Abstract
Purpose
Active surveillance represents a strategy to address the overtreatment of prostate cancer, yet uncertainty regarding individual patient outcomes remains a concern. We evaluated outcomes in a prospective multi-center study of active surveillance.
Methods
We studied 905 men in the prospective Canary Prostate cancer Active Surveillance Study (PASS) enrolled between 2008 to 2013. We collected clinical data at study entry and at pre-specified intervals and determined associations with adverse reclassification defined as increased Gleason grade or greater cancer volume on follow-up biopsy. We also evaluated the relationships of clinical parameters with pathology findings in participants who underwent surgery after a period of active surveillance.
Results
During a median follow-up of 28 months, 24% of participants experienced adverse reclassification, of whom 53% underwent treatment while 31% continued active surveillance. Overall, 19% of participants received treatment, 68% with adverse reclassification while 32% opted for treatment without disease reclassification. In multivariate Cox proportional hazards modeling, percent of biopsy cores with cancer, BMI, and PSA density were associated with adverse reclassification (P = 0.01, 0.04, 0.04). Of 103 participants subsequently treated by radical prostatectomy, 34% had adverse pathology, defined as primary pattern 4–5 or non-organ confined disease, including two with positive lymph nodes, with no significant relationship between risk category at diagnosis and findings at surgery (P = 0.76).
Conclusion
Most men remain on active surveillance at five years without adverse reclassification or adverse pathology at surgery. However, clinical factors had only modest association with disease reclassification, supporting the need for approaches that improve prediction of this outcome.
Keywords: prostatic neoplasms, prospective studies, active surveillance
INTRODUCTION
The prostate-specific antigen (PSA) era has been associated with stage migration towards lower grade and stage prostate cancers, such that the majority of newly diagnosed prostate neoplasms are apparently indolent.1,2 The number of prostate cancers (PCa) identified each year far exceeds the number of lethal cases; there is overdiagnosis of those cancers that may never progress or cause harm if left untreated.3 In the US, most men diagnosed with low risk PCa undergo curative therapy,2,4,5 resulting in substantial overtreatment.
Active surveillance (AS) is a management strategy for PCa that can mitigate overtreatment by delaying intervention in patients whose tumors initially have features consistent with a low-risk cancer and treating only when a more clinically-significant malignancy is identified. Patients managed with AS undergo serial monitoring with serum PSA measurements, clinical exams, and repeat biopsies. Intervention is only recommended for evidence of a more aggressive tumor, usually based on changes in biopsy characteristics or PSA values.
In 2008, in response to the growing evidence of PCa overtreatment and the need for a prospective platform for the discovery and validation of biomarkers of PCa outcomes, we established a multi-institutional AS cohort.6 We present the first analysis of clinical factors associated with outcomes in 905 participants enrolled in the Canary Prostate Active Surveillance Study (PASS) and provide detailed pathologic data in a subset of the cohort who underwent radical prostatectomy after initial AS.
METHODS
Canary Prostate Active Surveillance Study (PASS) Cohort
Canary PASS opened for enrollment in 2008.6 The protocol (clinicaltrials.gov NCT00756665) was approved by institutional review boards at each of nine clinical sites and a coordinating center. All men provided written informed consent for entry into this prospective, observational, AS study.
In order to sample the full spectrum of men using AS, broad eligibility criteria were used, including histologically confirmed adenocarcinoma of the prostate, cT1–2 disease, no previous treatment for PCa, and willingness to undergo serial monitoring while providing biospecimens for subsequent analysis. Participants must have undergone ≥10-core biopsy within a year before enrollment or ≥two biopsies one of which was in the two years prior to enrollment. Although there is no restriction to the time between diagnosis and enrollment, the median time was 8.4 months (IQR=14.4 months) with 67% of the participants enrolled after their diagnostic biopsy and 22% enrolled after their first surveillance biopsy.
Participants were followed with serum PSA measurements every 3 months, clinical and digital rectal examination (DRE) every 6 months, and repeat prostate biopsy 6–12, 24, 48, 72 months after diagnosis. At least 10-core regimens are required; 91% of study biopsy regimens were ≥12-core.
Participants were deemed to have adverse disease reclassification (hence referred to only as reclassification) upon any increase in Gleason grade (primary or sum) on repeat biopsy and/or an increase in biopsy tumor volume defined as an increase in the ratio of number of biopsy cores containing cancer to total number of cores, from <34% to ≥34%. Participants with disease reclassification were offered treatment; those declining treatment were allowed to remain on-study. Biopsies and radical prostatectomies were evaluated for Gleason score by GU-trained pathologists at each site using the 2005 WHO/ISUP modified Gleason system.7
De-identified demographic, clinical, and pathologic data is maintained in a central data repository. A collaboration agreement governing study conduct and data use was executed at participating institutions.
Statistical Analysis
We use PASS data collected through May, 2013 when 909 participants were enrolled. Four participants enrolled >10 years after initial diagnosis were excluded. Age, race, Gleason score, and tumor volume (ratio of number of cores containing cancer to total number of biopsy cores) were from the time of diagnosis. PSA was measured prior to PCa diagnosis. PSA density was calculated from the diagnostic PSA and the first available prostate volume. Clinical T-stage and BMI were from study enrollment.
Participants were stratified by NCCN risk criteria at diagnosis using the following criteria:8 very low risk: cT1, PSA density<0.15, Gleason score≤6, ≤2 cores containing cancer, ≤50% of any core containing cancer; low risk: cT1/T2a, PSA<10ng/ml, Gleason score≤6; intermediate risk: cT2b/T2c, PSA 10–20ng/mL, Gleason Score 7; high risk: participants met intermediate risk criteria except PSA >20ng/ml. There were 462 participants with insufficient data to classify them as very low risk but who met low risk criteria. Fisher’s Exact test was used to evaluate the relationship between risk classification and pathologic outcome.
Continuous variables were categorized for meaningful clinical interpretation. Categorical variables were summarized using frequencies and percentages. Outcomes included time from diagnosis to grade reclassification, any pathologic disease reclassification (grade and/or volume), or curative treatment. Participants without the event of interest were censored at the date of last study contact. A Kaplan-Meier curve was used to present the probability of disease reclassification or treatment over time. Median survival probabilities and their confidence intervals were reported. Cox proportional hazards models were used to assess the association of clinical variables with reclassification or treatment. Both univariate and multivariate models were applied to compare the unadjusted and adjusted hazards ratio for each variable. Statistical analysis was performed using SAS version 9.3 and R studio version 0.98.501.
RESULTS
Demographics of the cohort are displayed in Table 1. Median age was 63 (IQR=9). Although PASS uses broad eligibility criteria, most participants (87%) met NCCN criteria for very low risk or low risk cancer at diagnosis. Over 99% of the cohort was stage ≤cT2a, 93% had PSA <10 ng/mL, and 94% had a Gleason score ≤6.
Table 1. Participant demographics at diagnosis.
Clinical T-stage and BMI are from study entry.
| Characteristic (N, % of known) | PASS Cohort (n = 905) | Reclassified (n = 216) | No Reclassification & Repeat Biopsy (n = 560) | Treatment (n = 170) | Treated with Surgery (n = 103) |
|---|---|---|---|---|---|
| Race | |||||
|
| |||||
| Caucasian | 816 (91) | 194 (90) | 507 (91) | 152 (89) | 94 (91) |
| African American | 52 (6) | 12 (5) | 29 (5) | 10 (6) | 3 (3) |
| Asian | 24 (3) | 8 (4) | 15 (3) | 7 (4) | 6 (6) |
| Other | 7 (<1) | 2 (1) | 4 (1) | 1 (<1) | 0 |
| Unknown | 6 | 0 | 5 | 0 | 0 |
|
| |||||
| Age | |||||
|
| |||||
| <50 | 42 (5) | 5 (2) | 30 (6) | 8 (5) | 8 (8) |
| 50–60 | 291 (32) | 66 (31) | 193 (34) | 51 (30) | 35 (34) |
| 61–70 | 471 (52) | 113 (52) | 284 (51) | 88 (52) | 50 (49) |
| >70 | 101 (11) | 32 (15) | 53 (9) | 23 (13) | 10 (10) |
|
| |||||
| PSA | |||||
|
| |||||
| 0 – 3.99 | 268 (29) | 47 (22) | 179 (32) | 34 (20) | 22 (21) |
| 4.0 – 10.0 | 576 (64) | 154 (71) | 344 (61) | 123 (72) | 73 (71) |
| >10.0 | 61 (7) | 15 (7) | 37 (7) | 13 (8) | 8 (8) |
|
| |||||
| Clinical T-stage | |||||
|
| |||||
| T1 | 804 (89) | 188 (87) | 492 (88) | 146 (86) | 92 (89) |
| T2a | 96 (10) | 26 (12) | 65 (12) | 21 (12) | 11 (11) |
| T2b/c | 5 (<1) | 2 (<1) | 3 (<1) | 3 (2) | 0 |
|
| |||||
| Gleason Score | |||||
|
| |||||
| ≤6 | 846 (94) | 205 (95) | 528 (94) | 162 (95) | 101 (98) |
| 7 (3+4) | 56 (6) | 11 (5) | 29 (5) | 8 (5) | 2 (2) |
| 7 (4+3) | 3 (<1) | 0 | 3 (1) | 0 | 0 |
|
| |||||
| Tumor Volume, % cores containing cancer | |||||
|
| |||||
| 1 – 10 | 414 (53) | 60 (33) | 283 (58) | 45 (33) | 29 (35) |
| 11 – 33 | 340 (43) | 109 (59) | 189 (39) | 86 (62) | 51 (61) |
| ≥34 | 34 (4) | 14 (8) | 16 (3) | 7(5) | 3 (4) |
| Unknown | 117 | 33 | 72 | 32 | 20 |
|
| |||||
| PSA density | |||||
|
| |||||
| 0 – 0.15 | 508 (70) | 97 (56) | 348 (74) | 69 (52) | 41 (51) |
| 0.151 – 0.30 | 176 (24) | 63 (36) | 97 (21) | 48 (36) | 29 (36) |
| >0.30 | 43 (6) | 14 (8) | 25 (5) | 16 (12) | 10 (13) |
| Unknown | 178 | 42 | 90 | 37 | 23 |
|
| |||||
| BMI | |||||
|
| |||||
| <25 | 230 (25) | 57 (26) | 154 (27) | 43 (25) | 29 (28) |
| 25 – 29.9 | 457 (50) | 96 (44) | 283 (50) | 84 (49) | 50 (49) |
| 30 – 34.9 | 154 (18) | 42 (20) | 92 (17) | 27 (16) | 16 (16) |
| ≥35 | 64 (7) | 21 (10) | 31 (6) | 16 (9) | 8 (8) |
|
| |||||
| Family history | |||||
|
| |||||
| Yes | 229 (27) | 55 (27) | 140 (26) | 45 (28) | 28 (29) |
| No | 634 (73) | 147 (73) | 393 (74) | 115 (72) | 70 (71) |
| Unknown | 42 | 14 | 27 | 10 | 5 |
|
| |||||
| NCCN Prostate Cancer Classification | |||||
|
| |||||
| Very Low Risk | 284 (31) | 41 (19) | 208 (37) | 31 (18) | 24 (23) |
| Low Risk | 503 (56) | 150 (69) | 284 (51) | 115 (68) | 69 (67) |
| Intermediate Risk | 115 (13) | 25 (12) | 66 (12) | 23 (14) | 9 (9) |
| High Risk | 3 (<1) | 0 | 2 (<1) | 1 (<1) | 1 (1) |
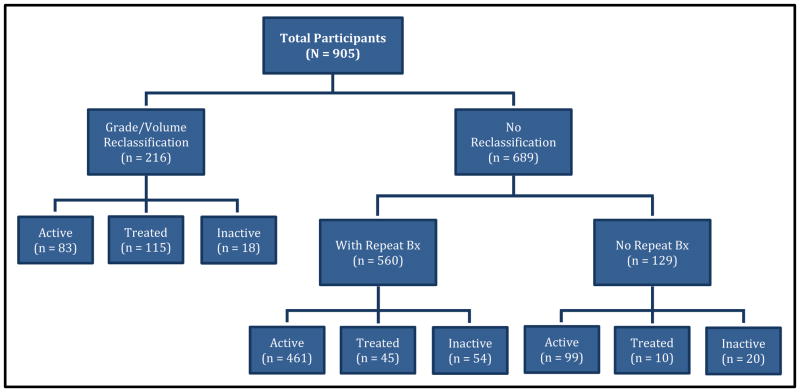
The status of participants in the cohort is shown in Figure 1; the median follow-up from diagnosis was 28 months (IQR=33.5 months). Of the 905 participants enrolled, 216 (24%) experienced tumor grade and/or volume reclassification; increased grade was the most common type of disease reclassification, seen in 188/216 (87%) of men with reclassification (Table 2). Of 216 participants with disease reclassification, 83 remained on AS or were considering treatment, 115 received curative treatment, and 18 dropped out of PASS without confirmed treatment; reclassification type did not differ in those treated/not treated. Of 689 without reclassification, 560 participants underwent repeat biopsy, while 129 had not yet undergone repeat biopsy. Of these 689 participants who did not experience disease reclassification, 560 remained on AS, 55 received treatment, and 74 dropped from study follow-up. Overall, 170 (19%) participants received treatment, 115 (68%) of whom had associated disease reclassification while 55 (32%) opted for treatment without study-defined reclassification; of these approximately 40% had increasing tumor volume yet did not meet the definition for volume reclassification, while the remainder had no identifiable reason for treatment. Of the 92 total participants who were inactive, 32 moved, 41 were lost to follow-up, 13 refused future contact, and 6 died of causes other than PCa. There were no distant metastases or PCa deaths.
Figure 1. Status of PASS participants.
Participants are grouped according to whether they experienced adverse reclassification (an increase in biopsy Gleason grade and/or ratio of number of cores containing cancer to total number of cores from <34% to ≥34%) or had no reclassification. Participants with no reclassification are further divided into participants who had at least one repeat biopsy, and thus able to be reclassified, and those who had not yet had a repeat biopsy, and thus not able to be reclassified. Participants are further divided into those that are active (continuing to use active surveillance), had documented treatment, or inactive (left the study with no documentation of treatment).
Table 2. Participants by reclassification type and status of treatment.
Of the 216 participants that were reclassified by biopsy Gleason grade or tumor volume, 115 (53%) had documented treatment, 34 (16%) were in the process of scheduling treatment (16) or inactive (18; 7 of which likely received treatment, 1 died from causes other than prostate cancer, 10 are lost to follow-up) and 67 (31%) remained on active surveillance. A total of 170 participants had been treated, 115 (68%) with associated reclassification and 55 (32%) without study-defined reclassification.
| Number of participants | |||||
|---|---|---|---|---|---|
| Reclassification type | Reclassified (%) | Reclassified, Treated | Reclassified, Scheduling Treatment or Inactive | Reclassified, Continue AS | No Reclassification, Treated |
| Grade | 138 (64%) | 69 | 21 | 48 | - |
| Volume | 28 (13%) | 13 | 6 | 9 | - |
| Grade and Volume | 50 (23%) | 33 | 7 | 10 | - |
| None | - | - | - | - | 55 |
| TOTAL | 216 | 115 | 34 | 67 | 55 |
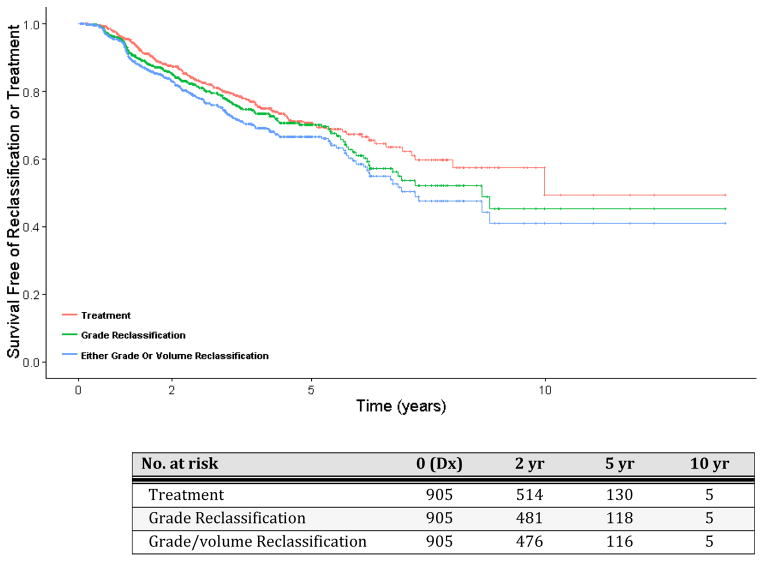
Kaplan-Meier estimates of time to disease reclassification or treatment are shown in Figure 2. The probability of a patient remaining on AS at 2, 5, and 10 years after diagnosis was 88%, 71% and 50%, respectively. Median time free of treatment, grade reclassification, or any biopsy reclassification (grade and/or volume) was 10.0 years (95%CI 8.0,-), 8.6 years, (95%CI 6.7,-), and 7.2 years (95%CI 6.2,-) respectively.
Figure 2. Kaplan-Meier estimates of survival free of outcome.
Outcomes are a.) any increase in biopsy Gleason score (grade reclassification); b.) either an increase in biopsy Gleason score or volume to ≥34% of cores with cancer (grade/volume reclassification), c.) treatment. Time zero was defined as the time of diagnosis. Participants without event were censored at the date of last study contact.
The univariate and multivariate association of clinical variables at diagnosis with time to grade reclassification are in Table 3 (and Supplementary Table 1). In multivariate Cox proportional hazards modeling (n=605), percent of cores containing cancer at diagnosis, BMI, and PSA density were significantly associated with grade reclassification (P=0.01, 0.04, 0.04, respectively). Analysis excluding individuals who used 5ARI did not alter the conclusion, and the rate of reclassification was the same in the participants excluded from multivariable analysis due to missing variables as in the full cohort. Modeling for the outcomes of either grade or volume reclassification or of treatment are similar (Supplementary Tables 2&3).
Table 3. Cox Proportional Hazards Models for time to grade reclassification.
Modeling for the outcomes of time to either grade or volume reclassification or time to treatment are similar and are shown in Supplementary Material (Tables 2 and 3).
| Time to grade reclassification
|
||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
|
| ||||||
| Variable | N | Adjusted Haz. Ratio (95%CI) | P-Value | N | Adjusted Haz. Ratio (95%CI) | P-Value |
|
| ||||||
| Age | ||||||
|
| ||||||
| < 55 | 137 | reference | 93 | reference | ||
| 55 – 65 | 464 | 1.03 (0.66,1.62) | 0.45 | 310 | 1.08 (0.59,1.97) | 0.82 |
| > 65 | 304 | 1.24 (0.78,1.98) | 202 | 1.19 (0.64,2.23) | ||
|
| ||||||
| Clinical T-Stage | ||||||
|
| ||||||
| T1 | 804 | reference | 541 | reference | ||
| T2a | 96 | 1.06 (0.68,1.64) | 0.29 | 60 | 0.91 (0.50,1.65) | 0.26 |
| T2b/c | 5 | 3.05 (0.76,12.32) | 4 | 3.31 (0.77,14.16) | ||
|
| ||||||
| PSA | ||||||
|
| ||||||
| 0 – 3.99 | 266 | reference | 166 | reference | ||
| 4.0 – 10.0 | 578 | 1.78 (1.25,2.52) | <0.01 | 387 | 0.96 (0.58,1.59) | 0.98 |
| >10.0 | 61 | 1.56 (0.83,2.91) | 52 | 0.91 (0.38,2.17) | ||
|
| ||||||
| Percent Cores Containing Cancer | ||||||
|
| ||||||
| 1 – 10 | 414 | reference | 314 | reference | ||
| 11 – 30 | 315 | 1.87 (1.32,2.64) | <0.01 | 243 | 1.81 (1.21,2.73) | 0.01 |
| >30 | 59 | 2.57 (1.52,4.35) | 48 | 2.13 (1.14,3.95) | ||
|
| ||||||
| Family History | ||||||
|
| ||||||
| No | 634 | reference | 446 | reference | ||
| Yes | 229 | 0.95 (0.68,1.34) | 0.78 | 159 | 0.98 (0.65,1.49) | 0.92 |
|
| ||||||
| Race | ||||||
|
| ||||||
| Caucasian | 816 | reference | 545 | reference | ||
| African American | 52 | 1.31 (0.73,2.36) | 0.19 | 38 | 1.39 (0.70,2.76) | 0.50 |
| Other | 31 | 1.70 (0.89,3.22) | 22 | 1.43 (0.57,3.61) | ||
|
| ||||||
| BMI | ||||||
|
| ||||||
| <25 | 230 | reference | 155 | reference | ||
| 25 – 29.9 | 457 | 0.97 (0.68,1.39) | 318 | 1.15 (0.72,1.83) | ||
| 30 – 34.9 | 154 | 1.29 (0.84,1.97) | 0.03 | 92 | 1.71 (0.96,3.03) | 0.04 |
| >35 | 64 | 1.93 (1.15,3.24) | 40 | 2.38 (1.2,4.70) | ||
|
| ||||||
| PSA Density | ||||||
|
| ||||||
| 0 – 0.10 | 296 | reference | 235 | reference | ||
| 0.101 – 0.15 | 212 | 1.19 (0.78,1.80) | 181 | 0.97 (0.57,1.62) | ||
| 0.151 – 0.30 | 176 | 2.12 (1.44,3.11) | <0.01 | 153 | 1.85 (1.09,3.13) | 0.04 |
| >0.30 | 43 | 1.28 (0.63,2.61) | 36 | 1.08 (0.41,2.80) | ||
Surgery was the most common form of treatment: 105 men underwent radical prostatectomy, 59 received radiation, 3 received hormones and 1 was treated with cryotherapy. Data were available for 103 participants who underwent radical prostatectomy (Table 4 and Supplementary Table 4). Prior to surgery, these men had undergone a mean of 2.5 biopsies (range 1–7). The biopsy most proximal to surgery had the highest Gleason score in all but 3 participants who had a negative biopsy and then underwent surgery. Table 4 shows the distribution of participants according to the highest biopsy Gleason score and corresponding Gleason score at prostatectomy; participants are stratified by their NCCN risk category at the time of diagnosis. Overall, there were 34 participants (33%) who were pathologically upgraded at prostatectomy, 14 (14%) who were downgraded. A total of 35 (34%) had adverse pathologic features at surgery including primary Gleason pattern 4–5, extraprostatic extension, seminal vesicle invasion, or lymph node metastasis. Importantly, there was no significant relationship between risk classification at diagnosis and adverse pathology at surgery: 9/24 (37%) very low risk had adverse pathology, 22/69 (32%) low risk participants had adverse pathology, and 4/10 (40%) diagnosed with intermediate or high risk disease had adverse pathology at surgery (P=0.76).
Table 4. Pathology results from participants undergoing surgery, stratified by NCCN risk category at diagnosis.
The biopsy with the highest Gleason score prior to surgery is shown (the biopsy immediately preceding surgery in all but 3 participants), along with the Gleason score at the time of surgery. All but 2 participants were diagnosed with Gleason 3+3 disease. Adverse pathology was defined as primary pattern 4 or 5, extraprostatic extension (EPE), positive lymph nodes (N1), and/or seminal vesicle invasion (SVI), at the time of surgery.
| Biopsy | Prostatectomy Gleason | Adverse pathology | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Highest Gleason | N | 3+3 (%) | 3+4 (%) | 3+5 (%) | 4+3 (%) | 4+4 (%) | 4+5 (%) | 5+4 (%) | N (%) | Description |
| NCCN Very Low Risk at Diagnosis | ||||||||||
| 3+3 | 13 | 5 (38) | 6 (46) | - | 1 (8) | 1 (8) | - | - | 3 (20) |
2 primary pattern 4 1 EPE & N1 |
| 3+4 | 6 | - | 5 (83) | - | 1 (17) | - | - | - | 1 (17) | 1 primary pattern 4 |
| 4+3 | 4 | - | - | - | 2 (50) | - | 1 (25) | 1 (25) | 4 (100) |
2 primary 4 or 5 1 primary 4 & EPE 1 primary 4 & EPE & SVI |
| 4+4 | 1 | - | - | - | 1 (100) | - | - | - | 1 (100) | 1 primary pattern 4 |
| TOTAL | 24 | 9 (37) | ||||||||
| NCCN Low Risk at Diagnosis | ||||||||||
| 3+3 | 24 | 9 (37) | 15 (63) | - | - | - | - | - | 2 (9) | 2 EPE |
| 3+4 | 29 | 4 (14) | 22 (76) | 1 (3) | 1 (3) | 1 (3) | - | - | 9 (31) |
2 primary pattern 4 7 EPE |
| 3+5 | 2 | - | - | - | 2 (100) | - | 2 (100) | 2 primary pattern 4 | ||
| 4+3 | 11 | - | 5 (45) | - | 5 (45) | - | 1 (9) | - | 7 (64) |
3 primary pattern 4 1 EPE 2 primary 4 & EPE 1 primary 4 & N1 |
| 4+4 | 2 | - | 1 (50) | - | 1 (50) | - | - | - | 1 (50) | 1 primary 4 & EPE & SVI |
| 4+5 | 1 | - | 1 (100) | - | - | - | - | - | 1 (100) | 1 EPE & SVI |
| TOTAL | 69 | 22 (32) | ||||||||
| NCCN Intermediate or High Risk at Diagnosis | ||||||||||
| 3+3 | 4 | 3 (75) | 1 (25) | - | - | - | - | - | 0 | |
| 3+4 | 2 | - | 1 (50) | - | 1 (50) | - | - | - | 1 (50) | 1 primary pattern 4 |
| 4+3 | 2 | - | 1 (50) | - | 1 (50) | - | - | - | 1 (50) | 1 primary 4 & EPE |
| 4+4 | 1 | - | - | - | 1 (100) | - | - | - | 1 (100) | 1 primary 4 & EPE & SVI |
| 4+5 | 1 | - | - | - | - | - | 1 (100) | - | 1 (100) | 1 primary 4 |
| TOTAL | 10 | 4 (40) | ||||||||
| Overall TOTAL | 103 | 21 (20) | 58 (57) | 1 (1) | 17 (16) | 2 (2) | 3 (3) | 1 (1) | 35 (34) | |
DISCUSSION
Overtreatment for low risk prostate cancer(PCa) is one of the most important issues in PCa management and was a large factor in the US Preventive Services Task Force’s recommendation against PSA screening.9 Due to the relatively indolent natural history of low risk disease, active surveillance(AS) is an effective strategy to mitigate overtreatment by delaying or avoiding primary therapy; multiple series have demonstrated no or very low PCa-specific mortality. Nonetheless, current monitoring tools lack the specificity and sensitivity needed for many clinicians and patients to more broadly embrace AS for localized PCa, resulting in persistently high curative treatment rates in the U.S.2
In our prospective, multi-center cohort with participants from nine sites throughout North America, we demonstrate that in a diverse clinical setting, AS delays or avoids active treatment with a median time free of treatment of over five years, consistent with results from single-center studies.10–13 Interestingly, a substantial proportion of patients who experience disease reclassification on AS do not opt for primary treatment, while many patients without reclassification opt for curative treatment over a relatively short period of follow-up.
The primary endpoint in our analysis is detection of higher grade or volume cancer on repeat prostate biopsy. This finding during AS may be due to actual disease evolution or, most often, to the presence of a higher grade or volume tumor that was missed due to undersampling of the prostate during biopsy; we use the term ‘adverse reclassification’ rather than ‘progression’ to describe our endpoint. Higher grade tumors are a more aggressive phenotype and generally have worse outcomes.14–16 Similarly, a higher proportion of biopsy cores involved with cancer is correlated with stage of disease and worse outcomes after primary treatment.17,18 While many series use 2 cores with cancer to define higher volume cancer, we chose a conservative threshold for reclassification of 34% of total biopsy cores containing cancer.19 The use of PSA kinetics to define disease progression while on AS is controversial,20 and although PSA kinetics are not currently used to define disease progression in PASS, PSA data are collected for evaluation as the cohort matures.
Our study shows that while clinical factors are related to disease reclassification, such associations are modest. We found significant but modest associations between adverse disease reclassification and PSA density, tumor volume, and BMI. PSA density has been associated with time to treatment, progression, and adverse pathological features in other AS cohorts,10,13,21,22 and is an eligibility criterion in some series.12 In our experience, prostate volume was inconsistently collected, suggesting that it is generally not used to influence AS decisions. Volume of cancer, defined by proportion of total cores involved with cancer, is a surrogate measure for overall disease volume, which is correlated with worse disease-specific outcomes.23 Likewise, obesity has been associated with less favorable outcomes in a variety of cancers, including PCa.24
The PASS cohort included 103 participants who underwent radical prostatectomy after initial surveillance. Of these 103, 101 (98%) were initially diagnosed with Gleason 3+3 disease. Sixty-one (59%) experienced upgrading on biopsy prior to surgery, presumably leading to the decision to treat the cancer, while 41 men were treated with 3+3 disease. Interestingly, 24/41 (58%) participants who underwent prostatectomy for 3+3 disease were found to have higher grade disease at surgery. Some of this upgrading is likely due to the previously-described rates of intra- and inter-observer variability.25
Our pathologic data demonstrate a poor correlation of initial risk group with adverse surgical pathology. Using a definition of adverse pathology of primary Gleason pattern 4–5 and/or non-organ confined disease, participants who fulfilled the NCCN definition of very low risk, low risk, or intermediate/high risk disease at diagnosis had adverse pathology at surgery in 37%, 32%, and 40% respectively, after a period of AS. In our cohort, 2 participants had positive lymph nodes, both of whom had surgery less than a year after cancer diagnosis. One met NCCN criteria for very low risk disease at initial diagnosis (2/12 cores of 3+3, <50% tumor per core), had pattern 3+3 carcinoma in 3/12 cores >50% tumor in 1 core on repeat biopsy, and at surgery had pT3a, 3+4 disease with one positive node. The other was diagnosed with low risk Gleason 3+3 disease, had 4+3 disease in 4/14 cores on repeat biopsy, and at surgery had pT2c, 4+3 disease with a single positive node. While the interpretation of these observations is limited by small numbers, primarily patients who experienced reclassification on follow-up, these data suggest that clinical characteristics alone are not sufficient to accurately distinguish indolent cancers from those that may be more aggressive. There is a clear need to move beyond PSA, stage, and biopsy characteristics to a more biologically-based assessment of risk, both at diagnosis and during periodic re-evaluation. The serial biospecimens collected in PASS will allow us to evaluate genomic and molecular diagnostic tests designed to distinguish aggressive cancers from those that will not cause harm if left untreated.26
There are limitations of this study. The impact of AS on the more established disease-specific endpoints, such as PCa metastasis or mortality, is not possible with our short follow-up. However, previous studies including randomized clinical trials27 have found that low risk disease is associated with low long-term disease mortality.11 Similarly, cancer reclassification in AS to higher grade or volume disease often represents undersampling at original prostate biopsy. However, the detection of intermediate risk disease in a man on AS would then permit therapeutic interventions that are more likely beneficial to the patient.14,19 Finally, central pathologic review was not performed for the primary endpoint of biopsy reclassification. A benefit of this approach is that pathologic evaluation in our multi-center study better reflects community practice.
CONCLUSION
At the time of diagnosis, clinical characteristics alone do not completely distinguish indolent prostate cancers from those cancers that may benefit from early intervention, as evidenced by equal rates of adverse prostatectomy pathology between very low, low, and intermediate risk disease at diagnosis. Better tools are needed to improve risk stratification. The PASS biorepository will allow for validation of biomarkers to identify patients who may be better-managed with treatment, versus men whose long-term prognosis allows a less-intensive follow-up schedule and provide greater confidence in the appropriateness of a non-treatment management strategy.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by the Canary Foundation; the National Cancer Institute at the National Institutes of Health’s Early Detection Research Network (grant number U01 CA086402); and the National Institutes of Health (grant numbers P30 CA054174, and the Pacific Northwest Prostate Cancer SPORE P50 CA097186).
We thank all PASS participants for their dedicated contributions. We also thank a large and dedicated team of coordinating center staff, coordinators, lab staff, and physicians who have made this study possible.
ABBREVIATIONS
- AS
active surveillance
- DRE
digital rectal examination
- FHCRC
Fred Hutchinson Cancer research Center
- PASS
Prostate Active Surveillance Study
- PSA
prostate specific antigen
- PSADT
PSA doubling time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
References
- 1.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Broering JM, Kantoff PW, et al. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178(3 Pt 2):S14–19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller DC, Gruber SB, Hollenbeck BK, et al. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98(16):1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 6.Newcomb LF, Brooks JD, Carroll PR, et al. Canary Prostate Active Surveillance Study: design of a multi-institutional active surveillance cohort and biorepository. Urology. 2010;75(2):407–413. doi: 10.1016/j.urology.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29(9):1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 8.Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 1.2014. J Natl Compr Canc Netw. 2013;11(12):1471–1479. doi: 10.6004/jnccn.2013.0174. [DOI] [PubMed] [Google Scholar]
- 9.Moyer VA on behalf of the USPSTF. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 10.Dall’Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112(12):2664–2670. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 11.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33(3):272–277. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 12.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29(16):2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 13.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63(4):597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Cooperberg MR, Freedland SJ, Pasta DJ, et al. Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer. 2006;107(10):2384–2391. doi: 10.1002/cncr.22262. [DOI] [PubMed] [Google Scholar]
- 15.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111(1):58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 16.Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90(10):766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 17.Freedland SJ, Aronson WJ, Terris MK, et al. Percent of prostate needle biopsy cores with cancer is significant independent predictor of prostate specific antigen recurrence following radical prostatectomy: results from SEARCH database. J Urol. 2003;169(6):2136–2141. doi: 10.1097/01.ju.0000065588.82511.06. [DOI] [PubMed] [Google Scholar]
- 18.Tsuzuki T, Hernandez DJ, Aydin H, et al. Prediction of extraprostatic extension in the neurovascular bundle based on prostate needle biopsy pathology, serum prostate specific antigen and digital rectal examination. J Urol. 2005;173(2):450–453. doi: 10.1097/01.ju.0000151370.82099.1a. [DOI] [PubMed] [Google Scholar]
- 19.Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938–1942. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62(6):976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 21.Warlick C, Trock BJ, Landis P, et al. Delayed versus immediate surgical intervention and prostate cancer outcome. J Natl Cancer Inst. 2006;98(5):355–357. doi: 10.1093/jnci/djj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong M, Slezak JA, Lin CP, et al. Development and multi-institutional validation of an upgrading risk tool for Gleason 6 prostate cancer. Cancer. 2013;119(22):3992–4002. doi: 10.1002/cncr.28303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein JI. Prognostic significance of tumor volume in radical prostatectomy and needle biopsy specimens. J Urol. 2011;186(3):790–797. doi: 10.1016/j.juro.2011.02.2695. [DOI] [PubMed] [Google Scholar]
- 24.Bhindi B, Kulkarni GS, Finelli A, et al. Obesity is associated with risk of progression for low-risk prostate cancers managed expectantly. Eur Urol. 2014;66(5):841–848. doi: 10.1016/j.eururo.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 25.McKenney JK, Simko J, Bonham M, et al. The potential impact of reproducibility of Gleason grading in men with early stage prostate cancer managed by active surveillance: a multi-institutional study. J Urol. 2011;186(2):465–469. doi: 10.1016/j.juro.2011.03.115. [DOI] [PubMed] [Google Scholar]
- 26.Lin DW, Newcomb LF, Brown EC, et al. Urinary TMPRSS2:ERG and PCA3 in an Active Surveillance Cohort: Results from a Baseline Analysis in the Canary Prostate Active Surveillance Study. Clin Cancer Res. 2013;19(9):2442–2450. doi: 10.1158/1078-0432.CCR-12-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.