Abstract
Background
Macrotyloma uniflorum Linn (Fabaceae) is a herbaceous plant with annual branches. It is used in kidney stones, inflamed joints, fever, musculoskeletal disorders, sinus wounds and localized abdominal tumors. It is reported as an antioxidant and nutraceutical (forage and food). GC-MS analysis of ethanol extract has led to identification of twenty-eight compounds from M. uniflorum by comparison of their retention indices and mass spectra fragmentation patterns with those stored on the GC-MS computer library.
Results
The main constituents identified were mome inositol, ethyl alpha-d-glucopyranoside, n- hexadecanoic acid, linoleic acid (9, 12-octadecadienoic acid), its esters and ethyl derivatives, Vitamin E, stigmasterol and 3-beta-stigmast-5-en-3-ol.
Conclusions
The extracts are rich in linoleic acid and its esters, mome inositol and ethyl alpha-d-glucopyranoside; therefore, this plant can be medicinally beneficial as an antioxidant, in diabetes and its related disorders.

Electronic supplementary material
The online version of this article (doi:10.1186/s13588-014-0013-y) contains supplementary material, which is available to authorized users.
Keywords: GC-MS, Macrotyloma uniflorum, Chemical constituents, Retention indices
Background
Macrotyloma uniflorum (synonym: Dolichos biflorus Linn.) belonging to the family Fabaceae is commonly known as Kulthi in Hindi and horse gram in English. It is a herbaceous plant with annual branches, sub-erect or twining, leaflets of 2.5 to 5 cm. Its seed is 6 to 8 mm long and 3 to 4 mm broad. The seeds are trapezoidal oblong or somewhat rounded in shape and pale to dark reddish brown or orange brown or all black in colour. The genus Macrotyloma comprises about 25 species, most of which are restricted to Africa of which four have been identified as M. uniflorum, M. stenocarpum, M. verrucosum and M. benadirianum[1]. Traditionally, it has been widely used in the treatment of kidney stones, inflamed joints, fever, musculoskeletal disorders, sinus wounds and localized abdominal tumors [2],[3]. Experimentally, the seeds are reported as hepato-protective, diuretic and antioxidant [4]–[6]. To the best of the authors' knowledge, no published literature exists about the chemical contents of the ethanol extract of M. uniflorum. Thus it was planned to carry out GC-MS analysis of ethanol extract of the seeds of M. uniflorum.
Methods
Preparation of crude extract
The seeds (1 Kg) were coarsely powdered and defatted with petroleum ether (60°C to 80°C) for 7 days by cold maceration. The fat-exhausted drug was further extracted with ethanol (95% v/v) by soxhlation for 72 h. The extract was concentrated in a rotary vacuum evaporator to yield 25.0% w/w of dark-brown-coloured extract. The ethanol extract of seeds was diluted with ethanol and filtered with Whatman No. 42 to obtain a particle-free extract for analysis by GC-MS.
GC-MS analysis
The extract was directly used for the analysis. GC-MS was carried out on a GCMS-QP2010 Plus (Shimadzu, Kyoto, Japan) system with head space sampler (AOC-20s) and auto injector (AOC-20i), equipped with mass selective detector, having ion source temperature of 230°C, interface temperature of 260°C, a solvent cut time of 2.50 min threshold of 1,000 eV and mass range of 40 to 650 m/z. Compounds were separated using a Rtx 5 MS capillary column (Restek Company, Bellefonte, USA: crossbond 5% diphenyl/ 95% dimethyl polysiloxane) having dimensions 30 m (length) × 0.25 mm (diameter) × 0.25 μm (film thickness). The split mode was used at a ratio of 10:1. The temperature of the injector was initialized to 250°C, having a split injection mode. The temperature was programmed from 100°C (3 min), then further increased to 280°C at a ramp rate of 10°C/min (19 min hold).
Helium (>99.999%) was used as the carrier gas at a linear flow velocity of 40.9 cm/s. The debit of gas (helium) vector was fixed to 16.3 mL/min, with a total flow of 1. 21 mL/min. The volume of injected sample was 1.0 μL of ethanol extract. The components were identified by comparison of their retention indices (RI) relative to homologous alkane series (purchased from Sigma, St. Louis, USA) and by comparison of their mass spectral fragmentation patterns with those data provided in WILEY8.LIB, NIST08.LIB, NIST08s.LIB and NIST.LIB. Identification was assumed when a good match of mass spectrum and RI was achieved.
Results and discussion
The seeds were purchased from Bhagalpur district, Bihar, India, and identified by Dr. K. C. Bhatt, NBPGR, New Delhi. A voucher specimen (PGS-13-02) has been deposited in the Department of Pharmaceutical Sciences (Pharmacognosy Division), Guru Jambheshwar University of Science and Technology, Hisar, Haryana, India.
Chemical composition
GC-MS analysis of ethanol extract led to the identification of twenty-eight compounds from M. uniflorum (Table 1, Figure 1). The main constituents identified were two polysaccharides namely mome inositol and ethyl alpha-d-glucopyranoside. A number of fatty acids and their esters have also been identified; they were n- hexadecanoic acid, 9, 12-octadecadienoic acid and its esters. Phytosterols, namely stigmasterol and 3-β-stigmast-5-en-3-ol, were present in traces. Other compounds present were 3-cyclopentylpropionic acid, 2-dimethylaminoethyl ester, heneicosane and vitamin E. literature survey showed that Dolichin A and B and pyroglutaminylglutamine along with some flavonoids were isolated from this plant. Seeds of M. uniflorum contain lectins, glycoprotein, agglutinin, an anti-A phytoagglutinin, four glycosidase enzymes, allantoinase and a strong diuretic dipeptide, pyroglutamylglutamine. The seeds are rich source of the enzyme urease and also contain β-sitosterol [7].
Table 1.
Chemical composition of ethanol extract of M. uniflorum seed
| Peak | Retention time | Retention indices | Area% | Name |
|---|---|---|---|---|
| 1 | 3.645 | 1,056 | 0.67 | Benzeneacetaldehyde |
| 2 | 4.465 | 1,116 | 0.51 | Benzeneethanamine |
| 3 | 10.051 | 1,499 | 0.27 | L-Phenylalanine, ethyl ester |
| 4 | 11.076 | 1,580 | 0.53 | 2-(1-methyl-2-propenyl)bicyclo[2.2.1]heptane |
| 5 | 11.331 | 1,602 | 0.39 | 1H-Pyrrole, 2-(2,4,6-cycloheptatrienyl) |
| 6 | 12.007 | 1,660 | 11.14 | Ethyl .alpha.-d-glucopyranoside |
| 7 | 13.027 | 1,750 | 23.24 | Mome inositol |
| 8 | 15.312 | 1,971 | 2.76 | n-Hexadecanoic acid |
| 9 | 15.568 | 1,996 | 0.49 | Heptadecanoic acid, ethyl ester |
| 10 | 17.015 | 2,151 | 19.79 | 9,12-Octadecadienoic acid (Z,Z) |
| 11 | 17.176 | 2,169 | 1.61 | Ethyl (9Z,12Z)-9,12-octadecadienoate |
| 12 | 18.319 | 2,300 | 0.12 | Heptadecane, 3-methyl |
| 13 | 18.543 | 2,327 | 1.88 | Octanamide, N-(2-hydroxyethyl) |
| 14 | 19.678 | 2,465 | 3.18 | 3-Cyclopentylpropionic acid, 2-dimethylaminoethyl ester |
| 15 | 19.833 | 2,484 | 0.60 | (R)14-Methyl-8-hexadecyn-1-ol |
| 16 | 20.158 | 2,526 | 1.77 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester |
| 17 | 21.207 | 2,662 | 1.27 | 1-Cyclohexyldimethylsilyloxybutane |
| 18 | 21.483 | 2,698 | 0.22 | Eicosane |
| 19 | 21.607 | 2,712 | 19.49 | 9,12-Octadecadienoic acid (Z,Z)-, 2,3-dihydroxypropyl ester |
| 20 | 22.342 | 2,798 | 0.50 | Heneicosane |
| 21 | 22.414 | 2,805 | 0.33 | 9-Methyl-10,12-hexadecadien-1-ol acetate |
| 22 | 23.334 | 2,897 | 0.19 | Hexatriacontane |
| 23 | 26.396 | 3,116 | 2.42 | i-Propyl 9,12-octadecenadienoate |
| 24 | 26.966 | 3,154 | 0.72 | Vitamin E |
| 25 | 27.432 | 3,181 | 1.76 | Tricyclo[20.8.0.0(7,16)]triacontane, 1(22),7(16)-diepoxy |
| 26 | 29.695 | 3,290 | 0.97 | Stigmasterol |
| 27 | 31.064 | 3,355 | 2.42 | Stigmast-5-en-3-ol, (3 beta) |
| 28 | 32.328 | 3,413 | 0.74 | (−)-Isolongifolol, acetate |
Figure 1.
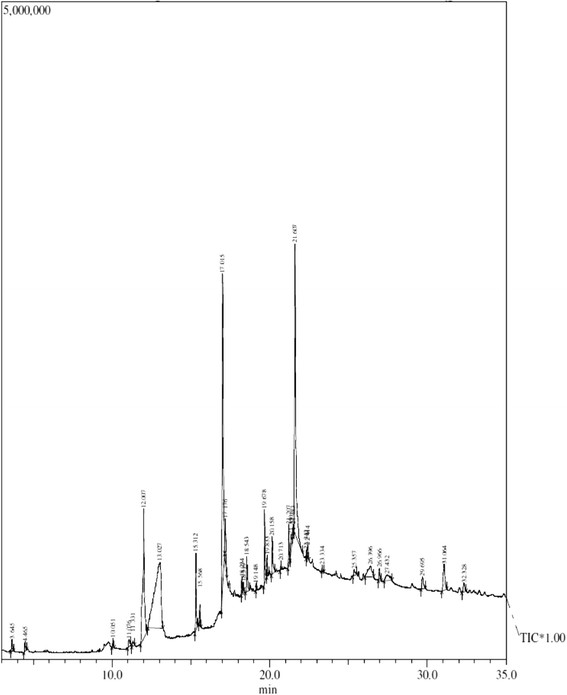
A typical GC-MS chromatogram of the constituents of ethanol extract of seeds of M. uniflorum .
Conclusions
Mome inositol, one of the major components of extract of M. uniflorum, is reported as anti-alopecic, anti-cirrhotic, anti-neuropathic, cholesterolytic, lipotropic and a sweetener. n-hexadecanoic acids act as a 5-alpha-reductase inhibitor, a hemolytic agent and an antioxidant [8]. (3β)-stigmast-5-en-3-ol has shown an insulin-like effect, that is, stimulating glucose transport apart from its existing cholesterol-lowering efficacy. Therefore, it can play a beneficial role as an antidiabetic agent [9]. Animal studies have revealed that linoleic acid is converted to gamma linoleic acid in the body and can prevent chemically induced diabetes while restoring normal antioxidant status in tissues [10]. It can also prevent diabetic neuropathy, a painful condition resulting from exposure of nerves to high glucose levels [11]. Hence, the plant can be utilized as a natural sweetener, anti-alopecic, anti-cirrhotic, anti-neuropathic, cholesterolytic, lipotropic, antioxidant and antidiabetic.
Authors’ information
Sneha Das is PhD research scholar pursuing her doctorate under supervision of Professor Neeru Vasudeva and Professor Sunil Sharma at Department of Pharmaceutical Sciences, Guru Jambheshwar University of Science and Technology, Hisar, Haryana, India, 125001.
Acknowledgements
We are grateful to Department of Science and Technology, New Delhi, India, under the Ministry of Science and Technology, New Delhi, India, and the Department of Pharmaceutical Sciences, Guru Jambheshwar University of Science and Technology, Hisar, Haryana, for providing fellowship and infrastructure, respectively to Sneha Das for carrying out the research. Also we would like to thank Dr. Ajay Kumar, scientist at AIRF, JNU, Delhi, for helping us perform GC-MS.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SD has carried out all practical work and wrote the manuscript. NV and SS supervised all the work including the designing and drafting of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Sneha Das, Email: pharma.sneha@gmail.com.
Neeru Vasudeva, Email: neeruvasudeva@gmail.com.
Sunil Sharma, Email: sunilsgju@gmail.com.
References
- 1.Mehra A, Upadhyaya M. Macrotyloma uniflorum Lam traditional crop of kumaun himalaya and ethnobotanical perspectives. Int J Agric Food Sci. 2013;3(4):148–150. [Google Scholar]
- 2.Muthu AK, Sethupathy S, Manavalan R, Karar PK. Hypolipidemic effect of methanolic extract of Dolichos biflorus Linn in high fat diet fed rats. Ind J Exp Biol. 2005;43:522. [PubMed] [Google Scholar]
- 3.Muthu AK, Sethupathy S, Manavalan R, Karar PK. Antioxidant potential of methanolic extract of Dolichos biflorus Linn. in high fat diet fed rabbits. Ind J Pharmacol. 2006;38:131. doi: 10.4103/0253-7613.24620. [DOI] [PubMed] [Google Scholar]
- 4.Parmar HB, Das SK, Gohil KJ. Hepatoprotective activity of Macrotyloma uniflorum seed extract on paracetamol and d-galactosamine induced liver toxicity in Albino rats. IJPR. 2012;2(2):86–91. [Google Scholar]
- 5.Ravishankar K, Priya PSVV. Evaluation of diuretic effect of ethanolic seed extracts of Macrotyloma uniflorum and Cucumis melo in rats. Int J Pharm Bio Sci. 2012;3(3):251–255. [Google Scholar]
- 6.Ravishankar K, Priya PSVV. In vitro antioxidant activity of ethanolic seed extracts of Macrotyloma uniflorum and Cucumis melo for therapeutic potential. IJRPC. 2012;2(2):442–445. [Google Scholar]
- 7.Ghani A. Medicinal Plants of Bangladesh with Chemical Constituents and Uses. Dhaka, Bangladesh: Asiatic Society of Bangladesh; 2003. [Google Scholar]
- 8.Kumar NR, Reddy JS, Gopikrishna G, Solomon KA. GC-MS determination of bioactive constituents of Cycas beddomei cones. Int J Pharm Bio Sci. 2012;3(3):344–350. [Google Scholar]
- 9.Sujatha S, Anand S, Sangeetha KN, Shilpa K, Lakshmi J, Balakrishnan A, Lakshmi BS. Biological evaluation of (3β)-STIGMAST-5-EN-3-OL as potent anti-diabetic agent in regulating glucose transport using in vitro model. Int J Diabetes Mellitus. 2010;2:101–109. doi: 10.1016/j.ijdm.2009.12.013. [DOI] [Google Scholar]
- 10.Suresh Y, Das UN. Long-chain polyunsaturated fatty acids and chemically induced diabetes mellitus: effect of omega-6 fatty acids. Nutrition. 2003;19(2):93–114. doi: 10.1016/S0899-9007(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 11.Pitel S, Raccah D, Gerbi A, Pieroni G, Vague P, Coste TC. At low doses, a gamma-linolenic acid-lipoic acid conjugate is more effective than docosahexaenoic acid-enriched phospholipids in preventing neuropathy in diabetic rats. J Nutr. 2007;137(2):368–372. doi: 10.1093/jn/137.2.368. [DOI] [PubMed] [Google Scholar]


