate DhT and also considerable intraspecific variability in DhT. For this study we used plants collected from populations located along a moisture availability gradient and subsequently grown in a common garden. Due to selection pressures, we predicted that M. inflexa would show lower DhT in plants originating from mesic habitats compared with less mesic habitats because plants originating from a moist environment would have little use for DhT. Based on previous studies (Stieha et al., 2014), females were expected to be more DhT than males, offering a potential explanation for the female-biased sex ratios seen in this and many bryophyte populations. Additionally, we tested the predicted population difference in DhT in the field by using plants collected directly from field habitats.
MATERIALS AND METHODS
Study organism, sampling and growth conditions
Marchantia inflexa is a New World liverwort with unisexual individuals, found from northern Venezuela to the southern USA (Bischler, 1984). Marchantia inflexa exhibits genetic variation in DhT in some developmental stages (gemmae) (Stieha et al., 2014), and its ecology and physiology have been the subject of multiple relevant studies (McLetchie and Puterbaugh, 2000; Groen et al., 2010a, b; Brzyski et al., 2014; Stieha et al., 2014).
Plants for this study were collected from Trinidad, the Republic of Trinidad and Tobago and maintained in greenhouse conditions at The University of Kentucky, Lexington, KY, USA. Specimens were vouchered at the Missouri Botanical Garden (St Louis, MO, USA, specimen numbers M092113 and M092115) and at the National Herbarium of the Republic of Trinidad and Tobago (St Augustine, Trinidad, specimen number TRIN34616, D. N. McLetchie, collector). Species identification was verified by Alan Whittemore (New York Botanical Garden, Bronx, New York, USA). Plants were collected from populations in two distinct habitats along a moisture availability gradient: streams (native habitat) and roads (novel habitat). The streams are humid and shaded by trees, representing a high-moisture habitat while the roads are exposed and are expected to be less humid. Furthermore, the presence of a dry season (although intermittent rainfall occurs) causes the roads to experience intervals of drying.
Male and female plants were collected from randomly chosen locations (patches) along one stream site (East Turure River) and along one road site (Cumaca Road). Each patch was physically separated from the other to guarantee that individuals were genetically different, and uniqueness of each isolate was confirmed by PCR analysis (Brzyski et al., 2014). Eighteen genotypes were selected for use in the current study: four males from the stream site, four males from the road site, five females from the stream site and five females from the road site.
For this study, thalli of stock plants with their meristematic region intact were transplanted onto steam-sterilized soil (collected from the North Farm, University of Kentucky, Lexington, KY, USA) in 12-well plug trays (3·5 × 4 cm). At least 36 clones (three sets) of each genotype were maintained in a randomized layout in the greenhouse. Trays were placed on capillary mats that were kept wet by daily watering with deionized water and covered with a shade cloth to mimic field light conditions.
Dehydration tolerance assay and recovery
Dehydration conditions of differing intensity were generated using saturated salt solutions to modify the relative humidity (RH) in dehydration chambers. Experimental RHs, water vapour pressure deficits (VPDs) and corresponding treatment levels are shown in Table 1. Water vapour deficit was calculated to enable comparison of treatment intensity with our field assay (Anderson, 1936). Each experimental RH was calculated based on an equilibrium RH chart (Wexler and Hasegawa, 1954) and verified using a HOBO™ humidity sensor attached to a data logger (Onset Computer Corporation, Bourne, MA, USA).
Table 1.
Treatment conditions for laboratory dehydration assays. Temperature was maintained in a growth chamber on a 12-h light/12-h dark cycle. Relative humidity was verified using a HOBO™ data logger with sensors attached. All assays were 22 h long. Vapour pressure deficit indicates the relative intensity of the combined temperature and relative humidity during the assay
| Treatment level | Temperature (°C) | Relative humidity (%) | Vapour pressure deficit (kPa) | Salt |
|---|---|---|---|---|
| Level 1 | 13 | 95·41 | 0·08 | KNO3 |
| Level 2 | 13 | 85·92 | 0·21 | KCl |
| Level 3 | 13 | 75·61 | 0·37 | NaCl |
| Level 4 | 13 | 55·87 | 0·66 | MgN2O6 |
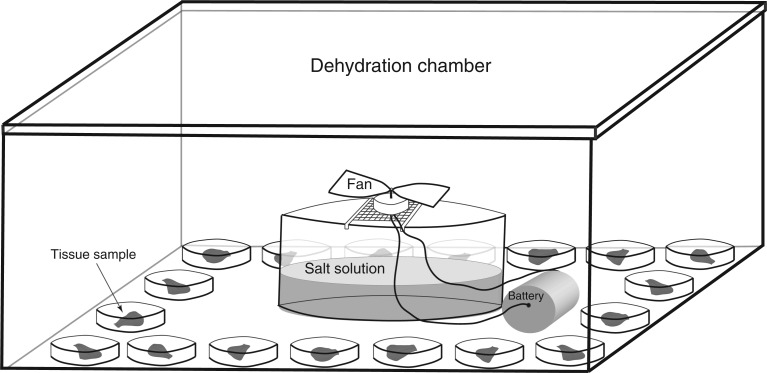
Dehydration assays were conducted in dehydration chambers (airtight plastic boxes 24 × 10 × 32 cm) and were placed in a growth chamber with a 12-h light/12-h dark cycle period and a constant temperature of 13 °C. Healthy vegetative thallus tips (∼7 mm in length) from each of the 18 genotypes were collected from the greenhouse, saturated with distilled water and placed in the growth chamber for 24 h to ensure that each sample was fully hydrated before the assay began. After 24 h, each thallus tip was randomly assigned to one of four treatment groups and blotted to remove external water. Each thallus tip was then placed in a 35 × 10 mm Falcon™ brand Petri dish along with a single filter paper disc and 200 μL of distilled water. These Petri dishes were placed on the internal perimeter of the dehydration chamber, and a bowl of saturated salt solution (differed between treatments) was placed in the centre of the box. A small battery-powered fan sat on a wire mesh stand above the salt solution (Fig. 1). Plants were dehydrated for 22 h, after which the fan was stopped and the plants were rehydrated with distilled water and maintained in the same growth chamber for 1 week. Treatments were replicated through time across all 18 genotypes: level 1 = three replications; level 2 = ten replications; level 3 = nine replications; level 4 = three replications.
Fig. 1.
Schematic of the dehydration assay set-up. Each box contained one tissue sample from each of the 18 genetic lines. Each sample was placed in a Petri dish along with a single filter paper disk and 200 μL of distilled water. The dehydration chamber was placed in a growth chamber with a constant temperature of 13 °C and a 12-h light/12-h dark cycle.
Recovery of each individual thallus tip was analysed by chlorophyll fluorescence after 1 week of recovery to determine the condition of photosystem II. Chlorophyll fluorescence is a common method used to assay plant recovery after dehydration stress (Krause and Weis, 1984; Csintalan, 1999). Maximum potential quantum yield (Fv/Fm) readings of dark-adapted leaves (20 min) were taken using an OS5-FL modulated chlorophyll fluorometer (Opti-Sciences, Tyngsboro, MA, USA). Recovery was also assessed by monitoring plant growth over 2 months, during which time the plants were maintained in a hydrated state in the same growth chamber.
Relative water content
The relative water content (RWC) of tissue was measured to determine the percentage of water lost by samples in each treatment level. Vegetative tissue of M. inflexa was collected from the greenhouse and hydrated for 24 h in the growth chamber. Following hydration, the tissue was blotted dry to remove external water. Each plant was then weighed to the nearest nanogram using a Chan 29 electrobalance to determine the turgid mass (Mt), randomly assigned to one of the four treatment groups, and subjected to the dehydration assay described above. However, following completion of the assay at 22 h, the tissue was not rehydrated. Instead, samples were weighed to determine sample fresh mass (Mf), and the tissue was placed in a drying oven (80 °C) for 3 d, after which its dry mass (Md) was taken. The RWC was calculated for each sample with the following formula:
Field study
A study was conducted to investigate corresponding patterns of DhT at the field sites in Trinidad during March 2015. Vegetative thallus tips (∼7 mm in length) were collected from plants growing in two habitat types (stream and road). The samples were collected from moist substrate and appeared hydrated when collected. Sampled thallus tips were saturated in stream water for 24 h, blotted dry, and then placed randomly into one of two dehydration treatment levels (Table 2). Three trails were conducted with ten thallus tips in each. Half of the thallus tips in each trial were from the stream habitat and half were from the road habitat. The assay was conducted following the same protocol as the laboratory dehydration assay (described above) with a few minor changes. First, because there was no access to a temperature-regulated growth chamber in Trinidad the dehydration assay was conducted under a raised building to avoid direct sunlight. Ambient temperatures ranged from 26 °C (night) to 30 °C (day). The corresponding VPDs and RHs for these treatments are listed in Table 2. The VPD was needed to compare the relative intensity of field and laboratory treatments because it accounts for the effect of temperature (Anderson, 1936). The thallus tips were allowed to dehydrate until they were noticeably dry, as indicated by visual changes in thallus curvature, after which they were rehydrated with stream water. Recovery was assessed by chlorophyll fluorescence 1 week after rehydration.
Table 2.
Treatment conditions for dehydration assays conducted in the field. Temperature and humidity were confirmed using a HOBO™ data logger with sensors attached. The temperature varied with ambient air temperature in Trinidad and the assay was conducted in a location to avoid direct sunlight. Vapour pressure deficit indicates the relative intensity of the combined temperature and relative humidity during the assay
| Treatment level | Temperature (°C) | Relative humidity (%) | Vapour pressure deficit (kPa) | Salt |
|---|---|---|---|---|
| Field level 1 | 26–30 | 83·63 | 0·50–0·64 | KCl |
| Field level 2 | 26–30 | 75·09 | 0·80–1·07 | NaCl |
Verification of equilibration and rate of drying
To confirm that samples had equilibrated to the RH in the dehydration chambers after 22 h, vegetative thalli were collected from a single randomly selected genotype (1F) and subjected to the laboratory dehydration assay described above. Samples were weighed as in the RWC experiment and then randomly assigned to one of the four dehydration treatment levels. In order to minimize disturbance of the samples and potential changes to the treatment conditions, samples were not weighed for the first 16 h of the dehydration assay (except in treatment level 4 because these samples dried much faster than the others). Samples were then weighed at 16, 18, 20, 21 and 22 h using the Chan 29 electrobalance. When mass did not decline for three consecutive measurements, we considered tissues to be equilibrated to the RH in the chamber. If the samples were not equilibrated to experimental RH at 22 h the assay was extended for an additional hour. After completion, samples were oven-dried and weighed as described above. The RWC for each sample was calculated based on final Md, Mt and Mf at each time point using the equation for RWC.
Physiological changes
Because the RHs used in this study were higher than those typical for bryophyte dehydration studies, we tested the physiological consequence of these treatments by measuring specific physiological changes (photosynthesis and respiration). We focused this effort on changes in gas exchange during dehydration at 75 % RH. The higher experimental RHs (85 and 95 %) were not used because plants retained high RWC at equilibrium with these RHs, and the lowest RH (55 %) was not used because all M. inflexa samples died from this treatment.
Thallus tips (∼7 mm long) were collected from greenhouse-grown specimens of one randomly selected male (8X) and female (2X) and placed in distilled water. Sample area was measured using ImageJ (Schneider et al., 2012). Prior to placement in the gas exchange chamber, the thallus tip was blotted to remove external water and 20 μL of distilled water was added to make the thallus tip adhere to the plastic sheet used to support it in the gas exchange chamber.
Gas exchange was measured with an open-flow system (LI-6400, Li-Cor, Lincoln, NE, USA) equipped with a fluorescence attachment (6400-40, Li-Cor). Air temperature was allowed to vary with the ambient laboratory temperature (22–24 °C), and RH ranged from 72 to 80 %. Thus, the VPD varied from 0·53 to 0·84 MPa. Airflow rate was set at 100 µmol s−1 and CO2 was set at 450 µmol mol−1. Infrared gas analysers (sample and reference) were matched prior to the assays.
To obtain baseline CO2 assimilation rates (µmol CO2 m−2 s−1), we first took three readings 15 min after equilibrium at a light intensity of 150 µmol m−2 s−1. To measure respiration, light intensity was set at 0 µmol m−2 s−1 and three readings were taken after 15 min. In M. inflexa, gas exchange rates usually equilibrate in 5–6 min after a change in light intensity (unpubl. res. DNM). After taking baseline measurements, each plant was immediately subjected to the assay to measure changes in either photosynthesis or respiration, but never both because these assays damage tissues. For photosynthesis, plants were assayed using the AUTO program of the LI-6400, at a light intensity of 150 µmol m−2 s−1 at 20-min intervals for 5 h, then assayed at 0 µmol m−2 s−1 to confirm that photosynthesis had ceased (i.e. no change in gas exchange rates between 150 and 0 µmol m−2 s−1). For respiration, plants were assayed using the AUTO program of the LI-6400 at a light intensity of 0 µmol m−2 s−1 at 30-min intervals for 7·5 h, then assayed at 150 µmol m−2 s−1 to confirm that photosynthesis was not contributing to the gas exchange rates (i.e. no increase in carbon fixation from 0 to 150 µmol m−2 s−1). The difference between input and output air mmol H2O mol−1 at each time point was used as a measure of the dehydration state of the tissue, where decreasing positive values indicated that the tissue was still drying and zero indicated an equilibrium dehydrated state. The interval and duration of the photosynthesis and respiration assays were based on trials showing that plants in the light (150 μmol m−2 s−1) took 3–4 h and those in the dark took 6–7 h to equilibrate to the RH.
Statistical analyses
All statistical analyses were done in JMP®, Version 10 (SAS Institute Inc., Cary, NC). The complete data set for this project has been archived on Figshare.
Dehydration tolerance assay
To account for genotypic variation we estimated initial Fv/Fm of healthy tissues of each genotype using five thallus tips per genotype. The mean Fv/Fm for each genotype was used as the initial Fv/Fm value and the recovery Fv/Fm value for each thallus tip was subtracted from the mean initial Fv/Fm value for that genotype. Thus, the measure of recovery used for analysis of dehydration response was (initial Fv/Fm − recovery Fv/Fm =ΔFv/Fm). Larger values indicate less recovery. The difference in recovery across treatment level was analysed using a mixed linear model with ΔFv/Fm as the dependent variable. The fixed effects tested were treatment level, sex, habitat and the sex × habitat interaction. Genotype (nested within sex) and trial (replications) were random effects. Post hoc comparisons were made with a Tukey honest significant difference (HSD) test.
Because of the strong treatment differences (high recovery for levels 1 and 2, and no recovery for level 4), level 3 was selected for further use in elucidating differences in responses to dehydration between the sexes and habitats. That is, responses were most variable at this treatment level. The dependent variable analysed was ΔFv/Fm. The fixed effects tested were sex, habitat and the sex × habitat interaction. Genotype (nested within sex) and trial (replications) were random effects. Post hoc comparisons were made with a Tukey HSD test.
Relative water content
Differences in RWC for each treatment level were tested for significance using a mixed linear model. The dependent variable was RWC and the fixed effect tested was treatment level. Genotype (nested within sex) and trial (replications) were random effects. Post hoc comparisons were made with a Tukey HSD test.
Field study
Differences between habitat types in ability to recover from dehydration were analysed exclusively for field treatment level 1 using the mixed linear model. Field treatment level 2 was not used because samples did not recover from this treatment. We did not take initial Fv/Fm readings for these samples, so recovery was assessed using only the recovery Fv/Fm values. The dependent variable was Fv/Fm and the fixed effect analysed was habitat. Plant identity and trial (replications) were random effects. The sex of these plants was unknown and therefore sex effects were not investigated.
Verification of equilibration and rate of drying
Differences in the rate of change in RWC were investigated by repeated measures multivariate analysis of variance (MANOVA) with a full factorial design, repeated through time. We considered no change in RWC over three time points to be an indication of equilibration to treatment RH. Thus, the final three time points were analysed separately to ascertain if the RWC had stopped changing using repeated measures MANOVA.
Physiological changes
The difference between initial and final photosynthetic rate, as well as the verification of photosynthetic cessation, were investigated using 95 % confidence intervals (CIs) calculated for both mean initial and mean final photosynthetic rate across trials and genotypes. Respiration rates were too low to detect a change over time. Thus respiration levels were not analysed further.
The response variable of photosynthetic rate was examined using a mixed linear model in which photosynthetic rate was the dependent variable, time and genotype were fixed effects, and trial (replications) was a random effect. The response variable of dehydration state was examined by a similar mixed linear model where dehydration state was the dependent variable, time and genotype were fixed effects and trial (replications) was a random effect. The relationship between time to stop photosynthesis and the time to reach dehydration equilibrium was investigated using the mixed linear model where time was the dependent variable, process (photosynthesis or dehydration equilibrium) and genotype were fixed effects, and trial (replications) was a random effect.
RESULTS
Dehydration tolerance assay and recovery
Treatment intensity
The effects of treatment were significant (F3,21 = 21·39, P <0·001). Samples in level 1 were the least damaged and those in level 4 were the most damaged by dehydration (Fig. 2). Estimation of survival by long-term observation and mortality tracking over 2 months was consistent with the Fv/Fm data. All samples survived the level 1 treatment and 96 % of plants survived level 2 treatment. Sixty-three percent of plants survived level 3 treatment, but growth of these plants was substantially reduced. All plants died in the level 4 treatment.
Fig. 2.
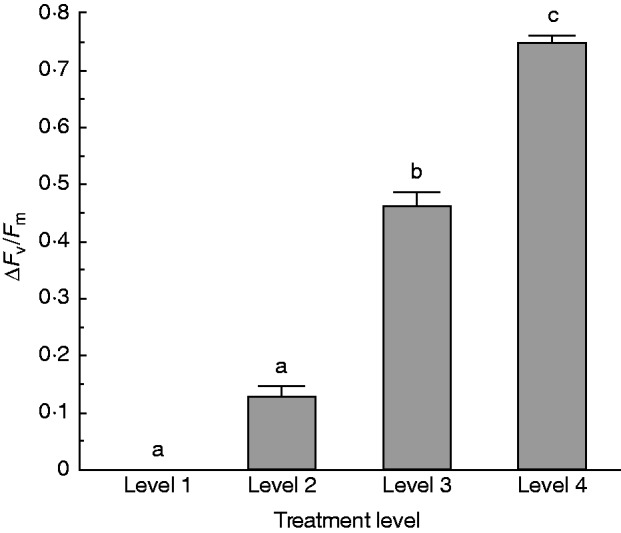
ΔFv/Fm of samples for each of the four levels of dehydration treatment. Recovery was measured after 1 week. Sample sizes used to calculate survival were: level 1 = three; level 2 = ten; level 3 = nine; level 4 = three. Higher ΔFv/Fm indicates more damage. Bars with different lowercase letters are significantly different at P < 0·05.
Sex and habitat patterns
Analysis of recovery from treatment level 3 revealed a sex effect (F1,13·37 =7·60, P = 0·016) and a sex × habitat interaction (F1,13·37 = 4·94, P =0·044). Females were less damaged by dehydration than males (Fig. 3A). More specifically, females were less damaged than males from the stream populations, but this sex difference was not evident in plants from road populations (Fig. 4). No overall population difference was found between individuals from stream or road habitats (Fig. 3B).
Fig. 3.
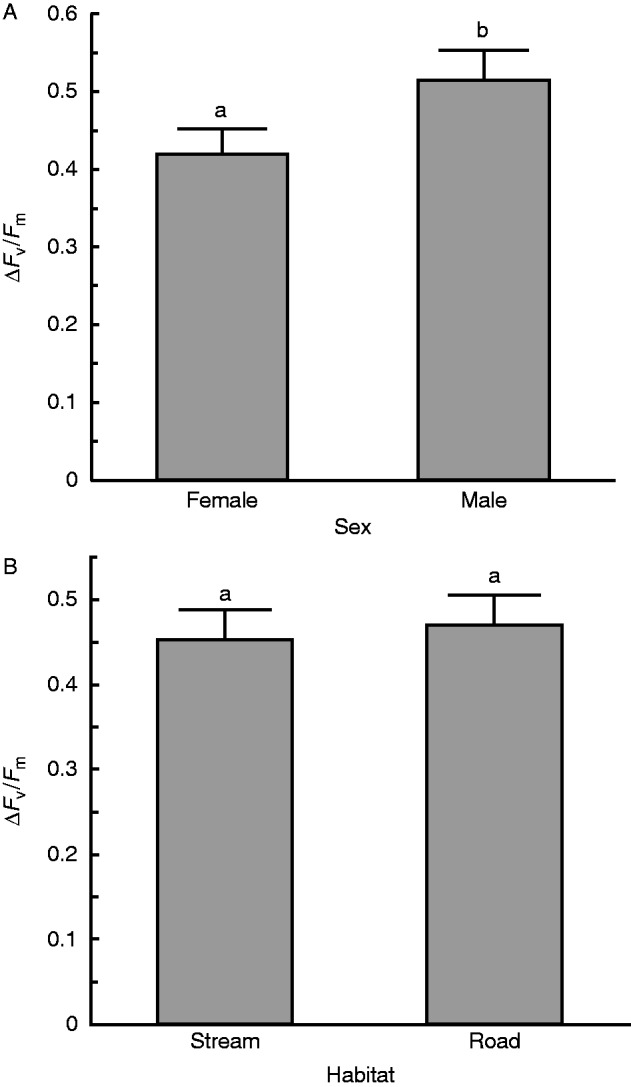
(A) Difference in damage incurred from dehydration at level 3 between the sexes was significant, with females being less damaged than males (P =0·016). (B) Difference in damage incurred for plants from wet versus dry source habitats at level 3 is not significant. Recovery was measured after 1 week. There were nine trials. Bars with different lowercase letters are significantly different at P < 0·05.
Fig. 4.
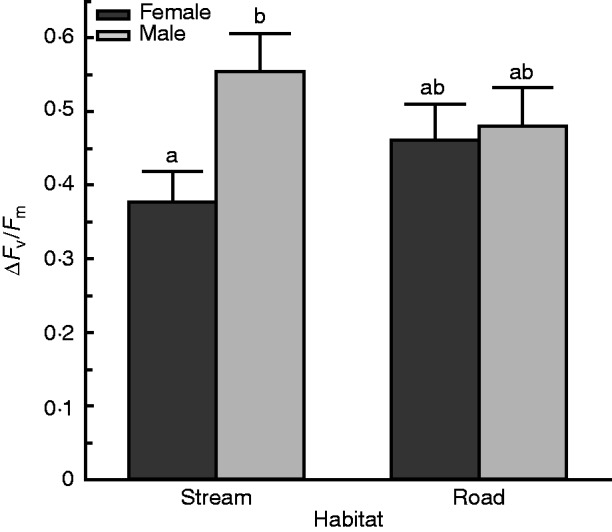
Females from the stream were less damaged than males from the stream, whereas females and males from the road did not differ from one another. This interaction was statistically significant at treatment level 3 (P =0·044). Recovery was measured after 1 week. There were nine trials. Bars with different lowercase letters are significantly different at P < 0·05.
Relative water content
Differences in mean RWC across treatment level were significant (F3,195 = 278·25, P <0·001; Fig. 5). Tissue in the level 1 treatment maintained full saturation. Tissue in the level 2 treatment lost some cellular water (RWC = 79·9 ± 4·4 %) and that in the level 3 treatment lost a substantial amount of cellular water (RWC = 19·7 ± 2·9 %). Tissue in the level 4 treatment lost almost all cellular water.
Fig. 5.
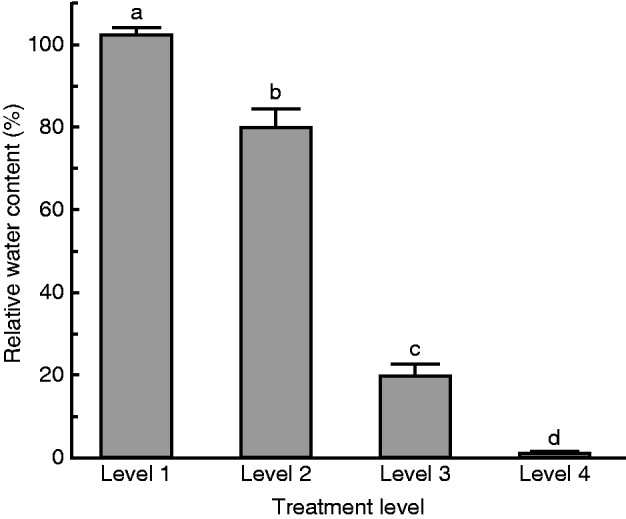
Mean RWC of M. inflexa tissue after treatment at each of the four dehydration intensities (Table 1). Sample size for each treatment group was three replicated dehydration chambers each containing 18 individuals. Bars with different lowercase letters are significantly different at P < 0·05.
Field study
Plants sampled from the dry road habitat recovered more completely from dehydration treatment than those collected from the moist stream habitat (F1,26 = 4·49, P = 0·044, Fig. 6).
Fig. 6.
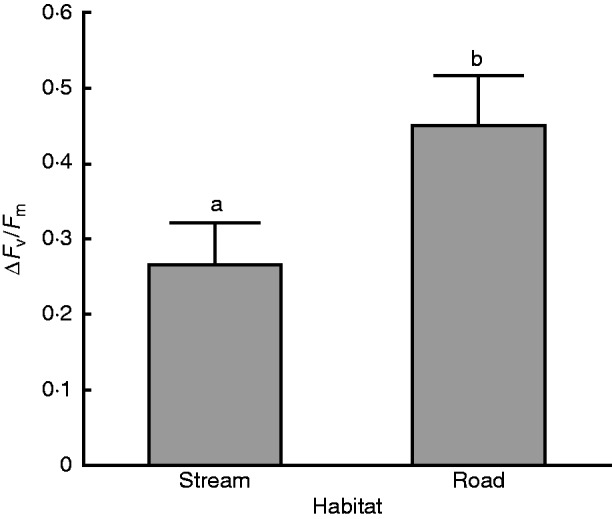
The difference in recovery Fv/Fm in plants collected directly from the field at stream or road sites and subjected to dehydration conditions was significant (P =0·0438). Recovery was measured after 1 week. There were three trials with ten thallus tips in each. Bars with different lowercase letters are significantly different at P < 0·05.
Verification of equilibration and rate of drying
There was a significant effect of time in all treatment levels (F5,3 = 529·77, P <0·001) as well as a significant time × treatment interaction (Wilks’ λ, F15,8·68 = 17·69, P <0·001), indicating that the rate of drying differed across treatments (Fig. 7). Samples in both treatment levels 3 and 4 equilibrated to the treatment RH well within the timeframe of our assay, as evidenced by a lack of change in RWC during the last 4 h of the assay for treatment level 3 and the last 12 h for level 4. Samples in treatment levels 1 and 2 took the entire 22 h to equilibrate. During the final 3 h of the assay there was no significant time effect on RWC at any treatment level (level 1: F2,1 = 96·53, P = 0·072; level 2: F2,1 = 3·36, P = 0·36; level 3: F2,1 = 0·38 P = 0·75; level 4: F2,1 = 0·55, P = 0·4), demonstrating that tissue was equilibrated to treatment conditions during this final time period.
Fig. 7.
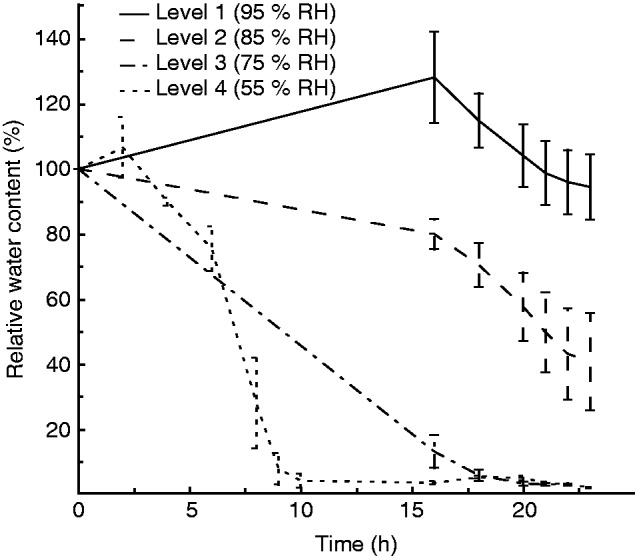
Drying rate as change in RWC over time for the four treatment levels. Three samples of a single genotype (1F) were used in each treatment level. RWC was calculated using the fully hydrated sample mass, the mass measured over the course of the assay and the final oven-dry mass. The slight initial increase in RWC seen in level 1 and level 4 treatments is a result of the samples picking up external water prior to drying. The equilibration RWC here is not consistent with that seen in Fig. 5 because in Fig. 5 there was a single level 3 trail that did not equilibrate and level 2 trails contained substantial variation.
Physiological changes
Tissue samples in the light equilibrated to ∼75 % RH after ∼3 h, as indicated by the lack of difference in mmol H2O mol−1 of input and output air (Fig. 8A). Samples stopped assimilating CO2 after ∼3 h (Fig. 8B).
Fig. 8.
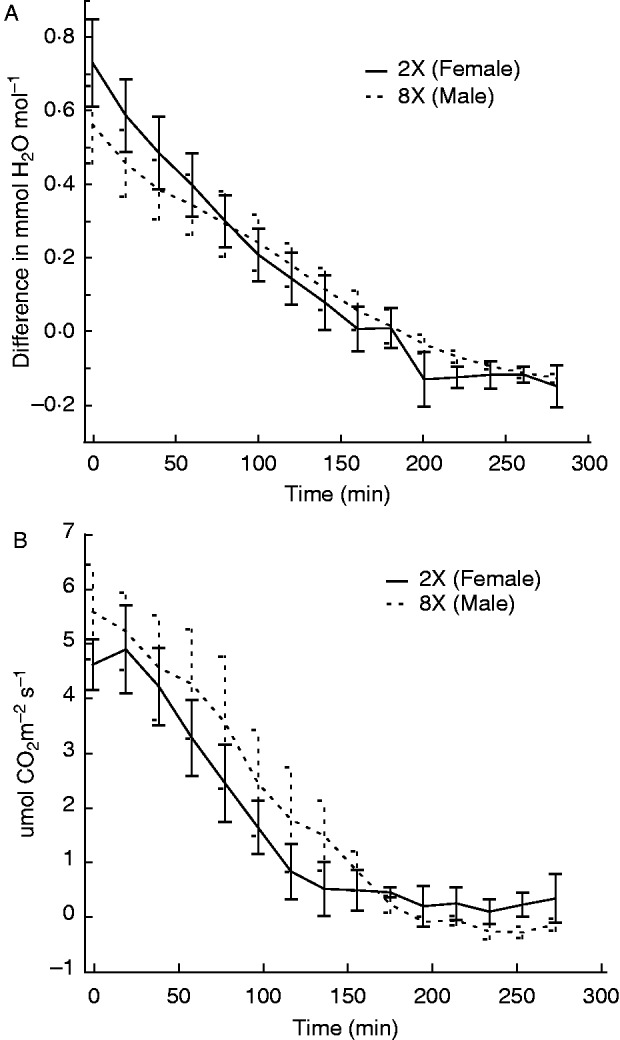
(A) The dehydration state of samples over time was measured as difference in mmol H2O mol−1 input to mmol H2O mol−o output. (B) The change in photosynthetic rate was measured over the same time period. Photosynthetic rates were measured as µmol CO2 m−2 s−1 and decreased to 0 between 2 and 3 h of drying at ∼75 % RH. There was no difference between genotypes in their dehydration state or photosynthetic rate (n=6).
Neither initial (F1,4 = 0·85, P = 0·408) nor final (F1,4 = 3·37, P = 0·14) photosynthetic rates were significantly different between genotypes, but there was a significant change in photosynthetic rate during the process of equilibration to ∼75 % RH (Fig. 8B). The mean initial photosynthetic rate was 5·48 µmol CO2 m−m s−s (95 % CI 5·10 to 5·86 µmol CO2 m−m s−s). The mean final photosynthetic rate was −0·02 µmol CO2 m−m s−s (95 % CI −0·19 to 0·16 µmol CO2 m−m s−s). Because the confidence intervals do not overlap, we conclude that the initial and final photosynthetic rates are significantly different from one another. Furthermore, the 95 % CI for the final photosynthetic rate includes 0, suggesting that photosynthesis was likely shutdown at this point. We found that initial respiration levels were too low to detect a change over time.
There was no significant difference detected in the time to dehydrate to equilibrium at ∼75 % RH and the time to cease photosynthesis (F1,5 = 0·72, P = 0·435), nor any genotype effect (F1,4 = 0·14, P = 0·729), indicating that both processes happen simultaneously, and consistently across genotypes.
DISCUSSION
Our results indicate that M. inflexa displays a moderate degree of DhT, which confirms our expectation and extends existing knowledge of the diversity of DhT phenotypes within bryophytes. Marchantia inflexa also exhibits a sex difference in DhT, with females showing a higher degree of DhT than males. This sex differences may be caused by differences in male and female reproductive strategies, which in turn may explain biased population sex ratios. The sex difference is particularly intriguing because it was only observed for plants originating from the moist stream habitat. We did not find evidence of genetic adaptation to dry habitats, but we did detect plasticity in DhT as plants showed acclimation to different environments.
Recovery
No M. inflexa samples were able to recover from the most intense dehydration treatment (level 4), implying that M. inflexa is not highly DhT (or desiccation tolerant), compared with other species that can easily recover from this degree of dehydration (Proctor et al., 2007). However, many samples recovered from the level 3 treatment, and this treatment did dehydrate samples considerably, as indicated by the relatively low cellular water content and the cessation of photosynthesis at equilibration. Moderate DhT provides an important link between desiccation-sensitive crops and the highly desiccation-tolerant model organisms and may provide valuable insight into the acquisition of DhT.
Marchantia inflexa females exhibited a higher degree of DhT than males (Fig. 3A), which suggests contrasting male and female reproductive strategies or functions. This contradicts the typical expectation that females of dioecious plants should be less stress-tolerant due to higher resource investment in reproduction (Juvany and Munné-Bosch, 2015). However, there is mixed evidence on the direction of sex-specific stress tolerance differences in dioecious plants. While most studies indicate that males have higher water stress tolerance than females (Juvany and Munné-Bosch, 2015), multiple examples of higher tolerance in females have been observed as well (Ward et al., 2002; Sánchez-Vilas and Retuerto, 2009; Melanie et al., 2013). Further complicating matters, some studies have found that sex-linked differences in stress tolerance depend on life stage and environmental conditions (Rakocevic et al., 2009; Juvany and Munné-Bosch, 2015). Notably, higher DhT has been observed for females versus males in some bryophyte species (Newton, 1972; Stieha et al., 2014). In M. inflexa, like other bryophytes (McLetchie, 1992; Stark et al., 2000), males likely allocate more resources to pre-fertilization sexual investment than females, possibly at the expense of traits related to survival. Conversely, females may invest more in self-preservation than males as they must persist for longer during offspring maturation. Although plants were in a non-reproductive stage in our study, the underlying differences in male and female reproductive strategies could be important. For instance, M. inflexa males consistently reach sexual maturity more readily than females (Brzyski et al., 2014), and this precocious trait might trade off with DhT. This could explain the higher degree of DhT in females compared with males, but resolving this will require more systematic investigation of sex-specific trade-offs in DhT and sexual reproduction.
We predicted that plants collected from dry habitats (roadsides) would show higher recovery from dehydration than those collected from moist habitats (streams). However, we found no difference in the DhT of these two groups when plants were grown in a common garden. This suggests that there are no adaptive differences in DhT between plants from these divergent habitat types (Fig. 3B), but the road habitats are novel and thus it is not particularly surprising that we did not detect evidence of DhT adaptation to this environment. However, when plants were collected directly from these two habitat types (field study) and subjected to a dehydration treatment, samples from dry habitats had higher DhT than plants from moist habitats (Fig. 6). This pattern fits our initial prediction but contradicts our laboratory study, and suggests a strong potential for acclimation. In other words, it implies that DhT in M. inflexa is a plastic trait. The ability of M. inflexa to acclimate to dry conditions corroborates pervious work on protoplasts showing that slight decreases in water potential can lead to increased DhT and multiple studies demonstrating hardening in vegetative tissue (Schonbeck and Bewley, 1981; Beckett, 1999; Walters et al., 2002; Beckett et al., 2005; Cruz de Carvalho et al., 2011). We conclude that genetic adaptations are not responsible for the habitat difference in DhT, but rather that acclimation to dry conditions can increase DhT in M. inflexa.
The sex × habitat interaction detected in the common garden study was driven by plants derived from the stream habitat (Fig. 4). Females from the stream showed higher DhT than males, whereas plants from roadsides had similar responses across the sexes. While this pattern is complex, it is consistent with previous studies on M. inflexa showing habitat × sex interactions for life history traits. For example, the pattern of habitat-specific sexually dimorphic DhT is similar to what Brzyski et al. (2014) found for growth rate. In that study there was no overall habitat difference for growth rate, but stream-collected plants had a sexually dimorphic growth rate and road-collected plants did not have a dimorphic growth rate. One possible explanation for this pattern is that because the stream populations are native and well established, there has been more time for selection to generate the dimorphic patterns observed. Conversely, the roads are novel habitats and there has been less time for selection to act on secondary sexual dimorphism. In addition, the road populations engage in sexual reproduction much more frequently than those along the streams (Brzyski et al., 2014), which could lead to more mixing of male and female traits. Coupled with reduced evolutionary time, this mixing may explain the more homogeneous DhT of the sexes in road versus stream populations.
Verification
In bryophyte dehydration studies, equilibration to different RHs is often used as a measure of dehydration stress (Proctor et al., 2007; Wood, 2007). Thus, it was necessary for us to demonstrate that the length of our assay was sufficient for tissue to equilibrate to each treatment RH. In doing so we showed that samples achieved equilibration during our assay, and also that the rate of drying varied across treatments (Fig. 7). Samples dehydrated at the lower RHs not only dried more completely, but also dried faster, which very likely influenced their survival. A great deal of work has shown that drying rate strongly affects survival and recovery from dehydration, with faster drying typically being more damaging for intact vegetative tissues (Farrant, 1999; Cruz de Carvalho et al., 2012, 2015), although in many seeds slow drying is more damaging (Pammenter et al., 1998).
Most of the work on bryophyte DhT has been done using equilibration to lower RHs than were used in our study (Wood, 2007). The standard RH used for dehydration treatment is ∼50 %, which has been very informative in studying highly desiccation-tolerant species, but for understanding moderate levels of DhT we find it useful to use less intense dehydration treatments. Although other studies have used similarly less intense treatments (Pardow and Lakatos, 2013), there may be some concern as to the physiological effect of our treatments. To address this concern, we demonstrated that photosynthetic cessation is synchronized with equilibration to ∼75 % RH in M. inflexa (Fig. 8A, B). Although the cessation of photosynthesis does not imply that all metabolic activity has ceased, it is a central plant process, and its shutdown indicates that substantial physiological changes are associated with this level of dehydration (Dilks and Proctor, 1979; Deltoro et al., 1998).
Conclusions
Taken together, the results of this study demonstrate that M. inflexa exhibits moderate levels of DhT and that, depending on the habitat, females are more DhT than males. By combining a common garden and field study, we also show that acclimation, not adaptation, is responsible for habitat differences in DhT in these M. inflexa populations. Although it long been known that many bryophytes are highly desiccation-tolerant, this study provides a rare empirical example demonstrating that some bryophytes are moderately DhT. These findings are particularly relevant in an agricultural context, where greater understanding of moderate DhT could inform strategies for developing DhT crop species. Furthermore, with climate change predicted not only to increase temperatures on a global scale, but also to drive more variation in weather patterns (and more extreme events, such as droughts), these results also provide an important step towards better predictions of the response of M. inflexa and similar species to environmental changes.
ACKNOWLEDGEMENTS
We thank the University of Kentucky, College of Agriculture, Food and Environment for greenhouse space; the Wildlife Section, Forestry Division, Ministry of Agriculture Land and Marine Resources of Trinidad and Tobago for collection and export permits; the Water and Sewage Authority for allowing access to the research site; and Andrea and Darryl McLetchie for logistical support while in Trinidad. Craig Sargent provided statistical support; Helen Rogers piloted the dehydration assay; and Scott Hotaling, Jonathan Moore and Carol Baskin provided comments that improved the manuscript.
LITERATURE CITED
- Alpert P. 2000. The discovery, scope, and puzzle of desiccation tolerance in plants. Plant Ecology 151: 5–17. [Google Scholar]
- Alpert P. 2005. The limits and frontiers of desiccation-tolerant life. Integrative and Comparative Biology 45: 685–695. [DOI] [PubMed] [Google Scholar]
- Alpert P. 2006. Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? Journal of Experimental Biology 209: 1575–1584. [DOI] [PubMed] [Google Scholar]
- Anderson DB. 1936. Relative humidity or vapor pressure deficit. Ecology 17: 277–282. [Google Scholar]
- Bader MY, Reich T, Wagner S, González González AS, Zotz G. 2013. Differences in desiccation tolerance do not explain altitudinal distribution patterns of tropical bryophytes. Journal of Bryology 35: 47–56. [Google Scholar]
- Beckett R. 1999. Partial dehydration and ABA induce tolerance to desiccation-induced ion leakage in the moss Atrichum androgynum. South African Journal of Botany 65: 212–217. [Google Scholar]
- Beckett R, Hoddinott N. 1997. Seasonal variations in tolerance to ion leakage following desiccation in the moss Atrichum androgynum from a KwaZulu-Natal afromontane forest. South African Journal of Botany 63: 276–279. [Google Scholar]
- Beckett RP, Mayaba N, Minibayeva F V, Alyabyev AJ. 2005. Hardening by partial dehydration and ABA increase desiccation tolerance in the cyanobacterial lichen Peltigera polydactylon. Annals of Botany 96: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. 1979. Physiological aspects of desiccation tolerance. Annual Review of Plant Physiology 30: 195–238. [Google Scholar]
- Billi D, Potts M. 2002. Life and death of dried prokaryotes. Research in Microbiology 153: 7–12. [DOI] [PubMed] [Google Scholar]
- Bischler H. 1984. Marchantia L: the new world species. Bryophytorum Bibliotheca 26: 1–228. [Google Scholar]
- Black M, Pritchard HW. 2002. Desiccation and survival in plants: drying without dying. Wallingford: CABI Publishing. [Google Scholar]
- Brzyski JR, Taylor W, McLetchie DN. 2014. Reproductive allocation between the sexes, across natural and novel habitats, and its impact on genetic diversity. Evolutionary Ecology 28: 247–261. [Google Scholar]
- Clegg JS. 2005. Desiccation tolerance in encysted embryos of the animal extremophile, Artemia. Integrative and Comparative Biology 45: 715–724. [DOI] [PubMed] [Google Scholar]
- Cruz de Carvalho RC, Branquinho C, da Silva JM. 2011. Physiological consequences of desiccation in the aquatic bryophyte Fontinalis antipyretica. Planta 234: 195–205. [DOI] [PubMed] [Google Scholar]
- Cruz de Carvalho R, Catalá M, Marques da Silva J, Branquinho C, Barreno E. 2012. The impact of dehydration rate on the production and cellular location of reactive oxygen species in an aquatic moss. Annals of Botany 110: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz de Carvalho R, Bernardes DA Silva A, et al. 2014. Differential proteomics of dehydration and rehydration in bryophytes: evidence towards a common desiccation tolerance mechanism. Plant, Cell & Environment 37: 1499–1515. [DOI] [PubMed] [Google Scholar]
- Cruz de Carvalho R, Bernardes da Silva A, Branquinho C, Marques da Silva J. 2015. Influence of dehydration rate on cell sucrose and water relations parameters in an inducible desiccation tolerant aquatic bryophyte. Environmental and Experimental Botany 120: 18–22. [Google Scholar]
- Csintalan Z. 1999. Chlorophyll fluorescence during drying and rehydration in the mosses Rhytidiadelphus loreus (Hedw.) Warnst., Anomodon viticulosus (Hedw.) Hook. & Tayl. and Grimmia pulvinata (Hedw.) Sm. Annals of Botany 84: 235–244. [Google Scholar]
- Deltoro V, Calatayud A, Gimeno C, Abadai A, Barreno E. 1998. Changes in chlorophyll a fluorescence, photosynthetic CO2 assimilation and xanthophyll cycle interconversions during dehydration in desiccation-tolerant and intolerant liverworts. Planta 207: 224–228. [Google Scholar]
- Dilks TJK, Proctor MCF. 1976. Seasonal variation in desiccation tolerance in some British bryophytes. Journal of Bryology 9: 239–247. [Google Scholar]
- Dilks TJK, Proctor MCF. 1979. Photosynthesis, respiration and water content in bryophytes. New Phytologist 82: 97–114. [Google Scholar]
- Farrant JM. 1999. The effect of drying rate on the survival of three desiccation-tolerant angiosperm species. Annals of Botany 84: 371–379. [Google Scholar]
- Farrant JM, Moore JP. 2011. Programming desiccation-tolerance: From plants to seeds to resurrection plants. Current Opinion in Plant Biology 14: 340–345. [DOI] [PubMed] [Google Scholar]
- Farrant JM, Lehner A, Cooper K, Wiswedel S. 2009. Desiccation tolerance in the vegetative tissues of the fern Mohria caffrorum is seasonally regulated. Plant Journal 57: 65–79. [DOI] [PubMed] [Google Scholar]
- Gaff DF. 1971. Desiccation-tolerant flowering plants in southern Africa. Science 174: 1033–1034. [DOI] [PubMed] [Google Scholar]
- Gaff DF. 1997. Mechanisms of desiccation tolerance in vascular resurrection plants In: Basra A, ed. Mechanisms of Environmental Stress Resistance in Plants. Boca Raton, FL, USA: CRC Press, 43–58. [Google Scholar]
- Gaff DF, Oliver M. 2013. The evolution of desiccation tolerance in angiosperm plants: a rare yet common phenomenon. Functional Plant Biology 40: 315–328. [DOI] [PubMed] [Google Scholar]
- Gechev TS, Dinakar C, Benina M, Toneva V, Bartels D. 2012. Molecular mechanisms of desiccation tolerance in resurrection plants. Cellular and Molecular Life Sciences 69: 3175–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grene R, Vasquez-Robinet C, Bohnert HJ. 2011. Molecular biology and physiological genomics of dehydration stress In: Lüttge U, Beck E, Bartels D, eds. Plant desiccation tolerance. Berlin: Springer, 255–287. [Google Scholar]
- Groen KE, Stieha CR, Crowley PH, McLetchie DN. 2010a. Sex-specific plant responses to light intensity and canopy openness: implications for spatial segregation of the sexes. Oecologia 162: 561–570. [DOI] [PubMed] [Google Scholar]
- Groen KE, Stieha CR, Crowley PH, McLetchie DN. 2010b. Sex-specific plant responses to two light levels in the liverwort Marchantia inflexa (Marchantiaceae). Bryologist 113: 81–89. [Google Scholar]
- Gusev O, Suetsugu Y, Cornette R, et al. 2014. Comparative genome sequencing reveals genomic signature of extreme desiccation tolerance in the anhydrobiotic midge. Nature Communications 5: 4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra F, Golovina E. 1999. Membrane behavior during dehydration: implications for desiccation tolerance. Russian Journal of Plant Physiology 46: 295–306. [Google Scholar]
- Hsiao TC. 1973. Plant responses to water stress. Annual Review of Plant Physiology 24: 519–570. [Google Scholar]
- Juvany M, Munné-Bosch S. 2015. Sex-related differences in stress tolerance in dioecious plants: a critical appraisal in a physiological context. Journal of Experimental Botany 66: 6083–6092. [DOI] [PubMed] [Google Scholar]
- Koster KL, Leopold AC. 1988. Sugars and desiccation tolerance in seeds. Plant Physiology 88: 829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster KL, Balsamo RA, Espinoza C, Oliver MJ. 2010. Desiccation sensitivity and tolerance in the moss Physcomitrella patens: assessing limits and damage. Plant Growth Regulation 62: 293–302. [Google Scholar]
- Krause GH, Weis E. 1984. Chlorophyll fluorescence as a tool in plant physiology: II. Interpretation of fluorescence signals. Photosynthesis Research 5: 139–157. [DOI] [PubMed] [Google Scholar]
- Le TN, McQueen-Mason SJ. 2006. Desiccation-tolerant plants in dry environments. Reviews in Environmental Science and Biotechnology 5: 269–279. [Google Scholar]
- Leprince O, Deltour R, Thorpe PC, Atherton NM, Hendry GAF. 1990. The role of free radicals and radical processing systems in loss of desiccation tolerance in germinating maize (Zea mays L.). New Phytologist 116: 573–580. [Google Scholar]
- Lüttge U, Beck E, Bartels D. 2011. Plant desiccation tolerance. Berlin: Springer Science & Business Media. [Google Scholar]
- McLetchie DN. 1992. Sex ratio from germination through maturity and its reproductive consequences in the liverwort Sphaerocarpos texanus. Oecologia 92: 273–278. [DOI] [PubMed] [Google Scholar]
- McLetchie DN, Puterbaugh MN. 2000. Population sex ratios, sex-specific clonal traits and tradeoffs among these traits in the liverwort Marchantia inflexa. Oikos 90: 227–237. [Google Scholar]
- Melanie M, Oñate M, García MB, Munné-Bosch S. 2013. Photo-oxidative stress markers reveal absence of physiological deterioration with ageing in Borderea pyrenaica, an extraordinarily long-lived herb. Journal of Ecology 101: 555–565. [Google Scholar]
- Newton ME. 1972. Sex-ratio differences in Mnium hornum Hedw. and M. undulatum Sw. in relation to spore germination and vegetative regeneration. Annals of Botany 36: 163–178. [Google Scholar]
- Oliver MJ, Tuba Z, Mishler BD. 2000. The evolution of vegetative desiccation tolerance in land plants. Plant Ecology 151: 85–100. [Google Scholar]
- Oliver MJ, Velten J, Mishler BD. 2005. Desiccation tolerance in bryophytes: a reflection of the primitive strategy for plant survival in dehydrating habitats? Integrative and Comparative Biology 45: 788–799. [DOI] [PubMed] [Google Scholar]
- Oliver MJ, Cushman JC, Koster KL. 2010. Dehydration tolerance in plants. Methods in Molecular Biology 639: 3–24. [DOI] [PubMed] [Google Scholar]
- Osakabe Y, Osakabe K, Shinozaki K, Tran L-SP. 2014. Response of plants to water stress. Frontiers in Plant Science 5: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammenter N, Greggains V, Kioko J, Wesley-Smith J, Berjak P, Finch-Savage W. 1998. Effects of differential drying rates on viability retention of recalcitrant seeds of Ekebergia capensis. Seed Science Research 8: 463–471. [Google Scholar]
- Pardow A, Lakatos M. 2013. Desiccation tolerance and global change: implications for tropical bryophytes in lowland forests. Biotropica 45: 27–36. [Google Scholar]
- Proctor MCF, Oliver MJ, Wood AJ, Stark LR, Cleavitt NL, Mishler BD. 2007. Desiccation-tolerance in bryophytes: a review. Bryologist 110: 595–621. [Google Scholar]
- Rakocevic M, Medrado MJS, Martim SF, Assad ED. 2009. Sexual dimorphism and seasonal changes of leaf gas exchange in the dioecious tree Ilex paraguariensis grown in two contrasted cultivation types. Annals of Applied Biology 154: 291–301. [Google Scholar]
- Robinson S, Wasley J, Popp M, Lovelock C. 2000. Desiccation tolerance of three moss species from continental Antarctica. Australian Journal of Plant Physiology 27: 379–388. [Google Scholar]
- Sánchez-Vilas J, Retuerto R. 2009. Sex-specific physiological, allocation and growth responses to water availability in the subdioecious plant Honckenya peploides. Plant Biology (Stuttgart, Germany) 11: 243–254. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbeck MW, Bewley JD. 1981. Responses of the moss Tortula ruralis to desiccation treatments. II. Variations in desiccation tolerance. Canadian Journal of Botany 59: 2707–2712. [Google Scholar]
- Stark LR, Mishler BD, McLetchie DN. 2000. The cost of realized sexual reproduction: assessing patterns of reproductive allocation and sporophyte abortion in a desert moss. American Journal of Botany 87: 1599–1608. [PubMed] [Google Scholar]
- Stark LR, Nichols L, McLetchie DN, Smith SD, Zundel C. 2004. Age and sex-specific rates of leaf regeneration in the Mojave Desert moss Syntrichia caninervis. American Journal of Botany 91: 1–9. [DOI] [PubMed] [Google Scholar]
- Stark LR, Oliver MJ, Mishler BD, McLetchie DN. 2007. Generational differences in response to desiccation stress in the desert moss Tortula inermis. Annals of Botany 99: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LR, Greenwood JL, Brinda JC, Oliver MJ. 2013. The desert moss Pterygoneurum lamellatum (Pottiaceae) exhibits an inducible ecological strategy of desiccation tolerance: effects of rate of drying on shoot damage and regeneration. American Journal of Botany 100: 1522–1531. [DOI] [PubMed] [Google Scholar]
- Stieha CR, Middleton AR, Stieha JK, Trott SH, Mcletchie DN. 2014. The dispersal process of asexual propagules and the contribution to population persistence in Marchantia inflexa (Marchantiaceae). American Journal of Botany 101: 348–356. [DOI] [PubMed] [Google Scholar]
- Toldi O, Tuba Z, Scott P. 2009. Vegetative desiccation tolerance: is it a goldmine for bioengineering crops? Plant Science 176: 187–199. [Google Scholar]
- Walters C, Farrant JM, Pammenter N, Berjak P. 2002. Desiccation stress and damage In: Black M, Pritchard HW, eds. Desiccation and survival in plants: drying without dying. Wallingford: CABI, 263–291. [Google Scholar]
- Ward JK, Dawson TE, Ehleringer JR. 2002. Responses of Acer negundo genders to interannual differences in water availability determined from carbon isotope ratios of tree ring cellulose. Tree Physiology 22: 339–346. [DOI] [PubMed] [Google Scholar]
- Wexler A, Hasegawa S. 1954. relative humidity-temperature relationships of some saturated salt solutions in the temperature range 0 °C to 50 °C. Journal of Research of the National Bureau of Standards 53: 19–26. [Google Scholar]
- Wood AJ. 2007. The nature and distribution of vegetative desiccation-tolerance in hornworts, liverworts and mosses. Bryologist 110: 163–177. [Google Scholar]