Abstract
Introduction:
Conventional methods to estimate the time of death are adequate, but a histological method is yet unavailable to assess postmortem interval (PMI). The autolytic changes that occur in an unfixed antemortem gingival tissue which reflects histologically at an early stage are similar to changes that occur in postmortem tissue. These histological changes can be used and applied in a postmortem tissue as a method to assess PMI.
Aims:
The aim of the study is to assess the histological changes in a gingival tissue left unfixed for various time intervals and to correlate the findings with duration.
Materials and Methods:
Sixty gingival tissues obtained from patients following therapeutic extractions, impactions, gingivectomy and crown lengthening procedures were used. Each tissue obtained was divided into two pieces and labeled as “A”, the control group and “ B” the study group. Tissues labeled “A” were fixed in 10% formalin immediately and tissues labeled“B” were placed in closed containers and fixed after 15, 30, 45 min, 1, 2, and 4 h time interval. Of the sixty tissues in the study group “ B”, ten tissues were used for each time interval under investigation. All the fixed tissues were processed, stained, assessed, and analyzed statistically using Pearson correlation and regression analysis.
Results:
Histological changes appear at 15 min in an unfixed antemortem tissue. At 2 h interval, all layers with few cells in basal cell layer are involved. At 4 h interval, loss of stratification and complete homogenization of cells in the superficial layers with prominent changes in basal layer is evident. There was a positive correlation (<1.0) between the time interval and the appearance of the histological changes.
Conclusion:
Histological changes such as complete homogenization of cells in superficial layers and loss of epithelial architecture at 4 h in unfixed antemortem tissue may be used as a criterion to estimate PMI, after further studies on postmortem tissues.
Keywords: Autolysis, homogenization, postmortem interval
Introduction
Postmortem interval (PMI) describes the period elapsed from the time of death. Estimation of the PMI is of prime importance in the forensic arena.[1] Usually, the PMI is estimated by assessing the postmortem morphological changes that include algor mortis, rigor mortis, livor mortis, postmortem clotting, putrefaction, and adipocere formation.[2] Although there are various methods adopted to estimate the PMI, newer methods are being investigated as an additional aid so that a combination of different methods can give a accurate value.[1]
A tissue after removal from the body has to be fixed in formalin immediately for histological study. When it is left unfixed, it shows both morphological and histological changes but the histological changes precede the morphological change. Many authors have studied the histological changes in antemortem gingival tissue left unfixed for various time intervals and compared with postmortem tissue before freezing.[2,3] The present study deals with identification of early histological changes in antemortem gingival tissues which are left unfixed for various time intervals and the changes when correlated with duration can be helpful in estimating PMI.
Aims
To study the histological changes in a gingival tissue left unfixed for various time intervals
To analyze and correlate the histological findings with duration of unfixation
To compare the results with the previous studies conducted at postmortem level.
Materials and Methods
The ethical clearance was obtained from the Institutional Ethical Committee, and informed consent was obtained from the patients. The present study was conducted on gingival tissues obtained from patients following therapeutic extractions, impactions, gingivectomy, and crown lengthening procedures from the Department of Oral Surgery and Periodontics, C.S.I. College of Dental Sciences and Research, Madurai. Sixty gingival tissues were collected, in person, and the time of removal was noted down. The mean age of the patients selected for the study ranged from 21 to 35 years. Of sixty patients, thirty-five were male and the remaining twenty-five were female patients.
Inclusion criteria: Patients with clinically healthy gingival tissues.
Exclusion criteria: Patients with systemic diseases such as diabetes, hypertension, anemia, and patients with lesions involving gingiva.
The obtained tissues were washed well and each tissue was divided into two pieces and labeled as “A” the control group and “B” the study group. The tissues named “A” were fixed in 10% formalin immediately after removal and tissues named “B” were placed in closed containers at room temperature and fixed in formalin after 15 min, 30 min, 45 min, 1 h, 2 h, and 4 h time interval. Of the sixty samples in the study group “B”, ten samples were used for each time interval under investigation.
All the fixed tissues both in control and study group were processed and stained using hematoxylin and eosin stains. The sections were assessed for changes at the epithelial level by studying the nuclear and cytoplasmic details and the epithelial architecture at ×40, ×100, and ×200 magnifications using a Olympus trinocular research microscope (model BX53). Statistical analysis was done by Pearson correlation and regression analysis using SPSS version 20 (IBM, Armonk, NY, United States of America), to analyze the correlation between the histological changes and time intervals.
Results
The following histological changes were observed in all the tissues under the study group at different time intervals:
Chromatin clumping: The chromatin is fragmented forming visible clumps within the nucleus
Nuclear vacuolation
Karyopyknosis: Shrinkage of the nucleus and increased basophilia
Prominent and widened intercellular junction
Eosinophilia: Bright pink staining of the cytoplasm
Homogenization: Merging of cellular outlines leading to a glossy, homogenous appearance
Loss of epithelial architecture.
Changes at 15 min
Isolated cells showing chromatin clumping were seen scattered in superficial and prickle cell layers of the epithelium. Nuclear vacuolation was evident in a few cells. Prominent and widened intercellular bridges were seen in the superficial layers of the epithelium [Figure 1].
Figure 1.
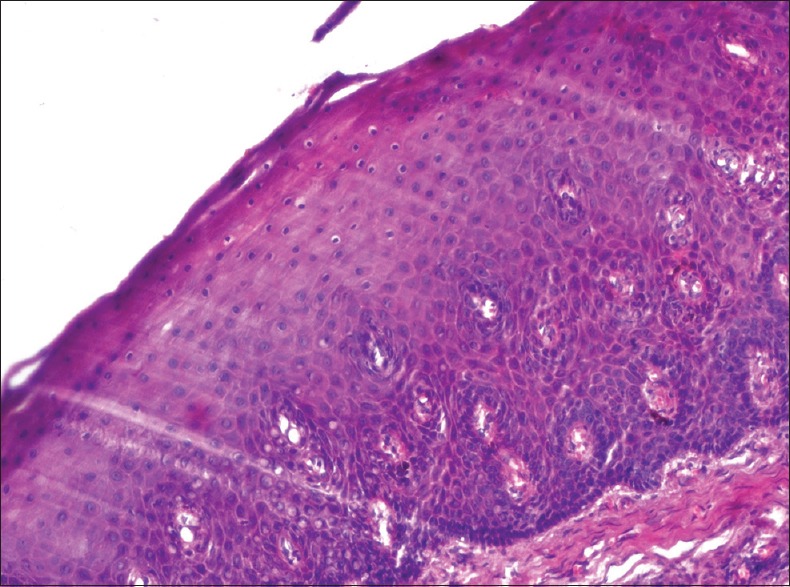
Photomicrograph showing histological changes in an antemortem unfixed gingival tissue at 15 min (H and E, ×200) isolated cells showing chromatin clumping, nuclear vacuolation, and prominent and widened intercellular bridges
Changes at 30 min
The histological changes observed were the same as seen in 15 min, except that numerous cells were involved. Pyknosis of nuclei was observed in the upper layers of the epithelium.
Changes at 45 min
Similar changes observed at 30 min interval, but very prominent and numerous in the upper layers of the epithelium.
Changes at 1 h
Similar changes observed at 30 min interval, involving all the suprabasal layers of the epithelium.
Changes at 2 h
Eosinophilia and loss of intercellular bridges, called homogenization appears in the superficial layer and prickle cell layer. The nuclear changes were seen involving the suprabasal layers and few cells in basal cell layer [Figure 2].
Figure 2.
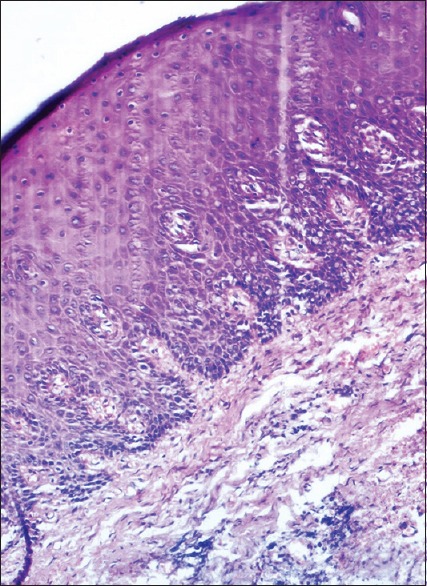
Photomicrograph showing histological changes in an antemortem unfixed gingival tissue at 2 h (H and E, ×100) nuclear changes such as vacuolation and pyknosis involving basal cell layer and homogenization of cells in superficial layer
Changes at 4 h
Homogenization and loss of epithelial architecture were evident in the superficial layers of epithelium [Figures 3 and 4]. Nuclear changes were very prominent involving the basal cell layer [Figure 5].
Figure 3.
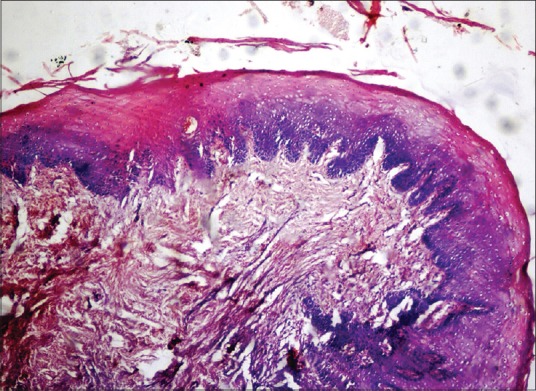
Photomicrograph showing histological changes in an antemortem unfixed gingival tissue at 4 h (H and E, ×40) homogenization and loss of epithelial architecture in the superficial layers
Figure 4.
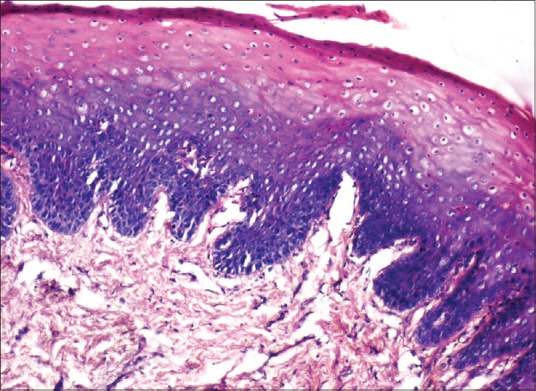
Photomicrograph showing histological changes in an antemortem unfixed gingival tissue at 4 hours (H and E, ×100)
Figure 5.
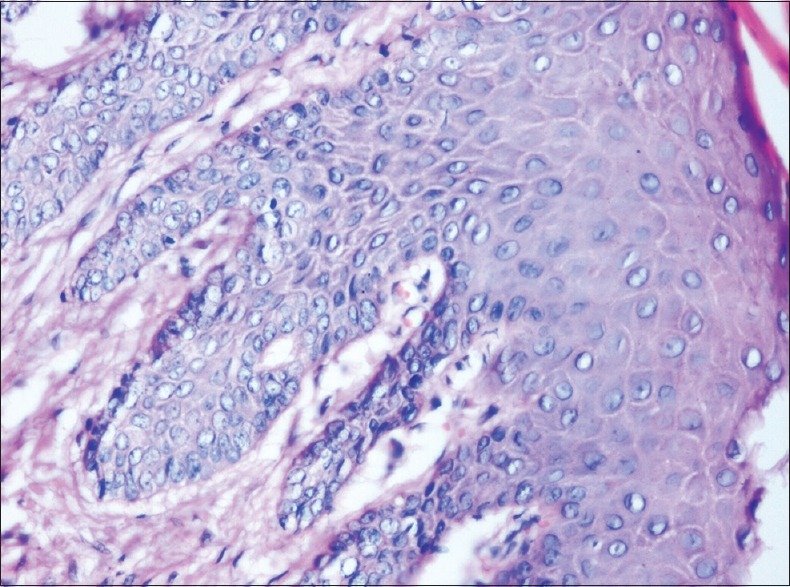
Photomicrograph showing histological changes in an antemortem unfixed gingival tissue at 4 hours (H and E, ×200) nuclear changes involving the cells of the basal layer
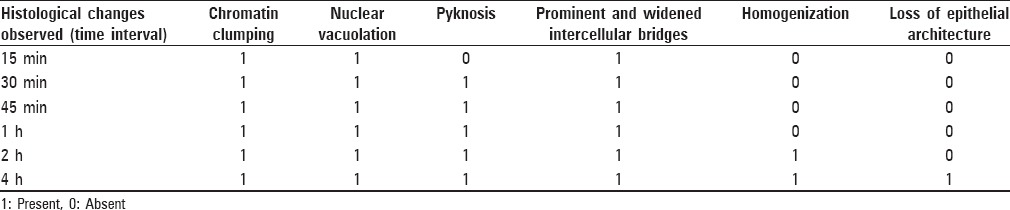
From the above-mentioned results, it is seen that autolytic changes begin as early as 15 min in an unfixed antemortem tissue and the changes increase proportionately with time. There is no much difference in the changes observed in the sections between 15 min and 1 h, but at 2 h interval, few cells of the basal cell layer are involved. At 4 h interval, changes are evident in all the layers of the epithelium. Complete homogenization of cells and loss of stratification and epithelial architecture above the basal layer of the epithelium are evident at 4 h [Table 1].
Table 1.
Presence or absence of histological changes at different time intervals
Statistical analysis showed that there was a positive correlation (correlation coefficient, r < 1.0) between the time intervals and the appearance of the histological changes [Figure 6].
Figure 6.
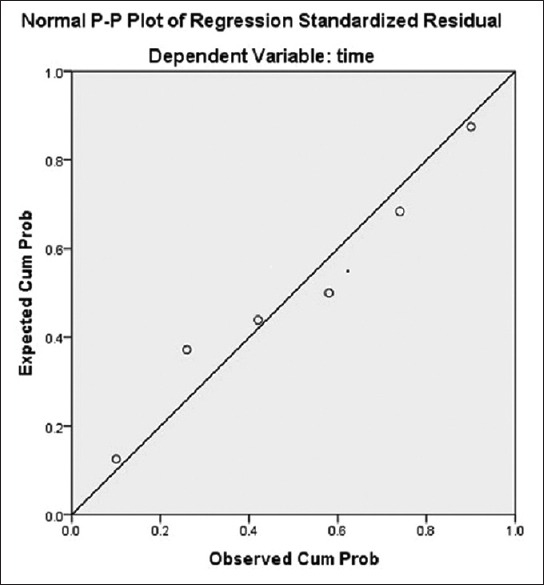
The regression line is close to the observed value which shows there was a strong correlation and regression between the time intervals and appearance of the histological changes
Discussion
Estimation of time elapsed since death (PMI) is an important requisite in many forensic cases[4] The various approaches to assess the PMI include physical (algor mortis, livor mortis), physicochemical (rigor mortis), biochemical (electrolyte concentration, enzyme activity), microbiological (decomposition), entomological and botanical processes.[1,4] Clinical findings such as algor mortis, rigor mortis, and livor mortis appear at around 6 h postmortem.[4] Most of these methods remain relatively inaccurate and cannot be applied to the very early postmortem period.[1] The various morphological or clinical changes that occurred in a dead individual will be preceded by the changes at histological level in tissues at an early stage. There is no specific histological method available or followed so far, to assess PMI. A few authors have studied the histological changes in unfixed antemortem tissues and compared them with changes in postmortem tissues.[2,3] However, all the available previous studies failed to report very early changes. Hence, in the present study, histological changes in antemortem gingival tissues left unfixed for various time intervals after removal were evaluated as it can help in estimating PMI.
When a tissue is removed from the living body, hypoxia occurs, leading to anoxia and cell death occurs. Following death of the cell, initiation of autolysis occurs by liberation of hydrolytic enzymes from lysosomes. This leads to disintegration of the cell resulting in changes occurring in the nucleus and cytoplasm.[5,6] Changes that occur in the nucleus after death are pyknosis, karyolysis, and karyorrhexis. Pyknosis is the shrinkage of nucleus due to the loss of water from the nucleus to the cytoplasm. If the nuclear substance is soluble in the cytoplasm, it results in karyolysis and if it is insoluble, it results in karyorrhexis.[7] The enzymes responsible for autolysis disintegrate the intracellular organelles very quickly, and this gives the cytoplasm a homogenous look, which becomes intensively eosinophilic, resulting in a loss of cell details and tissue architecture.[8]
The mechanisms and histological features of autolysis are the same as for necrosis since the cause is anoxia and absence of life support.[2] It may be quite difficult to distinguish in a histological section, autolysis from necrosis and the only difference is the absence of an inflammatory reaction.[2,5,6]
Fixation is an important step in histological processing of tissues. A tissue whether it is healthy or pathological when it is fixed in a fixative (formalin) immediately after removal from the body, the structural details of the cells are well preserved.[9] When the tissue is left unfixed, it undergoes autolytic changes that may be assessed both histologically and morphologically.[3] Thus, any tissue whether it is obtained antemortem or postmortem shows changes that reflect histologically at an early stage and morphologically at a later stage.
The present study was conducted on healthy unfixed, antemortem gingival samples which were fixed at regular time intervals and processed further. The hematoxylin and eosin stained sections showed characteristic features of autolysis such as chromatin clumping, eosinophilia, homogenization, nuclear pyknosis, and nuclear vacuolation in all the samples. Samples fixed at 2 h interval show nuclear changes involving all layers of epithelium, including a few cells of the basal cell layer. At 4 h interval, changes are evident in all the layers of the epithelium including basal layer. In our study, in entire study group, specific histological changes such as homogenization, loss of epithelial architecture and autolytic changes involving the basal layer occurring in antemortem gingival tissue at 4 h after removal has been identified. Differentiation between changes at 15 min, 30 min, 45 min, and 1 h is very minimal. However, changes at 4 h can be very well differentiated from changes at 15 min. There was a positive correlation (<1.0) between the time interval and the appearance of the histological changes.
Various authors have studied the PMI at histological level in gingival tissue at both antemortem and postmortem level. Gururaj and Sivapathasundharam in 2004 studied the initiation of decomposition at cellular level in antemortem and postmortem gingival samples and concluded that decomposition process is initiated in a dead person within 24 h after death and the other clinical features of decomposition occur subsequently.[2] Pradeep et al. in 2009 studied the initiation of decomposition changes in antemortem and postmortem gingival samples and have concluded that decomposition sets in early in the antemortem samples compared to postmortem samples.[3] Yadav et al. in 2012 studied the degenerative changes in the labial mucosa from 31 nonrefrigerated cadavars and evaluated the nuclear and cytoplasmic changes at varying PMIs.[10]
The earlier studies were able to determine the various changes occurring in the antemortem and postmortem tissue between 10 and 24 h but fail to determine the early change that occurs immediately after death. Also, the previous studies have discussed all the nuclear and cytoplasmic changes that occur in an unfixed antemortem tissue, but the correlation between the histological changes and the duration after death which is essential to determine PMI was not discussed. Our results in relation to histological changes are similar to the studies conducted previously. The specific histological changes that occurred at 4 h interval in unfixed antemortem tissues may be considered as criteria to analyze further in a larger study group both in antemortem and postmortem tissues to assess PMI.
Various factors influence the rate of autolysis.[1,11,12] Autolysis matches with the activity of certain enzymes, called autolytic enzymes, present in lysosomes. The mechanism of enzyme action is temperature – dependent and increase in temperature speeds up autolysis.[2,12,13] In the present study, tissues were obtained from healthy persons and the average room temperature where the tissues were placed was 38°–42°. The obtained gingival tissues were stored in closed containers until fixation, to avoid dehydration and control the availability of moisture and thus preventing mummification of tissue.[6] The process of fixation was standardized in that the same preparation of 10% formalin was used for all the tissues, both control and the study group. Various authors have studied the effect of size of tissue on the rate of autolysis.[14] In our study, factors such as size of tissue and body mass index were not considered.
This is the first study of its kind to determine the early changes in an unfixed antemortem gingival tissue occurring after its removal. The histological changes in our study are similar to previous studies conducted on both antemortem and postmortem gingival tissues. With the results obtained in our study, we assume that the initiation of decomposition occurs in 15 min and it progresses with time. The tissue that we obtained in our study from a living individual differs from the tissue obtained from a cadaver, and we do not know the difference between the metabolism that occurs immediately following a loss of blood supply in an antemortem and a postmortem gingival tissue, which may be studied at molecular level only. We assume that in dead individual the stagnated blood with the reserve oxygen and nutrients may allow the cell to thrive for some more time, at least in practice that may affect the initiation of autolytic changes when compared to antemortem tissue, in which there will be a sudden complete loss of blood supply following removal which may allow the autolytic changes to occur immediately. Also, in practical, scenario, for any individual who dies in a hospital or at residence, it may take few minutes to confirm the death clinically by a physician.
In our study, at 15 min of time interval following the removal of tissue, the autolytic changes appear only in the superficial layers but the basal layer did not show any autolytic change and as the time increases the changes involves the basal layer which is evident in the sections studied at 4 h of time interval. Basal cell layer has an increased nuclear content compared with the other superficial layers of the epithelium. It has high mitotic potential and is considered to be composed of stem cells and progenitor cells.[15] Also, the basal layer is arranged in a manner such that the superficial cells above the basal layer are meant for protection which prevents the environmental factors such as temperature affecting it at an early stage. We assume that this may be the reason for the delay in involvement of the basal cell layer by autolytic change.
Although the histological changes are similar in both antemortem and postmortem tissues, further studies on a large scale are needed. Studying the histological changes by removing the tissue at varying time intervals from a same dead individual will standardize the procedure and may give better accurate results.
Conclusion
The unfixed antemortem gingival tissues show features of autolysis at an early stage which may be assessed histologically and the changes can be correlated with duration. The studies conducted on postmortem gingival tissues also reflect the similar histological changes. Thus, the unfixed gingival tissues may be used to predict PMI by histological method in the forensic aspects, and its routine applications in practice may be considered after a further study in a large scale by this particular method.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sampaio-Silva F, Magalhães T, Carvalho F, Dinis-Oliveira RJ, Silvestre R. Profiling of RNA degradation for estimation of post morterm interval. PLoS One. 2013;8:e56507. doi: 10.1371/journal.pone.0056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gururaj N, Sivapathasundharam B. Post mortem findings in the gingiva assessed by histology and exfoliative cytology. J Oral Maxillofac Pathol. 2004;8:18–21. [Google Scholar]
- 3.Pradeep GL, Uma K, Sharada P, Prakash N. Histological assessment of cellular changes in gingival epithelium in ante-mortem and post mortem specimens. J Forensic Dent Sci. 2009;1:61–5. [Google Scholar]
- 4.Payne J, Busuttil JA, Smock W. Forensic Medicine Clinical and Pathological Aspects. San Francisco, London: GMM Publishers; 2003. [Google Scholar]
- 5.Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotrans Pathological Basis of Disease. 7th ed. St. Louis, Missouri: Elsevier; 2004. [Google Scholar]
- 6.Walter JB, Talbot IC. Walter and Israel General Pathology. 7th ed. Haryana, India: Elsevier; 1996. [Google Scholar]
- 7.Wells HG. The relation of autolysis to the histological changes occurring in necrotic areas. J Med Res. 1906;15:149–65. [PMC free article] [PubMed] [Google Scholar]
- 8.Zdravkovic M, Kostov M, Stojanovic M. Identification of postmortem autolytic changes on the kidney tissue using PAS stained method. Med Biol. 2006;13:181–4. [Google Scholar]
- 9.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 5th ed. Philadelphia, USA: Churchill Livingstone; 2002. [Google Scholar]
- 10.Yadav A, Angadi PV, Hallikerimath S, Kale A, Shetty A. Applicability of histologic post-mortem changes of labial mucosa in estimation of time of death – A preliminary study. Aust J Forensic Sci. 2012;44:343–52. [Google Scholar]
- 11.Nery LR, Moreira CR, Cestari TM, Taga R, Damante JH. Postmortem acinar autolysis in rat sublingual gland: A morphometric study. J Appl Oral Sci. 2010;18:509–14. doi: 10.1590/S1678-77572010000500014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehendiratta M, Jain K, Boaz K, Bansal M, Manaktala N. Estimation of time elapsed since the death from identification of morphological and histological time-related changes in dental pulp: An observational study from porcine teeth. J Forensic Dent Sci. 2015;7:95–100. doi: 10.4103/0975-1475.154594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kushwaha V, Yadav M, Srivastava AK, Agarwal A. Time since death from degenerative changes in the kidney. J Indian Acad Forensic Med. 2010;32:37–41. [Google Scholar]
- 14.Matuszewski S, Konwerski S, Fratczak K, Szafalowicz M. Effect of body mass and clothing on decomposition of pig carcasses. Int J Legal Med. 2014;128:1039–48. doi: 10.1007/s00414-014-0965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanci A. Ten Cate's Oral Histology: Development, Structure, and Function. 7th ed. St. Louis: Harcourt Health Sciences; 2003. [Google Scholar]


