Abstract
Bronchiolitis obliterans syndrome (BOS) is a significant post-transplant complication with low survival. BOS stage 0p (BOS 0p) is a parameter detected on pulmonary function tests (PFTs) after lung transplantation to identify patients at risk to develop BOS. We performed a retrospective study on 442 patients who underwent allogeneic stem cell transplant from 2007 to 2011 to evaluate whether development of BOS 0p is a risk factor in this population for BOS. Patients who met criteria for BOS 0p were significantly more likely to develop BOS (hazard ratio [HR], 3.22; P < .001). BOS 0p was significantly associated with a history of lung disease pretransplant (HR, 2.48; P =.001) and chronic graft-versus-host disease (GVHD) outside the lung post-transplant (HR, 23; P < .001). Finally, BOS 0p criteria were adequately sensitive in predicting BOS (85%), with a high negative predictive value (98%). Our findings suggest a routine PFT screening strategy with the intent of detecting BOS 0p, especially among patients with prior lung disease and who developed chronic GVHD, could suitably identify an at-risk population for the development of BOS.
Keywords: Chronic GVHD, Bronchiolitis obliterans, syndrome, BOS 0p
INTRODUCTION
Chronic graft-versus-host disease (GVHD) of the lung manifests as bronchiolitis obliterans syndrome (BOS), one of the most serious pulmonary complications after allogeneic hematopoietic stem cell transplantation (HSCT). Pathologically, BOS is characterized by progressive fibroproliferation within the terminal bronchioles in the lungs, resulting in obliteration of airway lumens [1]. Clinically, BOS appears as fixed airflow obstruction on spirometry, and at the time of diagnosis most patients present with moderate to severe disease [1,2]. After diagnosis, BOS typically follows an irreversible and progressive decline in lung function despite therapeutic intervention, with 5-year survival rates as low as 13% [1–3].
In this setting, anticipating irreversible lung damage from BOS is an essential strategy to improve outcomes through earlier preventive or therapeutic interventions. Previous studies have shown that patients who experience an annualized decrease in forced expiratory volume in 1 second (FEV1) by 5% or more at 1-year after allogeneic HSCT are at an increased mortality risk, particularly among patients with chronic GVHD [4]. Presumably, detecting early airflow obstruction may identify patients at risk of death due to BOS, among other causes.
BOS stage 0p (BOS 0p) is a spirometric parameter applied to identify patients with the potential to develop BOS after lung transplantation where it also represents a significant complication, with clinical and pathologic similarities to chronic GVHD of the lung [5]. BOS 0p is met when a decline in FEV1 of 10% to 19% of predicted normal or a decline in predicted forced expiratory flow between 25% and 75% of vital capacity (FEF25-75) > 25% is observed on consecutive pulmonary function tests (PFTs); in lung transplantation patients it can predict the development of BOS [5–7]. In this single-institution study, we evaluated BOS 0p as a predictor for BOS in a cohort of allogeneic transplant patients.
METHODS
We retrospectively evaluated all patients age 18 years and older who underwent allogeneic HSCT at the University of Michigan Blood and Marrow Transplantation Program between January 1, 2007 and December 31, 2011. Four hundred forty-two consecutive patients were identified, and we reviewed their outcomes through December 31, 2013. Transplants beyond the first 1 were excluded from the study. Data were obtained from the University of Michigan Blood and Marrow Transplantation database. This study was performed under institutional review board approval at the University of Michigan Health System.
The diagnosis of BOS was determined using clinical criteria established by the chronic GVHD Working Group at the National Institutes of Health requiring the following: (1) FEV1/forced vital capacity ratio <.7 and FEV1 <75% of predicted, (2) evidence of airway obstruction on high-resolution computed tomography or a residual volume on PFTs >120%, (3) absence of infection in the respiratory tract, and (4) histologic diagnosis of BOS or at least 1 other manifestation of chronic GVHD in an additional organ system [8]. Infection was assessed in patients by clinical examination, microbiologic assessment, and radiologic assessment with either chest radiograph or computed tomography of the chest. The date of detection of BOS was noted as the date of the PFT performed that met criteria for BOS.
BOS 0p was determined using similar criteria applied to lung transplantation patients. Thus, the diagnostic criteria for BOS 0p were as follows: (1) a decline of ≥10% predicted FEV1 or (2) a decline of ≥25% predicted FEF25-75 but (3) not otherwise meeting criteria for BOS. These findings had to be present in at least 2 consecutive PFTs. The date of detection of BOS 0p was noted as the date of the first PFT that demonstrated BOS 0p. PFTs were examined from pretransplant through last date of follow-up, death, or relapse, whichever occurred first.
All spirometry was performed according to American Thoracic Society guidelines. The University of Michigan Health System–predicted equations for adults are derived from a combination of sources of data in the literature established approximately 15 years ago. For our study, we recorded forced vital capacity, vital capacity, FEV1, and FEF25-75, all computed according to the equations of Morris et al. [9]. Per institutional guidelines, spirometry was obtained pretransplant, defined as spirometry performed within 1 month of transplant, at day 100, at day 180, and at 1 year. After 1 year, measurement interval varied based on transplantation physician preference and patients’ clinical states. In most cases, the interval between PFTs was 3 months, and the largest interval between PFTs was 6 months. After 2 years, patients with chronic GVHD continued to have spirometry performed annually at a minimum. Patients without chronic GVHD underwent spirometry at the discretion of the primary transplant physician.
Clinical data analyzed in this study were collected prospectively in a database for long-term transplant follow-up. Pretransplant characteristics collected included age, gender, stem cell source, conditioning regimen, and tobacco use history. Additionally, we identified whether a patient had a history of lung disease before transplant. Pulmonary conditions included in this history were a history of chronic obstructive pulmonary disease, asthma, pulmonary embolism, or persistent pleural effusion. Finally, diagnosis and staging of acute and chronic GVHD were collected, with determination and staging assigned using previously established criteria [8,10].
A competing risks regression model according to the methods of Fine and Gray [11] and Gray [12] were used to assess the association of patient characteristics with BOS and BOS 0p, treating death and relapse as competing risks. Cox regression without competing risks was used to assess the association of acute and chronic GVHD with BOS and BOS 0p, treating acute and chronic GVHD as time-varying covariates. Cox regression without competing risks was used to assess the association of BOS and BOS 0p with overall survival, treating BOS and BOS 0p as time-varying covariates.
Sensitivity, specificity, and predictive values of BOS 0p, FEV1 criterion, and FEF25-75 criterion to detect BOS were calculated. Patients included in these analyses had a minimum 180 days of follow-up. Additionally, we only included patients who developed BOS 0p within 2 years. Finally, upon development of BOS 0p, we included patients with a minimum 1-year follow-up after detection of BOS 0p. All analyses were done in the statistical package R (R Development Core Team, Vienna, Austria).
RESULTS
Patient Characteristics
Four hundred forty-two patients ages 18 years and older underwent allogeneic HSCT between 2007 and 2011; baseline patient characteristics are outlined in Table 1. Most patients were men (n = 259, 59%) with a median age of 54 years (range, 18 to 73 years). Most patients underwent myeloablative conditioning before transplant (n = 301, 68%), and most patients had a matched-sibling allogeneic SCT. Stem cell source in nearly all patients was peripheral blood (n = 403, 91%). At the end of our follow-up interval, 175 patients were alive, with a median follow-up of 3.95 years (range, 2.01 to 6.96 years).
Table 1.
Patient Characteristics
| Clinical Characteristics | Value |
|---|---|
| Median age, yr (range) | 54 (18–73) |
| Sex | |
| Male | 259 (59%) |
| Female | 183 (41%) |
| Disease risk | |
| Low | 147 (33%) |
| Intermediates | 121 (28%) |
| High | 174 (39%) |
| Donor type | |
| Sibling | 224 (51%) |
| Unrelated | 218 (49%) |
| HLA status | |
| Matched | 345 (78%) |
| Mismatched | 78 (18%) |
| Stem cell source | |
| Peripheral blood | 403 (91%) |
| Bone marrow | 23 (5%) |
| Cord blood | 16 (4%) |
| Conditioning intensity | |
| Myeloablative | 301 (68%) |
| Reduced intensity | 141 (32%) |
| Conditioning regimen | |
| Myeloablative, busulfan | 216 (49%) |
| TBI | 65 (15%) |
| Acute GVHD | |
| Grades I–IV | 277 (63%) |
| Grades III–IV | 87 (20%) |
| Chronic GVHD | |
| Mild | 67 (15%) |
| Moderate | 87 (20%) |
| Severe | 60 (14%) |
| Baseline lung function | |
| FEV1, mean (range) | 97 (39–144) |
| FEF25-75, mean (range) | 83 (12–165) |
| Previous tobacco use | |
| Yes | 189 (43%) |
| No | 253 (57%) |
| Prior lung disease | |
| Yes | 46 (10%) |
| No | 396 (90%) |
TBI indicates total body irradiation.
All patients in this study had pretransplant spirometry. Eighty-two patients (19%) underwent spirometry once, with relapse or death limiting additional tests. Remaining patients underwent a median of 6 PFT studies (range, 2 to 28), and, in total, of 2358 PFTs were performed on all patients.
Diagnosis and Risk Factors for BOS
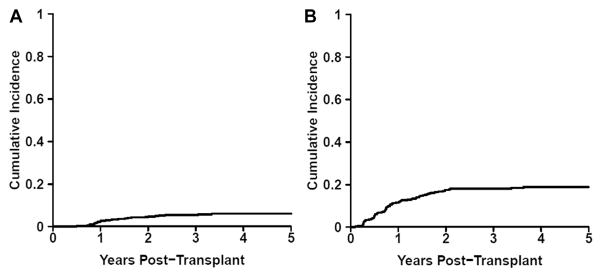
Twenty-six patients developed BOS by the end of our follow-up interval. The cumulative incidence of BOS was 5% (95% confidence interval, 3% to 6%) at 2 years post-transplant (Figure 1A). Median time to detection of BOS was 401 days (range, 178 to 1218). Upon detection of BOS, median percent-predicted FEV1 was 52% (range, 25% to 75%), indicating moderate to severe airflow obstruction, and median percent-predicted FEF25-75 was 21% (range, 8% to 42%). Finally, development of BOS was significantly associated with increased mortality by the end of the study period (hazard ratio [HR], 1.92; P = .035).
Figure 1.
Cumulative incidence of (A) BOS and (B) BOS 0p.
Multivariate analysis of pretransplant characteristics revealed that only a history of lung disease was associated with development of BOS. Patients with history of lung disease pretransplant were 4 times more likely to develop BOS compared with patients without lung disease (HR, 4.05; P = .001). In our study, stem cell source, total body irradiation, myeloablative conditioning, prior tobacco use, or history of grades III–IV acute GVHD post-transplant were not associated with the development of BOS (Table 2) [13–16].
Table 2.
Associations with BOS
| Risk Factor | HR | P |
|---|---|---|
| Age | .99 | .69 |
| Sex | ||
| Male | 1.00 (ref) | .61 |
| Female | 1.21 | |
| Stem cell source | ||
| Bone marrow | 1.00 (ref) | .87 |
| Peripheral blood | 1.14 | |
| Myeloablative, busulfan | 1.43 | .38 |
| TBI | .48 | .3 |
| Previous tobacco use | .98 | .97 |
| Previous lung disease | 4.05 | .001 |
| Grades III–IV acute GVHD | 1.93 | .14 |
Post-transplant, patients who met criteria for BOS 0p were significantly more likely to develop BOS (HR, 3.22; P < .001). Detection of BOS 0p preceded BOS in 22 patients (85%). In these patients, BOS 0p was detected within 1 year in 19 patients (86%) and between 1 and 2 years in 3 patients (14%). Further, criteria for BOS 0p were met at a median of 222 days (range, 21–1039) before BOS. Progression from BOS 0p to BOS on spirometry occurred within 3 months in 4 patients, within 3 and 6 months in 4 patients, within 6 and 9 months in 9 patients, and over 1 year in 5 patients.
Four remaining patients (15%) developed BOS without first meeting criteria for BOS 0p because of a significant decline in lung function between consecutive measurements of lung function that met criteria for BOS. These 4 patients developed BOS within 1 year.
Diagnosis and Risk Factors for BOS 0p
Seventy-nine patients met criteria for BOS 0p, with an estimated 2-year cumulative incidence of 16% (95% confidence interval, 13% to 20%) (Figure 1B). The median time to detection of BOS 0p was 273 days (range, 32 to 1910 days). Detection of BOS 0p occurred within 1 year in 51 patients (65%), within 1 and 2 years in 22 patients (28%), and after 2 years in 6 patients (7%). The median percent-predicted FEV1 on development of BOS 0p was 81% (range, 30% to 114%), and the median percent-predicted FEF25-75 was 65% (range, 11% to 128%).
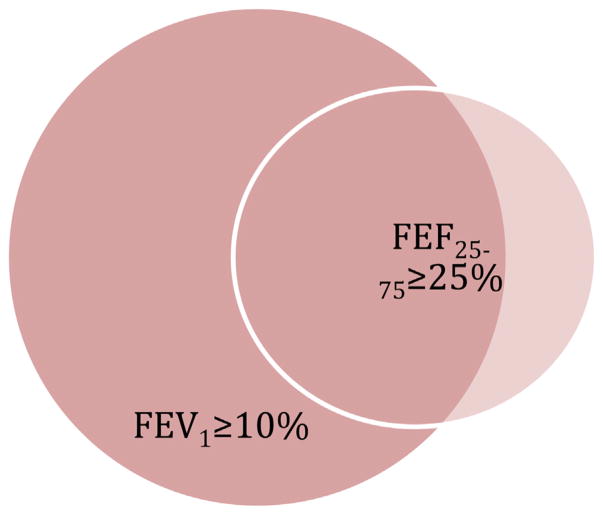
In most patients, we detected significant changes to the percent-predicted FEV1 at the time of diagnosis of BOS 0p. At diagnosis of BOS 0p, 39 patients (49%) had both >10% decline to the percent-predicted FEV1 and >25% decline to FEF25-75; 31 patients (39%) had >10% decline in their percent-predicted FEV1 only, and 9 of these patients (12%) experienced a >25% decline in their percent-predicted FEF25-75 only (Figure 2). The development of BOS 0p was not associated with a higher mortality rate.
Figure 2.
Venn diagram illustrating the PFT change responsible for detection of BOS 0p.
As in the case with BOS, multivariate analysis revealed that BOS 0p was significantly associated with a history of lung disease pretransplant (HR, 2.48; P = .001). Sixteen patients (20%) with BOS 0p had a pretransplant history of lung disease. Eight of these patients (50%) progressed to BOS. Age, sex, conditioning regimen, or cell source were not associated with BOS 0p. Analysis of time-varying characteristics revealed that development of grades III to IV acute GVHD and chronic GVHD outside the lung were strongly associated with the development of BOS 0p (HR, 1.85; P = .038 and HR, 23; P < .001, respectively) (Table 3). Seventy-two patients (91%) with BOS 0p had a history of chronic GVHD. None of the patients who met criteria for BOS 0p without chronic GVHD later met spirometric criteria for BOS.
Table 3.
Associations with BOS 0p
| Risk Factor | HR | P |
|---|---|---|
| Age | .99 | .13 |
| Sex | ||
| Male | 1.00 (ref) | .43 |
| Female | .84 | |
| Stem cell source | ||
| Bone marrow | 1.00 (ref) | .61 |
| Peripheral blood | 1.25 | |
| Myeloablative, busulfan | 1.27 | .30 |
| TBI | 1.06 | .84 |
| Previous tobacco use | 1.04 | .86 |
| Previous lung disease | 2.29 | .004 |
| Grades III–IV acute GVHD | 1.85 | .03 |
| Chronic GHVD | 22.8 | <.001 |
Predictive Characteristics of BOS 0p
The performance of the FEV1 criterion, FEF25-75 criterion, and BOS 0p criteria as predictors of BOS are outlined in Table 4. Two hundred seventy-five patients were included for these analyses. BOS 0p criteria overall had the best performance characteristics. Our results indicated that the false-positive rate for the BOS 0p criteria was high, reflected by a low positive predictive value (29%) and fair specificity (78%). However, the overall sensitivity for the detection of BOS by the BOS 0p criteria was good (85%). Furthermore, the BOS 0p criteria had a very good negative predictive value (98%). The FEV1 criterion had comparable performance characteristics with similar sensitivity (77%) and a similar negative predictive value (97%). The false-positive rate was essentially no different from the BOS 0p criteria (positive predictive value = 30%, specificity = 81%). The FEF25-75 criterion demonstrated a marginally lower false-positive rate, reflected by a higher specificity (88%) and higher positive predictive value (37%), but at the expense of sensitivity (65%).
Table 4.
Predictive Measurements for BOS 0p
| Criterion | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| FEV1 0p | 77% | 81% | 30% | 97% |
| FEF25-75 0p | 65% | 88% | 37% | 96% |
| FEV1 or FEF25-75 0p (BOS 0p) | 85% | 78% | 29% | 98% |
PPV indicates positive predictive value; NPV, negative predictive value.
Predictive measurements of BOS 0p by FEV1 criterion, FEF25-75 criterion, or FEV1 or FEF25-75 criterion.
DISCUSSION
BOS represents a rare but significant post-transplant complication, with very low survival rates after diagnosis [2]. The syndrome is usually diagnosed at a relatively advanced stage, where the higher likelihood of irreversible lung damage limits the efficacy of available therapies and survival [2,3]. So far, lung transplantation is the only curative treatment of severe BOS after allogeneic HSCT, where the primary disease process leading to allogeneic transplant was otherwise controlled [17]. Thus, predicting BOS could allow for early intervention, potentially preventing irreversible lung damage and improving outcomes.
In this study, we proposed that through serial pulmonary function testing after HSCT, detection of early spirometric changes could identify patients at risk for BOS. Before this study, evidence for this strategy was lacking. Accordingly, less than half of the transplant centers perform regular PFT screening for BOS [18]. At our institution, we performed routine spirometry every 3 months during the first year post-transplant and every 3 to 6 months beyond 1 year post-transplant. We retrospectively reviewed all spirometry data to identify BOS 0p, defined as a decrease in the percent-predicted FEV1 of ≥10% or a decrease in the percent-predicted FEF25-75 of ≥25% and not meeting criteria for BOS, similar to BOS stage 0p in lung transplantation patients [5]. To ensure these changes were not transient, we only identified patients as meeting BOS 0p if they had a second PFT performed that demonstrated a persistent decline in lung function.
Based on our data, we conclude that BOS 0p may be a suitable method to identify patients at risk for BOS. Patients who met criteria for BOS 0p were significantly more likely to develop BOS (HR, 3.22; P < .001). Further, the sensitivity for the detection of BOS by the BOS 0p criteria was good (85%), and a negative result within 2 years indicates that BOS is unlikely (negative predictive value = 98%). Finally, criteria for BOS 0p were met at a median of 222 days (range, 21 to 1039) before detection of BOS.
Patients with chronic GVHD were at an overwhelmingly higher risk for BOS 0p (HR, 23; P < .001). Further, patients with a history of lung disease (chronic obstructive pulmonary disease, asthma, pulmonary embolism) pretransplant were at a higher risk for BOS 0p (HR, 2.48; P = .001). These patients may warrant closer screening for BOS 0p compared with other patients post-transplant.
The operating characteristics of BOS 0p in allogeneic HSCT patients were similar to the results reported in the lung transplantation population [6,7]. In allogeneic HSCT patients, BOS 0p criteria operated with good sensitivity (85%) and specificity (78%), which is consistent with the operating characteristics reported for BOS stage 0p within a retrospective cohort study of double-lung transplantation patients (sensitivity = 79%, specificity = 80%) [6]. There was a difference in our patient population: We found a lower positive predictive value (29%) compared with the positive predictive value of BOS stage 0p in lung transplantation patients (77%). The higher false-positive rate leading to this result in our patient population can likely be attributed to the lower prevalence of BOS (5%) after allogeneic HSCT compared with the higher prevalence of BOS after lung transplantation (50%) [6]. The trade-off to achieve a lower false-positive rate in our population would likely be lower sensitivity, which is undesirable given the high morbidity associated with BOS.
There are several potential limitations to this study. First, the small number of patients who developed BOS 0p and BOS limited our ability to assess baseline characteristics as risk factors for either BOS 0p or BOS. Second, there was the lack of standardization in screening methods after 1 year, potentially introducing bias. Although most patients underwent spirometry every 3 months between years 1 and 2, several underwent spirometry every 6 months, especially if they appeared to be healthy and if they did not have chronic GVHD. Increased screening in patients with chronic GVHD may partially contribute to the increased detection of BOS 0p compared with patients without chronic GVHD. Third, variability in screening interval between 1 and 2 years may have biased the reported operating characteristics for BOS 0p. We attempted to minimize bias in our reported sensitivity and negative predictive value by only evaluating patients who underwent a minimum of 3 PFTs and by only looking at spirometry data up to 2 years to detect BOS 0p. A minimum of 3 PFTs were necessary to detect BOS 0p according to the methods we used. Limiting our detection period to 2 years ensured all patients were screened over a uniform period of time for BOS 0p. However, BOS 0p conceivably could have been under-reported in individuals screened less frequently. Fourth, because BOS 0p was mostly detected in patients with either active acute or chronic GVHD, the use of immunosuppression for GVHD at other sites may have affected the time from detection of BOS 0p to BOS.
Future studies designed to actively detect BOS 0p and BOS are essential to confirm and build on our findings. A prospective study with defined screening intervals would eliminate some of the bias encountered in this study and provide a more accurate incidence of BOS 0p. Further, larger patient cohorts need to be examined to help further define risk factors for both BOS 0p and BOS. If these findings can be confirmed prospectively, patients found to develop BOS 0p could be more closely monitored to identify additional risk factors for BOS or studied in early intervention trials with the goal to prevent or delay progression to BOS.
In summary, our findings provide evidence that through routine spirometry, detection of BOS 0p within allogeneic HSCT patients may adequately identify patients at risk for the development of BOS. Our findings also suggest screening for BOS 0p should particularly be performed in patients with a history of lung disease pretransplant and with chronic GVHD at sites outside the lung. Upon further confirmation of our findings, detection of BOS 0p could ultimately identify patients who would benefit from early interventions to delay or prevent progression to BOS.
Acknowledgments
Financial disclosure: There are no financial disclosures to report.
Conflict of interest statement: There are no conflicts of interest to report.
References
- 1.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 5.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 6.Hachem RR, Chakinala MM, Yusen RD, et al. The predictive value of bronchiolitis obliterans syndrome stage 0-p. Am J Crit Care Med. 2004;169:468–472. doi: 10.1164/rccm.200307-1018OC. [DOI] [PubMed] [Google Scholar]
- 7.Lama VN, Murray S, Mumford JA, et al. Prognostic value of bronchiolitis obliterans syndrome stage 0-p in single-lung transplant recipients. Am J Respir Crit Care Med. 2005;172:379–383. doi: 10.1164/rccm.200501-097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy non-smoking adults. Am Rev Respir Dis. 1971;103:57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR severity index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 11.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 12.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 13.Ringdén O, Remberger M, Ruutu T, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Blood. 1999;93:2196–2201. [PubMed] [Google Scholar]
- 14.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant. 2003;9:657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 15.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation—an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:S106–S114. doi: 10.1016/j.bbmt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazourian L, Rogers AJ, Ibanga R, et al. Factors associated with bronchiolitis obliterans syndrome and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Am J Hematol. 2014;89:404–409. doi: 10.1002/ajh.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogl UM, Nagayama K, Bojic M, et al. Lung transplantation for bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation: a single-center experience. Transplantation. 2013;95:623–628. doi: 10.1097/TP.0b013e318277e29e. [DOI] [PubMed] [Google Scholar]
- 18.Greinix HT, Loddenkemper C, Pavletic SZ, et al. Diagnosis and staging of chronic graft-versus-host disease in the clinical practice. Biol Blood Marrow Transplant. 2011;17:167–175. doi: 10.1016/j.bbmt.2010.07.017. [DOI] [PubMed] [Google Scholar]