Abstract
Background and Aims Retranslocation of iron (Fe) from source tissues enhances plant tolerance to Fe deficiency. Previous work has shown that silicon (Si) can alleviate Fe deficiency by enhancing acquisition and root to shoot translocation of Fe. Here the role of Si in Fe mobilization in older leaves and the subsequent retranslocation of Fe to young leaves of cucumber (Cucumis sativus) plants growing under Fe-limiting conditions was investigated.
Methods Iron (57Fe or naturally occurring isotopes) was measured in leaves at different positions on plants hydroponically growing with or without Si supply. In parallel, the concentration of the Fe chelator nicotianamine (NA) along with the expression of nicotianamine synthase (NAS) involved in its biosynthesis and the expression of yellow stripe-like (YSL) transcripts mediating Fe–NA transport were also determined.
Key Results In plants not receiving Si, approximately half of the total Fe content remained in the oldest leaf. In contrast, Si-treated plants showed an almost even Fe distribution among leaves with four different developmental stages, thus providing evidence of enhanced Fe remobilization from source leaves. This Si-stimulated Fe export was paralleled by an increased NA accumulation and expression of the YSL1 transporter for phloem loading/unloading of the Fe–NA complex.
Conclusions The results suggest that Si enhances remobilization of Fe from older to younger leaves by a more efficient NA-mediated Fe transport via the phloem. In addition, from this and previous work, a model is proposed of how Si acts to improve Fe homeostasis under Fe deficiency in cucumber.
Keywords: Cucumber (Cucumis sativus), iron retranslocation, leaves, nicotianamine, nicotianamine synthase (NAS), phloem transport, silicon, yellow stripe-like (YSL) transporters
INTRODUCTION
Silicon (Si) and iron (Fe) are the second and the fourth most abundant minerals, respectively, in the earth’s crust. While the essentiality of Fe for plants was discovered in the middle of the 19th century (see Römheld and Nikolic, 2006), Si is still not accepted as an essential element. However, its beneficial effect on plant growth and development, especially under stress conditions, is well documented in the literature (for a review, see Liang et al., 2015). In aerated soils, only a small proportion of Fe is available for higher plants, and in calcareous soils, which account for one-third of the world’s agricultural soils, the availability of Fe does not even satisfy plant requirements (Vose, 1982; Guerinot and Yi, 1994; Lindsay, 1995). Iron deficiency is a major nutritional disorder responsible for reduction in both yield and quality of a wide range of crops (Grusak and Della Penna, 1999; Alloway, 2008). Iron deficiency in crops thus has a strong negative impact on human health worldwide (Welch, 2004; White and Broadley, 2009).
In recent years, considerable progress has been made in understanding the physiological and molecular mechanisms by which plants increase uptake and bioavailability of Fe (for reviews, see Walker and Connolly, 2008; Jeong and Guerinot, 2009; Thomine and Vert, 2013). However, information regarding long-distance transport of Fe and its distribution within shoots is still lacking. Several studies indicate that during senescence and/or Fe deprivation, substantial mobilization of Fe and its subsequent retranslocation via the phloem may occur from vegetative tissues such as leaves and stems (Zhang et al., 1995, 1996; Shi et al., 2011; Waters and Sankaran, 2011). Retranslocation of Fe is a complex process that involves Fe solubilization and phloem loading in the source tissue, phloem transport and phloem unloading in the sink tissue (Shi et al., 2012). Phloem transport of Fe is suggested to be of particular importance for the growth and quality of crops under Fe-limiting conditions because young leaves, fruits and seeds acquire Fe almost exclusively through the phloem. However, the means by which the molecular machinery functions to remobilize Fe to the shoot and redistribute it between source and sink tissues is much less understood.
Nicotianamine (NA) is a plant-specific non-proteinogenic amino acid synthesized by the condensation of three molecules of S-adenosylmethionine in a reaction catalysed by nicotianamine synthase (NAS). As a hexadentate metal chelator, NA complexes both Fe2+ and Fe3+, with a higher affinity for Fe3+, but forming a more stable complex with Fe2+ (von Wirén et al., 1999). In graminaceous (Strategy 2) roots, NA is the direct precursor of phytosiderophores of the mugineic acid family (Mori, 1999) that strongly chelate Fe3+ in the rhizosphere thereby enabling Fe acquisition (Römheld and Marschner, 1986). It has also been proposed that NA plays a key role in Fe complex formation for phloem loading and translocation of Fe in plants, as well as in intracellular Fe chelation and short-distance transport (Scholz et al., 1992; Hell and Stephan, 2003). In tomato (Solanum lycopersicum), NA increases within both root and leaf cells in response to increasing Fe levels (Pich et al., 2001). Three arabidopsis (Arabidopsis thaliana) NAS genes expressed in leaves showed varied patterns of Fe regulation; AtNAS1 was not Fe regulated, whereas AtNAS3 was downregulated and AtNAS4 upregulated by Fe deficiency (Klatte et al., 2009).
The phloem-based long-distance Fe transport to sink tissues (e.g. young leaves) involves loading of Fe into the companion cell/sieve element complex and its unloading into corresponding sink tissues (Zhai et al., 2014). However, the contribution of phloem-specific transporters to loading/unloading of Fe has long been a subject of debate and remains insufficiently clear. The main contributors to this process are several yellow stripe-like (YSL) proteins, which are members of the oligopeptide transporter (OPT) family. It has been demonstrated that arabidopsis AtYSL1 andAtYSL3, and rice (Oryza sativa) OsYSL2 mediate Fe–NA transport and facilitate Fe loading into seeds (Waters et al., 2006; Chu et al., 2010; Ishimaru et al., 2010). Zhai et al. (2014) recently demonstrated that another member of the OPT family in arabidopsis, AtOPT3, also mediates Fe loading into the phloem in leaves as well as regulating Fe transport to developing tissues. The relevant contributors to Fe remobilization from source leaves in cucumber (Cucumis sativus), however, are as yet unknown.
It has recently been reported that Si nutrition can alleviate Fe deficiency stress in Strategy 1 species such as cucumber and soybean (Glycine max) (Gonzalo et al., 2013; Pavlovic et al., 2013). Pavlovic et al. (2013) demonstrated that application of Si increased the root apoplastic Fe pool and enhanced the expression of proteins involved in reduction-based Fe uptake. Moreover, Si influenced the expression of genes involved in biosynthesis of Fe-mobilizing compounds, thus resulting in enhanced accumulation of organic acids and phenolics which mediate increased Fe availability in the rhizosphere and mobilization of root apoplastic Fe (Pavlovic et al., 2013). It has also been shown that application of Si can facilitate mobility and xylem translocation of Fe towards the shoot, along with the accumulation of Fe-chelating compounds such as citrate in xylem sap and leaf tissues (Pavlovic et al., 2013; Bityutskii et al., 2014).
The objective of the present work was to gain further insight into the mechanisms involved in Si-mediated remobilization of Fe from older to younger leaves of cucumber plants under Fe-limiting conditions. We conducted stable isotope experiments with 57Fe in order to trace Fe distribution in the shoot. In addition, we focused on the distribution of NA as a potential Fe chelator relevant to Fe mobilization in source leaves and its subsequent retranslocation to the young leaves. Total NA content and the expression of NAS, a key enzyme involved in NA biosynthesis, as well as the expression of YSL transporters for the Fe–NA complex were therefore also determined at different developmental stages of the leaves.
MATERIALS AND METHODS
Plant materials and growth conditions
After soaking in 1 mm CaSO4 overnight, seeds of cucumber (Cucumis sativus L. cv. Chinese long) were germinated for 4 d between two sheets of filter paper moistened with saturated CaSO4. The seedlings were then transferred to an Fe-free nutrient solution (four plants per 2·5 L plastic pot) containing (in mm): 0·7 K2SO4, 0·1 KCl, 2·0 Ca(NO3)2, 0·5 MgSO4, 0·1 KH2PO4, and (in μm): 0·5 MnSO4, 0·5 ZnSO4, 0·2 CuSO4, 0·01 (NH4)6Mo7O24 and 10 H3BO3. After 7 d of pre-culture, the cotyledons were removed and plants were transferred to a complete nutrient solution supplied with 10 μm FeIII-EDTA, either 57Fe enriched or with natural abundance of Fe isotopes (here named Fe). After 3 d, Fe was again withheld from the nutrient solution, and plants were grown for another 11 d in Fe-free nutrient solution, either without (–Si) or with (+Si) supply of 1·5 mm monosilicic acid [Si(OH)4] freshly prepared and monitored according to Pavlovic et al. (2013). The first green leaf (L0), fully expanded during the pre-culture, was removed at the time of withdrawal of Fe from the nutrient solution (for a schematic presentation of the experimental set-up, see Supplementary Data Fig. S1). In addition, to minimize further mobilization of Fe from the root apoplast and subsequent enzymatic FeIII reduction preceding Fe2+ uptake, the pH of the nutrient solution was kept above 7·0 by buffering with CaCO3 (0·2 g L–1) and checked daily. The nutrient solutions were renewed completely every 2 d and continuously aerated.
Plants were grown under controlled environmental conditions in a growth chamber with a light/dark regime of 16:8 h, temperature regime of 24:20 °C, photon flux density of 450 μmol m–2 s–1 at plant height, and relative air humidity of about 70 %.
At harvest, root apoplastic Fe was removed by a reductive extraction as described below. Subsequently, plants were divided into the following parts: root, stem (together with leaf petioles and main midrib) and leaves (blades devoid of main midrib) collected from four different positions (from the base to the youngest leaf): L1 (developed during 3 d Fe treatment), L2, L3 and L4 (including apex), which all appeared after the Fe treatment. Samples were oven dried at 70 °C for 48 h, weighed and pulverized in a ceramic grinder.
Spectral plant analysis diagnostic (SPAD) measurement
The chlorophyll content in leaves was estimated non-destructively as SPAD units, using a portable Chlorophyll Meter SPAD-502 device (Minolta Camera Co., Osaka, Japan).
Determination of Si
Dry plant material (0·2 g) was digested with 3 mL of concentrated HNO3 + 2 mL of H2O2 in a microwave oven (Speedwave MWS-3+; Berghof Products + Instruments GmbH, Eningen, Germany) for 1 h. Samples were diluted with about 15 mL of H2O, transferred into 25 mL plastic flasks, 1 mL of hydrofluoric acid was added and samples were then left overnight. After addition of 2·5 mL of 2 % (w/v) H3BO3, the flask volume was adjusted to 25 mL with deionized H2O, and Si was determined by inductively coupled plasma optical emission spectrometry (ICP-OES; SpectroGenesis EOP II, Spectro Analytical Instruments GmbH, Kleve, Germany) after a final dilution of the samples of 1:100 (v/v) with deionized H2O.
Determination of Fe in root apoplast and plant tissues
After washing in a solution containing 0·5 mm CaSO4 and 5 mm MES (pH 5·5) for 10 min, intact roots of each plant were transferred into a 40 mL incubation solution containing 5 mm MES (pH 5·5), 0·5 mm CaSO4 and 1·5 mm 2,2′-bipyridyl, followed by incubation for 10 min under reductive conditions by adding 0·5 g of solid sodium dithionite under continuous N2 bubbling through the solution. Apoplastic Fe was removed as the red FeII[bipyridyl]3 complex and determined by measuring the absorbance at 520 nm using a extinction coefficient of 8·65 mm–1 (Bienfait et al., 1985).
For Fe determination in plant tissue, dry plant material (0·2 g) was microwave digested in 3 mL of concentrated HNO3 + 2 mL of H2O2 for 1 h. Samples were then transferred into 25 mL plastic flasks and the volume was adjusted to 25 mL with deionized H2O. Fe was determined by ICP-OES. The analytical accuracy of total concentrations was evaluated using certified reference material (GBW 10015 Spinach, Institute of Geophysical and Geochemical Exploration, Langfang, China).
Preparation of 57FeIIIEDTA and 57Fe determination in leaf tissue
57FeIIIEDTA was freshly prepared from 57Fe2O3 (96·64 % isotopic enrichment; Isoflex, San Francisco, CA, USA) as previously described by Pavlovic et al. (2013).
Dry leaf material (0·5 g) was microwave digested in 8 mL of concentrated HNO3 for 1 h. 57Fe was determined by quadrupole inductively coupled plasma-mass spectrometry (ICP-MS; Agilent 7500ce, Agilent Technologies, Manchester, UK). The instrument was equipped with a PFA microflow nebulizer and was used in hydrogen mode to eliminate spectral interference(s). Instrument settings were as described previously (Laursen et al., 2009); however, prior to analysis, the hydrogen flow of the octopole ion guide was optimized to give maximum interference-free 56Fe and 57Fe signals, lowest possible backgrounds and accurate 56Fe/57Fe values according to the natural abundance ratio of 43·3 (IUPAC values). This was evaluated by hydrogen ramping on non-enriched samples and standards, which yielded accuracies of 100 ± 5 % of the true 56Fe/57Fe ratio at the optimal hydrogen flow rate (7 mL min–1). All samples were diluted to 3·5 % (v/v) HNO3 prior to analysis, and external calibration was conducted to obtain total 56Fe and 57Fe concentrations using a commercially available standard solution (P/N 4400-132565, CPI International, Amsterdam, The Netherlands). The analytical accuracy of total concentrations was evaluated using certified reference material (Spinach NCS ZC73013, China National Analysis Center for Iron and Steel, Beijing, China). Concentration data were accepted if the accuracy exceeded 90 % of the certified reference value. The accuracy of 56Fe/57Fe isotope ratios was evaluated by analysis of four non-enriched samples, resulting in an average isotope ratio of 44·3 ± 2·3. Data were processed by the Masshunter Workstation Software, version B.02.01 (Agilent Technologies, Manchester, UK).
NA extraction and analysis
Deep-frozen leaf samples were homogenized using a mortar and pestle in liquid N2 and extracted in deionized water at 80 ºC for 30 min. After centrifugation at 5000 g for 30 min at 4 °C, the supernatant was collected and subjected to a liquid chromatography–mass spectrometric (LC-MS) analysis of NA, according to a modification of the method of Yamaguchi and Uchida (2012).
The samples were separated using a Syncronis C18 column (100 × 2·1 mm, 1·7 μm particle size) (Thermo Fisher Scientific Inc., Bremen, Germany). The mobile phase consisted of (A) water + 0·01 % acetic acid and (B) acetonitrile. A linear gradient program at a flow rate of 0·25 mL min–1 was used: 0·0–1·0 min 5 % B, 1·0–5·0 min from 5 to 95 % (B), 5·0–7·0 min 95 % B, 7·0–7·1 min from 95 to 5 % (B), then 5 % (B) for 8 min. The injection volume was 5 μL. The Thermo Scientific Orbitrap LC-MS system consisted of a quaternary pump (Accela 600), an autosampler and a linear hybrid ion trap-orbitrap MS (LTQ Orbitrap XL) with heated electrospray ionization (HESI). A standard 100 mg L–1 stock solution of NA (Toronto Research Chemicals, North York, Canada) was prepared by dissolution in ultrapure water (0·055 μS cm–1) and was further diluted at concentrations of 0·050, 0·075, 0·100, 0·250, 0·500, 0·750 and 1·000 mg L–1 for the calibration curve.
The mass spectrometer was operated in positive mode; HESI-source parameters were as follows: source voltage 5 kV, capillary voltage 30 V, tube lens voltage 90 V, capillary temperature 300 °C, sheath and auxiliary N2 flow 232 and 8 (arbitrary units). The mass spectra were acquired by full range acquisition covering 230–1000 m/z. The normalized collision energy of the collision-induced dissociation cell was set at 35 eV.
Thermo Xcalibur software (version 2.1) was used for instrument control, data acquisition and data analysis. Nicotianamine was quantified according to the corresponding spectral characteristics: mass spectra, accurate mass, characteristic fragmentation and characteristic retention time. Quantification was carried out according to the exact mass search method by comparing the retention times and exact mass of the available standard.
RNA extraction and real-time quantitative PCR
Leaf tissue samples (0·5–1 g f. wt) were frozen in liquid nitrogen and ground thoroughly using a mortar and pestle. RNA was isolated using the RNeasy® Mini Kit (Qiagen) as described in the RNeasy® Mini Handbook.
Prior to cDNA synthesis, DNA was removed from RNA samples using Ambion DNA-free DNase Treatment and Removal Reagents. First-strand cDNA was synthesized from 5 μg of RNA with M-MuLV reverse transcriptase (Fermentas, Vilnius, Lithuania) and random hexamer primers (Applied Biosystem, Foster City, CA, USA) according to the manufacturer’s instructions. The cDNAs were diluted 1:5 with nuclease-free water and aliquots were used for real-time PCR with primers designed for cucumber. Real-time PCRs were performed in a 25 μL volume containing 500 nm of each primer and 1× SYBER Green PCR master mix (Applied Biosystems). Real-time PCR was performed on the ABI Prism 7500 Sequence Detection System (Applied Biosystem) using parameters recommended by the manufacturer (i.e. 2 min at 50 °C, 10 min at 95 °C and 40 cycles of 95 °C for 15 s and 60 °C for 1 min). Accumulation of PCR products was detected in real time and the results were analysed with 7500 System Software (Applied Biosystems).
The primers used in this study are: for NAS1, 5'-GGAGTTCGAGGTGGTGTTTC-3' and 5'-CCACCACCGGATAAACAAAC-3'; for NAS4, 5'-TCCCAAAAACCGAGTTTCAC-3' and 5'-GAAACACCACCTCGAACTCC-3'; for YSL1, 5'-TGCTTTTGCTTTCTTGACCTC-3' and 5'-TAGGAGCCATCTTATCTTATGGC-3'; for YSL3, 5'-tccatgcttttaagccaagc-3' and 5'-cgggaccttgtactcaccat-3'; and for ACTIN (ACT), 5'-GCTGGCATATGTTGCTCTTG-3' and 5'-CGATGGTGATGACTTGTCCA-3'. Levels of transcription were calculated with the 2−ΔCt method using ACT as an internal control. Each PCR was done in triplicate and included no template controls. To determine the amplification efficiency of real-time PCRs, cDNAs were diluted 5-, 10-, 20- and 40-fold. The calculated PCR efficiency [E(%) = (10 − 1/slope − 1) × 100] was between 90 and 100 % (–3·6 > slope > –3·1).
Sequence data from this article can be found in the GenBank/EMBL data libraries and EST database (http://www.icugi.org/) under the following accession numbers (in parentheses): CsNAS1 (XM004158701), CsNAS4 (XP004144812), CsYSL1 (XM004163525), CsYSL3 (XP004150025) and CsACT (AB010922).
Statistical analysis
Each independent experiment with four replications (individual plants) per treatment was repeated 2–3 times. Data from a representative experiment were subjected to analysis of variance using the statistical software Statistica 6 (StatSoft, Inc., Tulsa, OK, USA), and means were compared by Tukey’s test at the 5 % significance level (P ≤ 0·05).
RESULTS
Effect of experimental conditions on plant growth and Fe movement during experimentation
To study the effect of Si nutrition on Fe mobility in leaves, we carried out an experiment with Fe-deprived cucumber plants which were supplied with Fe (57Fe-enriched or naturally occurring isotopes with 56Fe prevailing) for a short period (3 d) and then transferred to Fe-free nutrient solution with or without Si supply for 11 d (see Fig. S1). The Si concentration in the leaves of Si-treated plants was 6-fold higher than that of those grown in the absence of Si (Table 1). Addition of Si did not affect root or stem dry biomass, but strongly stimulated leaf growth, resulting in > 30 % higher total plant dry biomass in Si-fed plants (Table 2). Buffering the pH of the nutrient solution above 7·0 markedly decreased mobilization of Fe from the root apoplast (Table 3); hence, the total Fe content in the root apoplast remained at the same level in both Si-treated and untreated plants at the end of the experiment (Table 4). Furthermore, the concentration of Fe in the xylem sap was very low during the first 2 d when Fe was excluded from the nutrient solution, while afterwards throughout the experiment no Fe was detected (Supplementary Data Table S1). Addition of CaCO3 did not per se show any negative effect on plant growth, which was inhibited exclusively by Fe deficiency and amended by supply of Si (Table S2).
Table 1.
Silicon concentrations in different cucumber tissues
| Treatment | Si concentration (mg g–1 d. wt) |
||
|---|---|---|---|
| Root | Stem | Leaves | |
| –Si | 4·0 ± 0·5a | 7·5 ± 1·5a | 3·4 ± 0·2a |
| +Si | 5·9 ± 0·8b | 12·0 ± 1·4b | 21·5 ± 1·8b |
Seven-day-old Fe-deprived plants were supplied with 10 μm Fe for 3 d and then transferred to Fe-free nutrient solution with or without supply of 1·5 mm Si(OH)4 for 11 d.
Data shown are means ± s.d. (n = 4).
Significant differences (P < 0·05) between treatments are indicated by different letters.
Table 2.
Effect of Si nutrition on dry biomass of cucumber
| Treatment | Dry biomass (g per plant) |
|||
|---|---|---|---|---|
| Root | Stem | Leaves | Total | |
| –Si | 0·52 ± 0·04a | 0·9 ± 0·1a | 2·2 ± 0·2a | 3·6 ± 0·1a |
| +Si | 0·58 ± 0·07a | 1·0 ± 0·2a | 3·2 ± 0·1b | 4·8 ± 0·1b |
Seven-day-old Fe-deprived plants were supplied with 10 μm Fe for 3 d and then transferred to Fe-free nutrient solution with or without supply of 1·5 mm Si(OH)4 for 11 d.
Data shown are means ± s.d. (n = 4).
Significant differences (P < 0·05) between treatments are indicated by different letters.
Table 3.
Effect of the nutrient solution pH (–/+CaCO3) on the amount of Fe removed from the root apoplast of cucumber throughout the entire experiment
| Si treatment | Fe amount (μg per plant) |
Relative decrease (%) | |
|---|---|---|---|
| –CaCO3 (pH 5·0) | +CaCO3 (pH 7·0) | ||
| –Si | 66·2 ± 6·7b | 11·7 ± 2·1a | 92 |
| +Si | 87·8 ± 6·7c | 17·0 ± 2·3a | 81 |
Seven-day-old Fe-deprived plants were supplied with 10 μm Fe for 3 d and then transferred to Fe-free nutrient solution, unbuffered (pH < 5·0) or buffered with CaCO3, (0·2 g L–1; pH > 7·0), with or without supply of 1·5 mm Si(OH)4 for 11 d. Data shown are means ± s.d. (n = 4).
Significant differences (P < 0·05) between treatments are indicated by different letters.
Table 4.
Effect of Si supply on the Fe content in different cucumber tissues
| Treatment | Fe content (μg per plant) |
|||
|---|---|---|---|---|
| Root |
Stem | Leaves | ||
| Apoplast | Symplast | |||
| –Si | 103 ± 11a | 14 ± 2a | 28 ± 3a | 141 ± 16a |
| +Si | 92 ± 16a | 17 ± 3a | 31 ± 5a | 150 ± 13a |
Seven-day-old Fe-deprived plants were supplied with 10 μm Fe for 3 d and then transferred to Fe-free nutrient solution with or without supply of 1·5 mm Si(OH)4 for 11 d.
Data shown are means ± s.d. (n = 4).
Significant differences (P < 0·05) between treatments are indicated by different letters.
Si increases Fe remobilization from old to young leaves
Although there were no significant differences between Si treatments in total Fe content measured in the root symplast, stem and leaves (Table 4), Si supply successfully prevented symptoms of Fe chlorosis in young leaves, following 11 d of Fe deprivation (Fig. 1A). In contrast, plants grown in the absence of Si developed chlorotic leaves following withdrawal of Fe from the nutrient solution. The SPAD readings in younger leaves at positions L3 and L4 (for leaf positions, see Fig. 2A and Fig. S1) of Si-fed plants were significantly higher than in the older leaves at positions L1 and L2 (Fig. 2A). In these leaves, chlorophyll content was markedly lower as compared with the unfed Si plants.
Fig. 1.
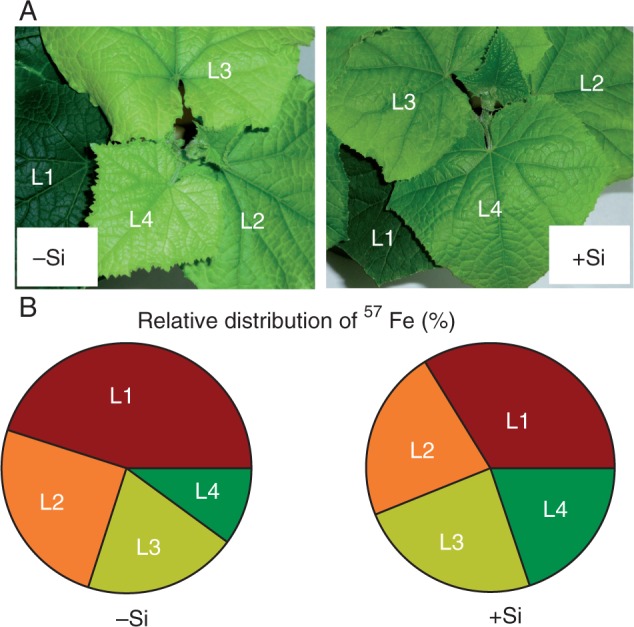
Effect of Si supply on redistribution of 57Fe in the leaves of cucumber plants 11 d after withdrawal of 57Fe from the nutrient solution. (A) Cucumber plants at the end of the experiment; (B) relative distribution of 57Fe. Leaf positions (from the base to the youngest leaf): L1, L2, L3 and L4. Plants were pre-cultured in Fe-free nutrient solution for 7 d, subsequently subjected to 10 μm 57FeIIIEDTA (96·64 % enriched) for 3 d and then transferred to an Fe-free nutrient solution with or without supply of 1·5 mm Si(OH)4 for 11 d. Data shown are means ± s.d. (n = 4). Significant differences (P < 0·05) between treatments are indicated by different letters.
Fig. 2.
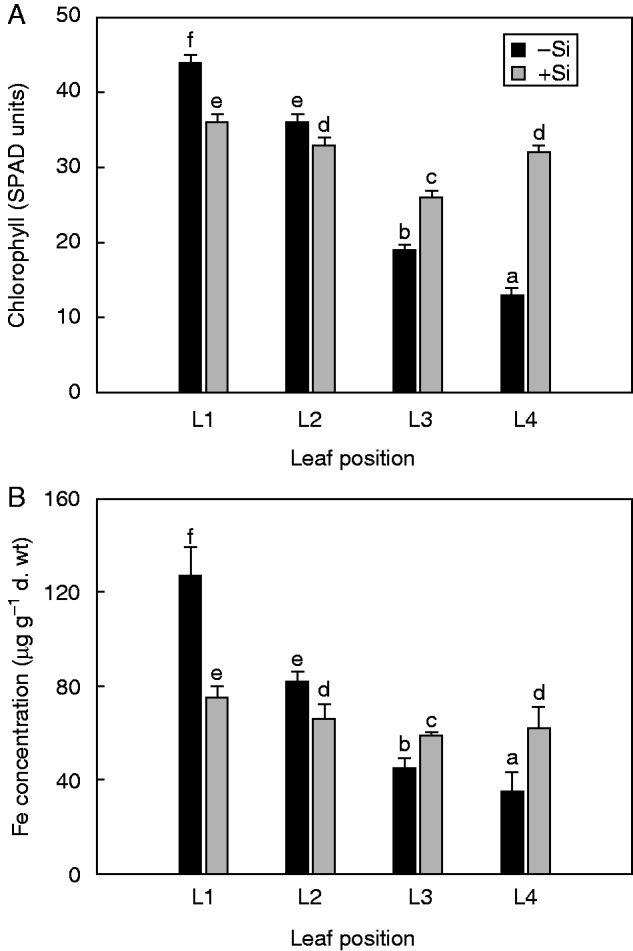
Effect of Si supply on spectral plant analysis diagnostic (SPAD) readings (A) and Fe concentration (B) in cucumber leaves at different positions. Leaf positions (from the base to the youngest leaf): L1, L2, L3 and L4. Iron-deprived 7-day-old plants were treated with 10 μm Fe for 3 d and then transferred to an Fe-free nutrient solution with or without supply of 1·5 mm Si(OH)4 for 11 d. Data shown are means ± s.d. (n = 4). Significant differences (P < 0·05) between treatments are indicated by different letters.
The Fe concentration in the youngest leaves (L4) of the Si-treated plants was about 2-fold higher than that in the untreated plants, whereas it was significantly lower in the oldest leaves (L1) (Fig. 2B). Silicon nutrition thus increased remobilization of Fe from old leaves to the youngest expanding leaves; this was further confirmed in the 57Fe experiment. Although total shoot Fe content remained the same in both –Si and +Si plants (Table 4), Si supply affected the relative leaf 57Fe distribution (Fig. 1B). For unfed Si plants, approx. 50 % of the total 57Fe content remained in the oldest leaf (L1), whereas those receiving Si showed an almost even 57Fe distribution between leaves at different positions, resulting in alleviation of Fe deficiency symptoms (Fig. 1A).
Si increases leaf NA concentration and affects the expression of NAS genes differently
The concentration of NA increased gradually from the older to younger leaves (Fig. 3). Silicon nutrition significantly increased the NA concentration in L1, L2 and especially in L3 leaves, while affecting NA concentrations less in the youngest (L4) leaves (Fig. 3). In the unfed Si plants, the expression levels of NAS1 showed a distinct profile depending on leaf position, with the lowest values observed in the oldest leaf (L1) (Fig. 4A). Supply of Si upregulated NAS1 expression in both the older (L1, L2) and younger (L3) expanded leaves. However, there was no significant difference between –Si and +Si treatments in NAS1 expression levels of the youngest (L4) expanding leaves (Fig. 4A). The expression of NAS4 was not influenced either by leaf developmental stages (unlike the clear leaf age-dependent expression pattern for NAS1; see Fig. 4A) or by Si supply (Fig. 4B).
Fig. 3.
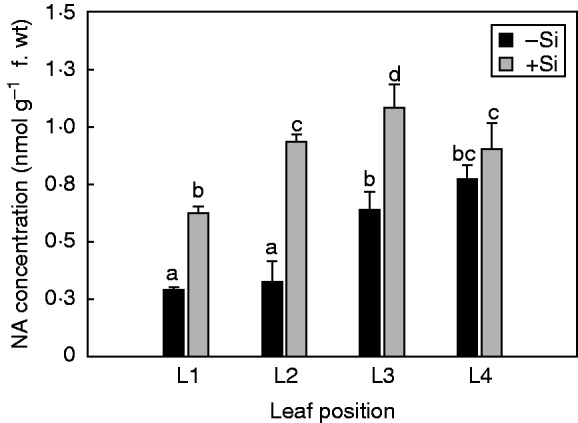
Effect of Si supply on the total nicotianamine (NA) concentration in cucumber leaves at different positions. Leaf positions (from the base to the youngest leaf): L1, L2, L3 and L4. The plants were treated as described in the legend of Fig. 2. Data shown are means ± s.d. (n = 4). Significant differences (P < 0·05) between treatments are indicated by different letters.
Fig. 4.
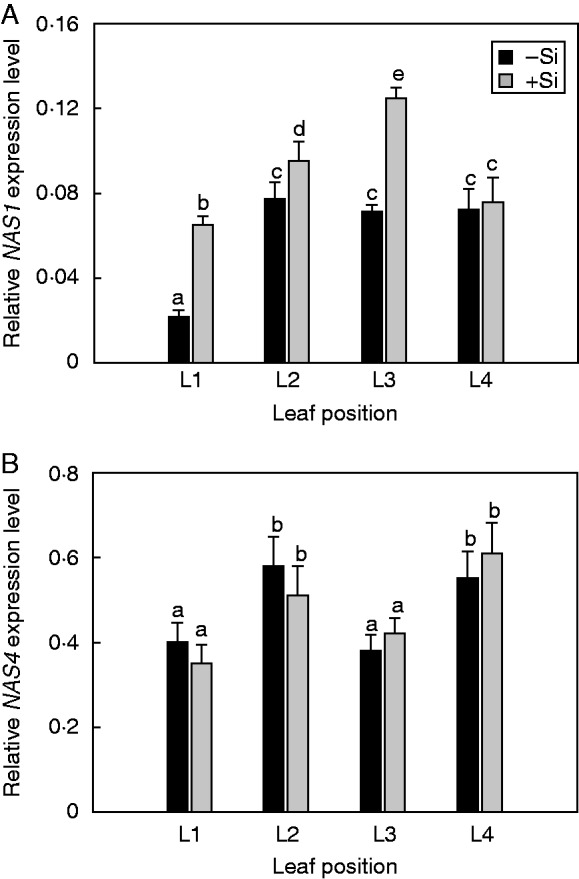
Effect of Si supplyon the relative expression level of NAS1 (A) and NAS 4 (B) in cucumber leaves at different positions. Leaf positions (from the base to the youngest leaf): L1, L2, L3 and L4. The plants were treated as described in the legend of Fig. 2. Data shown are means ± s.d. (n = 4). Significant differences (P < 0·05) between treatments are indicated by different letters.
Si upregulates YSL1, but not YSL3 leaf transporter for the Fe–NA complex
The expression of YSL1 was strongly upregulated in the leaves of Si-supplied plants, with the highest expression levels observed in the oldest (L1) leaf (Fig. 5A). The transcript abundance of YSL3 was highest in the oldest leaves and gradually decreased towards the shoot apex in both –Si and +Si plants. Silicon supplementation resulted in distinctly higher expression levels of YSL3 in younger fully expanded leaves (L2 and L3), while in the oldest expanded leaf (L1) it was lower, and in the youngest expanding (L4) leaf was at the level recorded in the –Si plants (Fig. 5B).
Fig. 5.
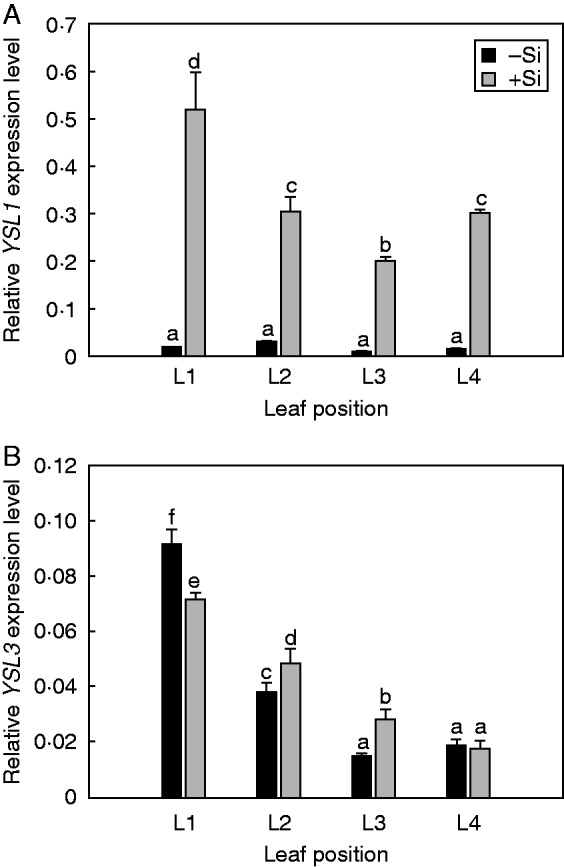
Effect of Si supply on the relative expression of YSL1 (A) and YSL3 (B) genes coding for Fe–NA transporters. Leaf positions (from the base to the youngest leaf): L1, L2, L3 and L4. The plants were treated as described in the legend of Fig. 2. Data shown are means ± s.d. (n = 4). Significant differences (P < 0·05) between treatments are indicated by different letters.
DISCUSSION
In the present work we tested and confirmed the hypothesis that Si supply enhances remobilization of Fe from older to younger cucumber leaves. We recently demonstrated that Si mitigates Fe deficiency in cucumber plants by increasing the apoplastic Fe pool in the roots and by accumulation of Fe-mobilizing compounds in roots (citrate and catechine) and leaves (citrate), thus enhancing Fe acquisition and translocation towards apical shoot parts (Pavlovic et al., 2013; Bityutskii et al., 2014). A major limitation in many retranslocation studies conducted so far is that the contribution of the apoplastic Fe pool in roots has not been adequately considered, as pointed out by Shi et al. (2012). As apoplastic Fe pools in cucumber roots are usually large (Cesco et al., 2002), only a small change of this pool during the long-term experiment may significantly contribute to Fe translocation to sink leaves. In the present study, mobilization of Fe from the root apoplastic pool and subsequent root to shoot translocation of Fe was controlled by maintaining a high pH in the Fe-free nutrient solution (Table 3; Table S1).
The experiment with enriched 57Fe clearly demonstrated that Si nutrition can induce mobilization of 57Fe from older leaves and can also enhance its retranslocation into the younger leaves developed after withdrawal of 57Fe from the nutrient solution (Fig. 1B). As a consequence of undeveloped xylem structures and a low transpiration rate, young expanding leaves are almost exclusively dependent on Fe import from the phloem. It is well documented that Fe can be mobilized from source organs and retranslocated to sink tissues, including young leaves via the phloem (Hell and Stephan, 2003; Römheld and Schaaf, 2004). For instance, Zhang et al. (1995) reported that under Fe deficiency up to 20 % of the total amount of both apoplastic and symplastic Fe can be mobilized from primary leaves of beans (Phaseolus vulgaris) and retranslocated towards young leaves and the shoot apex. Retranslocation of Fe can be even higher under conditions of induced senescence (Shi et al., 2012).
Shoot remobilization of Fe depends on the presence of NA, since the lack of Fe redistribution between older and younger leaves in the NA-free tomato mutant chloronerva can be overcome by application of NA (Scholz, 1989; Stephan and Scholz, 1993). In arabidopsis, using the mild and severe nas4x-1 and nas4x-2 mutants, Schuler et al. (2012) demonstrated that NA is involved in both long-distance transport of Fe to young leaves via the phloem and lateral transport of Fe from the vasculature to the mesophyll. Two members of the NAS protein family have so far been identified in cucumber (i.e. CsNAS1 and CsNAS4). However, no data exist in the literature on their expression pattern and specific functions in different tissues and organs of cucumber. A phylogenic tree of NAS amino acid sequences of cucumber and arabidopsis (Supplementary Data Fig. S2) suggests that NAS genes are most probably taxon specific, therefore leaving it as an open question whether or not their physiological function in cucumber is similar to that in arabidopsis. In the present work, we investigated the NA accumulation along with the expression of NAS1 and NAS4 genes in order to elucidate the mechanisms of Si-mediated distribution of Fe in cucumber leaves under limiting Fe conditions. Addition of Si upregulated NAS1 transcription and subsequently increased the NA concentration in fully expanded cucumber leaves (Fig. 4A), which resulted in enhanced remobilization of Fe from older leaves (L1, L2) and increased Fe concentration in younger leaves (L3, L4) without symptoms of Fe deficiency chlorosis (Fig. 2B; also see Fig. 1A). In contrast to NAS1, the NAS4 expression profile was unaffected by Si supply (Fig. 4B). The unaffected transcripts of NAS4 (upregulated by Fe deficiency; Klatte et al., 2009) appear to be attributable to the similar Fe status of the –Si and +Si plants (183 and 198 μg Fe per plant, respectively; calculated from Table 4). Therefore, Si upregulation of Fe-independent NAS1 transcripts seems to be crucial for increased leaf NA concentration in Si-fed plants, although the exact mechanisms for this remain for future investigation.
The expression pattern of arabidopsis AtYSL1 and AtYSL3 that mediate Fe–NA transport depends on the Fe status and leaf age, being more intensively expressed in the presence of Fe and in older leaves (Le Jean et al., 2005; Waters et al., 2006; Curie et al., 2009). The arabidopsis ysl1ysl3 double mutant displays strong interveinal chlorosis, and has a reduced leaf Fe concentration (Waters et al., 2006), suggesting that YSL1 and YSL3 act as key mediators in foliar loading/unloading of Fe. From the phylogenic tree of YSL sequences in cucumber and arabidopsis, cucumber CsYSL1 was most closely related to arabidopsis AtYSL3 as well as CsYSL3 to AtYSL3, indicating the similarity of their physiological functions (Supplementary Data Fig. S3). Our experiment in cucumber showed that Si supply did not influence expression of YSL1 and YSL3 genes in the same manner (Fig. 5). Waters et al. (2006) proposed that during vegetative growth, decreased YSL expression during Fe deficiency restricts delivery of Fe to source tissues, making it more available for younger actively growing tissues. Addition of Si increases YSL1 expression, especially in the oldest leaves, indicating that this transporter plays an important role in Fe translocation to the younger leaves (Fig. 5A). The expression pattern of YSL3 depended on the leaf developmental stage (decreasing from older to younger leaves) but was slightly upregulated by Si in younger expanded leaves (L2 and L3; see Fig. 5B), thus implying that increased expression levels of YSL3 transcripts is a consequence of increased Fe content in the leaves of Si-treated plants rather than a direct influence of Si. Interestingly, YSL1 transcripts were almost absent in all examined leaves of non Si-treated plants, and addition of Si increased the relative YSL1 expression dramatically (Fig. 5A). This suggests that the upregulation of YSL1 is not caused by Si-induced change in plant Fe status (Table 4), but by Si accumulation in the leaf tissues (Table 1). However, the question of how Si might affect transcriptional activation of YSL1 genes is still unresolved.
Whether and how Si acts in priming plants in response to biotic and perhaps abiotic stresses still remains unclear. A microarray study of Fauteux et al. (2006) in arabidopsis showed that Si supply did not impact expression of defence-related genes unless plants were powdery mildew stressed. These results suggest that Si treatment had no effect on the metabolism of unstressed plants but that it had beneficial properties attributable to modulation of a more efficient response to pathogen stress. However, until similar studies with high Si-accumulating plants can be carried out, the findings of previous work on arabidopsis have to be interpreted with caution because of a lack of the Lsi1 transporter in this species (Ma and Yamaji, 2015) and thus its inability to take up a large amount of Si. Also, Si showed an alleviating effect on plants stressed by abiotic factors such as drought and toxic metals, but had no effect on unstressed plants (e.g. Hattori et al., 2003; Liang et al., 2007). Relatively little is known concerning the possibility of an active role for Si in plant metabolism under abiotic stress conditions, particularly on the molecular aspects of Si-mediated nutrient efficiency (Liang et al., 2015). Our previous study showed the ability of Si to modulate root activity of Fe acquisition in cucumber at an early stage of Fe deficiency stress through regulation of gene expression levels of the proteins involved in this process (Pavlovic et al., 2013), which confirmed that Si played a limited role in the transcriptome of this plant species in the absence of stress. In the present study, Si nutrition modulated the expression of NAS1 involved in the biosynthesis of NA, resulting in more efficient remobilization of Fe from source to sink leaf tissues via Si-upregulated YSL1 transporter for the Fe–NA complex. Taken together, the benefits of Si are aligned with a response to transcriptomic changes induced by Fe deficiency at both root and leaf levels.
In conclusion, our results show for the first time that Si induces Fe mobilization in older leaves and increases its retranslocation to younger expanding leaves. Supply of Si enhances expression of the NAS1 gene responsible for NA biosynthesis and hence increased NA accumulation, which in turn enhances chelation of Fe and phloem loading of Fe–NA in the source (expanded) leaves to facilitate phloem transport of Fe and enhanced phloem unloading of Fe in the sink (expanding) leaves. Finally, summarizing the results presented in this and our previous studies (Pavlovic et al., 2013; Bityutskii et al., 2014), we propose a model of how Si acts to improve Fe homeostasis under Fe deficiency in cucumber (see Fig. 6).
Fig. 6.
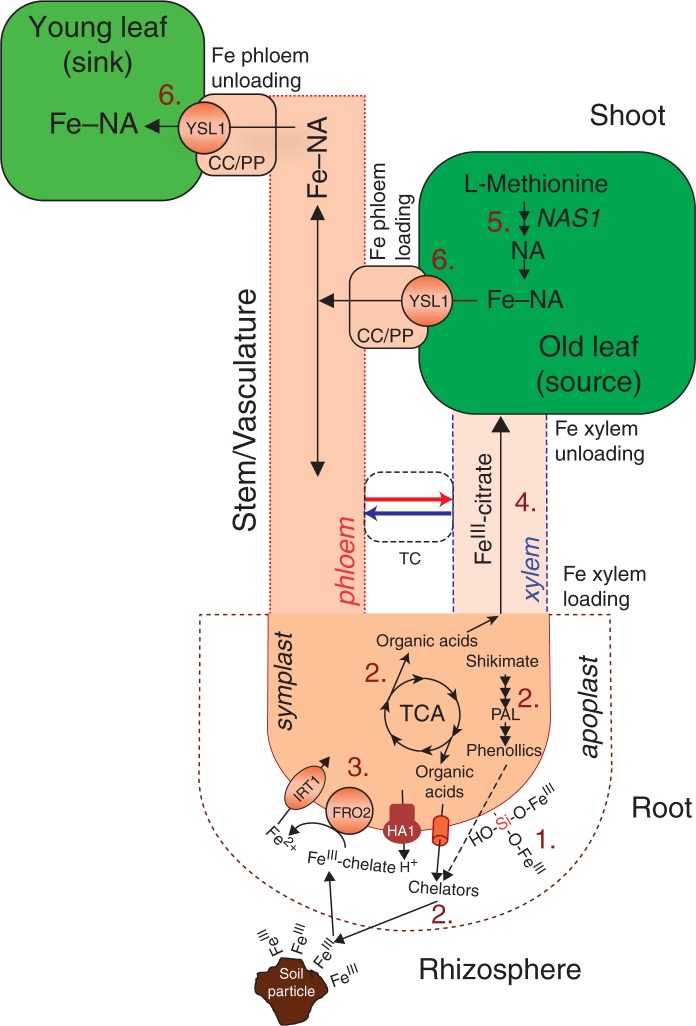
The proposed model of Si-mediated alleviation of Fe deficiency in cucumber. In roots, Si increases Fe pools in the root apoplast (1) and enhances the accumulation of Fe-chelating compounds (organic acids and phenolics) in roots by stimulating expression of genes related to their biosynthesis (TCA cycle; shikimate and phenylpropanoid pathway; see Pavlovic et al., 2013) for improved mobilization of Fe from the rhizosphere and reutilization of root apoplastic Fe (2), followed by the upregulated expression levels of the proteins involved in Strategy 1 reduction-based Fe uptake, i.e. HA1, FRO2 (in cucumber also known as CsFRO1; Waters et al., 2007) and IRT1 (3). Silicon also increases root to shoot movement of Fe complexed by citrate (and malate) via xylem (4). In leaves, Si enhances expression of NAS1 genes responsible for NA biosynthesis (see Fig. 4A) in sink leaves (5) and expression of YSL1 genes responsible for Fe phloem loading and phloem transport of NA-chelated Fe from sink to source leaves (see Fig. 5), as well as for unloading of the Fe–NA complex arriving via the phloem in young leaves (6). CC/SE, companion cell/sieve element; TC, transfer cell.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: concentration of Fe in the xylem sap of cucumber plants after withdrawal of Fe from the nutrient solution. Table S2: dry biomass of cucumber plants grown in the nutrient solution supplied with CaCO3. Figure S1: schematic presentation of the experimental set-up and movement of Fe within cucumber plants. Figure S2: the NJ phylogenetic tree of the NAS family in cucumber and arabidopsis. Figure S3: the NJ phylogenetic tree of cucumber and arabidopsis YSL proteins.
ACKNOWLEDGEMENTS
Our paper is dedicated to the memory of Professor Volker Römheld. We thank Dr Jelena Aleksic (Institute of Molecular Genetics and Genetic Engineering, University of Belgrade, Serbia) for phylogenetic analysis, and Dr Ernest A. Kirkby (Faculty of Biological Sciences, University of Leeds, UK) for a final revision of the English. This work was supported by the Serbian Ministry of Education, Science and Technological Development [OI173028] and in part by Innovation Fund Denmark [B21st: Biomass for the 21st Century].
LITERATURE CITED
- Alloway BJ. 2008. Micronutrients and crop production: an introduction In: Alloway BJ, ed. Micronutrient deficiency in global crop production. Dordrecht: Springer, 1–39. [Google Scholar]
- Bienfait HF, van den Briel W, Mesland-Mul NT. 1985. Free space iron pools in roots. Generation and mobilization. Plant Physiology 78: 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bityutskii N, Pavlovic J, Yakkonen K, Maksimovic V, Nikolic M. 2014. Contrasting effect of silicon on iron, zinc and manganese status and accumulation of metal-mobilizing compounds in micronutrient-deficient cucumber. Plant Physiology and Biochemistry 74: 205–211. [DOI] [PubMed] [Google Scholar]
- Cesco S, Nikolic M, Römheld V, Varanini Z, Pinton R. 2002. Uptake of 59Fe from soluble 59Fe–humate complexes by cucumber and barley plants. Plant and Soil 241: 121–128. [Google Scholar]
- Chu HH, Chiecko J, Punshon T, et al. 2010. Successful reproduction requires the function of Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 metal–nicotianamine transporters in both vegetative and reproductive structures. Plant Physiology 154: 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Cassin G, Couch D, et al. 2009. Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Annals of Botany 103: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauteux F, Chain F, Belzile F, Menzies JG, Bélanger RR. 2006. The protective role of silicon in the Arabidopsis-powdery mildew pathosystem. In: Proceedings of the National Academy of Sciences, USA 103: 17554–17559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo MJ, Lucena JJ, Hernández-Apaolaza L. 2013. Effect of silicon addition on soybean (Glycine max) and cucumber (Cucumis sativus) plants grown under iron deficiency. Plant Physiology and Biochemistry 70: 455–461. [DOI] [PubMed] [Google Scholar]
- Grusak MA, Della Penna D. 1999. Improving the nutrient concentration of plants to enchance human nutrition and health. Annual Review of Plant Physiology and Plant Molecular Biology 50: 133–161. [DOI] [PubMed] [Google Scholar]
- Guerinot ML, Yi Y. 1994. Iron: nutritious, noxious, and not readily available. Plant Physiology 104: 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Inanaga S, Tanimoto E, Lux A, Luxova M, Sugimoto Y. 2003. Silicon-induced changes in viscoelastic properties of sorghum root cell walls. Plant and Cell Physiology 44: 743–749. [DOI] [PubMed] [Google Scholar]
- Hell R, Stephan UW. 2003. Iron uptake, trafficking and homeostasis. Planta 216: 541–551. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Masuda H, Bashir K, et al. 2010. Rice metal–nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. The Plant Journal 62: 379–390. [DOI] [PubMed] [Google Scholar]
- Jeong J, Guerinot ML. 2009. Homing in on iron homeostasis. Trends in Plant Science 14: 280–285. [DOI] [PubMed] [Google Scholar]
- Klatte M, Schuler M, Wirtz M, Fink-Straube C, Hell R, Bauer P. 2009. The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiology 150: 257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen KH, Hansen TH, Persson DP, Schjoerring JK, Husted S. 2009. Multi-elemental fingerprinting of plant tissue by semi-quantitative ICP-MS and chemometrics. Journal of Analytical Atomic Spectrometry 24: 1198–1207. [Google Scholar]
- Le Jean M, Schikora A, Mari S, Briat JF, Curie C. 2005. A loss-of-function mutation in AtYSL1 reveals its role in iron and nicotianamine seed loading. The Plant Journal 44: 769–782. [DOI] [PubMed] [Google Scholar]
- Liang Y, Sun W, Zhu Y-G, Christie P. 2007. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environmental Pollution 147: 422–428. [DOI] [PubMed] [Google Scholar]
- Liang Y, Nikolic M, Bélanger R, Gong H, Song A. 2015. Silicon in agriculture. From theory to practice. Dordrecht: Springer. [Google Scholar]
- Lindsay WL. 1995. Chemical reactions in soils that affect iron availability to plants. A quantitative approach In: Abadía J, ed. Iron nutrition in soils and plants. Dordrecht: Kluwer Academic Publishers, 7–14. [Google Scholar]
- Ma JF, Yamaji N. 2015. A cooperative system of silicon transport in plants. Trends in Plant Science 20: 399–462. [DOI] [PubMed] [Google Scholar]
- Mori S. 1999. Iron acquisition by plants. Current Opinion in Plant Biology 2: 250–253. [DOI] [PubMed] [Google Scholar]
- Pavlovic J, Samardzic J, Maksimovic V, et al. 2013. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytologist 198: 1096–1107. [DOI] [PubMed] [Google Scholar]
- Pich A, Manteuffel R, Hillmer S, Scholz G, Schmidt W. 2001. Fe homeostasis in plant cells: does nicotianamine play multiple roles in the regulation of cytoplasmic Fe concentration? Planta 213: 967–976. [DOI] [PubMed] [Google Scholar]
- Römheld V, Marschner H. 1986. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiology 80: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V, Nikolic M. 2006. Iron In: Barker AV, Pilbeam DJ, eds. Handbook of plant nutrition. Boca Raton, FL: CRC Press, 329–350. [Google Scholar]
- Römheld V, Schaaf G. 2004. Iron transport in plants: future research in view of a plant nutritionist and a molecular biologist. Soil Science and Plant Nutrition 50: 1003–1012. [Google Scholar]
- Scholz G. 1989. Effect of nicotianamine on iron-remobilisation in de-rooted tomato seedlings. Biology of Metals 2: 89–91. [Google Scholar]
- Scholz G, Becker R, Pich A, Stephan UW. 1992. Nicotianamine: a common constituent of strategies I and II of iron acquisition by plants: a review. Journal of Plant Nutrition 15: 1647–1665. [Google Scholar]
- Schuler M, Rellán-Álvarez R, Fink-Straubec C, Abadía J, Bauer P. 2012. Transport of iron to sink organs, in pollen development and pollen tube growth in Arabidopsis. The Plant Cell 24: 2380–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Bäßler R, Zou C, Römheld V. 2011. Is iron phloem mobile during senescence in trees? A reinvestigation of Rissmüller’s finding of 1874. Plant Physiology and Biochemistry 49: 489–493. [DOI] [PubMed] [Google Scholar]
- Shi R, Weber G, Köster J, et al. 2012. Senescence-induced iron mobilization in source leaves of barley (Hordeum vulgare) plants. New Phytologist 195: 372–383. [DOI] [PubMed] [Google Scholar]
- Stephan UW, Scholz G. 1993. Nicotianamine: mediator of transport of iron and heavy metals in the phloem? Physiologia Plantarum 88: 522–529. [Google Scholar]
- Thomine S, Vert G. 2013. Iron transport in plants: better be safe than sorry. Current Opinion in Plant Biology 6: 1–6. [DOI] [PubMed] [Google Scholar]
- Vose PB. 1982. Iron nutrition in plants: a world overview. Journal of Plant Nutrition 5: 233–249. [Google Scholar]
- Walker E, Connolly EL. 2008. Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Current Opinion in Plant Biology 11: 530–535. [DOI] [PubMed] [Google Scholar]
- Waters BM, Sankaran RP. 2011. Moving micronutrients from the soil to the seeds: genes and physiological processes from a biofortification perspective. Plant Science 180: 562–574. [DOI] [PubMed] [Google Scholar]
- Waters BM, Chu HH, Didonato RJ, et al. 2006. Mutations in Arabidopsis yellow stripe-like1 and yellow stripe-like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiology 141: 1446–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters BM, Lucena C, Romera FJ, et al. 2007. Ethylene involvement in the regulation of the H+-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants. Plant Physiology and Biochemistry 45: 293–301. [DOI] [PubMed] [Google Scholar]
- Welch RM. 2002. The impact of mineral nutrients in food crops on global human health. Plant and Soil 247: 83–90. [Google Scholar]
- White PJ, Broadley MR. 2009. Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist 182: 49–84. [DOI] [PubMed] [Google Scholar]
- von Wirén N, Klair S, Bansal S, et al. 1999. Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants . Plant Physiology 119: 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Uchida R. 2012. Determination of nicotianamine in soy sauce and other plant-based foods by LC-MS/MS. Journal of Agricultural and Food Chemistry 60: 10000–10006. [DOI] [PubMed] [Google Scholar]
- Zhai Z, Gayomba SR, Jung H, et al. 2014. OPT3 is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis. The Plant Cell 26: 2249–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Römheld V, Marschner H. 1995. Retranslocation of iron from primary leaves of bean plants grown under iron deficiency. Journal of Plant Physiology 146: 268–272. [Google Scholar]
- Zhang C, Römheld V, Marschner H. 1996. Effect of primary leaves on 59Fe uptake by roots and 59Fe distribution in the shoot of iron sufficient and iron deficient bean (Phaseolus vulgaris L.) plants. Plant and Soil 182: 75–81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.