Abstract
Background and Aims Evidence suggests drought severity is increasing due to climate change, but strategies promoting severe drought survival in perennial grasses have been seldom explored. This is particularly true of summer dormancy, an adaptation common in summer-dry Mediterranean-type climates. In addition, though theory predicts superior drought survival results in lower potential productivity, studies rarely measure both drought survival and growth under optimal conditions.
Methods Physiological and functional ecological approaches were integrated to quantify interspecific variation in foliar and root traits in a suite of eight California perennial grass species. In a glasshouse experiment, summer dormancy, foliar functional trait variation, and seasonal growth and phenology under non-limiting water conditions and dehydration tolerance under progressive drought were quantified. In a second glasshouse study, root functional traits were quantified under non-limiting water conditions in rhizotrons.
Key Results Summer dormancy was associated with higher dehydration tolerance, and negatively associated with traits conferring dehydration avoidance. Species with greater summer dormancy were characterized by greater springtime productivity, earlier reproduction, and a shallow and fine root system, which are indicative of dehydration escape. Summer dormancy was associated with an acquisitive, competitive functional strategy in spring, and a conservative strategy in summer.
Conclusions Both the escape and acquisitive springtime strategies observed in summer dormant perennial taxa are typically associated with annual grasses. California grasslands were once dominated by perennial species, but have been overtaken by non-native Mediterranean annual grasses, which are expected to be further favoured by climate change. Owing to functional similarity with these exotic annuals, it is suggested that native summer dormant taxa may play an important ecological role in the future of both natural and restored California grasslands.
Keywords: Poaceae, summer dormancy, dehydration tolerance, dehydration avoidance, drought escape, functional traits, root traits, California grasslands, perennial grasses
INTRODUCTION
One of the anticipated effects of climate change is an increase in the frequency and intensity of extreme droughts (IPCC, 2014). In some regions, including in California, there is evidence that these changes are already beginning to occur (Diffenbaugh et al., 2015). Of critical importance in this context is how increasingly severe episodes of drought will impact plant species in both natural and managed communities (Smith, 2011). The impact of climate change on biodiversity in grasslands is rapidly increasing across the world (Millennium Ecosystem Assessment, 2005), so further study of drought survival in native grassland species is a timely endeavour.
Functional ecological theory predicts a growth–survival trade-off, such that the species best able to persist through periods of resource limitation are expected to be more ‘conservative’, i.e. slower growing, less productive and less competitive when resources are not limiting (Grime, 2001; Wright et al., 2004; Reich, 2014). In contrast, more ‘acquisitive’ species that are faster growing and better able to pre-empt available resources are expected to exhibit poorer survival under resource limitation. Functional traits are strong indicators of conservative and acquisitive strategies (Reich et al., 2003; Wright et al., 2004; Violle et al., 2007), and can be measured using standardized protocols (Cornelissen et al., 2003; Pérez-Harguindeguy et al., 2013) to identify the trade-offs governing resource allocation in both the foliar and root compartments (Freschet et al., 2010). In addition, they offer promise as predictors of larger scale population-, community- or ecosystem-level responses to environmental change (Lavorel and Garnier, 2002; Diaz et al., 2004; Shipley et al., 2006).
Though developed specifically to account for nutrient and light resources, there is growing evidence that the growth–survival trade-off applies to water as well (Reich, 2014). This evidence is strongest in woody taxa (Reich, 2014), as fewer studies have explored the relationship in herbaceous species. Likewise, though herbaceous species are known to exhibit varied drought survival strategies that may entail different resource requirements, drought survival and functional traits under non-limiting water conditions are rarely assessed in the same studies (Pérez-Ramos et al., 2013; Zwicke et al., 2015). Perennial herbaceous species from Mediterranean-type grasslands that experience a rainy and mild productive period followed by a yearly, severe summer drought are particularly interesting models for this type of study, as they are adapted to persist across seasons with highly contrasting resource availability.
Drought survival in perennial herbaceous species is achieved by combining the primary strategies of tolerance, avoidance and escape (Levitt, 1980; Ludlow, 1989). In addition, some herbaceous perennial taxa from Mediterranean-type climates also exhibit endogenous summer dormancy (Ofir and Koller, 1974; Volaire and Norton, 2006). The tolerance strategy is exemplified by plants that can withstand higher levels of tissue dehydration without additional water inputs, and is associated with the accumulation of water-soluble carbohydrates and proteins (Volaire, 1995; Volaire and Thomas, 1995). In contrast, avoidance is seen in taxa that are relatively sensitive to dehydration and must delay it through continued water uptake via a deep, efficient root system (Young et al., 2010). Escape is typically used to describe annual taxa that grow quickly, reproduce early and die before experiencing dehydration. Summer dormancy may be an analogous strategy for Mediterranean-type perennials in which the risk of drought-induced mortality is reduced by a period of foliar senescence that begins in spring and is not released until the dry season is nearing its end (Hoen, 1968; Ofir and Kigel, 2010). It is defined by either the full or partial senescence of mature foliage (which is more sensitive to dehydration than young meristematic tissue), reduced or suspended leaf growth and, in the case of complete dormancy, endogenous meristematic dehydration. Because it is both induced and released by photoperiod and temperatur, and is not a response to water deficit itself, it must be measured under non-limiting water conditions (Volaire and Norton, 2006; Norton et al., 2008).
Theory suggests that tolerance and avoidance should be associated with contrasting resource acquisition strategies and functional traits. Stress tolerance, including tolerance of drought, is expected to be associated with a conservative resource acquisition strategy (Grime, 2001). Conservative functional traits include denser leaves (leaf dry matter content, LDMC) and roots (root tissue density, RTD; root dry matter content, RDMC), delayed reproduction and lower productivity. On the other hand, avoidance mediated by extensive root development may be facilitated by acquisitive root traits, including faster elongation and production of longer roots with lower carbon investment (i.e. specific root length, SRL), due either to lower tissue density (i.e. lower RTD or RDMC) or to smaller diameter (Comas and Eissenstat, 2004; Hernández et al., 2010; White and Snow, 2012; Pérez-Ramos et al., 2013). An acquisitive root strategy may be promoted by an acquisitive foliar strategy (Pérez-Ramos et al., 2013), which includes a larger, less dense photosynthetic surface (specific leaf area, SLA), higher leaf elongation rate and greater above-ground productivity.
Summer dormancy is an adaptation to strong seasonal variation in water availability; as a result, it may be associated with seasonally contrasting resource acquisition strategies. Because the onset of dormancy in spring may reduce the window available for growth and reproduction, we expect the most summer dormant taxa to grow acquisitively and reproduce early in spring, similarly to dehydration-escaping annuals. Summer dormant species exhibit conservative growth in summer, which we expect to be associated with higher dehydration tolerance and lower investment in dehydration avoidance.
In the present study, we quantified degrees of summer dormancy and dehydration tolerance, as well as traits associated with dehydration avoidance, in eight species of California perennial grasses. We then related variation in these drought survival strategies to variation in functional traits indicative of conservative and acquisitive resource acquisition strategies. We asked the following questions. (1) Are more summer dormant species also more dehydration tolerant and less dehydration avoidant? (2) What are the seasonal patterns of growth and functional traits most associated with summer dormancy? In answering these questions, our aim is to develop a more holistic view of drought survival and associated functional strategies in wildland species from Mediterranean-type climates.
We also discuss the implications of our study in the context of perennial grassland ecology and management. In California’s Mediterranean-type region, native perennial bunchgrasses have been almost completely replaced by non-native annual grasses and forbs (D’Antonio et al., 2007), and management efforts now seek to preserve and restore native perennial grasslands. The success of non-native annuals may in part be due to their drought escaping strategy, in which they compete vigorously for resources in spring and ‘survive’ the summer drought as seed. Summer dormant native taxa may occupy a functionally similar role and, as a result, become more important for grassland management.
MATERIALS AND METHODS
Plant materials
We selected eight perennial grass species occurring in California’s Mediterranean-type climate zone. Seven of these species are native to California, while one, Poa bulbosa L., is non-native but naturalized and widespread in California. Four of the native species – Elymus glaucus Buckley subsp. glaucus, Melica californica Scribn., Poa secunda J. Presl subsp. secunda and Nassella pulchra (Hitchc.) Barkworth – were previously scored for dormancy by Laude (1953). In this earlier study, only P. secunda was found to retain no green tissue during summer drought. Both M. californica and N. pulchra retained some green tissue, but ceased vegetative growth, while E. glaucus continued to grow. The remaining three native species – Elymus elymoides (Raf.) Swezey, Festuca idahoensis Elmer and Koelaria macrantha (Ledeb.) Schult. – are common in California grasslands and are regarded as drought hardy, but their expression of summer dormancy has not been quantified. Poa bulbosa has been extensively studied in the context of summer dormancy and is known to express complete summer dormancy across its range (e.g. Ofir and Kigel, 2003). Because the level of summer dormancy expressed in California native grasses has seldom been explored, and to our knowledge has never been explored using the most current methods described in Norton et al. (2008), we included it as a reference species to ensure we captured functional trait values on at least one species that is known to express complete summer dormancy.
Seeds from all but three species were field collected in the early summer of 2012 from the University of California’s Stebbins Cold Canyon and Quail Ridge Natural Reserves in the foothills of the inner California Coast Range. Exceptions were Elymus glaucus, which was field collected in early summer 2011 from the UC Hopland Research and Extension Center, also in the interior Coast Range; P. secunda, which was obtained from a native seed grower (Hedgerow Farms, Winters, CA) after an initial field collection exhibited poor germination and survival; and K. macrantha, which was also obtained from Hedgerow Farms after a different field-collected species failed to germinate. Field-collected seed was obtained from 20–50 individual plants per species to avoid genetic uniformity.
Experimental design
Seeds of each species were sown in trays in winter 2013 at the CEFE-CNRS in Montpellier, France (43°38'N, 3°52'E). Upon germination, live tillers of each species were randomly assigned to either a (1) ‘short pot’ experiment to measure foliar traits and dehydration tolerance or (2) a rhizotron study to measure root and dehydration avoidance traits.
Short pot study design.
On 21 May 2013, four plants of each species were transplanted into short pots (18 cm wide by 22 cm deep) filled with a similar quantity (2610–2634 g) of potting mixture composed of 50 % clay and 50 % local soil. Shallow pots are recommended when quantifying dehydration tolerance because they minimize differences in water use among species arising from variation in rooting depth, and thus minimize the expression of dehydration avoidance traits (Volaire et al., 2014). From the time of transplant, pots were kept under full irrigation in a glass hoop-house on the CNRS campus to allow transplants to acclimate to ambient conditions and experience seasonal cues (temperature and photoperiod) necessary to induce summer dormancy. One pot per species was randomly assigned to each of 13 blocks. Positions of blocks and pots within blocks were re-randomized approximately every 2 weeks. One plant per pot was randomly selected and marked as the ‘target’ plant so that foliar traits could be measured on the same plant each season, and thus would represent change over time. Our unit of replication was the pot and, to maximize efficiency of limited resources, we elected not to measure leaf traits on multiple sub-sampled plants within pots. Seasonal biomass was measured on all plants in each pot and averaged so that values presented represent pot-level means. In June 2014, each short pot was randomly assigned to either a full summer irrigation treatment (three replicates per species) to assess summer dormancy or a progressive drought treatment (ten replicates) to quantify dehydration tolerance. We allocated more pots to the progressive drought treatment because it allowed us to assess drought recovery biomass (i.e. dehydration tolerance) at smaller increments of soil water depletion, which allows for clearer discrimination of critical dehydration thresholds and differences among species (see ‘Recovery after progressive drought’ below).
Rhizotron study design.
On 6 February 2014, we transplanted one plant of each species into each of six long (6 cm in diameter by 1 m long), clear plastic tubes, or ‘rhizotrons’, filled with 3108 g of substrate composed of 75 % sand and 25 % local soil. Insulated tubes were kept in a greenhouse and fully irrigated throughout the observation period. Mean air temperature within the greenhouse was maintained between 16·9 °C (March) and 20·6 °C (May), monthly mean relative humidity ranged from 46·7 % to 55·3 %, and radiation (photon flux) ranged from 42·7 to 86·7 μmol m–2 s–1. Rhizotrons were placed in random order along one wall in the greenhouse, and the order was re-randomized weekly. We positioned the rhizotrons at a slight angle (around 20°) from the vertical to encourage visible root growth against the tube walls.
Measurements
Foliar growth dynamics and functional traits under non-limiting water conditions (short pots).
We measured aerial dry mass production and leaf elongation seasonally from autumn 2013 to autumn 2014. At the start of each season, all plants were clipped and their initial heights (all between 5 and 8 cm) recorded. The maximum vegetative height of the target plant was then measured two or three times each week, and these values (after subtracting the initial height) were used to estimate leaf elongation rates. After leaf elongation began to taper, plants were clipped again, and leaf elongation monitoring was recommenced. Maximum leaf elongation rates (Emaxleaf) were estimated for each pot by regressing the length of the longest fully extended leaf on the number of growing days using a logistic growth function and solving for the maximum rate of increase.
From autumn 2013 to spring 2014, seasonal aerial biomass of the target plant was collected separately from that of the other three plants in each pot, and all plant biomass was oven-dried at 65 °C for 48 h (i.e. sufficient time to achieve constant weight). Cuts were performed on 15 October 2013 (autumn), 20 December 2013 (winter) and 6 March 2014 (early spring). Aerial productivity potential (APP) was calculated by dividing dry mass by the number of growing days. We calculated APP for the target plant only and as an average of all individual plants within each pot, and elected to present the latter.
In late spring, we monitored reproductive phenology, recording the dates on which reproductive culm elongation began and the first fully emerged inflorescence was observed for each target plant. Following emergence of the first full inflorescence, the number of culms was recorded two or three times each week. After culm elongation was observed in a target plant, leaf elongation measurements for that plant were stopped, as the plant had transitioned from the vegetative to reproductive stage of development. All pots of a species were clipped when roughly 50 % of elongating culms on target plants had fully emerged. Late spring clipping for the earliest flowering species (P. bulbosa) occurred on 16 April 2014, and had been completed for all species by 11 June 2014. To account for differences in reproductive phenology, we separated spring biomass clippings into vegetative and reproductive tissue components, and calculated APP using only vegetative tissues.
Leaf traits were measured on each target plant in autumn 2013 and spring 2014. The SLA (leaf area per unit leaf dry mass) and LDMC (leaf dry mass per unit leaf fresh mass) were determined for one fully expanded leaf from each target plant according to the protocols described by Garnier et al. (2001). Leaf area and width were measured with a ΔT area meter (model MK2, Cambridge, UK). All leaf samples were oven-dried at 65 °C for 48 h.
Root growth and functional traits under non-limiting water conditions (rhizotrons).
We marked the depth of the deepest visible root tip on the surface of each rhizotron twice weekly. After elongation had ceased for most species, we destructively sampled 2–4 tubes per species on 19 May 2014 for further trait measurement. To sample, we carefully removed soil and roots from each tube, taking care not to disturb the depth distribution of roots in the column. We then cut each column into 25 cm segments and froze the segments in clear plastic bags. For E. glaucus, we further divided the 0–25 cm segment into two equal 12·5 cm segments for use in a concurrent study on intraspecific variability (trait values for E. glaucus were later averaged or summed, as appropriate). After the soil samples were completely frozen, we carefully washed the roots to remove as much soil as possible, and refroze the cleaned roots suspended in water.
Once frozen, cleaned root samples were prepared for trait analysis using WinRHIZO software (ver. 2003b, Regent Instruments Inc., Quebec, Canada). Frozen samples were thawed and a representative sub-sample of root tissue (with no roots exceeding 2 mm in diameter) was taken and scanned at 400 dpi (Bouma et al., 2000). After thawing, all sub-samples used for WinRHIZO analysis were immediately weighed, scanned then oven-dried for 48 h at 65 °C, and reweighed. Remaining root tissue was immediately oven-dried and weighed. WinRHIZO was used to assess length, mean diameter, area and volume of fine root tissue. These values were then used to calculate specific root length (SRL; root length per unit dry root mass), specific root area (SRA; root area per unit of dry mass), RTD (the ratio of root dry mass to fresh volume) and RDMC (root dry mass per unit root fresh mass).
We estimated maximum root elongation rates (Emaxroot) by regressing the depth of the deepest visible root tip onto the number of growing days according to a logistic growth model and solving for the maximum rate of increase. We estimated the 95 % rooting depth for each tube by regressing the cumulative root dry mass recovered from each soil segment onto the square of the maximum depth of each segment. The regression coefficients were then used to solve for the depth above which 95 % of root biomass was located.
Summer growth dynamics (summer dormancy) under non-limiting water conditions (short pots).
On all short pots assigned to the full summer irrigation treatment, we measured leaf elongation and seasonal vegetative dry matter production using the same methods as described above. We also recorded visual estimates of vegetative senescence twice per week. To assess summer dormancy, we calculated Emaxleaf, APP, and relative summer growth potential (RSGP; summer dry mass standardized by species-specific spring dry mass production). We calculated RSGP in addition to APP to account for intrinsic interspecific variation in productivity. Summer dormancy is characterized by cessation or reduction of summer growth, so RSGP can be understood as its opposite.
Recovery after progressive drought (dehydration tolerance) (short pots).
Short pots receiving the progressive drought treatment were watered once to full soil saturation on 11 June 2014 and weighed to obtain their initial soil water content (SWC). The field capacity of the substrate was reached at 66 % SWC, while the wilting point was reached at 12 %. Pots were re-weighed 1–3 times weekly to assess the percentage of soil moisture remaining. When approx. 12 % soil moisture remained, we rehydrated two pots per species at roughly 2 % SWC increments. Once rehydrated, pots were moved into a 20 °C glasshouse to break dormancy and facilitate new growth. Once a species stopped producing recovery biomass upon rehydration, we rehydrated all remaining pots of that species. To allow for relaxation of summer dormancy, biomass was harvested 35 d after rehydration for incompletely dormant species and 49 d after rehydration for completely dormant species. Dormancy was assessed by visual estimates of vegetative senescence under non-limiting water conditions; completely dormant species were P. bulbosa, P. secunda and M. californica. Biomass was collected for each plant individually and prepared and weighed as above. We used standardized recovery biomass (SRB; dry matter produced upon rehydration after drought divided by dry matter produced during the same time frame under non-limiting water conditions) as a measurement of dehydration tolerance.
Statistical analyses
All analyses were conducted in R (R Core Team, 2014). Species differences in growth and trait values under non-limiting water conditions were estimated using linear models followed by post-hoc tests using Tukey’s correction for multiple comparisons in the lsmeans package (Lenth and Hervé, 2015). For both studies, species was included as a predictor. For the short pot study, block was never a significant predictor, and was excluded from final models. To conform to the assumptions of linear models, Emaxleaf, APP, SLR, SLA, RTD and RDMC were log-transformed, and flowering phenology and autumn LDMC were square-root-transformed. For all growth and trait values measured from autumn to spring in the short pot study, there were 13 replicate measures taken per species (i.e. one target plant per pot in each of 13 blocks). For RSGP, which was measured in a sub-set of the short pots under non-limiting summer water conditions, there were three replicates per species (i.e. one target plant per pot in each of three blocks). For all root traits, all plants surviving in rhizotrons by the end of the experiment were sampled, resulting in between two and four replicate measures per species.
We evaluated SRB using a linear mixed effects model in the lme4 package (Bates et al., 2014). Species and SWC at rehydration were included as fixed effects; SWC was measured as a continuous variable and thus was included in models as such. We measured SRB in ten replicate pots per species. Because we measured SRB on all four plants in each pot and not only on the target individual, we included pot as a random effect to account for sub-sampling within pots. We estimated SRB, and thus dehydration tolerance, at two thresholds: 9·3 % and 7·9 % soil moisture. The upper threshold is the lowest SWC at which there were data for all species, while the lower threshold is the lowest SWC achieved by a species with intermediate dormancy and SRB at 9·3 % SWC (E. elymoides). We present the lower threshold with the caveat that it requires projecting beyond the scope of our empirical data for some species. We lack data at lower SWC for some species because we elected to rehydrate all remaining pots after a species consistently stopped recovering; because species consistently failing to recover at higher SWC are highly unlikely to recover at lower SWC, we feel that presenting the lower threshold is valid.
We also calculated pairwise and multivariate associations among traits. Pairwise correlations were calculated as Pearson’s R, and multivariate associations were assessed with principal components analysis (PCA) in the vegan package (Oksanen et al., 2015). Because our studies used different levels of replication, we used a matrix of species mean trait values for multivariate analysis. Thus, results of PCA are intended to aid in visualizing complex, multitrait syndromes, and were not used to make further statistical comparisons among individual species.
RESULTS
Foliar growth dynamics and functional traits measured during the growing season
Productivity (APP) differed significantly among species in all seasons except winter (Table 1). In autumn, APP ranged from 1·09 mg d–1 in E. elymoides to 14·16 mg d–1 in K. macrantha. In spring, the most productive species were K. macrantha, P. bulbosa and P. secunda, each producing >10 mg of dry mass per day. The Emaxleaf differed significantly among species in autumn and winter growing seasons, but not in spring. Average APP (calculated from autumn to spring) was strongly correlated with autumn APP, but not with winter or spring (Table 2).
Table 1.
Species differences in foliar traits measured under non-limiting water conditions
| Nassella pulchra | Koelaria macrantha | Elymus glaucus | Elymus elymoides | Festuca idahoensis | Melica californica | Poa secunda | Poa bulbosa | ||
|---|---|---|---|---|---|---|---|---|---|
| SLA (m2 kg–1) | Autumn | 14·49 ± 1·09b | 19·73 ± 1·05a | 23·76 ± 1·05a | 22·83 ± 1·14a | 12·72 ± 1·05b | 19·75 ± 1·05a | 36·42 ± 2·19c | 22·74 ± 1·14a |
| Spring | 21·51 ± 1·13a | 20·15 ± 1·08a | 24·87 ± 1·08ab | 20·87 ± 1·08a | 8·3 ± 1·08c | 27·75 ± 1·08b | 36·78 ± 1·13d | 9·69 ± 1·13c | |
| LDMC (mg g–1) | Autumn | 348·31 ± 18·22 n.s. | 302·75 ± 16·32 n.s. | 317·18 ± 16·7 n.s. | 291·03 ± 17·39 n.s. | 293·04 ± 16·05 n.s. | 302·37 ± 16·3 n.s. | 245·13 ± 30·57 n.s. | 293·3 ± 17·46 n.s. |
| Spring | 298·57 ± 8·55d | 267·34 ± 8·22abd | 258·81 ± 8·22ab | 255·43 ± 8·22a | 143·76 ± 8·22c | 293·61 ± 8·22bd | 242·28 ± 8·55a | 147·97 ± 8·55c | |
| Emaxleaf (cm d–1) | Autumn | 0·68 ± 0·04b | 0·58 ± 0·04b | 1·5 ± 0·11a | 2·03 ± 0·13a | 0·77 ± 0·05b | 1·06 ± 0·07c | – | 0·71 ± 0·05b |
| winter | 0·3 ± 0·03a | 0·34 ± 0·03ab | 0·47 ± 0·04bc | 0·39 ± 0·04ab | 0·28 ± 0·03a | 0·3 ± 0·03a | 0·7 ± 0·07c | 0·37 ± 0·04ab | |
| spring | 0·63 ± 0·04 n.s. | 0·71 ± 0·05 n.s. | 0·73 ± 0·05 n.s. | 0·75 ± 0·05 n.s. | 0·72 ± 0·05 n.s. | 0·8 ± 0·05 n.s. | 0·82 ± 0·06 n.s. | 0·81 ± 0·06 n.s. | |
| APP (mg d–1) | Autumn | 13·5 ± 1·98d | 14·16 ± 2·08d | 4·1 ± 0·6ab | 1·09 ± 0·16c | 2·61 ± 0·38a | 7·19 ± 1·06b | – | 0·55 ± 0·08e |
| Winter | 6·4 ± 0·79a | 5·24 ± 0·65a | 5·4 ± 0·67a | 3·83 ± 0·47a | 4·26 ± 0·53a | 3·7 ± 0·48a | 6 ± 2·68a | 4·87 ± 0·63a | |
| Spring | 5·34 ± 0·36ab | 10·66 ± 0·71c | 6·88 ± 0·46a | 5·23 ± 0·35ab | 9·34 ± 0·63c | 4·78 ± 0·32b | 12·19 ± 0·85c | 10·03 ± 0·7c | |
| Growing season | 7·96 ± 0·49cd | 10·28 ± 0·63d | 6·05 ± 0·37a | 4·03 ± 0·25a | 6·95 ± 0·43bc | 5·51 ± 0·35b | – | 5·7 ± 0·36b | |
| Flowers (Julian days) | First | 98·09 ± 3·01c | 155 ± 3·78b | 118·77 ± 3·31a | 119·21 ± 3·32a | 146·03 ± 3·67b | 101·34 ± 3·06c | 94·94 ± 3·08c | 66·07 ± 2·57d |
| 50 % | 107·8 ± 1·83c | 155 ± 2·19b | 139·15 ± 2·08a | 132·81 ± 2·03a | 155 ± 2·19b | 110·39 ± 1·85c | 105·06 ± 1·88c | 71·15 ± 1·55d |
Values are marginal means ± s.e.; letters indicate significant differences as determined by Tukey’s post-hoc tests.
SLA, specific leaf area; LDMC, leaf dry matter content; Emaxleaf, maximum leaf elongation rate; APP, aerial productivity potential.
Table 2.
Correlations between foliar traits, drought recovery biomass (SRB) and relative summer growth potential (RSGP)
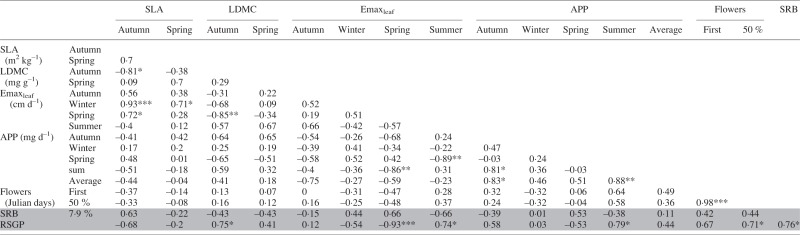 |
Significance of Pearson’s R: ***P < 0·001, **P < 0·01, *P < 0·05.
SLA, specific leaf area; LDMC, leaf dry matter content; Emaxleaf, maximum leaf elongation rate; APP, aerial productivity potential.
The date of first inflorescence emergence was strongly correlated to the date at which 50 % of total inflorescences produced had fully emerged (Table 2). Interspecific variation in both measures was statistically significant (Table 1). The three most dormant species were among the earliest to flower by both metrics: P. bulbosa achieved 50 % inflorescence emergence by 12 March 2014, while P. secunda and M. californica were slightly later at 5 April and 11 Apriul 2014, respectively. Nassella pulchra, the least dormant species, was also among the earliest to flower, achieving 50 % inflorescence emergence by 17 April.
Mean SLA ranged from 12·72 m2 kg–1 in F. idahoensis to 36·42 m2 kg–1 in P. secunda in autumn, and from 8·3 to 36·78 m2 kg–1 in P. bulbosa in spring (Table 1). Species differences in LDMC were not significant in autumn, but ranged from 245·13 mg g–1 in P. secunda to 348·31 mg g–1 in N. pulchra. In spring, LDMC ranged from 143·76 mg g–1 in F. idahoensis to 298·57 mg g–1 in N. pulchra. Autumn SLA and LDMC were negatively correlated, but spring measures of SLA and LDMC were not (Table 2).
Root growth dynamics and functional traits measured during the growing season
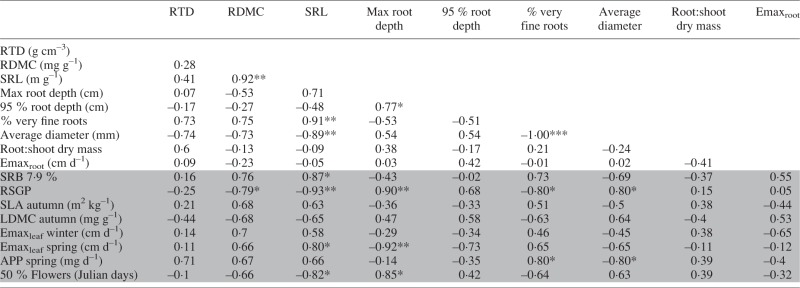
Variation among species was statistically significant for nearly all of the root traits measured (Table 3). The percentage of very fine roots varied greatly among species, ranging from roughly 48 % in E. elymoides to > 95 % in both P. secunda and P. bulbosa. Likewise, the average root diameter was roughly 0·12 mm in both Poa species, and around 0·25 mm in E. elymoides and E. glaucus. A greater percentage of very fine roots, and thus a lower average root diameter, was strongly correlated with SRL (Table 4). Both Poa species exhibited significantly higher SRL than all other species, and SRL and RDMC were correlated.
Table 3.
Species differences in root traits measured under non-limiting water conditions
| Nassella pulchra | Koelaria macrantha | Elymus glaucus | Elymus elymoides | Festuca idahoensis | Melica californica | Poa secunda | Poa bulbosa | |
|---|---|---|---|---|---|---|---|---|
| RTD (g cm–3) | 0·27 ± 0·05ab | 0·34 ± 0·04a | 0·16 ± 0·02b | 0·22 ± 0·02ab | 0·23 ± 0·03ab | 0·22 ± 0·03ab | 0·3 ± 0·04ab | 0·28 ± 0·03a |
| RLD (cm cm–3) | 2·24 ± 1·04abc | 7·94 ± 2·14a | 3·12 ± 0·84ab | 0·77 ± 0·21c | 2·23 ± 0·74abc | 1·55 ± 0·51bc | 9·98 ± 3·3a | 1·06 ± 0·25bc |
| RDMC (mg g–1) | 227·74 ± 28·6abc | 185·61 ± 13·46ab | 208·25 ± 15·1abc | 198·44 ± 14·39abc | 193·27 ± 17·16abc | 164·55 ± 14·61a | 268·93 ± 23·88bc | 262·81 ± 16·5c |
| SRL (m g–1) | 88·67 ± 16·75a | 118·47 ± 12·92a | 116·73 ± 12·73a | 77·16 ± 8·42a | 98·84 ± 13·21a | 112·44 ± 15·02a | 337·41 ± 45·08b | 359·77 ± 33·99b |
| Max depth (cm) | 70 ± 15·86ab | 69·8 ± 6·46a | 48·12 ± 4·88abc | 46·96 ± 4·34abc | 43·42 ± 4·02bcd | 34·85 ± 5·58bcd | 28·89 ± 2·67d | 30·84 ± 3·12cd |
| 95 % depth (cm) | 72·05 ± 7·87a | 45·44 ± 4·54abc | 48·72 ± 4·54abc | 52·02 ± 4·54ab | 31·09 ± 5·57bc | 35·48 ± 5·57bc | 25 ± 5·57c | 38·28 ± 3·94bc |
| Very fine roots (%) | 81·27 ± 4·19ab | 77·78 ± 2·42a | 51·47 ± 2·42cd | 48·01 ± 2·42c | 58·59 ± 2·97cd | 62·03 ± 2·97d | 95·72 ± 2·97b | 96·59 ± 2·1b |
| Average diameter (mm) | 0·19 ± 0·02abcd | 0·17 ± 0·01ab | 0·25 ± 0·01c | 0·26 ± 0·01c | 0·22 ± 0·01ac | 0·21 ± 0·01ac | 0·12 ± 0·01bd | 0·12 ± 0·01d |
| Root:shoot dry mass | – | 0·33 ± 0·03a | 0·12 ± 0·03b | 0·14 ± 0·03bc | 0·1 ± 0·04b | 0·16 ± 0·04bc | 0·29 ± 0·04ac | 0·02 ± 0·03b |
| Emaxroot (cm d–1) | 1·15 ± 0·16a | 0·79 ± 0·09ab | 0·64 ± 0·09ab | 0·76 ± 0·09ab | 0·56 ± 0·11ab | 0·81 ± 0·11ab | 0·39 ± 0·11b | 0·95 ± 0·08a |
Values are marginal means ± s.e.
Letters indicate significant differences as determined by Tukey’s post-hoc tests (P < 0·05).
RTD, root tissue density; RDMC, root dry matter content; SRL, specific root length; SRA, specific root area; Emaxroo, maximum root elongation rate.
Table 4.
Correlations between root traits, select foliar traits, drought recovery biomass (SRB) and relative summer growth potential (RSGP)
 |
Significance of Pearson’s R: ***P < 0·001, **P < 0·01, *P < 0·05.
RTD, root tissue density; RLD, root length density; RDMC, root dry mass content; SRL, specific root length; SRA, specific root area; Emaxroot, maximum root elongation rate; SLA, specific leaf area; LDMC, leaf dry mass content; Emaxleaf, maximum leaf elongation rate; APP, aerial productivity potential (summer dry mass/growing days); RSGP, relative summer growth potential (summer dry mass/spring dry mass); SRB, standardized recovery biomass (dry mass produced upon rehydration after drought/dry mass in irrigated controls).
Interspecific variation in 95 % rooting depth was statistically significant (Table 3). The shallowest-rooting species was P. secunda, with an estimated 95 % rooting depth within the shallowest soil partition (0–25 cm) we collected. The deepest-rooting species was E. elymoides, with an estimated 95 % rooting depth deeper than 50 cm. Rooting depth was highly variable across tubes of most species.
Summer dormancy and dehydration tolerance
Interspecific variation in both RSGP and SRB was statistically significant (Table 5; Fig. 1). Both P. bulbosa and P. secunda expressed complete dormancy and produced no new biomass during summer. Complete senescence of all mature foliage had occurred by mid May in all individuals of P. bulbosa, and by the first week in June for P. secunda. Regrowth in both species did not recommence until plants were moved to a glasshouse at 20 °C (optimal conditions to break summer dormancy). For P. bulbosa, the first individual broke dormancy on 17 July 2014, and most had green tissue by the end of the month. Only one out of three pots of P. secunda under full summer irrigation recovered from dormancy beginning on 25 July 2014. Dormancy in M. californica was nearly complete: all pots had senesced between 85 and 90 % of mature foliage by the end of June 2014. At least one individual plant per pot maintained a small amount of green tissue (approx. 2 % of spring growth potential, or 0–1 tillers per individual plant) throughout the duration of the summer growth period; more vigorous regrowth was noticeable shortly after plants were moved to the 20 °C glasshouse. All other species expressed incomplete dormancy, with RSGP ranging from 15 % in F. idahoensis to 76 % in N. pulchra, the least dormant species in our study.
Table 5.
Species differences in summer growth dynamics under non-limiting water conditions and standardized drought recovery biomass (SRB) measured after progressive drought (shaded in grey)
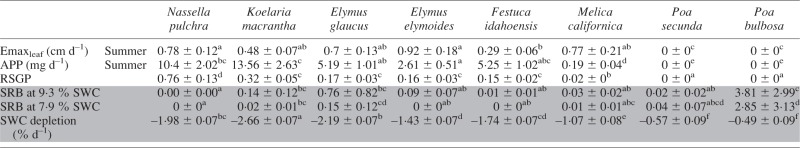 |
Values are marginal means ± s.e.; letters indicate significant differences as determined by Tukey’s post-hoc tests (P < 0·05).
Emaxleaf, maximum leaf elongation rate; APP, aerial productivity potential; RSGP, relative summer growth potential; SRB, standardized recovery biomass.
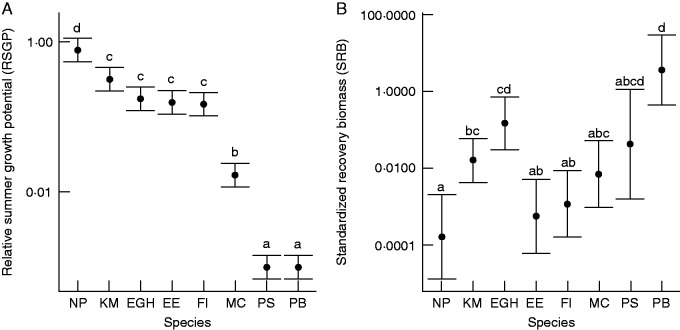
Fig. 1.
Mean relative summer growth potential and standardized recovery biomass after dehydration to 7·9 % soil water content as predicted by linear model fit. Bars are 95 % confidence intervals; letters indicate significant differences among species corrected for multiple comparisons using Tukey’s post-hoc test (P < 0·05). An RSGP of 1 indicates that summer productivity under non-limiting water conditions equalled optimal growing season productivity, while 0 indicates full summer dormancy. An SRB of 1 indicates that productivity after drought equalled productivity in non-water-limited pots of the same species. NP, Nassella pulchra; KM, Koeleria macrantha; EGH, Elymus glaucus; EE, Elymus elymoides; FI, Festuca idahoensis; MC, Melica californica; PS, Poa secunda; PB, Poa bulbosa.
In P. bulbosa exposed to progressive drought, SRB after rehydration predicted at both 9·6 and 7·9 % SWC was over twice as great as biomass produced by fully irrigated pots during the same growth period (Table 5). In contrast, N. pulchra, the least dormant species, did not recover at SWC <10 %. Predicted mean SRB for P. secunda was among the highest in our study, but was much more variable than P. bulbosa, and in five of the nine rehydrated pots no plants recovered. Melica californica had relatively low SRB; however, all plants recovered in nine out of ten pots.
Associations between growing season and summer growth dynamics and functional traits
A negative correlation between SRB and RSGP was statistically significant (Table 2). Correlations between RSGP, SRB and foliar traits are presented in Table 2; correlations between root traits, selected foliar traits, SRB and RSGP are presented in Table 4. Because P. secunda exhibited poor survival in both fully irrigated and progressive drought treatments, it was excluded from calculations of pairwise correlations with SRB.
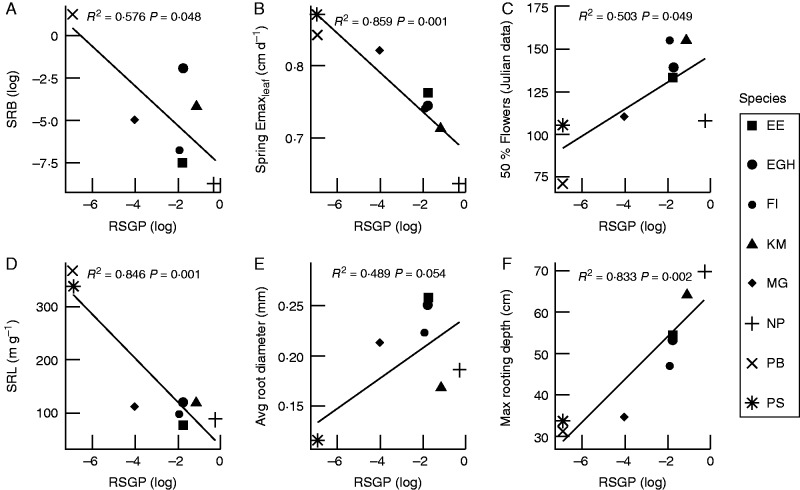
Later flowering phenology was correlated with RSGP, while spring Emaxleaf was negatively correlated with RSGP (Fig. 2). The root traits SRL, RDMC and the percentage of very fine roots were all negatively correlated with RSGP, while average root diameter and RSGP were positively correlated (Table 4). SRL was also correlated with higher SRB and spring Emaxleaf, and negatively correlated to the time to 50 % flowering.
Fig. 2.
Trait correlations with relative summer growth potential. Traits include drought recovery biomass (SRB), summer Emaxleaf (an indicator of summer dormancy), springtime growth dynamics (spring Emaxleaf and date of 50 % inflorescence emergence) and root traits (SRL and average root diameter). NP, Nassella pulchra; KM, Koeleria macrantha; EGH, Elymus glaucus; EE, Elymus elymoides; FI, Festuca idahoensis; MC, Melica californica; PS, Poa secunda; PB, Poa bulbosa.
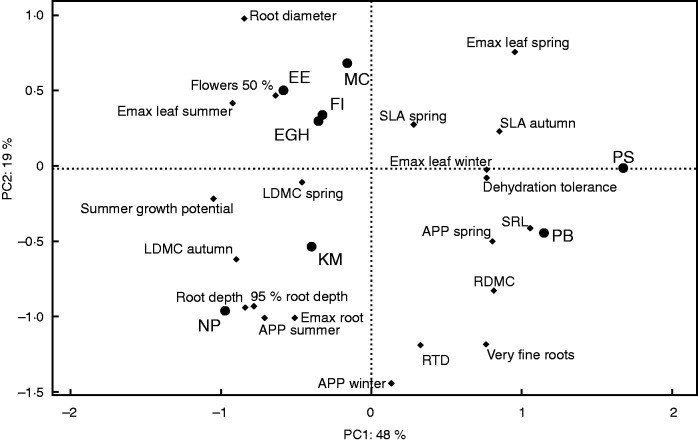
In a PCA including growing season and summer growth traits, the first two axes explained 67 % of the total variance (Fig. 3). The first axis (PC1, accounting for 48 % of variance) opposed RSGP and SRB, indicating a trade-off between summer growth and dehydration tolerance. Higher spring Emaxleaf, spring APP and autumn SLA all loaded positively onto the first axis with higher dehydration tolerance (SRB). In contrast, higher autumn LDMC loaded negatively with summer activity (RSGP, summer Emaxleaf). In the root compartment, higher SRB and summer dormancy were associated with high SRL, while deeper roots with a larger diameter were associated with summer activity.
Fig. 3.
Principal components analysis of foliar and root functional traits and seasonal growth dynamics under non-limiting water conditions. Both SRB and RSGP were log-transformed prior to analysis. NP, Nassella pulchra; KM, Koeleria macrantha; EGH, Elymus glaucus; EE, Elymus elymoides; FI, Festuca idahoensis; MC, Melica californica; PS, Poa secunda; PB, Poa bulbosa. For other abbreviations and units, see Tables 1 and 3.
DISCUSSION
Large variation in summer dormancy is associated with higher dehydration tolerance
We found strong variation in dehydration tolerance, which was associated with increasing summer dormancy. The species in our study are all native to or (in the case of P. bulbosa) naturalized in California, and all plant materials were obtained from extremely summer-dry interior grasslands or woodlands, so it is not surprising that dehydration tolerance in our study was high. For example, at the 12 % wilting point, survival was near 100 % in our species (305 of 312 total plants). In contrast, survival at 12 % SWC plummeted in populations of Dactylis glomerata from the Mediterranean Basin (F. A. Volaire, pers. comm.), with its less drought-severe summers (Clary, 2008). Nevertheless, dehydration tolerance at very low soil moisture content varied significantly among our species (see Fig. 1), ranging from full recovery after prolonged drought approaching 7 % SWC in P. bulbosa, to lower SRB (but still, high survival) at roughly 10 % SWC in N. pulchra.
In our study, summer dormancy under non-limiting water conditions was associated with higher dehydration tolerance. This result confirm the existence of the classical trade-off between allocation to growth during a given period (here summer) and ability to survive stress during the same period (Sibly and Calow, 1989; Reich, 2014). Drought recovery biomass (SRB) produced by the most dormant species, P. bulbosa, exceeded that produced by all other species (Fig. 1). Likewise, the least dehydration-tolerant species, N. pulchra, was also the least summer dormant, which expressed a summer growth potential similar to its growth potential in spring.
In P. secunda, SRB was highly variable (Fig. 1): while recovery biomass was high in surviving plants, many plants in both summer drought and full irrigation treatments did not survive. It is possible that P. secunda requires different conditions to break dormancy. However, an early study using P. secunda (then called P. scabrella) demonstrated that rapid release of summer dormancy can be achieved under similar conditions up to 20 months after induction (Laude, 1953). We propose that summer water abundance, rather than deficit, may explain high variability in P. secunda recovery. Laude (1953) noted that summer dormancy release in P. scabrella was slower in plants receiving summer irrigation. Our initial field collection of P. secunda experienced high mortality, probably induced by summer overwatering (see the Materials and Methods); our control pots were fully irrigated during summer and SWC in pots of P. secunda under progressive drought decreased slowly relative to other species. In addition, to ensure nutrient retention during our 2 year study, we used a potting substrate with relatively high clay content, causing SWC to decline at a slower rate than anticipated. We suspect that if SWC more closely matched field conditions, SRB for P. secunda would be more consistent, and dehydration tolerance would be higher.
Summer dormancy is associated with an acquisitive strategy in spring, and is not associated with dehydration avoidance traits
Summer dormancy was associated with an acquisitive foliar strategy in spring characterized by faster springtime vegetative growth and earlier reproduction (see Fig. 2). Some individuals of fully dormant P. bulbosa began reproduction as early as late March, and all individuals of P. bulbosa and P. secunda were completely dormant by mid May when most other species were only beginning to flower. In multivariate analysis, higher SLA was also associated with summer dormancy, indicating that during the growing season summer dormant taxa exhibit a more resource acquisitive growth strategy (see Fig. 3). This trend was driven by P. secunda, but had SLA been measured earlier in P. bulbosa (i.e. prior to the start of foliar senescence), we would anticipate a significant correlation between spring SLA and summer dormancy.
Stress tolerance is expected to confer lower growth rates and productivity (Grime, 1977), but, in our study, this trade-off was not apparent. We did not find dehydration tolerance or summer dormancy to be associated with lower seasonal productivity or Emaxleaf, though P. bulbosa did exhibit relatively low productivity in autumn (Table 1), perhaps suggesting that a less productive autumn compensates for rapid springtime growth. Whether this relationship was true in P. secunda could not be determined in our study due to high mortality during the first summer after sowing.
Summer dormancy was also associated with an acquisitive below-ground strategy, including higher SRL and greater percentage of very fine roots in the upper soil layer (Comas and Eissenstat, 2004; Tjoelker et al., 2005). Notably, SRL of both P. bulbosa and P. secunda was substantially higher than for all other species, exceeding that of the next highest species by > 200 m g–1 (see Table 3). Roots with high SRL achieve greater length with lower carbon investment, either via reduced carbon investment per unit fresh matter (RDMC) or volume (RTD), or by producing thinner roots (Pérez-Harguindeguy et al., 2013). In our study, higher SRL resulted from thinner roots, not lower RTD or RDMC (see Table 4).
That summer dormancy was associated with an acquisitive foliar strategy in spring and a shallow root system with efficient, acquisitive fine root traits is consistent with the expectation that it is analogous to drought escape, the strategy most often associated with annual taxa adapted to severe, yet predictable, Mediterranean summer droughts (Volaire and Norton, 2006). Fine root traits, including high SRL, indicate that both P. bulbosa and P. secunda are adapted to forage and compete optimally for water and nutrients in spring, prior to the onset of drought when resources are plentiful (Fort et al., 2014). In these species, efficient foraging and soil moisture uptake in spring coupled with rapid springtime vegetative growth facilitate a quick progression to reproductive maturity before the transition into summer dormancy. While Mediterranean annuals escape the summer drought as seed, summer dormant perennials escape via a high dehydration tolerance of meristematic tissues, while senescing all foliar tissue (and, thus, ceasing water uptake) until dormancy is released (Volaire et al., 2009).
Our study suggests that summer dormant taxa do not rely strongly on dehydration avoidance. They invested relatively little in traits that would promote access to deep soil moisture during summer drought, including a deep root system, which may in part be facilitated by thicker roots with greater soil penetration ability (Chimungu et al., 2015). In contrast, the most summer active species had deeper, thicker roots and lower dehydration tolerance. Though our rhizotron study did not include a drought treatment, these traits suggest that more summer active species rely on access to deep soil moisture, lending further support to a tolerance–avoidance trade-off (Ludlow, 1989). Our results are in agreement with earlier studies finding that both N. pulchra and E. glaucus maintain some green foliage and continue to access deep soil moisture during the summer drought (Holmes and Rice, 1996). The same study also found that N. pulchra and E. glaucus allocate more root biomass to deep soil layers during dry years than in wet years. To determine the extent of reliance on continued water uptake during drought (and, thus, dehydration avoidance), uptake and root plasticity should be measured in conditions allowing for full root system expression, and under both limiting and non-limiting water conditions.
Ecological implications for California’s Mediterranean-type grasslands
In California’s Mediterranean climate grasslands, native perennial bunchgrasses have been almost completely supplanted by exotic (and often invasive) annual species, many of which originated in the Mediterranean Basin (D’Antonio et al., 2007). Dominance of non-native annuals has been attributed, in part, to increasing drought severity. A field survey of California grasslands found that native perennial cover declined (and, thus, exotic annual cover increased) as one moved away from the milder coast toward the harsher inland Central Valley (Clary, 2012). Similarly, a greenhouse study found that California perennial grasses were physiologically intermediate to perennial and annual grasses originating in the Mediterranean Basin (Vaughn et al., 2011). The authors attributed the intermediacy to the relative severity of California’s summer droughts, which are similar in intensity to regions in the Mediterranean Basin that support more annuals than perennials (Clary, 2008). Several of our species were among those included in this earlier comparison, and our study demonstrates that, particularly when considering summer dormancy, there is a range of drought survival strategies and expression of ‘annual-like’ habits within the broader guild of California wildland perennials.
Drought-escaping perennial species that are more ‘annual like’ (i.e. that have acquisitive traits in spring and are more summer dormant) should be better adapted to regions with more severe drought stress, and may be better competitors against functionally similar invasive annual grasses. Indeed, in the arid Great Basin east of California, native perennial grasses (including P. secunda and Elymus multisetus) with more ‘annual-like’ phenology have been shown to be more effective competitors against the highly invasive and noxious annual grass, Bromus tectorum (Goergen et al., 2011). In contrast, N. pulchra, which is commonly assumed to have been dominant in California’s harsh interior grasslands prior to agricultural conversion and invasion (but see Holstein, 2001), would seem to have less functional similarity to annuals. This may in part explain the poor performance of N. pulchra observed in competition with exotic annual species (Dyer and Rice, 1997).
Both temperature and drought stress are expected to increase in California as a result of climate change (Cayan et al., 2008; Diffenbaugh et al., 2015). A recent analysis of California native grass species found that the distributions of species with higher SLA are highly correlated with higher temperatures, suggesting that invasive exotic annuals will probably further replace native perennial grasses (Sandel and Dangremond, 2012). Our study suggests that more summer dormant native perennials may be better suited to withstand both the changing climate and associated increases in invasion pressure. Management of California grasslands would benefit from further studies of competitive dynamics between summer dormant perennial species such as P. secunda and M. californica, and non-native annual grasses.
Conclusion
This study demonstrates the importance of analysing functional traits and adaptive plant strategies taking into account seasonal patterns. While grasses with complete summer dormancy actively acquire nutrients and light in spring, they conserve the water resource during summer when a severe drought is expected. In contrast, Mediterranean grasses with lower summer dormancy have less contrasting seasonal growth patterns, and exhibit a more opportunistic strategy, growing at any time when the resources are available owing in particular to their ability to avoid dehydration. Lastly, owing to their acquisitive functional strategy in spring and, thus, their similarity to annual taxa, our study suggests that the most summer dormant native perennial grasses may be better suited to the changing climate than others, and may become more abundant in future native plant communities.
ACKNOWLEDGEMENTS
We thank Pascal Chapon for his dedicated technical help, and the ‘Terrain d’experience’ platform at CEFE-CNRS for providing all facilities and technical support. Thanks to Jeffrey Clary and Taraneh Emam for providing seeds, Tom Hare and Christie Smith for assistance collecting data, and Truman Young and Kevin Rice for helpful comments on the manuscript. This work was supported by LabEx CeMEB, Mediterranean Centre for Environment and Biodiversity, www.labex-cemeb.org; a Fulbright fellowship [to J. B.]; and a travel award from the National Science Foundation REACH Integrative Graduate Education and Research Traineeship (NSF-DGE#0801430) [to J. B.].
LITERATURE CITED
- Bates D, Maechler M, Bolker BM, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Bouma TJ, Nielsen KL, Koutstaal B. 2000. Sample preparation and scanning protocol for computerised analysis of root length and diameter. Plant and Soil 218: 185–196. [Google Scholar]
- Cayan DR, Maurer EP, Dettinger MD, Tyree M, Hayhoe K. 2008. Climate change scenarios for the California region. Climatic Change 87: 21–42. [Google Scholar]
- Chimungu JG, Loades KW, Lynch JP. 2015. Root anatomical phenes predict root penetration ability and biomechanical properties in maize (Zea mays). Journal of Experimental Botany 66: 3151–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary J. 2008. Rainfall seasonality determines annual/perennial grass balance in vegetation of Mediterranean Iberian. Plant Ecology 195: 13–20. [Google Scholar]
- Clary J. 2012. Determinants of perennial and annual grass distribution in Mediterranean-climate California. Plant Ecology 213: 1203–1208. [Google Scholar]
- Comas LH, Eissenstat DM. 2004. Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Functional Ecology 18: 388–397. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335. [Google Scholar]
- D’Antonio CM, Malmstrom C, Reynolds S, Gerlach J. 2007. Ecology of invasive non-native species in California grasslands In: Stromberg MR, Corbin JD, D’Antonio CM, eds. California grasslands: ecology and management. Berkeley, CA: University of California Press, 67–86. [Google Scholar]
- Diaz S, Hodgson JG, Thompson K, et al. 2004. The plant traits that drive ecosystems: evidence from three continents. Journal of Vegetation Science 15: 295–304. [Google Scholar]
- Diffenbaugh NS, Swain DL, Touma D. 2015. Anthropogenic warming has increased drought risk in California. In: Proceedings of the National Academy of Sciences, USA 112: 3931–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AR, Rice KJ. 1997. Intraspecific and diffuse competition: the response of Nassella pulchra in a California grassland. Ecological Applications 7: 484–492. [Google Scholar]
- Fort F, Cruz P, Jouany C. 2014. Hierarchy of root functional trait values and plasticity drive early-stage competition for water and phosphorus among grasses. Functional Ecology 28: 1030–1040. [Google Scholar]
- Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R. 2010. Evidence of the ‘plant economics spectrum’ in a subarctic flora. Journal of Ecology 98: 362–373. [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. 2001. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology 15: 688–695. [Google Scholar]
- Goergen EM, Leger EA, Espeland EK. 2011. Native perennial grasses show evolutionary response to Bromus tectorum (cheatgrass) invasion. PLoS One 6: e18145. doi:10.1371/journal.pone.0018145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist 111: 1169–1194. [Google Scholar]
- Grime JP. 2001. Plant strategies, vegetation processes, and ecosystem properties. New York: John Wiley and Sons. [Google Scholar]
- Hernández EI, Vilagrosa A, Pausas JG, Bellot J. 2010. Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant Ecology 207: 233–244. [Google Scholar]
- Hoen K. 1968. Summer dormancy in Phalaris tuberosa. Australian Journal of Agricultural Research 19: 227–239. [Google Scholar]
- Holmes TH, Rice KJ. 1996. Patterns of growth and soil-water utilization in some exotic annuals and native perennial bunchgrasses of California. Annals of Botany 78: 233–243. [Google Scholar]
- Holstein 2001. Pre-agricultural grassland in central California. Madrono 48: 253–264. [Google Scholar]
- IPCC. 2014. Climate change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge and New York: Cambridge University Press. [Google Scholar]
- Laude HM. 1953. The nature of summer dormancy in perennial grasses. Botanical Gazette 114: 284–292. [Google Scholar]
- Lavorel S, Garnier E. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisting the Holy Grail. Functional Ecology 16: 545–556. [Google Scholar]
- Lenth R, Hervé M. 2015. lsmeans: least-squares means.
- Levitt J. 1980. Responses of plants to environmental stresses. New York: Academic Press. [Google Scholar]
- Ludlow MM. 1989. Strategies of response to water stress In: Kreeb KH, Richter H, Hinckley TM, eds. Structural and functional responses to environmental stress. The Hague, The Netherlands: SPB Academic Publishing, 269–281. [Google Scholar]
- Millennium Ecosystem Assessment. 2005. Ecosystems and human well-being: synthesis. Washington, DC, Island Press. [Google Scholar]
- Norton MRA, Lelièvre FB, Fukai SC, Volaire FB. 2008. Measurement of summer dormancy in temperate perennial pasture grasses. Australian Journal of Agricultural Research 59: 498–509. [Google Scholar]
- Ofir M, Kigel J. 2003. Variation in onset of summer dormancy and flowering capacity along an aridity gradient in Poa bulbosa L., a geophytic perennial grass. Annals of Botany 91: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir M, Kigel J. 2010. Ecotypic variation of summer dormancy relaxation associated with rainfall gradient in the geophytic grass Poa bulbosa. Annals of Botany 105: 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir M, Koller D. 1974. Relationship between thermoinduction and photoinduction of flowering and dormancy in Hordeum bulbosum L., a perennial grass. Australian Journal of Plant Physiology 1: 259–270. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, et al. 2015. vegan: Community ecology package. R package.
- Pérez-Harguindeguy N, Díaz S, Lavorel S, et al. 2013. New handbook for standardized measurment of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- Pérez-Ramos IM, Volaire F, Fattet M, Blanchard A, Roumet C. 2013. Tradeoffs between functional strategies for resource-use and drought-survival in Mediterranean rangeland species. Environmental and Experimental Botany 87: 126–136. [Google Scholar]
- R Core Team. 2014. R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
- Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, et al. 2003. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences 164: S143–S164. [Google Scholar]
- Sandel B, Dangremond EM. 2012. Climate change and the invasion of California by grasses. Global Change Biology 18: 277–289. [Google Scholar]
- Shipley B, Vile D, Garnier É. 2006. From plant traits to plant communities: a statisitcal mechanistic approach to biodiversity. Science 314: 812–814. [DOI] [PubMed] [Google Scholar]
- Sibly RM, Calow P. 1989. A life-cycle theory of responses to stress. Biological Journal of the Linnean Society 37: 101–116. [Google Scholar]
- Smith MD. 2011. The ecological role of climate extremes: current understanding and future prospects. Journal of Ecology 99: 651–655. [Google Scholar]
- Tjoelker M, Craine J, Wedin D, Reich P, Tilman D. 2005. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytologist 167: 493–508. [DOI] [PubMed] [Google Scholar]
- Vaughn KJ, Biel C, Clary JJ, et al. 2011. California perennial grasses are physiologically distinct from both Mediterranean annual and perennial grasses. Plant and Soil 345: 37–46. [Google Scholar]
- Violle C, Navas M-L, Vile D, et al. 2007. Let the concept of trait be functional! Oikos 116: 882–892. [Google Scholar]
- Volaire F. 1995. Growth, carbohydrate reserves and drought survival strategies of contrasting Dactylis glomerata populations in a Mediterranean environment. Journal of Applied Ecology 32: 56–66. [Google Scholar]
- Volaire F, Norton M. 2006. Summer dormancy in perennial temperate grasses. Annals of Botany 98: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F, Thomas H. 1995. Effects of drought on water relations, mineral uptake, water-soluble carbohydrate accumulation and survival of two contrasting populations of Cocksfoot (Dactylis glomerata L.). Annals of Botany 75: 513–524. [Google Scholar]
- Volaire F, Norton MR, Lelièvre F. 2009. Summer drought survival strategies and sustainability of perennial temperate forage grasses in Mediterranean areas. Crop Science 49: 2386. [Google Scholar]
- Volaire F, Barkaoui K, Norton M. 2014. Designing resilient and sustainable grasslands for a drier future: adaptive strategies, functional traits and biotic interactions. European Journal of Agronomy 52: 81–89. [Google Scholar]
- White TA, Snow VO. 2012. A modelling analysis to identify plant traits for enhanced water-use efficiency of pasture. Crop and Pasture Science 63: 63–76. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Young SL, Kyser GB, Barney JN, Claassen VP, DiTomaso JM. 2010. Spatio-temporal relationship between water depletion and root distribution patterns of Centaurea solstitialis and two native perennials. Restoration Ecology 18: 323–333. [Google Scholar]
- Zwicke M, Picon-Cochard C, Morvan-Bertrand A, Prud’homme M-P, Volaire F. 2015. What functional strategies drive drought survival and recovery of perennial species from upland grassland? Annals of Botany 116: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]