Abstract
Food allergy is increasing in prevalence; as a result, there is intense focus on developing safe and effective therapies. Current methods of specific immunotherapy include oral, sublingual, and epicutaneous, while nonspecific methods that have been investigated include: Chinese herbal medicine, probiotics, and anti-IgE antibodies. Although some studies have demonstrated efficacy in inducing desensitization, questions regarding safety and the potential for achieving immune tolerance remain. Although some of these therapies demonstrate promise, further investigation is required before their incorporation into routine clinical practice.
Introduction
The increase in food allergy in the United States and throughout the world is a growing public health concern. From 1997 to 2007, the prevalence of food allergy in children increased by 18%.1 An estimated 15 million Americans have a food allergy, accounting for approximately 8% of children and 5% of adults.2 Although most reactions from accidental ingestions are typically mild and self-limited, severe cases of anaphylaxis are associated with peanut, tree nuts, and shellfish and have resulted in fatalities.3 Appropriately, there has been an increase in research for food allergy treatments. Current guidelines for food allergy management include education, strict avoidance, nutritional monitoring, appropriate treatment of anaphylaxis with injectable epinephrine, and regular follow-up with an allergy specialist.4 In this review, the authors seek to update the previous article on food allergy therapy from this series, highlighting key clinical trials and emerging approaches for the treatment of food allergies.5
Standard of care
At present, there is no cure for food allergy. Diagnosis and management are focused on identification of triggers and targeted dietary elimination.4 Patients are encouraged to read ingredient labels, avoid cross-contamination, and consult with a nutritionist to ensure adequate growth. They are taught to recognize anaphylaxis and administer injectable epinephrine. In principle, strict dietary elimination should protect an individual from immunoglobulin (Ig) E–mediated reactions; however, in practice, patients with food allergies experience multiple exposures following diagnosis from both accidental and nonaccidental ingestions.6,7 Constant vigilance and perpetual risk produce significant anxiety. Quality-of-life surveys among children with food allergies and their parents suggest this anxiety leads to restriction of daily activities.8,9 Avoidance measures can also result in nutritional deficits and growth impairment.10
Immunotherapy background
Oral immunotherapy (OIT) is not conceptually new, as instructions for treatment of egg sensitivity with egg white are recorded in the Babylonian Talmud.11 There are reports of physicians attempting food desensitization published as early as 1905 with varied success (Table 1). Investigators demonstrated that patients were able to tolerate foods after a period of gradual incremental exposure, usually occurring over a period of months to years.12 Clinical texts through the mid-twentieth century reference hyposensitization to foods as a treatment for food allergy, although its effectiveness remained in question. In the 1980s, European investigators renewed interest in OIT after publishing positive results from small case series.13,14 These early reports and studies paved the way for more systematic investigation of immunotherapy as an active treatment for food allergy. An understanding of the pathogenesis of IgE-mediated disease and mechanisms of desensitization has elucidated pathways for targeted approach. Interestingly, many questions raised by early investigators continue to elude researchers today (Box 1).
Table 1.
Early history of allergen immunotherapy for food allergy
| Year | Summary of Key Findings |
|---|---|
| 1905 | Finkelstein conceptualizes OIT. He successfully desensitizes nurslings with “milk idiosyncrasy” by gradually administering increasing drops of milk.15 |
| 1908 | Schofield treats a 13-year-old patient with egg allergy over an 8-mo period by incrementally increasing small amounts of raw egg disguised in pill form.13 |
| 1912 | Schloss describes a patient with allergies to egg, oat, and almond, which he orally desensitizes to egg. Sensitivity to almond and oat also decreases during this treatment. He uses skin testing to guide up-dosing during therapy.16 |
| 1920 | Schloss reports 5 patients with egg allergy treated with subcutaneous injection of ovomucoid. He also describes 12 children with food allergies successfully desensitized using OIT.17 |
| 1920 | Park describes oral desensitization of a child with hypersensitiveness to cow’s milk. Pallor and drowsiness were noted during build-up phase.18 |
| 1926 | Stuart and Farnham advise treatment of food allergies by the oral method at an early age. In cases of milk or egg sensitization, their recommendation is enthusiastic because treatments are “simple and regularly successful.”19 |
| 1930 | Freeman performs rush inoculation over 8 d with cod fish juices in a 7-y-old boy with fish sensitivity. The patient is subsequently started on a fish diet and prescribed an ounce of cod-liver oil daily. A concurrent egg allergy resolves with this therapy.20 |
| 1935 | Keston, Waters, and Hopkins report effectively desensitizing 50 cases of food allergy to milk, wheat, egg, orange, tomato, or cocoa and publish oral desensitization protocols to each of these foods.21 |
| 1940 | Edwards describes successful oral desensitization of 11 of 12 patients with milk allergy using protocols published by Keston et al.22 |
Box 1.
-
*
Can true immune tolerance be achieved through food desensitization?
-
*
What is the preferred route for antigen administration?
-
*
Do clinical outcomes improve if immunotherapy is started earlier in life?
-
*
How long must therapy continue in order to achieve a permanent effect?
-
*
What differences exist between children who naturally outgrow food sensitivity and those who are desensitized?
-
*
Does immunotherapy hasten the development of immunologic tolerance in children who will ultimately outgrow a food allergy?
Immunotherapy mechanisms
Immunotherapy is based on the principle that incremental exposure to a given antigen can render an individual temporarily less reactive to that antigen (eg, desensitization) and eventually result in longer-lasting changes. Although the exact mechanisms are unknown, some of the immunologic changes that occur have been elucidated (Fig. 1).23 Early on, repeated administration of increasing immunotherapy doses suppressed basophil and mast cell reactivity. Interleukin-10 is produced, presumably by lymphocytes, which is thought to suppress allergic responses and drive production of IgG4 antibody. Initially, immunotherapy increased the production of antigen-specific IgA, IgG1, IgG4, and IgE, although IgE tends eventually to decline to below baseline values in response to therapy (around 12–18 months). Ultimately, these changes result in a decrease in tissue mast cells and eosinophils, accounting for clinical hyporesponsiveness to antigen exposure and diminished skin prick test reactivity.
Figure 1.
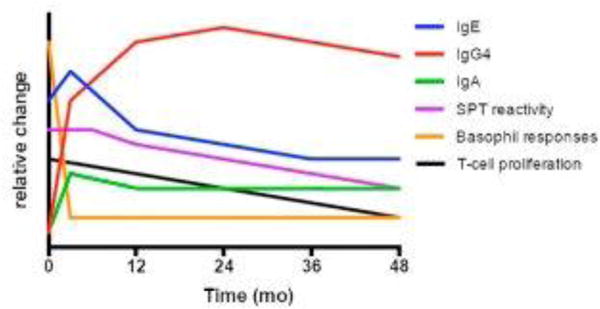
Approximate changes seen in immunologic parameters for food allergy while undergoing immunotherapy. Notably, basophil reactivity declines relatively quickly. IgG4 and IgE increase, although ultimately IgE will decrease. SPT, skin prick test.
Desensitization versus tolerance
Interpretation of food allergy literature requires understanding of an important distinction between the concepts of clinical desensitization and tolerance. Desensitization refers to a reversible state induced by short-term exposure to an allergen. Once administration of the allergen is discontinued, the previous level of clinical reactivity returns. An analogy would be the brief desensitization protocols widely used to manage patients with certain drug allergies. On the other hand, therapeutic tolerance would suggest that the immunotherapy treatment has induced disease-modifying changes that will persist even after the treatment is discontinued. Importantly, some individuals, regardless of treatment, will spontaneously develop immune tolerance and naturally outgrow their food sensitivity. Although eventual tolerance to milk and egg are relatively common, peanut, tree nut, and seafood allergies tend to persist over time.2
Incomplete understanding of the immunologic changes induced by immunotherapy, and whether these changes truly reflect immune tolerance, has led to the emergence of the term sustained unresponsiveness (SU). SU refers to the ability to successfully consume the treated allergen during an oral food challenge (OFC) performed typically 1 to 4 weeks after stopping active treatment. Importantly, many of the studies addressed in this review have only measured desensitization as a primary outcome. Only a few trials have addressed whether OIT results in SU, and none have done so in a rigorous, placebo-controlled fashion.23
Oral immunotherapy
Milk, egg, and peanut are the most studied foods in OIT trials. Most modern studies have been single-food OIT trials; as a result, these 3 major foods will be discussed separately. Overall, OIT trials have a dropout rate of 15% to 20%.24 Moreover, several studies have observed spontaneous resolution of food allergy among subjects receiving placebo. This finding is consistent with observational studies of the natural history of peanut allergy, for example, which have found spontaneous resolution in about 20% of individuals.25
General conclusions regarding efficacy are difficult to draw from the current literature because success rates for OIT have been defined differently. Ideally, subjects completing a course of immunotherapy should be able to incorporate culprit foods into their diets, consuming them ad libitum without symptoms. However, most studies only approximate the inclusion of the culprit food ad libitum in subjects’ diets. Studies often define success in terms of reaching a target maintenance dose or passing an endpoint challenge; therefore, it is important to interpret results in the context of the selected outcome measures. Overall safety of immunotherapy is also somewhat variable among studies, with adverse events not always being categorized in the same way (eg, many exclude oral itching). The lack of natural history or control groups as comparisons in many studies has also limited the determination of safety. Safety and efficacy are discussed further with regards to specific studies.
In addition to significant heterogeneity and varied primary endpoints, published trials have other scientific shortcomings. Most clinical protocols incorporate a crossover design that allows subjects in the placebo group to be reassigned to the treatment arm after a desensitization challenge. Although this may facilitate subject recruitment and retention and satisfy ethical concerns associated with repeatedly challenging subjects treated with placebo, it does not allow for comparisons between subjects who successfully complete immunotherapy and those who might have developed natural tolerance. Table 2 provides a general overview of study design for OIT.
Table 2.
Overview of oral immunotherapy
| Phase | Length | Description |
|---|---|---|
| Modified rush | 1 d | Minute quantities given and dose is escalated over a period of several hours (6–8 doses) |
| Build-up period | 6–9 mo | Daily doses taken at home with gradual increases performed under clinical observation every 1–2 |
| Maintenance | Months to years | Target dose of allergen consumed daily at home |
Despite the paucity of data supporting tolerance induction, some argue that OIT protects against accidental ingestions and improves quality of life, thus justifying its incorporation into clinical practice.26,27 It is clear from multiple studies that OIT increases reaction thresholds, but there are no good data to prove this protects from reactions due to accidental ingestions. OIT has also not been thoroughly studied in subjects who are the most sensitive. A history of severe anaphylaxis is often a criterion for exclusion, while these patients might benefit the most from food immunotherapy.
Milk
Cow’s milk (CM) is the most common food allergy in early childhood. It is also one of the most important foods to reintroduce into the diets of children with food allergies, because children who must avoid milk are at an increased risk for significantly decreased weight, height, and body mass index–for-age percentiles.28,29 A recent systematic review and meta-analysis examined 6 randomized, controlled trials conducted between 2007 and 2012. Pooled data demonstrated a 10-fold increased likelihood of achieving desensitization to CM in children undergoing CM OIT than in non-OIT-treated patients. Subjects with CM allergy who underwent milk OIT tended to tolerate more milk protein, although these data did not reach statistical significance. A wide range of children received treatment with epinephrine during OIT trials (6.7%–30.8% of children); however, most reactions were mild.30
Maintenance of desensitization after CM OIT has been evaluated in 2 recent studies. A study published in 2013 evaluated 2 different CM maintenance regimens. Thirty-two children with milk allergy were randomized after successfully completing CM OIT and achieving desensitization, defined as consumption of 200 mL of CM. For 1 year, 16 patients continued daily maintenance ingestion of 150 to 200 mL of CM (group A), while the other 16 ingested the same amount twice weekly (group B). Both groups were also allowed to consume CM ad libitum. The primary outcome was maintenance of desensitization without symptoms on CM ingestion. Three subjects were lost to follow-up (1 in group A and 2 in group B). Adverse events were similar between groups, and none discontinued therapy due to adverse events or noncompliance. The authors concluded that twice-weekly ingestion of a maintenance dose seems to be equally effective at safely maintaining desensitization as the daily regimen, with 9 of 15 subjects in group A and 9 of 15 subjects in group B consuming CM without symptoms. It is important to note the absence of a placebo group or a group exclusively consuming CM ad libitum. Moreover, differences in the quantity of CM consumed ad libitum between groups were not assessed.31
In contrast, a retrospective study examined 32 patients who had successfully completed CM OIT under 2 different protocols (16 from each), with follow-up ranging from 1.3 to 5.3 years. Twenty-two percent of subjects limited their consumption of CM due to symptoms, and only 31% of subjects tolerated full servings of CM with minimal or no symptoms. One subject required multiple doses of epinephrine for ongoing symptoms. Both studies provide important data that must be considered before starting OIT. Strict adherence to daily maintenance dosing may be difficult for children and families for a variety of reasons (eg, illness, travel, strenuous exercise, menses, food aversion). The same may be true for regular incorporation of trigger foods after OIT. It is also clear from these data that satisfactory long-term outcomes after CM immunotherapy appear to be dependent on ongoing exposure, which is difficult for some individuals. Importantly, some subjects who had been classified as protocol-defined successes in the trial seemed to have relapsed completely.32
Egg
In 2012, a multicenter study by the Consortium for Food Allergy Research (CoFAR) randomized 55 subjects with egg allergy (placebo 15, treatment 40). After 22 months of OIT using a daily maintenance dose of 2 g of egg white powder, 30 subjects (75%) passed a desensitization challenge (10 g of egg powder). After 6 to 8 weeks off treatment, just 11 of 29 subjects tolerated this same amount of egg. Using intention-to-treat analysis, 28% had developed SU. This study revealed differences in rates of desensitization after OIT for 10 months (55% passed a 5-g challenge) and 22 months (75% passed a 10-g challenge), suggesting that continued allergen administration over a prolonged period might improve efficacy. None of the subjects in the placebo arm passed the challenge at 10 months.33 In a more recent, smaller open trial of egg OIT, Meglio and colleagues34 achieved desensitization rates of 80% (8/10, active treatment) versus 20% (2/10, placebo) after 6 to 9 months of OIT.
A 2014 Cochrane Review analyzed 4 randomized, controlled trials of egg OIT. Only the CoFAR trial mentioned earlier included a placebo arm, whereas the other 3 compared subjects on OIT versus standard avoidance diet. Of the 100 patients receiving OIT in the 4 studies, 69% experienced adverse events, with 5% requiring epinephrine. Overall, 44% could tolerate a partial serving of egg, while 39% could tolerate a whole egg. However, because of the small sample size, low quality of evidence, and methodological differences, the only conclusion that could be drawn was that desensitization seems to be possible in a large number of egg-allergic patients, but adverse events remain a significant concern.35
Peanut
Although less common than milk and egg allergy, peanut allergy is less likely to resolve spontaneously; as a result, it may be easier to study the effects of treatment in peanut-allergic subjects. The only double-blind, placebo-controlled (DBPC) trial of OIT for peanut allergy was conducted in 2011 and assessed desensitization. Twenty-eight subjects (ages 1–16) were randomized to receive peanut OIT (n = 19) or placebo (n = 9). Three subjects (16%) in the OIT group discontinued treatment because of side effects, but all remaining participants tolerated a maintenance dose of 4000 mg of peanut protein and completed 12 months of therapy. Each of the treatment subjects passed an OFC with 5000 mg of peanut protein after 1 year of therapy, compared with 280 mg in the placebo group (range, 0–1900 mg; P <.001).36
The first study to investigate SU to peanut was published in 2014. In this open, non-placebo-controlled trial of peanut OIT, 24 of 39 (61.5%) subjects completed therapy for up to 5 years. Of these 24, 12 (50% per protocol, and 31% by intention to treat) were able to consume 5000 mg of peanut protein and 8000 to 10,000 mg of peanut butter after stopping OIT for 4 weeks. Thus, 12 patients were considered to achieve SU. These patients were subsequently instructed to incorporate peanut into their diet ad libitum at least several days per week. Questionnaire follow-up completed by 87.5% of the SU patients revealed that none experienced any allergic reactions due to peanut exposure, with a median follow-up time of 40 months.37
Another study in 2014 evaluated immunologic parameters and the possibility of SU to peanut after OIT or SLIT. Gorelik and colleagues38 found a greater effect for OIT than SLIT, but both demonstrated suppression of basophil activity and decreased Th2 responses to peanut. However, these changes were not preserved in some patients while on maintenance and waned in others after stopping immunotherapy. Despite the lack of a placebo group and its crossover design, the data strongly suggest that immunologic changes associated with SU may not be truly sustained in most patients.
The recent STOP II trial conducted in the United Kingdom was a 2-phase, randomized, controlled crossover trial of 99 children with peanut allergy (ages 7–16). During phase I, subjects were allocated to active treatment (n = 49) or elimination (n = 50). After an OFC at 6 months, placebo subjects were reallocated to the treatment arm for phase II. The primary outcome measure of the study was the proportion of subjects able to tolerate 1400 mg of peanut protein following 6 months of OIT with 800 mg of peanut protein daily. Despite the lower maximum daily dose than that used in other OIT trials, 62% (24/39) of the treatment group was able to tolerate 1400 mg of peanut protein (approximately 10 peanuts) and were considered to be desensitized, while none of the placebo group passed the OFC. By the end of phase II, however, 84% of the active group and 91% of the control group were able to tolerate the daily maintenance doses. The study did not have participants stop OIT and thus did not assess for SU. Adverse reactions were higher in the treatment group as expected, although they were mild, primarily consisting of oral pruritus and abdominal pain.39
The findings described have led some clinicians to incorporate OIT into routine clinical practice.40 A retrospective chart review of peanut OIT used in clinical practice, not as research, following different protocols in multiple clinical sites was recently published. Results for 352 patients showed that 85% reached maintenance dosing, but actual dose and length of maintenance therapy differed by site. The authors argue that OIT is safe for use in clinical practice given the low rate of epinephrine administration (0.7 per 1000 escalation doses, 0.2 per 1000 maintenance doses). However, other adverse reactions were not reported, and there is no evidence that accidental exposures were reduced or any other benefits were achieved.41 In addition, other studies have reported significantly higher rates of allergic reactions among OIT participants compared with those avoiding the food.42 Quality-of-life changes with OIT have also not been significantly observed, despite the assumption of improved quality of life with OIT. The Food Allergy Quality of Life Questionnaire was used before and after egg OIT in 22 children and their parents. They found only minimal improvement in health-related quality of life as rated by the parents, whereas children reported a benefit in terms of dietary restriction, but a negative impact by allergic reactions due to OIT.43 More data conclusively demonstrating safety and efficacy are needed in order to justify routine clinical use of OIT outside of a research setting.44
Extensively heated milk and egg as oral immunotherapy
Baking egg or milk proteins decreases their allergenicity, likely due to alteration of conformational epitopes. However, heating alone does not reduce the allergenicity of all proteins; in fact, with limited heat application, some foods can form neoepitopes, increasing their allergenicity.45 Baking food proteins with wheat may also confer a matrix effect in which the wheat-protein complex alters sequential IgE-binding epitopes that are unaffected by heat alone. This decreased allergenicity is sufficient for many patients to tolerate the trigger food on a regular basis.46 There is evidence to suggest that regular consumption of baked egg- and milk-containing products may help patients outgrow these allergies more quickly, although this evidence is weakened by the lack of an appropriate control group. Several studies have demonstrated that regular ingestion of baked egg or milk may hasten and increase rates of SU to nonbaked egg or milk when compared with strict avoidance. These studies have also shown that consumption of baked egg or milk decreases skin prick test size and increases IgG4. As seen in OIT, specific IgE increases initially and ultimately decreases with time. Although tolerance to baked goods may be associated with a milder clinical phenotype, these data suggest that introduction of baked allergens may actually alter the natural history of egg and milk allergies.47
Introduction of baked egg or milk into the diets of allergic children should be performed cautiously. Although some studies suggest that some patients can perform these OFCs at home, passage rates range from 60%48 to 85%, and up to 20% of failures to baked egg require epinephrine.49 Ideally, patients should be evaluated by an allergist and undergo a medically supervised OFC before introducing baked allergens.
Multiple food oral immunotherapy
Although OIT for single allergens shows promise, up to 30% of children with food allergy are sensitive to more than one allergen50 and in highly atopic populations, this proportion may be greater than 50%.51 Results from a phase I trial of multifood OIT were recently published. Forty participants (ages 4–46) were recruited, and a DBPC OFC to peanut was performed at enrollment. Additional reported food allergies were documented with subsequent DBPC OFCs. Twenty-five subjects were started on multifood OIT after demonstrating 1 or more food allergies in addition to peanut allergy. The remaining 15 subjects were diagnosed with peanut allergy alone and allocated to single allergen OIT. Doses of each allergen were escalated until a maintenance dose of 4000 mg was reached. Adverse reaction rates did not differ significantly between groups, and the dropout rates were similar. Two subjects from each group required treatment with epinephrine for OIT dose-related reactions.52 This study suggests the safety of multifood OIT is comparable to peanut OIT, but further randomized trials are needed to demonstrate efficacy.
Sublingual immunotherapy
The first study evaluating food SLIT was performed in Europe for hazelnut allergy. SLIT for pollen allergy had been widely used in Europe before this study, and the US Food and Drug Administration (FDA) recently approved its use with tablets for grass and ragweed allergies (dust mite remains in development). Like OIT, SLIT takes advantage of allergen exposure through the oral mucosa, which is thought to be tolerogenic. Liquid doses are delivered to the surface under the tongue, where antigen-presenting cells (ie, Langerhans cells) take up the antigen.53 Extract concentrations and the volume of liquid that can be held under a patient’s tongue have limited the utility of SLIT. Unlike OIT doses that range from milligrams to grams, SLIT doses typically range from micrograms to milligrams at maintenance. Although the quantity of antigen delivered is smaller with SLIT, the oral mucosa is exposed to undigested antigen, in contrast to OIT, which exposes the antigen to gastric digestion.54 Clinical trials investigating the safety and efficacy of SLIT in the treatment of food allergies have been limited to studies on hazelnut55 and peach allergy,56 which is not reviewed here, as well as milk and peanut, that are discussed later.
Results of DBPC, randomized trials examining the use of SLIT in peanut allergy have been published. In 2011, Kim and colleagues57 published a study in which 18 children (ages 1–11) completed 12 months of dosing followed by a DBPC OFC. Eleven subjects were randomized to active treatment with peanut with a goal dose of 2500μg daily, while 7 subjects were randomized to placebo. Compliance was similar between the groups, with only 0.26% of peanut doses requiring treatment with antihistamines and 0.02% of home doses requiring albuterol for minor wheeze. The median cumulative tolerated dose after 1 year of SLIT therapy in the peanut group was 1710 mg of peanut protein, compared with 85 mg for the placebo group.
The largest published SLIT study was performed in 40 peanut-allergic subjects (ages 12–37 years) by the CoFAR group in 2013. The first phase was a randomized, DBPC study for the first 44 weeks using a daily dose of 1386μg. After 44 weeks of therapy, subjects completed a 5 g DBPC OFC to peanut. No subjects were able to consume the full 5 g of peanut powder (~50% peanut protein), but 14 (70%) peanut SLIT subjects were able to consume at least 10-fold more peanut powder than at baseline (responders), compared with 3 (15%) placebo SLIT subjects. In the second phase, the placebo group crossed over to active treatment to a higher daily dose of peanut SLIT of 3696μg. This group completed a subsequent crossover 5 g DBPC OFC after 44 weeks. At this challenge, 7 of the 16 (44%) subjects who crossed over to active treatment were considered responders. After 68 weeks of treatment in the original active peanut SLIT group, the median successfully consumed dose increased from 496 mg at 44 weeks to 996 mg (P = .05); 5 subjects were able to consume the full 5 g, with one subject consuming 10 g, suggesting that longer-term therapy may confer increased dose threshold. There was a high dropout rate among the 40 children, but this was largely due to personal reasons rather than side effects. In the first phase, 40.1% of peanut SLIT doses resulted in mild symptoms, but this decreased to 3.3% when oropharyngeal reactions were excluded. One subject on peanut SLIT in phase 1 did require epinephrine during up-dosing and was withdrawn from active therapy. Side effects were similar after crossover: 35.8% of doses after week 44 elicited symptoms, but only 1.1% of doses elicited symptoms when oropharyngeal symptoms were excluded, and no reactions required epinephrine. Children with a history of life-threatening reactions were excluded for safety.25 This group may be the most likely to undertake SLIT and achieve clinically significant results.
SLIT may ultimately represent a safer method of immunotherapy for patients with a history of severe allergy who cannot tolerate OIT, or as a bridge to OIT to decrease side effects and improve safety, but it may never be the most effective method to induce clinically significant desensitization or SU given the lower daily maintenance doses used. Further investigation into this possibility is needed as well as the duration of therapy required and any potential lasting effects of SLIT. Unfortunately, recently published follow-up data from the previously cited CoFAR SLIT study demonstrate substantial rates of nonadherence and lower rates of SU (10.8%) than seen in OIT.58 Given that indefinite exposure is likely necessary to maintain whatever treatment benefits are achieved, this is a concerning finding. Studies directly comparing SLIT and OIT are discussed in the next section.
Sublingual immunotherapy versus oral immunotherapy
In a DBPC pilot study comparing SLIT and OIT, 21 children with peanut allergy were randomized to receive active SLIT/placebo OIT or active OIT/placebo SLIT. Although adverse reactions and withdrawal were less common among the active SLIT group, reaction thresholds were significantly higher in the OIT group (141-versus 22-fold, P = .01), and SLIT overall was not significantly superior to placebo.59 A retrospective comparison was carried out among peanut-allergic subjects who had completed 2 years of therapy, with 23 subjects in the OIT group and 27 subjects in the SLIT group. Safety was not directly compared, but the amount of peanut protein tolerated was higher in the OIT group and showed less variability, while changes in immunologic parameters (eg, IgE, IgG4, basophil reactivity) were greater in the OIT group.60
Another comparison of SLIT and OIT was performed for CM allergy as an open-label, randomized study. Thirty children were randomized to SLIT or SLIT converting to OIT at a lower and a higher dose, with a period off-therapy after about 15 months of maintenance in all groups. Adverse events led 2 children from the OIT group to withdraw. All children who reached maintenance were able to tolerate a higher amount of CM protein, and the amount increased with time. After 60 weeks, 60% of the SLIT group tolerated at least 10 times baseline CM protein, whereas 90% of the OIT group did the same, but this was not statistically significant (P = .053). After 6 weeks off therapy, only 1 in 10 children in the SLIT group maintained SU, whereas 8 of 20 in the OIT group had SU to CM, but again this was not significant (P = .09). Notably, 2 children reacted after only 1 week off therapy. Limitations to this study include small sample size and lack of power as well as lack of a placebo group.61 These early results suggest that despite a poorer safety profile, OIT is more effective than SLIT in inducing desensitization.
Epicutaneous immunotherapy
EPIT is an alternative approach to oral methods that is performed by repeated application of an allergen to intact skin. This approach is accomplished through a novel and proprietary epicutaneous delivery system (EDS) successfully developed in animal models.62 The EDS uses a circular disc spray-dried with allergen. Perspiration solubilizes the allergen, which is then disseminated into the stratum corneum. Food EPIT is ongoing in phase 1 and 2 trials, and it is important to note that there are few peer-reviewed results published in the literature to date. Pilot studies have found that it is relatively well-tolerated with very few systemic reactions and no reports of anaphylaxis. Reactions are primarily mild, cutaneous symptoms, including erythema, pruritus, and flares of atopic dermatitis.63,64
However, these studies have failed to show a statistically significant increase in the amount of food tolerated by milk- and peanut-allergic subjects treated with EPIT. The phase 1 CM EPIT pilot study was only performed for 3 months, which may not be long enough to show a significant effect. Nineteen subjects were randomized to milk EPIT (n = 10) or placebo (n = 9), with OFC before and after the use of EPIT. The mean cumulative tolerated dose was 12-fold higher in the active group versus 8% in the placebo, which was not statistically different (P = .13). Subjects in the active treatment group had a higher risk of local eczema at the site of antigen delivery. Interestingly, in one child, they observed a decrease in the minimum amount of CM protein that was tolerated after undergoing EPIT, compared with pre-EPIT CM OFC. 64 The potential for increased sensitization with cutaneous exposure is a concern with EPIT in light of the dual exposure hypothesis proposed by Lack.65 The hypothesis proposes that low-dose cutaneous exposure to a food can increase allergic sensitization, while oral/gastrointestinal exposure leads to tolerance.
The peanut EPIT pilot study followed children for 18 months of therapy and found a progressively increasing amount of peanut protein that was tolerated in the active group, but again it was not statistically significant.64 There are currently 3 clinical trials underway that will investigate various doses, safety, and efficacy, with 1 in Europe and 2 in the United States. If successful, these studies, and others performed for inhalant allergy, could suggest that EPIT may be a viable and safe treatment option for food allergy.
Nonspecific immunotherapy
Anti-Immunoglobulin E Therapy
Use of anti-IgE as monotherapy for food allergy was first investigated in a DBPC trial of the humanized, monoclonal, anti-IgE antibody TNX-901. Anti-IgE significantly increased reaction thresholds to peanut flour by 76% in the active group, although the trial was terminated early before completing subject recruitment.66 More recent phase I trials have used omalizumab as an adjunct to OIT, and these studies are discussed next. Subjects are generally pretreated with omalizumab for 2 to 5 months during a washout period and then continued on therapy until a maintenance dose of OIT is reached.
A pilot study in 13 children with peanut allergy demonstrated that omalizumab in conjunction with peanut OIT was effective at decreasing initial reactions and allowed for a more rapid build-up phase, with 92% of children achieving maintenance. Reactions were rare and mild during the study; however, after omalizumab was stopped, half of the children experienced reactions, and 17% required epinephrine. Notably, this small sample of patients had an overall higher total and peanut-specific IgE level than many other OIT studies.67
CM OIT was studied with omalizumab in 11 patients. After a 9-week lead-in on omalizumab, they underwent rush CM desensitization. Reactions were again rare and relatively mild, with only 4 reactions requiring epinephrine, which is similar to other studies. Unlike in the peanut study, increased reactions were not experienced after stopping omalizumab. The study is limited by a small sample size and lack of placebo group, and baseline OFCs were not performed, so the significance in the amount of CM protein tolerated after OIT cannot be established.68
A larger, phase I study examined the use of omalizumab as an adjunct to multiple food OIT. Twenty-five subjects were desensitized to up to 5 different foods. Doses of each allergen were escalated until a maintenance dose of 4000 mg was reached. Reactions were experienced by 5.3% of subjects, and this rate decreased over time. No subjects had serious adverse events while on omalizumab, although one patient did have a serious reaction requiring epinephrine off omalizumab in the maintenance phase.69
These studies suggest that omalizumab may be useful as an adjunct to other forms of immunotherapy. Aside from being relatively safe and well tolerated, it confers several benefits over immunotherapy alone, including decreased initial reactions, shorter escalation phases, and more patients reaching higher cumulative doses during rush desensitization. However, further investigation is needed to identify the appropriate dose, duration of pretreatment, and optimal length of use during therapy. Omalizumab is currently only FDA-approved in patients aged 12 years and older with severe atopic asthma or chronic idiopathic urticaria. The lack of a clinical indication for use in food allergy and cost has restricted its use to a research setting thus far.
Chinese Herbal Formula
Interest in complementary and alternative therapies and encouraging data from mouse models have led to clinical trials investigating the use of Chinese herbal therapy as a treatment of food allergy. Food allergy herbal formula-2 (FAHF-2), a compound containing 9 different traditional Chinese herbs, has been shown to be safe in preclinical and pilot studies. Some efficacy was seen in food-allergic mice treated with FAHF-2.70 Preliminary results of phase 2 trials in humans reveal that the treatment is safe. However, at the dose used in this study, there was no statistically significant benefit observed in the treatment group. Notably, there was poor adherence among the treatment group given the large number of capsules required.71 The major advantage of this therapy is that it is nonspecific, and could, therefore, be used to treat individuals with multiple food allergies if efficacy can be demonstrated with other herbal formulations and dosing regiments that are in preclinical studies.70
Table 3 provides an overview of the major forms of immunotherapy discussed earlier, with a summary of the advantages and disadvantages of each.
Table 3.
Immunotherapy summary: pro/con
| Method | Advantages | Disadvantages |
|---|---|---|
| OIT |
|
|
| SLIT |
|
|
| EPIT |
|
|
| Allergen-nonspecific therapies (eg, omalizumab, Chinese herbal formula) |
|
|
Probiotics
Probiotic supplementation has been examined in several allergic diseases, including asthma and atopic dermatitis, with mixed results. A recent meta-analysis including 21 studies found a significant decrease in the risk of atopic sensitization (positive skin prick test or elevated specific IgE) to common allergens, but not specifically to food. They found that prenatal and postnatal administration caused a significant reduction in sensitization. However, probiotics did not protect subjects from developing asthma or wheeze.72 Despite mixed results, probiotics remain an area of interest as a nonspecific form of immunotherapy.
One of the largest and most rigorous studies on probiotics was published in 2009, evaluating 119 infants with CM allergy. This randomized, DBPC study demonstrated no increase in tolerance to CM after 6 months of probiotic supplementation.73 More recently, a study of 55 infants with CM allergy was randomized to receive extensively hydrolyzed casein formula, with or without probiotic supplementation, for 6 months. They found statistically significantly higher rates of tolerance to CM at 6 and 12 months of therapy in the probiotic groups; however, greater differences were seen for those infants who had non-IgE-mediated allergy to CM.74 Probiotics have also been used as an adjunct to peanut OIT. A DBPC trial randomized 62 subjects with peanut allergy to receive probiotics plus peanut OIT (PPOIT) or placebo over an 18-month period. Maintenance therapy was then stopped for 2 to 5 weeks, and subjects underwent an OFC. Possible SU was achieved in 82.1% receiving PPOIT and 3.6% receiving placebo (P <.001). Nine children would need to be treated for 7 to achieve SU (1.27; 95% confidence interval, 1.06–1.59). Unfortunately, the PPOIT group was not compared with OIT alone, so it is impossible to determine the independent effect of probiotics.75 Carefully designed trials with appropriate controls are necessary for defining the effect of probiotic supplementation on food allergy.
Eosinophilic esophagitis and food immunotherapy
Although OIT has not been studied as a treatment of eosinophilic esophagitis (EoE) (rather, food avoidance is one treatment option), it deserves special consideration in the realm of OIT. The development of EoE has been observed in a small number of patients undergoing OIT for food allergy. Most of the current literature regarding this phenomenon consists of case reports and small case series. A recent systematic review with meta-analysis found one randomized clinical trial with 40 patients in which one child developed EoE. Three other full-length articles also met inclusion criteria, for a total of 6 of 179 patients developing EoE attributed to food OIT. According to their analysis, the incidence of EoE on OIT was 2.7%; however, they note considerable limitations. A significant drawback to these reports is that subjects are rarely screened for EoE before beginning clinical OIT trials due to the costs and risks associated with endoscopy. Given the frequency of comorbid IgE-mediated food allergy in patients with EoE, is it unclear whether the EoE was induced by OIT or existed as subclinical disease before treatment. Given available evidence, the risk of developing EoE while on OIT should not preclude further investigation, but it should be recognized as a potential adverse outcome and monitored in clinical research trials.76
Despite this risk of EoE while undergoing food OIT, baked milk may be safe to include in the diets of children with concomitant CM allergy and EoE. A small retrospective study in children with EoE, without IgE-mediated food allergy, found that 73% of patients maintained histologic remission of EoE while eating 2 to 3 servings per week of baked milk for 6 weeks.77 It remains to be seen if the same is true for baked egg.
Failed methods of immunotherapy
Several additional methods have been attempted as food allergy therapy, with significant safety concerns or lack of efficacy. Given the effectiveness of subcutaneous immunotherapy (SCIT) in treating venom and environmental allergies, researchers hypothesized that subcutaneous injection of food antigens would yield similar results. In a study published in 1997, peanut SCIT was performed in 12 patients. Although some efficacy was established, a high rate of systemic reactions prevented patients from reaching or continuing maintenance dosing. Following an anaphylaxis-related death of a patient who was mistakenly given a higher dose of peanut, it was concluded that peanut SCIT carried an unacceptable risk of severe reactions, preventing its continued clinical use.78
Escherichia coli (E-coli) encapsulated, recombinant Modified Peanut proteins Ara h 1, Ara h 2, Ara h 3 (EMP-123). EMP-123 is a novel form of immunotherapy that has not progressed beyond phase 1 trials due to safety concerns. EMP-123 is a rectally administered peanut vaccine comprising 3 recombinant modified peanut antigens (Ara h 1, 2, and 3) encapsulated within heat/phenol-inactivated Escherichia coli. Although the vaccine was safe in healthy control subjects, peanut-allergic subjects experienced significant adverse events; half of the 10 subjects terminated dosing early, and 2 suffered anaphylaxis requiring epinephrine.79 Both of these studies are important for historical consideration as well as potential future use if adjunctive therapies prove to minimize adverse reactions.
Preclinical studies
In addition to the ongoing studies mentioned previously, a variety of potential methods of immunotherapy are in development and preclinical trials. A summary is included in Fig. 2. Some of these methods may represent promising future directions in food allergy, requiring continued rigorous investigation.
Figure 2.
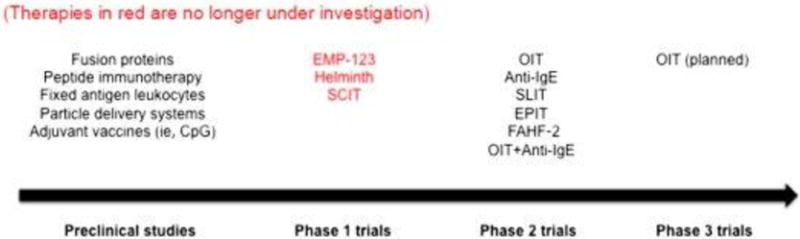
Many preclinical studies are in process as well as phase 2 trials as discussed. OIT will move into phase 3 trials. Other studies have not progressed beyond phase 1 studies due to safety or efficacy concerns. EMP-123, E-coli encapsulated, recombinant Modified Peanut proteins Ara h 1, Ara h 2, Ara h 3.
Key points.
-
*
The standard of care for the management of food allergies is education, avoidance of trigger foods, and treatment of allergic reactions due to accidental ingestion.
Oral, sublingual, and epicutaneous immunotherapy are all investigational treatment modalities primarily performed in research settings.
-
*
Evidence from clinical trials suggests that oral immunotherapy and possibly sublingual immunotherapy can effectively desensitize many subjects to trigger foods. A subset of desensitized subjects may achieve sustained unresponsiveness after withdrawal of therapy.
-
*
Nonspecific immunotherapy and other emerging therapies using modified food antigens may also be options for treatment, but they are currently limited to early clinical or preclinical trials.
Key questions.
* In an effort to answer these questions, researchers are now performing clinical trials in humans, predominantly with OIT, sublingual immunotherapy (SLIT), and epicutaneous immunotherapy (EPIT). It is important to note that these 3 most commonly considered methods of immunotherapy are allergen-specific, meaning that the therapy is only effective for the particular food given. Nonspecific therapies such as monoclonal antibodies to IgE have also been used to alter host immune responses. Second-generation OIT trials are beginning to use specific and nonspecific approaches in tandem to increase safety and efficacy.
Summary.
Immunotherapy for food allergy remains a promising area for future clinical application. Many of the questions posed by early investigators remain unanswered. Clinical trials have provided some insight as to viable treatment options for food allergy immunotherapy. At this point, the following conclusions can be made based on current evidence:
Desensitization can be achieved in most subjects receiving OIT and SLIT. Among desensitized individuals, a small subset may achieve SU.
Because of the absence of large controlled studies that include a natural history group, it cannot be definitively concluded that the number of subjects who achieve SU on OIT is greater than the number of individuals who would naturally outgrow their food allergy.
When compared with OIT, SLIT offers an enhanced safety profile at the expense of decreased efficacy.
Attempts at desensitization may be more effective and rapid if omalizumab is used as an adjunct.
Adverse effect profiles for all forms of food allergy immunotherapy are still being characterized, because the population that may benefit most from these therapies (those with a history of severe anaphylaxis) has been excluded from nearly all studies. Potential links between OIT and the development of EoE also require further investigation.
Continued investigation may reveal that among the variety of methods, certain forms may be best suited for certain foods or subsets of patients with or without adjunctive therapy. Given outstanding questions regarding safety and efficacy, food allergy immunotherapy requires further investigation before incorporation into routine clinical practice.
Acknowledgments
B.L. Wright’s fellowship is supported by an NIH training grant (T32AI007062). D.M. Fleischer is on the Research Advisory Board of Food Allergy Research and Education and the Medical Advisory Board of Food Allergy and Anaphylaxis Connection Team; has received research support from Monsanto Company and Receptos; is employed by University Physicians, Inc, University of Colorado Denver School of Medicine; has consultant arrangements with LabCorp; has received payment for lectures from Nestle Nutrition Institute; and has received royalties from UpToDate.
Footnotes
Disclosure Statement: B.J. Lanser, K.A. Orgel, and B.P. Vickery have nothing to disclose.
Contributor Information
Bruce J. Lanser, Department of Pediatric, University of Colorado Denver School of Medicine, Aurora, CO, USA and National Jewish Health, 1400 Jackson Street, J322, Denver, CO 80206, USA.
Benjamin L. Wright, Department of Pediatric, University of North Carolina at Chapel Hill School of Medicine, Campus Box #7231, Chapel Hill, NC 27599, USA and Allergy, Asthma & Clinical Immunology, Mayo Clinic, 13400 East Shea Boulevard, Scottsdale, AZ 85259, USA.
Kelly A. Orgel, Department of Pediatric, University of North Carolina at Chapel Hill School of Medicine, Campus Box #7231, Chapel Hill, NC 27599, USA.
Brian P. Vickery, Department of Pediatric, University of North Carolina at Chapel Hill School of Medicine, Campus Box #7231, Chapel Hill, NC 27599, USA.
David M. Fleischer, Department of Pediatric, University of Colorado Denver School of Medicine, Aurora, CO, USA and Children’s Hospital Colorado, 13123 E. 16th Ave, B518, Aurora, CO 80045, USA.
References
- 1.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;125:1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis and treatment. J Allergy Clin Immunol. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119:1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 4.Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134:1016–1025.e43. doi: 10.1016/j.jaci.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Nowak-Wegrzyn A, Muraro A. Food allergy therapy: is a cure within reach? Pediatr Clin North Am. 2011;58:511–530. doi: 10.1016/j.pcl.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Fleischer DM, Perry TT, Atkins D, et al. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics. 2012;130:e25–e32. doi: 10.1542/peds.2011-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampson MA, Munoz-Furlong A, Sicherer SH. Risk-taking and coping strategies of adolescents and young adults with food allergy. J Allergy Clin Immunol. 2006;117:1440–1445. doi: 10.1016/j.jaci.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Primeau MN, Kagan R, Joseph L, et al. The psychological burden of peanut allergy as perceived by adults with peanut allergy and the parents of peanut-allergic children. Clin Exp Allergy. 2000;30:1135–1143. doi: 10.1046/j.1365-2222.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 9.Sicherer SH, Noone SA, Munoz-Furlong A. The impact of childhood food allergy on quality of life. Ann Allergy Asthma Immunol. 2001;87:461–464. doi: 10.1016/S1081-1206(10)62258-2. [DOI] [PubMed] [Google Scholar]
- 10.Christie L, Hine RJ, Parker JG, et al. Food allergies in children affect nutrient intake and growth. J Am Diet Assoc. 2002;102:1648–1651. doi: 10.1016/s0002-8223(02)90351-2. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein GB, Heiner DC. Clinical and immunological perspectives in food sensitivity. A review. J Allergy. 1970;46:270–291. doi: 10.1016/0021-8707(70)90068-7. [DOI] [PubMed] [Google Scholar]
- 12.Schofield AT. A case of egg poisoning. Lancet. 1908;1:715. [Google Scholar]
- 13.Patriarca C, Romano A, Venuti A, et al. Oral specific hyposensitization in the management of patients allergic to food. Allergol Immunopathol (Madr) 1984;12:275–281. [PubMed] [Google Scholar]
- 14.Patriarca G, Schiavino D, Nucera E, et al. Food allergy in children: results of a standardized protocol for oral desensitization. Hepatogastroenterology. 1998;45:52–58. [PubMed] [Google Scholar]
- 15.Freier S, Kletter B. Milk allergy in infants and young children. Current knowledge Clin Pediatr. 1970;9:449–454. doi: 10.1177/000992287000900806. [DOI] [PubMed] [Google Scholar]
- 16.Schloss OM. A case of allergy to common foods. Am J Dis Child. 1912;3:341. [Google Scholar]
- 17.Schloss OM. Allergy in infants and children. Am J Dis Child. 1920;19:433–455. [Google Scholar]
- 18.Park EA. A case of hypersensitiveness to cow’s milk. Am J Dis Child. 1920;3:341. [Google Scholar]
- 19.Stuart HC, Farnham M. Acquisition and loss of hypersensitiveness. Am J Dis Child. 1926;32:341–349. [Google Scholar]
- 20.Freeman J. “Rush” inoculation, with special reference to hay-fever treatment. Lancet. 1930;215:744–747. [Google Scholar]
- 21.Keston BM, Waters I, Hopkins JG. Oral desensitization to common foods. J Allergy. 1935;6:431–436. [Google Scholar]
- 22.Edwards HE. Oral desensitization in food allergy. Can Med Assoc J. 1940;43:234–236. [PMC free article] [PubMed] [Google Scholar]
- 23.Rachid R, Umetsu DT. Immunological mechanisms for desensitization and tolerance in food allergy. Semin Immunopathol. 2012;34:689–702. doi: 10.1007/s00281-012-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoriaty E, Umetsu DT. Oral immunotherapy for food allergy: towards a new horizon. Allergy Asthma Immunol Res. 2013;5:3–15. doi: 10.4168/aair.2013.5.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleischer DM, Burks AW, Vickery BP, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131:119–127. doi: 10.1016/j.jaci.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansfield LE. Oral immunotherapy for peanut allergy in clinical practice is ready. Allergy Asthma Proc. 2013;34:205–209. doi: 10.2500/aap.2013.34.3666. [DOI] [PubMed] [Google Scholar]
- 27.Factor JM, Mendelson L, Lee J, et al. Effect of oral immunotherapy to peanut on food-specific quality of life. Ann Allergy Asthma Immunol. 2012;109:348–352.e2. doi: 10.1016/j.anai.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Robbins KA, Wood RA, Keet CA. Milk allergy is associated with decreased growth in US children. J Allergy Clin Immunol. 2014;134:1466–1468. doi: 10.1016/j.jaci.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobbs CB, Skinner AC, Burks AW, et al. Food allergies affect growth in children. J Allergy Clin Immunol Pract. 2015;3:133–134.e1. doi: 10.1016/j.jaip.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calatayud CM, Garcia AM, Aragones AM, et al. Safety and efficacy profile and immunological changes associated with oral immunotherapy for IgE-mediated cow’s milk allergy in children: systematic review and meta-analysis. J Investig Allergol Clin Immunol. 2014;24:298–307. [PubMed] [Google Scholar]
- 31.Pajno GB, Caminiti L, Salzano G, et al. Comparison between two maintenance feeding regimens after successful cow’s milk oral desensitization. Pediatr Allergy Immunol. 2013;24:376–381. doi: 10.1111/pai.12077. [DOI] [PubMed] [Google Scholar]
- 32.Keet CA, Seopaul A, Knorr S, et al. Long-term follow-up of oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2013;132:737–739. doi: 10.1016/j.jaci.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burks AW, Jones SM, Wood RA, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meglio P, Giampietro PG, Carello R, et al. Oral food desensitization in children with IgE-mediated hen’s egg allergy: a new protocol with raw hen’s egg. Pediatr Allergy Immunol. 2013;24:75–83. doi: 10.1111/j.1399-3038.2012.01341.x. [DOI] [PubMed] [Google Scholar]
- 35.Ramantsik O, Bruschettini M, Tosca MA, et al. Oral and sublingual immunotherapy for egg allergy. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD010638.pub2. undefined. [DOI] [PubMed] [Google Scholar]
- 36.Varshney P, Jones SM, Scurlock AM, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–660. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–475. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorelik M, Narisety SD, Guerrerio AL, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol. 2015;135:1283–1292. doi: 10.1016/j.jaci.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anagnostou K, Islam S, King Y, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised control trial. Lancet. 2014;383:1297–1304. doi: 10.1016/S0140-6736(13)62301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenhawt MJ, Vickery BP. Allergist-reported in the practice of food allergen oral immunotherapy. J Allergy Clin Immunol Pract. 2015;3:33–38. doi: 10.1016/j.jaip.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wasserman RL, Factor JM, Baker JW, et al. Oral immunotherapy for peanut allergy: multipractice experience with epinephrine-treated reactions. J Allergy Clin Immunol Pract. 2014;2:91–96. doi: 10.1016/j.jaip.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Keet CA, Wood RA. Emerging therapies for food allergy. J Clin Invest. 2014;124:1880–1886. doi: 10.1172/JCI72061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez-Ortiz M, Alvaro M, Piquer M, et al. Impact of oral immunotherapy on quality of life in egg allergic children. Pediatr Allergy Immunol. 2015;26:291–294. doi: 10.1111/pai.12355. [DOI] [PubMed] [Google Scholar]
- 44.Wood RA, Sampson HA. Oral immunotherapy for the treatment of peanut allergy: is it ready for prime time? J Allergy Clin Immunol Pract. 2014;2:97–98. doi: 10.1016/j.jaip.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Shin M, Lee J, Ahn K, et al. The influence of the presence of wheat flour on the antigenic activities of egg white proteins. Allergy Asthma Immunol Res. 2013;5:42–47. doi: 10.4168/aair.2013.5.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nowak-Wegrzyn A, Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009;9:234–237. doi: 10.1097/ACI.0b013e32832b88e7. [DOI] [PubMed] [Google Scholar]
- 47.Huang F, Nowak-Wegrzyn A. Extensively heated milk and egg as oral immunotherapy. Curr Opin Allergy Clin Immunol. 2012;12:283–292. doi: 10.1097/ACI.0b013e3283535bc3. [DOI] [PubMed] [Google Scholar]
- 48.Tan JW, Campbell DE, Turner PJ, et al. Baked egg food challenges – clinical utility of skin test to baked egg and ovomucoid in children with egg allergy. Clin Exp Allergy. 2013;43:1189–1195. doi: 10.1111/cea.12153. [DOI] [PubMed] [Google Scholar]
- 49.Bartnikas LM, Sheehan WJ, Larabee KS, et al. Ovomucoid is not superior to egg white testing in predicting tolerance to baked egg. J Allergy Clin Immunol Pract. 2013;1:354–360. doi: 10.1016/j.jaip.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–e17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 51.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100:444–451. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 52.Begin P, Winterroth LC, Dominguez T, et al. Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol. 2014;10:1. doi: 10.1186/1710-1492-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akdis CA, Barlan IB, Bahceciler N, et al. Immunological mechanisms of sublingual immunotherapy. Allergy. 2006;61:11–14. doi: 10.1111/j.1398-9995.2006.01159.x. [DOI] [PubMed] [Google Scholar]
- 54.Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. J Allergy Clin Immunol. 2008;121:1301–1308. doi: 10.1016/j.jaci.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enrique E, Pineda F, Malek T, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005;116:1073–1079. doi: 10.1016/j.jaci.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 56.Garrido-Fernandez S, Garcia BE, Sanz ML, et al. Are basophil activation and sulphidoleukotriene determination useful tests for monitoring patients with peach allergy receiving sublingual immunotherapy with a Pru p 3-enriched peach extract? J Investig Allergol Clin Immunol. 2014;24:106–113. [PubMed] [Google Scholar]
- 57.Kim EH, Bird JA, Kulis M, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–646.e1. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burks AW, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: long-term follow-up of a randomized multicenter trial. J Allergy Clin Immunol. 2015;135:1240–1248.e1–3. doi: 10.1016/j.jaci.2014.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. 2015;135:1275–1282.e1–6. doi: 10.1016/j.jaci.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chin SJ, Vickery BP, Kulis MD, et al. Sublingual versus oral immunotherapy for peanut-allergic children: a retrospective comparison. J Allergy Clin Immunol. 2013;132:476–478.e2. doi: 10.1016/j.jaci.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–455. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mondoulet L, Dioszeghy V, Ligouis M, et al. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy. 2010;40:659–667. doi: 10.1111/j.1365-2222.2009.03430.x. [DOI] [PubMed] [Google Scholar]
- 63.Dupont C, Kalach N, Soulaines P, et al. Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. 2010;125:1165–1167. doi: 10.1016/j.jaci.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 64.Dupont C, Bourrier T, de Blay F, et al. Peanut epicutaneous immunotherapy (EPIT) in peanut allergic children: 18 months treatment in the Arachild Study. J Allergy Clin Immunol. 2014;133:AB102. [Google Scholar]
- 65.Lack G. Update on risk factors for food allergy. J Allergy Clin Immunol. 2012;129:1187–1197. doi: 10.1016/j.jaci.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 66.Leung DY, Sampson HA, Yunginger JW, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 67.Schneider LC, Rachid R, LeBovidge J, et al. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut allergic patients. J Allergy Clin Immunol. 2013;132:1368–1374. doi: 10.1016/j.jaci.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nadeau KC, Schneider LC, Hoyte L, et al. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol. 2011;127:1622–1624. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Begin P, Dominguez T, Wilson SP, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin Immunol. 2014;10:7. doi: 10.1186/1710-1492-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Xiu-Min LI. Chinese herbal therapy for the treatment of food allergy. Curr Allergy Asthma Rep. 2012;12:332–338. doi: 10.1007/s11882-012-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J, Jones SM, Pongracic JA, et al. Safety, clinical and immunologic efficacy of a Chinese herbal medicine (Food Allergy Herbal Formula-2) for food allergy. J Allergy Clin Immunol. 2015;135:AB234. doi: 10.1016/j.jaci.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elazab N, Mendy A, Gasana J, et al. Probiotic administration in early life, atopy and asthma: a meta-analysis of clinical trials. Pediatrics. 2013;132:e666–e676. doi: 10.1542/peds.2013-0246. [DOI] [PubMed] [Google Scholar]
- 73.Se Soh Aw M, Chong YS, et al. Probiotic supplementation in the first 6 months of life in at risk Asian infants – effects on eczema and atopic sensitization at the age of 1 year. Clin Exp Allergy. 2009;39:571–578. doi: 10.1111/j.1365-2222.2008.03133.x. [DOI] [PubMed] [Google Scholar]
- 74.Canani RB, Nocerino R, Terrin G. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: a randomized trial. J Allergy Clin Immunol. 2012;129:580–582.e5. doi: 10.1016/j.jaci.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Tang MLK, Ponsonby A-L, Orsini F, et al. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol. 2015;135:737–744.e8. doi: 10.1016/j.jaci.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 76.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;113:624–629. doi: 10.1016/j.anai.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Leung J, Hundal NV, Katz AJ, et al. Tolerance of baked milk in patients with cow’s milk-mediated eosinophilic esophagitis. J Allergy Clin Immunol. 2013;132:1215–1216. doi: 10.1016/j.jaci.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nelson HS, Lahr J, Rule R, et al. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–751. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 79.Wood RA, Sicherer SH, Burks AW, et al. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy. 2013;68:803–808. doi: 10.1111/all.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]


