Abstract
Background and Aims Although silicon (Si) has been widely reported to alleviate plant nutrient deficiency, the alleviating effect of Si on potassium (K) deficiency and its underlying mechanism are poorly understood. Here, we examined whether Si-regulated putrescine (Put) metabolisms are involved in Si-alleviated K deficiency.
Methods Sorghum seedlings were grown in K deficiency solution with and without Si for 15 d. The influence of K deficiency and Si on leaf chlorosis symptoms, K+ concentration, polyamine (PA) levels, amine oxidase activities, the transcription of Put synthesis genes, antioxidant enzyme activities and H2O2 accumulation were measured.
Key Results Under K-sufficient conditions, plant growth was not affected by Si application. Si application significantly alleviated the growth inhibition induced by K-deficient stress, however. K deficiency induced leaf chlorosis and reduction in several leaf chlorosis-related metrics, including photosynthesis, efficiency of photosystem II photochemistry, chlorophyll content and chlorophyll a/b ratio; all of these changes were moderated by Si application. Si application did not influence the K+ concentration in leaves under K-sufficient or K-deficient conditions. It did, however, decrease the excessive accumulation of Put that was otherwise induced by K deficiency. Simultaneously, Put synthesis gene transcription and activation of amine oxidases were down-regulated by Si application under K-deficient conditions. In addition, Si reduced K-deficiency-enhanced antioxidant enzyme activities and decreased K-deficiency-induced H2O2 accumulation.
Conclusions These results indicate that Si application could reduce K-deficiency-induced Put accumulation by inhibiting Put synthesis and could decrease H2O2 production via PA oxidation. Decreased H2O2 accumulation contributes to the alleviation of cell death, thereby also alleviating K-deficiency-induced leaf chlorosis and necrosis.
Keywords: Silicon, K deficiency, leaf chlorosis, putrescine, arginine decarboxylase, H2O2, amine oxidase, sorghum
INTRODUCTION
Potassium (K) is the most abundant cation in plants and is associated or involved in several physiological processes supporting crop growth and development (Pettigrew, 2008; Wang and Wu, 2013, 2015). The importance of K+ in photosynthesis, osmoregulation, enzyme activation, protein synthesis, ion homeostasis and the maintenance of anion–cation balances in plants is well documented (Zhao et al., 2001; Kanai et al., 2011; Epron et al., 2016). K deficiency, therefore, can result in severe reduction in plant growth and yield (Hafsi et al., 2014; Wang and Wu, 2015).
As one of the most abundant elements in the Earth’s crust, silicon (Si) is reported to be beneficial for improving plant resistance to various stresses (Epstein, 2009; Yin et al., 2016). It has also been widely reported that Si application can alleviate plant nutrient deficiencies, including K deficiency (Miao et al., 2010; Hernandez-Apaolaza, 2014). Unlike several other mechanisms by which Si is known to alleviate other stresses, the ameliorative effect of Si on K deficiency and its underlying mechanism have rarely been investigated. Miao et al. (2010) concluded that Si application alleviates K deficiency in soybeans by enhancing the efficiency of the plant’s K use and thus reducing K-deficiency-induced oxidative damage. Yet K deficiency disturbs plant growth, development and yield in various ways, and it is not clear whether there are other mechanisms by which Si helps moderate K deficiency. In addition, a better understanding of the interaction between Si application and K deficiency would provide us with information that may prove relevant to the use of Si and K fertilizer in crop production.
In plants, typical external symptoms of K deficiency include curling of leaf tips as well as leaf chlorosis, which is directly linked to chlorophyll degradation (Tian et al., 2008). Often, K+ deficiency symptoms first appear on older leaves; this may represent the plant’s remobilization of K+ from mature and senescing leaves to the youngest ones, which contributes to plant survival under K-deficient conditions (Fageria et al., 2001; Cochrane and Cochrane, 2009; Hafsi et al., 2014). Cakmak (2005) suggested that K+-deficiency-induced leaf chlorosis could be associated with the oxidative degradation of chlorophyll by reactive oxygen species (ROS), production of which is enhanced by K+ deficiency.
Polyamines (PAs), including spermidine (Spd) and spermine (Spm) as well as their diamine obligate precursor putrescine (Put), are ubiquitous low-molecular-weight aliphatic amines that are implicated in the response to a wide range of abiotic and biotic stress conditions (Bouchereau et al., 1999; Alcázar et al., 2010; Liu et al., 2015). The accumulation of Put via arginine decarboxylase (ADC) activation is widespread among mono- and dicotyledonous species and may well be a universal response to K deficiency (Richards and Coleman, 1952; Bouchereau et al., 1999). It is established that the accumulation of Put plays a specific role in maintaining a cation–anion balance in plant tissues under K+-deficient conditions (Flores, 1991; Bouchereau et al., 1999). However, feeding Put to cut leaves of barley produced the same symptoms found in K+-deficient plants (Coleman and Richards, 1956). A similar pattern of severe leaf chlorosis and necrosis has been described in inducible ADC-overexpressing tobacco plants with high Put content (Masgrau et al., 1997; Panicot et al., 2002). Subsequently, additional evidence has suggested that either Put or its oxidation products, via diamine oxidase (DAO), are responsible factors for the deleterious effects observed in these transgenic plants (Panicot et al., 2002). Together, these results suggest that K deficiency induces the accumulation of Put to maintain the cation–anion balance, and the high content of endogenous Put is toxic for plant growth and results in leaf chlorosis.
In previous research, we found that Si can alter levels of PAs by regulating the transcription levels of PA synthesis genes under drought stress and salt stress; these results showed that PAs play an important role in Si-regulated stress resistance (Yin et al., 2014, 2016; Wang et al., 2015). Given the specific role of Put in mediating plant response to K deficiency, the hypothesis that Put is involved in Si-mediated K deficiency tolerance needs to be clarified. Therefore, the effects of Si application on leaf chlorosis symptoms, K+ concentration, PA levels, amine oxidase activities, Put synthesis gene expression levels and antioxidant enzyme activities were measured under K-deficient and Si application conditions.
MATERIALS AND METHODS
Seedling cultivation and Si and K deficiency treatment
Sorghum cultivar (Sorghum bicolor [L.] Moench. ‘Gadambalia’) was used in this experiment. The seedlings were planted in a growth chamber with a 14/10-h day/night cycle at a day/night temperature of 28/25 °C, 40–50 % relative humidity. Photosynthetically active radiation to the upper plant was about 600 μmol m−2 s−1.
Sterilized seeds were germinated in an incubator at 24 °C for 4 d under dark conditions. After germination, uniform healthy seedlings were transplanted into a plastic container (40 × 28 × 14 cm) with 8 litres of one-quarter strength Hoagland culture solution with KNO3 replaced by Ca(NO3)2 to control the K concentration. Four treatments were designed for this study: control, control + Si, low K and low K + Si. The K was supplied via KCl and the K concentration was set to 3 mm in the control (K sufficiency) treatments and 0·05 mm in the low-K (K deficiency) treatments. For the Si treatment, 1 mm H2SiO3 was added to each + Si container. H2SiO3 was produced according to Yin et al. (2013) by passing sodium silicate solution through a column filled with cation-exchange resins. The pH of the culture solution was adjusted to 6·0 (Yin et al., 2013) and the culture solution was continuously aerated and renewed every 3 d. Fifteen days after transplanting, the seedlings were harvested for the purpose of measuring the parameters listed below.
Dry weight
Six seedlings were harvested and dried at 75 °C for 72 h to measure the total weight.
Photosynthetic rate
The third leaf was selected to measure the photosynthetic rate using a portable photosynthesis system (Li-6400; LI-COR Inc., Lincoln, NE, USA) between 1000 and 1200 h. A 6-cm2 chamber was used and the photo flux density was set to 1000 μmol m−2 s−1. There were four replicates in each treatment.
Chlorophyll fluorescence
Photosystem II photochemistry efficiency (Fv/Fm) in the third leaves was analysed using a pulse amplitude modulated chlorophyll fluorescence system (Imaging PAM, Walz, Effeltrich, Germany) according to the method of Chen et al. (2015). The third leaves were dark-adapted for 30 min before measurement. Each treatment included four replicates.
Chlorophyll concentration
Total chlorophyll was extracted from frozen third leaf samples (approx. 0·2 g) using 80 % acetone on a shaker at room temperature until the tissue was completely bleached. The extract was centrifuged at 5000 g for 5 min, and the supernatant was gathered for absorbance measurement at 645 and 663 nm using a spectrophotometer (UV-2550, Shimadzu, Kyoto, Japan). The chlorophyll a and chlorophyll b concentrations were calculated using the formula developed by Lichtenthaler (1987). The total chlorophyll concentration (mg g−1 d. wt) and the ratio of chlorophyll a/b were then calculated. Each treatment included four replicates.
K+ concentration
For this measurement, the old leaves (leaves 1 and 2), the third leaves and the upper younger leaves (leaves 4 and 5) were examined separately. Dried leaf samples were milled to powder for ion analysis. The powder (approx. 0·5 g) was digested with 5 mL nitric acid in a glass tube at 320 °C for 5 h on a digital well-type digestion furnace (Foss Tecator Digester 20, Hoganas, Sweden). K+ concentrations were measured using an atomic adsorption spectrometer with a flame photometer (ZL5100, PerkinElmer, Inc., Waltham, MA, USA) according to the method of Wang et al. (2015). There were three replicates in each treatment. In all three leaf age categories, K+ concentration was not affected by Si under either control or K-deficient conditions; thus, only the K+ concentrations in the third leaves are shown.
Polyamine quantification
Frozen third leaf samples (approx. 0·5 g) were ground and homogenized in 5 mL of 5 % HClO4 and extracted for 24 h on a shaker at room temperature. After centrifuging, the supernatant was used to measure free-type polyamines. The polyamine concentration was analysed by high-performance liquid chromatography (HPLC: LC-10A, Shimadzu) according to Yin et al. (2014). There were three replicates in each treatment.
Gene expression analysis
Third leaf samples were collected to measure the transcription levels of PA synthesis genes. RNA was isolated and gene expression was analysed using quantitative RT-PCR (qRT-PCR) as described by Yin et al. (2016). Actin1 was used as a reference and the primer sequences are derived from Yin et al. (2016) as listed in Table 1. The relative quantity was calculated using the 2−ΔΔt method. There were three replicates in each treatment, and each replicate included two technical replicates.
Table 1.
Genes and oligonucleotides used in the real-time quantitative PCR experiment
| Gene | Loci | Encoded protein | Primers (5′–3′) |
|---|---|---|---|
| Actin1 | Sb01g010030 | Actin | TGTTCCCTGGGATTGCTG |
| GCCGGACTCATCGTACTCA | |||
| ADC | Sb10g002070 | Arginine decarboxylase | CGGGGAGAAGGGCAAGT |
| CTGGGAGCCAATGTGGAAGT | |||
| CPA | Sb04g021790 | N-carbamoylputrescine amidohydrolase | CTTGGTCCCTCTAGTTGC |
| TTCCTCGTCTTTGTCGTT | |||
| SPDS | Sb10g020570 | Spermidine synthase | ATCGCCTTACCAAGAAAT |
| CACCTCCAACAACCAAAA |
Arginine measurement
Third leaf samples were collected and arginine content was measured by HPLC analysis of fluorescent derivatives produced by a reaction with o-phthalaldehyde (OPA) according to the method of Yin et al. (2016). There were three replicates in each treatment.
Assays of DAO and PAO activities and protein content
Frozen third leaf samples (approx. 0·5 g) were ground with a mortar and pestle at 4 °C in 1·6 mL 100 mm sodium phosphate buffer (pH 6·5). Homogenates were centrifuged at 10^000 g for 20 min at 4 °C. Supernatants were used for the determination of protein concentration and DAO and polyamine oxidase (PAO) activities. DAO and PAO activities were determined according to Su et al. (2005). Three millilitres of reaction solution contained 2·5 mL sodium phosphate buffer (100 mm, pH 6·5), 0·1 mL crude enzyme extracts, 0·1 mL peroxidase (250 U mL–1) and 0·2 ml 4-aminoantipyrine/N,N-dimethylaniline reaction solutions. The reaction was initiated by the addition of 0·1 mL of 20 mm Put or Spd as a substrate. Enzyme activity was expressed in U (1 unit is the amount of enzyme that catalyses the oxidation of 1 μmol substrate min−1) on a protein basis. Protein content was measured following the method of Bradford (1976) with bovine serum albumin (BSA) as a standard. Each treatment included four replications.
H2O2 content analysis
Hydrogen peroxide levels were determined according to the method of Yin et al. (2010). Frozen third leaf samples (approx. 0·5 g) were homogenized in an ice bath with 5 mL 0·1 % (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 12^000 g for 20 min. Two millilitres of reaction solution contained 0·5 mL extracted supernatant, 0·5 mL 10 mm potassium phosphate buffer (pH 7·0) and 1 mL 1 m KI. The absorption at 390 nm was read, and the content of H2O2 was quantified based on the standard curve. There were three replicates in each treatment.
Antioxidant enzyme activities
Frozen third leaf samples (approx. 0·5 g) were homogenized in an ice bath with 50 mm sodium phosphate buffer (pH 7·0) containing 1 mm EDTA-Na2 and 2 % (w/v) polyvinylpolypyrrolidone (PVPP). The homogenate was centrifuged at 15^000 g for 20 min at 4 °C. Supernatants were used for the enzyme activity assays. Superoxide dismutase (SOD; EC 1·15·1·1) activity was estimated based on its ability to inhibit the photochemical reduction of nitro blue tetrazolium (NBT) at 560 nm (Beauchamp and Fridovich, 1973). Catalase (CAT; EC 1·11·1·6) activity was estimated by measuring the initial rate of the disappearance of H2O2 at 240 nm as described by Hamurcu et al. (2013). Ascorbate peroxidase (APX; EC 1·11·1·11) activity was estimated based on the decrease in absorbance at 290 nm as the ascorbate is oxidized as described by Nakano and Asada (1981). There were four replicates in each treatment.
Statistical analysis
Statistical analysis was performed using SPSS statistical software (Version 19·0 for Windows, SPSS, Chicago, IL, USA). Data were analysed by analysis of variance (ANOVA) using the least significant difference (LSD) post-hoc test and a P value < 0·05 was considered significant. In the figures, different letters indicate statistically significant differences at P < 0·05. All experiments were repeated at least three times.
RESULTS
Si application alleviated K-deficiency-induced growth inhibition in sorghum
Extreme K deficiency inhibits plant growth. In this study, Si application did not affect sorghum seedling growth under control conditions. Under K-deficient conditions, however, plant growth was significantly reduced and Si application alleviated this growth inhibition (Fig. 1). The dry weight of Si-treated plants was 35 % (P < 0·05) higher than that of Si-untreated plants under K-deficient conditions. This result showed that Si application alleviated K-deficiency-induced growth inhibition.
Fig. 1.
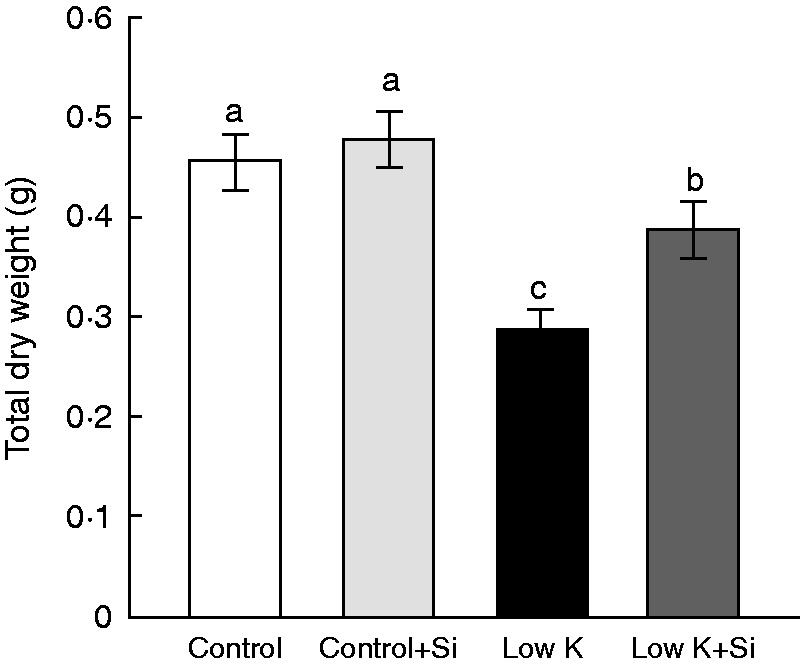
Effects of silicon (Si, 1 mm) on the growth of sorghum plants grown under control (3 mm KCl) and low-K (0·05 mm KCl) conditions for 15 d. Values are presented as the mean ± s.e. (n = 6). Different letters accompanying different values indicate statistically significant differences between the values at P < 0·05.
Si application alleviated K-deficiency-induced leaf chlorosis in sorghum
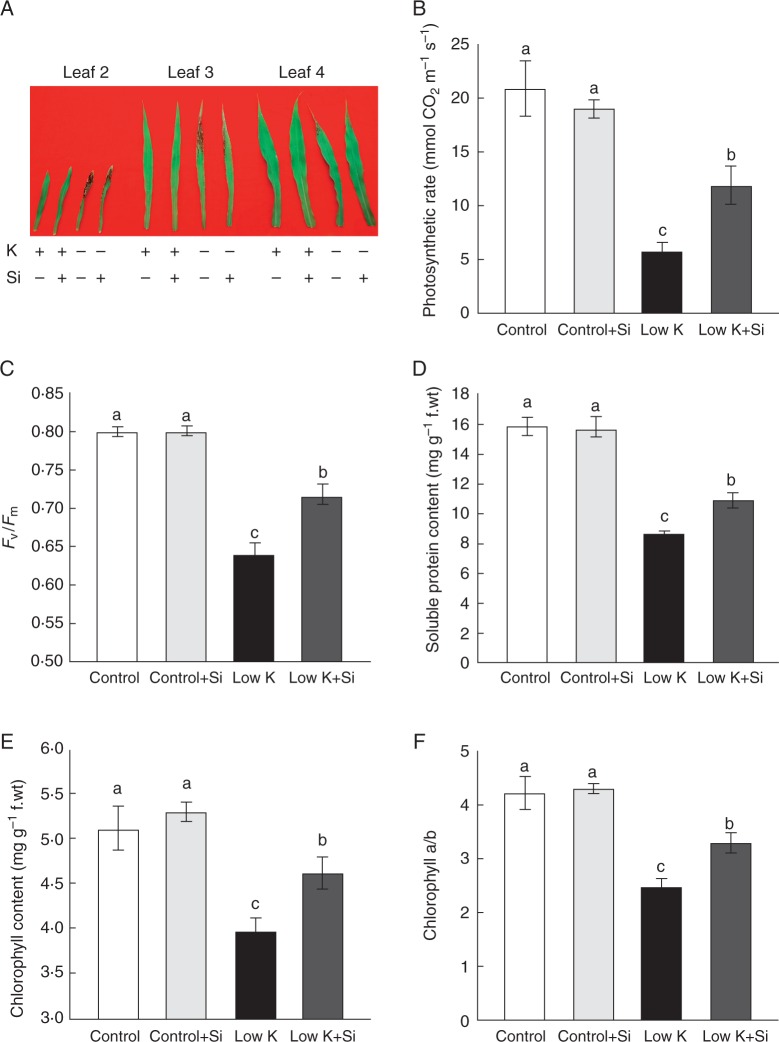
In plants, leaf chlorosis, which is directly bound up with chlorophyll degradation, is one of the typical external K+-deficiency-induced symptoms (Tian et al., 2008). In the present study, K deficiency resulted in obvious leaf chlorosis accompanied by anthocyanin accumulation and posterior necrosis in the older leaves. Si application alleviated this K-deficiency-induced leaf chlorosis and anthocyanin accumulation (Fig. 2A). Several leaf chlorosis-related symptoms in the third leaves were investigated. The leaf photosynthesis rate was decreased after 15 d of K deficiency, but Si significantly reversed this decrease (Fig. 2B). Similarly, the Fv/Fm, soluble protein content, chlorophyll content and chlorophyll a/b ratio also decreased after the 15-d K-deficiency treatment, but Si application significantly alleviated these K-deficiency-induced changes. These results indicated that Si application alleviated K-deficiency-induced leaf chlorosis.
Fig. 2.
Effects of silicon (Si, 1 mm) on leaf chlorosis of sorghum plants grown under control (3 mm KCl) and low-K (0·05 mm KCl) conditions for 15 d. (A) Phenotype of leaf chlorosis, (B) photosynthetic rate, (C) Fv/Fm, (D) soluble protein content, (E) total chlorophyll content and (F) chlorophyll a/b ratio. Values are presented as the mean ± s.e. (n = 4). Different letters accompanying different values indicate statistically significant differences between the values at P < 0·05.
Si application did not enhance leaf K+ concentration under K-deficient conditions
A major mechanism by which plants adapt to K starvation is the activation of K+ uptake through induction of the expression of high-affinity transporters (Siddiqi and Glass, 1987; Ashley et al., 2006; Ma et al., 2012). In the present study, the K+ concentration in the third leaves dramatically decreased after 15 d of K deficiency. Si application did not increase the K+ concentration in leaves under control or K-deficient conditions (Fig. 3). The similar K+ concentrations observed in Si-treated and Si-untreated plants indicates that Si’s protective effect against K-deficiency-induced leaf chlorosis was not due to increased leaf K+ concentration.
Fig. 3.
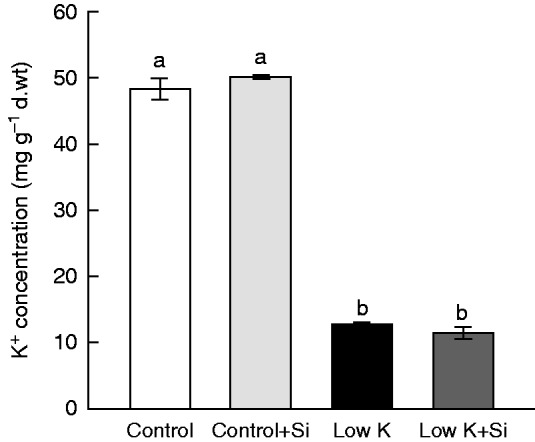
Effects of silicon (Si, 1 mm) on the third leaf K+ concentration of sorghum plants grown under control (3 mm KCl) and low-K (0·05 mm KCl) conditions for 15 d. Values are presented as the mean ± s.e. (n = 3). Different letters accompanying different values indicate statistically significant differences between the values at P < 0·05.
Si application reduced K-deficiency-induced excessive accumulation of Put
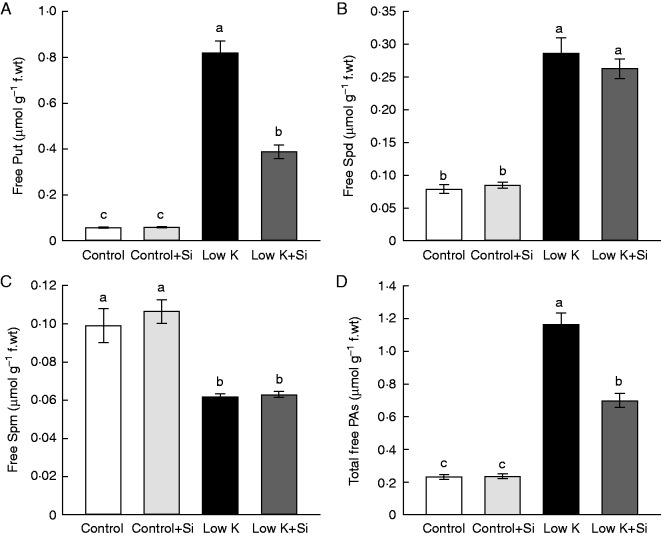
PAs are implicated in the response to a wide range of abiotic and biotic stress conditions (Bouchereau et al., 1999; Alcázar, et al., 2010; Liu et al., 2015). The accumulation of Put is considered to be a universal response to K deficiency (Richards and Coleman, 1952; Bouchereau et al., 1999). In this study, the free PAs were investigated in the third leaves after 15 d of K deficiency. As shown in Fig. 4, under control conditions, Si application did not change the PA levels. In accordance with previous studies, K deficiency induced a dramatic accumulation of Put, with the final Put concentration in K-deficient leaves 14 times that in normal-condition leaves. K deficiency also affected the levels of Spd and Spm, but less strongly than it affected Put. Under K-deficient conditions, the Spd concentration increased about three-fold, while the Spm concentration decreased by 38 % compared with normal conditions. Total PAs increased about four-fold under K-deficient conditions. Si application decreased the K-deficiency-induced Put accumulation. In Si-treated plants, the Put concentration in K-deficient leaves was 52 % lower than that in Si-untreated K-deficient plants. Si application did not change Spd and Spm levels, however. The total free PA level under K-deficient conditions was decreased by 40 % as a result of Si treatment. These results indicated that Si application decreased the K-deficiency-induced excessive accumulation of Put and that this effect could be partly responsible for Si’s ameliorative effect on leaf chlorosis induced by K deficiency.
Fig. 4.
Effects of silicon (Si, 1 mm) on the third leaf polyamine contents of sorghum plants grown under control (3 mm KCl) and low-K (0·05 mm KCl) conditions. (A) Putrescine (Put), (B) spermidine (Spd), (C) spermine (Spm) and (D) total polyamines content. All parameters were measured after 15 d of treatment. Values are presented as the mean ± s.e. (n = 3). Different letters accompanying different values indicate statistically significant differences between the values at P < 0·05.
Si application down-regulated the expression of Put-synthesis genes
The effect of Si on Put concentration might be caused by alterations in the expression patterns of genes encoding enzymes involved in Put biosynthesis. Therefore, the expression patterns of arginine decarboxylase (ADC), N-carbamoylputrescine amidohydrolase (CAP) and Spd synthase (SPDS) genes were analysed by qRT-PCR (Fig. 5). Under K-deficient conditions, mRNA levels of ADC and CAP increased 5·7- and 2·9-fold, respectively, compared with their levels under control conditions; under K-deficient conditions with Si treatment, they still increased, but to a lesser degree (1·5- and 0·5-fold). Regarding the SPDS gene, its expression level increased 2·3-fold under K-deficient conditions, but decreased significantly under K-deficient conditions with Si treatment. These results suggested that the down-regulation of these Put-synthesis genes by Si application under K-deficient conditions might be responsible for the ameliorative effect of Si application on the K-deficiency-induced excessive accumulation of Put.
Fig. 5.
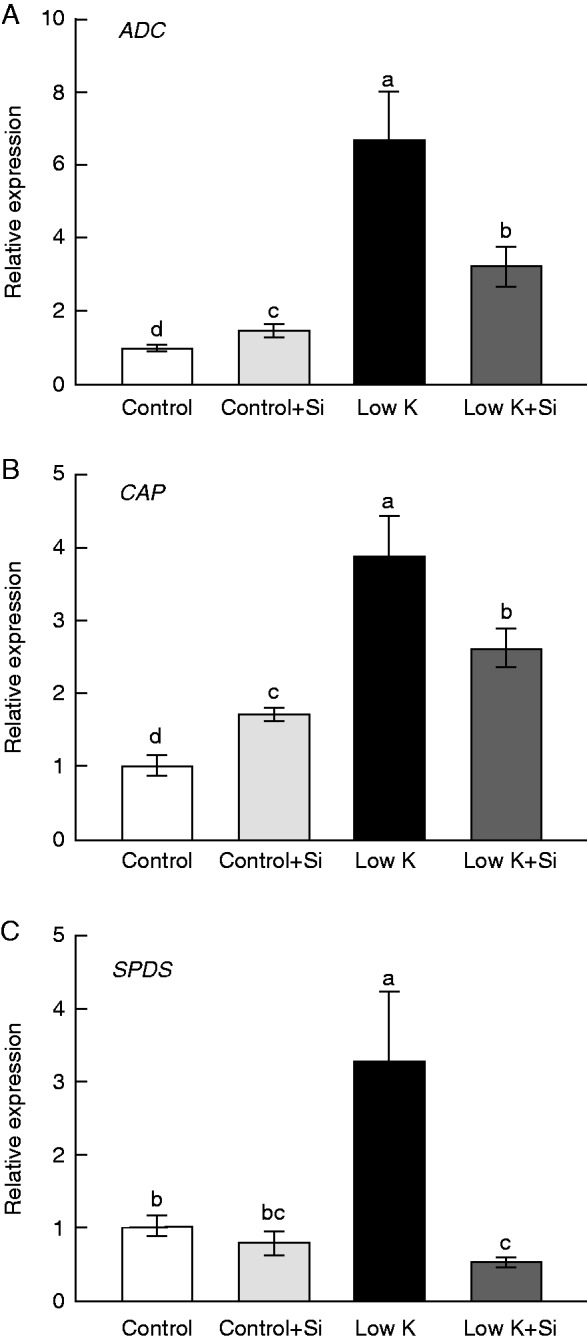
Effects of silicon (Si, 1 mm) on expression of putrescine synthesis-related genes encoding arginine decarboxylase (ADC) (A), N-carbamoylputrescine amidohydrolase (CAP) (B) and spermidine synthase (SPDS) (C) in third leaves of sorghum plants with and without Si application grown under control (3 mm KCl) and low-K (0·05 mm KCl) conditions. Each treatment includes three replications and each replication includes three technical replications. Different letters accompanying different values indicate statistically significant differences between the values at P < 0·05.
Si application decreased K-deficiency-induced arginine accumulation
To further clarify how Si affects Put synthesis, arginine, the substrate for Put synthesis, was investigated after 15 d of K deficiency and Si treatment. Under K-sufficient conditions, arginine concentrations were not affected by Si treatment (Fig. 6). K deficiency induced the accumulation of arginine in leaves to a concentration 37 % higher than that under normal growth conditions. Under K-deficient conditions with Si treatment, however, this increase did not occur. This result suggested that Si application may alleviate K-deficiency-induced excessive accumulation of Put by regulating arginine metabolism.
Fig. 6.
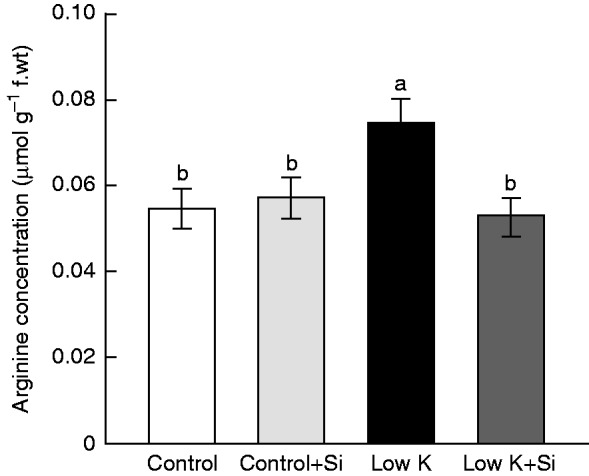
Effects of silicon (Si, 1 mm) on the third leaf arginine content of sorghum plants grown under control (3 mm KCl) and low-K (0·05 mm KCl) conditions for 15 d. Values are presented as the mean ± s.e. (n = 3). Different letters accompanying different values indicate statistically significant differences between the values at P < 0·05.
Si application reversed K-deficiency-induced elevation of DAO and PAO activities
It has been reported that PA catabolism, which is mediated by the amine oxidases DAO and PAO, leads to an accumulation of oxidation products (e.g. hydrogen peroxide), which may cause cellular damage (Cona et al., 2006; Moschou et al., 2008). Accordingly, DAO and PAO activities were measured in the present study (Fig. 7). Under control conditions, Si application had no influence on the activity of either DAO or PAO. Under K-deficient conditions, DAO and PAO activities increased by 42 and 35 %, respectively, but these increases were eliminated by Si application. This result showed that Si application decreases K-deficiency-induced activation of amine oxidases.
Fig. 7.
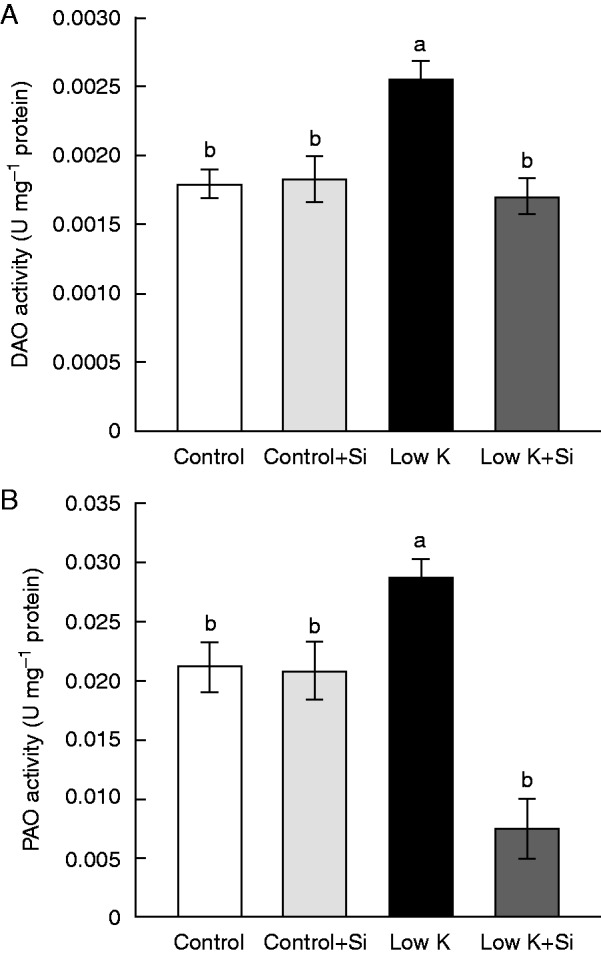
Effects of silicon (Si, 1 mm) on diamine oxidase (DAO) (A) and polyamine oxidase (PAO) (B) activities in third leaves of sorghum plants with and without Si application grown under control (3 mm KCl) and low-K (0·05 mm KCl) conditions. Values are presented as the mean ± s.e. (n = 4). Different letters accompanying different values indicate statistically significant differences between the values at P < 0·05.
Si application decreased K-deficiency-induced H2O2 accumulation
In addition, H2O2 content was determined after 15 d of K deficiency and Si treatment. As shown in Fig. 8, under control conditions, Si application did not change the H2O2 level. K deficiency induced a dramatic accumulation of H2O2 in leaves, with a final H2O2 concentration 6-fold greater than normal. In Si-treated K-deficient plants, however, this accumulation was decreased by half. This result showed that Si application alleviated the K-deficiency-induced accumulation of H2O2.
Fig. 8.
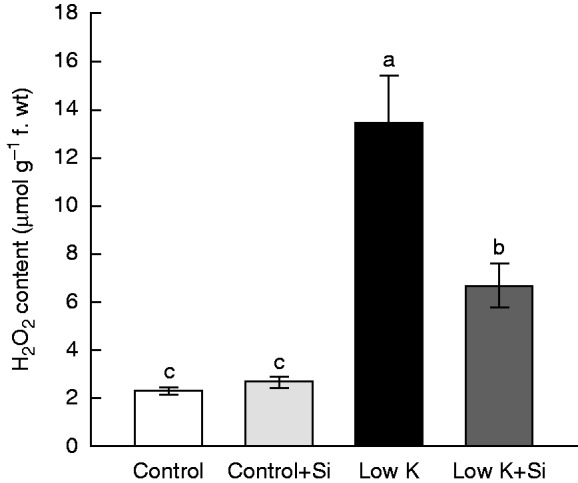
Effects of silicon (Si, 1 mm) on the third leaf H2O2 concentration of sorghum plants grown under control (3 mm KCl) and low-K (0·05 mm KCl) conditions for 15 d. Values are presented as the mean ± s.e. (n = 3). Different letters accompanying different values indicate statistically significant differences between the values at P < 0·05.
Si application reduced K-deficiency-enhanced antioxidant enzyme activities
Si application has been widely reported to influence antioxidant enzyme activities and to decrease ROS accumulation under various stresses (Liang et al., 2007). Accordingly, the effect of K deficiency on activities of antioxidant enzymes in the absence and presence of Si was investigated (Fig. 9). Under control conditions, Si application had no influence on the antioxidant enzyme activities. Under K-deficient conditions, SOD, CAT and APX activities increased by 207, 39 and 46 %, respectively, compared with control conditions. Under K deficiency with Si treatment, however, the increase in SOD activity was less dramatic (96 %) and the increases in CAT and APX activities were eliminated. This result indicated that Si application reduced K-deficiency-enhanced antioxidant enzyme activities.
Fig. 9.
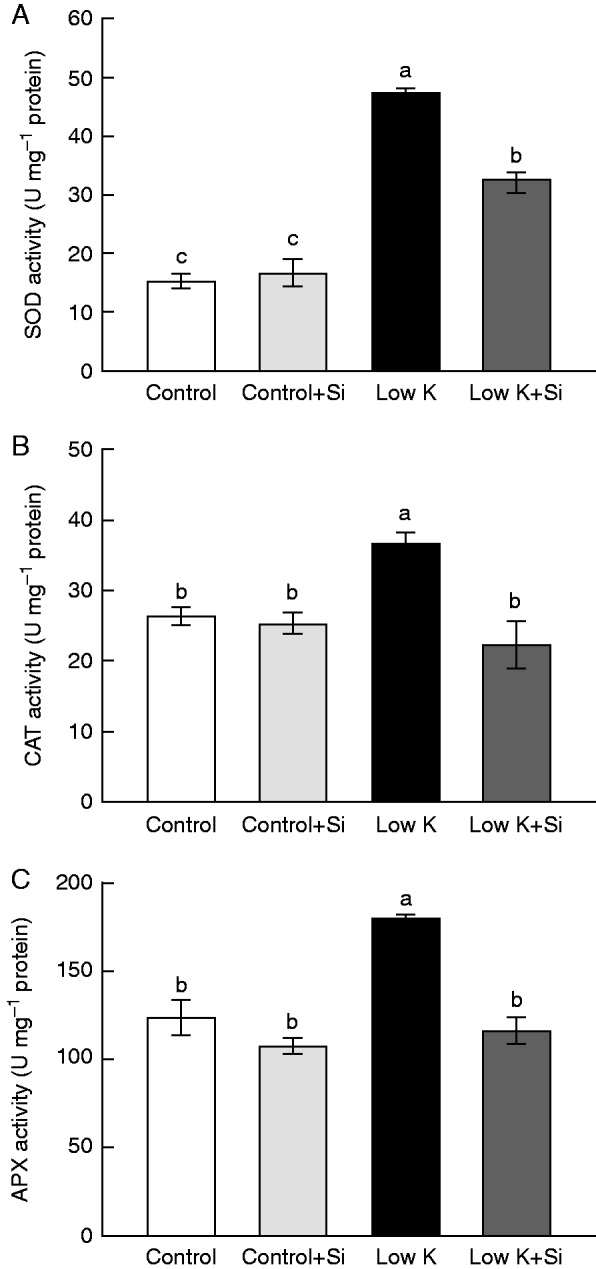
Effects of silicon (Si, 1 mm) on activities of (A) superoxide dismutase (SOD), (B) peroxidase (POD) and (C) catalase (CAT) in third leaves of sorghum plants with and without Si application grown under control (3 mm KCl) and low-K (0·05 mm KCl) conditions. Values are presented as the mean ± s.e. (n = 4). Different letters accompanying different values indicate statistically significant differences between the values at P < 0·05.
DISCUSSION
Severe K deficiency initially inhibits plant growth; with prolonged exposure time, leaf necrosis appears in old leaves (Mengel and Kirkby, 2001). In this study, Si-treated K-deficient plants maintained a higher dry weight than Si-untreated K-deficient plants (Fig. 1), indicating that Si application alleviated the growth inhibition associated with K deficiency. Severe K deficiency is also reported to induce leaf chlorosis in older leaves, which is directly bound up with chlorophyll degradation (Fageria et al., 2001; Tian et al., 2008). In this study, Si application alleviated K-deficiency-induced leaf chlorosis (Fig. 2). Activated K+ uptake by induction of high-affinity transporter expression is considered to be a major mechanism of plant adaptation to K starvation (Ashley et al., 2006; Ma et al., 2012). Yet the K+ concentration in leaves was not affected by Si application (Fig. 3), suggesting that Si’s ability to alleviate K-deficiency-induced leaf chlorosis is not due to increased leaf K+ concentration.
The accumulation of Put is considered to be a universal response to K deficiency (Bouchereau et al., 1999). High concentrations of endogenous Put are toxic to plant growth and result in leaf chlorosis (Masgrau et al., 1997; Panicot et al., 2002). In this study, leaf Put concentration increased 13-fold under K-deficient conditions, but increased only 6-fold under K-deficient conditions with Si treatment, indicating that Si application alleviated the K-deficiency-induced excessive accumulation of Put (Fig. 4). The results of this study suggest that Si application may alleviate K-deficiency-induced leaf chlorosis by reducing the excessive accumulation of Put.
It has been reported that the accumulation of Put in response to K deficiency occurs via the activation of ADC, which catalyses the decarboxylation of arginine for Put biosynthesis (Bouchereau et al., 1999). To investigate how Si mediates Put synthesis, the transcription levels of Put biosynthesis genes were analysed by qRT-PCR. The results revealed that ADC was up-regulated under K-deficient conditions and down-regulated by Si treatment under K-deficient conditions. Two other enzymes in the ADC pathway, agmatine iminohydrolase (AIH) and CAP, are also involved in Put biosynthesis (Alcázar et al., 2010). In this study, AIH expression was not investigated, but CAP, like ADC, was found to be down-regulated by Si treatment under K-deficient conditions. These results showed that Si may mediate Put synthesis through down-regulation of the ADC pathway. In addition, arginine concentration was enhanced by K deficiency, but not by K deficiency plus Si (Fig. 5), supporting the idea that Si application alleviated Put synthesis under K-deficient conditions.
It has been proposed that PAs are important substrates for H2O2 production, as they are degraded by DAO or PAO (Yoda et al., 2003, 2006). Yet the relationship between PAs and ROS is questionable because it has been suggested that PAs protect cells against ROS either by eliminating ROS (Das and Misra, 2004) or by inhibiting their formation (Papadakis and Roubelakis-Angelakis, 2005). At the same time, however, it is also postulated that PAs are ROS producers (H2O2) during their catabolism (Cona et al., 2006; Moschou et al., 2008). In this study, the activities of both DAO and PAO increased under K-deficient conditions, concomitant with an excessive degree of H2O2 accumulation (5·8-fold), while Si application inhibited the activation of amine oxidases and diminished the degree of H2O2 accumulation (2·5-fold) (Figs 7 and 8). Here, the activation of PAO was also inhibited by Si. This effect might be caused by the reduction of Spd biosynthesis by Si under K-deficient conditions as indicated by the down-regulation of the expression of SPDS, which catalyses the synthesis of Spd from Put (Fig. 5C). These results suggested that Si’s ability to decrease the K-deficiency-induced accumulation of H2O2 is caused, at least in part, by the inhibition of PA degradation via amine oxidases.
It has been widely reported that Si influences antioxidant enzyme activities and decreases ROS accumulation under various stresses, such as heavy metal toxicities, salt and freezing stress (Liang et al., 2007, 2008). In the present study, the activities of SOD, CAT and APX were increased by K deficiency, but these increases were markedly reduced by Si. This is consistent with the findings of a study on soybeans in which K-deficiency-induced accumulation of H2O2 and elevation of antioxidant enzyme activities were markedly decreased by Si (Miao et al., 2010). This result indicated that Si’s ability to decrease K-deficiency-induced H2O2 accumulation was not related to the improved scavenging due to increased antioxidant enzyme activities.
Hydrogen peroxide has long been considered a causative element for apoptotic cell death in plants (Hussain et al., 2011). Cakmak (2005) suggested that K-deficiency-induced leaf chlorosis and necrosis could be associated with the oxidative degradation of chlorophyll by ROS, whose production is enhanced by K+ deficiency. PAs have been postulated as inducers of plant cell death, with H2O2 suspected to be involved in the mechanism (Yoda et al., 2003, 2006; Iannone et al., 2013). It has also been reported that Put concentration is correlated with the severity of growth inhibition, leaf chlorosis and necrosis phenotype in ADC-overexpressing tobacco plants (Masgrau et al., 1997). In addition, phenotypic abnormalities have been observed in other studies with PA transgenic plants (Kumar et al., 1996; Kumria and Rajam, 2002; Waie and Rajam, 2003). Therefore, high concentrations of cellular PAs are cytotoxic. Another study has suggested that either Put or one of its oxidation products via DAO is a factor responsible for the deleterious effects observed in transgenic plants (Panicot et al., 2002). In this study, K deficiency induced leaf chlorosis in conjunction with excessive Put and H2O2 accumulation, but these changes were reversed by Si application, indicating that Si application may alleviate K-deficiency-induced leaf chlorosis by decreasing H2O2 via the inhibition of Put accumulation and oxidation.
In conclusion, the present study suggests that Si application could alleviate K-deficiency-induced leaf chlorosis by reducing the excessive accumulation of Put. It extends our current understanding of the interaction between Si application and K deficiency and will provide information about Si and K fertilizer use in crop production. Perhaps Si supplied by mineral soils or Si fertilizer will help plants grow in K-deficient soils. Combining the present findings with those of previous studies, a model describing how Si is involved in alleviating K deficiency by reducing the excessive accumulation of Put is proposed (Fig. 10). (1) Under K-deficient conditions, Si reduces the arginine content and depresses activation of the ADC pathway, suppressing excessive Put accumulation. (2) Si depresses the activation of amine oxidase activities. (3) These reductions in Put and amine oxidase activities contribute to decreasing the H2O2 production. (3) The decreased H2O2 accumulation contributes to the alleviation of cell death, thereby alleviating K-deficiency-induced leaf chlorosis and necrosis. To summarize, Si application may alleviate K-deficiency-induced leaf chlorosis by decreasing H2O2 via inhibiting Put synthesis and oxidation.
Fig. 10.
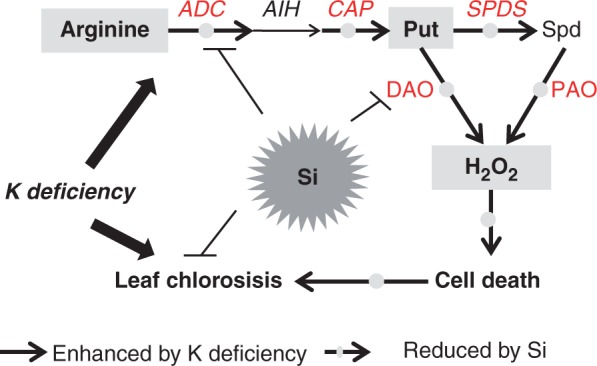
A model depicting how Si is involved in alleviating K deficiency by reducing the excessive accumulation of putrescine. Under K-deficient conditions, Si reduces the increase in arginine content and depresses activation of the ADC pathway by down-regulating the expression of arginine decarboxylase (ADC) and N-carbamoylputrescine amidohydrolase (CAP) (indicated by red and bold font), reducing the excessive accumulation of Put; Si also depresses the activation of diamine oxidase (DAO) and polyamine oxidase (PAO) activities. The reduced Put and depressed amine oxidase activities contribute to decreased H2O2 production, which contributes to the alleviation of cell death, leading to the alleviation of K-deficiency-induced leaf chlorosis and necrosis.
ACKNOWLEDGEMENTS
We thank Peng Liu, Meijuan Zhang and Binglin Xiong for their assistance with data collection. This work was supported by Youth Innovation Promotion Association of the Chinese Academy of Sciences (2013307), National Key Technology R&D Program (2015BAD22B01) and National Basic Research Program of China (2015CB150402).
LITERATURE CITED
- Alcázar R, Altabella T, Marco F, et al. 2010. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231: 1237–1249. [DOI] [PubMed] [Google Scholar]
- Ashley MK, Grant M, Grabov A. 2006. Plant responses to potassium deficiencies: a role for potassium transport proteins. Journal of Experimental Botany 57: 425–436. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. 1973. Isozymes of superoxide dismutase from wheat germ. Biochimica et Biophysica Acta 317: 50–64. [DOI] [PubMed] [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. 1999. Polyamines and environmental challenges: recent development. Plant Science 140: 103–125. [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Cakmak I. 2005. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. Journal of Plant Nutrition and Soil Science 168: 521–530. [Google Scholar]
- Chen D, Wang S, Xiong B, Cao B, Deng X. 2015. Carbon/nitrogen imbalance associated with drought-induced leaf senescence in Sorghum bicolor. PLoS One 10: e0137026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane TT, Cochrane TA. 2009. The vital role of potassium in the osmotic mechanism of stomata aperture modulation and its link with potassium deficiency. Plant Signaling & Behavior 4: 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RG, Richards FJ. 1956. Physiological studies in plant nutrition: XVIII. Some aspects of nitrogen metabolism in barley and other plants in relation to potassium deficiency. Annals of Botany 20: 393–409. [Google Scholar]
- Cona A, Rea G, Angelini R, Federico R, Tavladoraki P. 2006. Functions of amine oxidases in plant development and defence. Trends in Plant Science 11: 80–88. [DOI] [PubMed] [Google Scholar]
- Das KC, Misra HP. 2004. Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Molecular and Cellular Biochemistry 262: 127–133. [DOI] [PubMed] [Google Scholar]
- Epron D, Cabral OMR, Laclau JP, et al. 2016. In situ (CO2)-C-13 pulse labelling of field-grown eucalypt trees revealed the effects of potassium nutrition and throughfall exclusion on phloem transport of photosynthetic carbon. Tree Physiology 20: 9–15. [DOI] [PubMed] [Google Scholar]
- Epstein E. 2009. Silicon: its manifold roles in plants. Annals of Applied Biology 155: 155–160. [Google Scholar]
- Fageria NK, Filho M, Costa J. 2001. Potassium-use efficiency in common bean genotypes. Journal of Plant Nutrition 24: 1937–1945. [Google Scholar]
- Flores HE. 1991. Changes in polyamine metabolism in response to abiotic stress In: Slocum R, Flores HE, eds. The biochemistry and physiology of polyamines in plants. Boca Raton, FL: CRC Press, 214–225. [Google Scholar]
- Hafsi C, Debez A, Abdelly C. 2014. Potassium deficiency in plants: effects and signaling cascades. Acta Physiologiae Plantarum 36: 1055–1070. [Google Scholar]
- Hamurcu M, Sekmen AH, Turkan I, Gezgin S, Demiral T, Bell RW. 2013. Induced anti-oxidant activity in soybean alleviates oxidative stress under moderate boron toxicity. Journal of Plant Growth Regulation 70: 217–226. [Google Scholar]
- Hernandez-Apaolaza L. 2014. Can silicon partially alleviate micronutrient deficiency in plants? A review. Planta 240: 447–458. [DOI] [PubMed] [Google Scholar]
- Hussain SS, Ali M, Ahmad M, Siddique KH. 2011. Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnology Advances 29: 300–311. [DOI] [PubMed] [Google Scholar]
- Iannone MF, Rosales EP, Groppa MD, Benavides MP. 2013. H2O2 involvement in polyamine-induced cell death in tobacco leaf discs. Journal of Plant Growth Regulation 32: 745–757. [Google Scholar]
- Kanai S, Moghaieb RE, El-Shemy HA, et al. 2011. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Science 180: 368–374. [DOI] [PubMed] [Google Scholar]
- Kumar A, Taylor MA, Arif SAM, Davies HV. 1996. Potato plants expressing antisense and sense S-adenosylmethionine decarboxylase (SAMDC) transgenes show altered levels of polyamines and ethylene: antisense plants display abnormal phenotypes. Plant Journal 9: 147–158. [Google Scholar]
- Kumria R, Rajam MV. 2002. Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro morphogenesis and response to salt stress. Journal of Plant Physiology 159: 183–190. [Google Scholar]
- Liang Y, Sun W, Zhu Y, Christie P. 2007. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environmental Pollution 147: 422–428. [DOI] [PubMed] [Google Scholar]
- Liang Y, Zhu J, Li Z, et al. 2008. Role of silicon in enhancing resistance to freezing stress in two contrasting winter wheat cultivars. Environmental and Experimental Botany 64: 286–294. [Google Scholar]
- Lichtenthaler HK. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology 148: 350–382. [Google Scholar]
- Liu J, Wang W, Wu H, Gong X, Moriguchi T. 2015. Polyamines function in stress tolerance: from synthesis to regulation. Frontiers in Plant Science 6: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Wu W, Wang Y. 2012. Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biology 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masgrau C, Altabella T, Farras R. 1997. Inducible overexpression of oat arginine decarboxylase in transgenic tobacco plants. Plant Journal 11: 465–473. [DOI] [PubMed] [Google Scholar]
- Mengel K, Kirkby EA. 2001. Principles of plant nutrition. Dordrecht: Kluwer. [Google Scholar]
- Miao B, Han X, Zhang W. 2010. The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Annals of Botany 105: 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou PN, Delis ID, Paschalidis KA, Roubelakis-Angelakis KA. 2008. Transgenic tobacco plants overexpressing polyamine oxidase are not able to cope with oxidative burst generated by abiotic factors. Physiologia Plantarum 133: 140–156. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22: 867–880. [Google Scholar]
- Panicot M, Masgrau C, Borrell A, Cordeiro A, Tiburcio AF, Altabella T. 2002. Effects of putrescine accumulation in tobacco transgenic plants with different expression levels of oat arginine decarboxylase. Physiologia Plantarum 114: 281–287. [DOI] [PubMed] [Google Scholar]
- Papadakis AK, Roubelakis-Angelakis KA. 2005. Polyamines inhibit NADPH oxidase-mediated superoxide generation and putrescine prevents programmed cell death induced by polyamine oxidase-generated hydrogen peroxide. Planta 220: 826–837. [DOI] [PubMed] [Google Scholar]
- Pettigrew WT. 2008. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiologia Plantarum 133: 670–681. [DOI] [PubMed] [Google Scholar]
- Richards FJ, Coleman RG. 1952. Occurrence of putrescine in potassium-deficient barley. Nature 170: 460. [DOI] [PubMed] [Google Scholar]
- Siddiqi MY, Glass A. 1987. Regulation of K+ influx in barley: evidence for a direct control of influx by K+ concentration of root cells. Journal of Experimental Botany 38: 935–947. [Google Scholar]
- Su G, An Z, Zhang W, Liu Y. 2005. Light promotes the synthesis of lignin through the production of H2O2 mediated by diamine oxidases in soybean hypocotyls. Journal of Plant Physiology 162: 1297–1303. [DOI] [PubMed] [Google Scholar]
- Tian X, Wang G, Zhu R, Yang P, Duan L, Zhao H. 2008. Conditions and indicators for screening cotton (Gossypium hirsutum) genotypes tolerant to low-potassium. Acta Agronomica Sinica 34: 1435–1443. [Google Scholar]
- Wang S, Liu P, Chen D, Yin L, Li H, Deng X. 2015. Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Frontiers Plant Science 6: 759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu W. 2013. Potassium transport and signaling in higher plants. Annual Review of Plant Biology 64: 451–476. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu W. 2015. Genetic approaches for improvement of the crop potassium acquisition and utilization efficiency. Current Opinion in Plant Biology 25: 46–52. [DOI] [PubMed] [Google Scholar]
- Waie B, Rajam MV. 2003. Effect of increased polyamine biosynthesis on stress responses in transgenic tobacco by introduction of human S-adenosylmethionine gene. Plant Science 164: 727–734. [Google Scholar]
- Yin L, Wang S, Eltayeb AE, et al. 2010. Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231: 609–621. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang S, Li J, Tanaka K, Oka M. 2013. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiologiae Plantarum 35: 3099–3107. [Google Scholar]
- Yin L, Wang S, Liu P, et al. 2014. Silicon-mediated changes in polyamine and 1-aminocyclopropane-1-carboxylic acid are involved in silicon-induced drought resistance in Sorghum bicolor L. Plant Physiology and Biochemistry 80: 268–277. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang S, Li J, Tanaka K, et al. 2016. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant, Cell and Environment 39: 245–258. [DOI] [PubMed] [Google Scholar]
- Yoda H, Yamaguchi Y, Sano H. 2003. Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant Physiology 132: 1973–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda H, Hiroi Y, Sano H. 2006. Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiology 142: 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Oosterhuis DM, Bednarz CM. 2001. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica 38: 103–109. [Google Scholar]