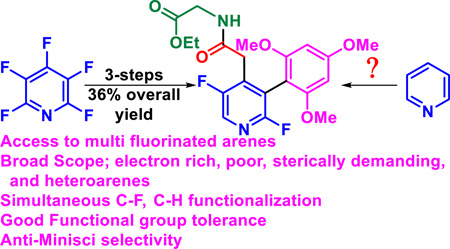
Abstract
Multifluorinated biaryls are challenging to synthesize and yet an important class of molecules. Owing to the difficulty associated with selective fluorination, this class of molecules represent a formidable synthetic challenge. An alternative approach to selective fluorination of biaryls is to couple an arene which already possess C–F bonds in the desired location. This strategy has been regularly utilized, relying heavily on traditional cross-coupling strategies which employ organometallics and halides (or pseudohalides) in order to achieve the coupling. Herein, we report conditions for the photocatalytic coupling via the direct functionalization of the C–F bond of a perfluoroarene and C–H bond of the other arene to provide an expedient route to multifluorinated biaryls. The mild conditions and good functional group tolerance enable a broad scope-including access to the anti-Minisci product of basic heterocycles. Finally, we demonstrate the value of the C–F functionalization approach by utilizing the high fluorine content to systematically build complex biaryls that contain between 2–5 Caryl–F bonds via synergistic use of photocatalysis and SNAr chemistry.
Graphical Abstract
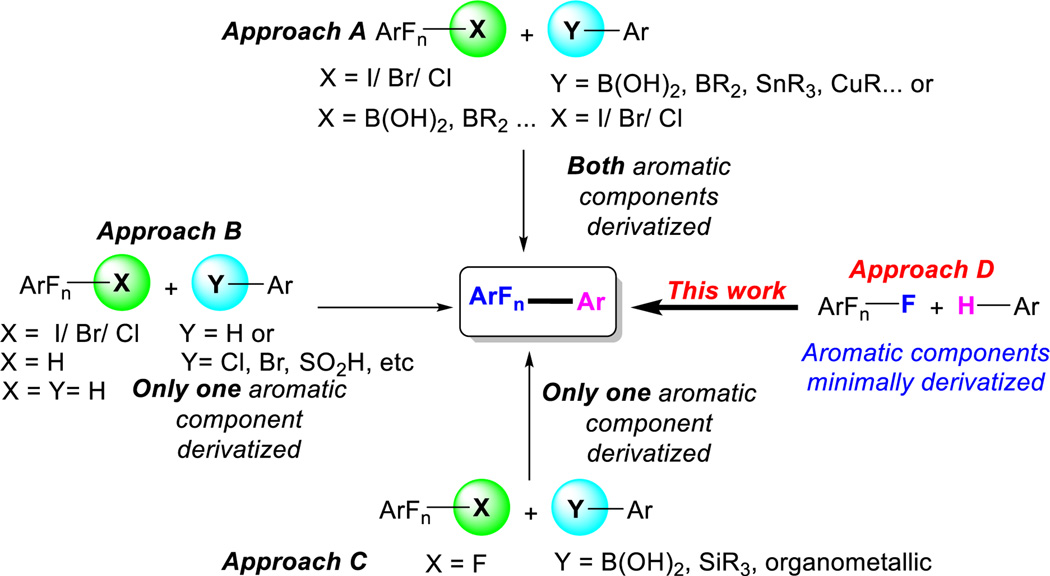
Fluorinated biaryls are an important class of molecules with many examples in drugs,1,2 agrochemicals,3,4 functional materials such as liquid crystals,5,6 organic light-emitting diodes (OLEDs),7,8 water splitting processes,9 as well as electron transport materials.10,11 While cross-coupling methods have made biaryls generally accessible, currently, far fewer methods exist which lead to the important class of partially fluorinated biaryls. Synthesis of fluorinated biaryls have been approached from several directions. Including, traditional cross-coupling of a fluorine containing arene that either possess a halogen or an organometallic group while the partner is substituted with a complimentary reactive group (Approach A, Scheme 1).12–19 Alternatively, methods characterized by the use of less functionalized molecules (i.e. H20 on one or both partners) have also been developed (Approach B).21–23 Typically, activated multifluorinated arenes are derived from the C–F bond in 1–3 steps, i.e. C–F to C–H24 to C–halogen25,26 to C–organometallic.27,28 Consequently, efforts have been made in the area direct CAr–F functionalization,29–32 which have been focused primarily with overcoming the difficulties associated with C–F functionalization (Approach C). To mention a few of the challenges; C–F bonds tend to be both kinetically and thermodynamically robust, they often form strong metal fluoride bonds which result in stable catalytic intermediates and lead to sluggish catalyst turnover. If all these issues are circumvented the method must contend with a C–F regioselectivity issue-since polyfluorinated arenes contain multiple C–F bonds. In this work (Approach D), we sought to form a new biaryl C–C bond directly from a C–F from the fluoroarenes and a C–H from the arene partner. It was expected that realizing this goal, would provide rapid access to new fluorinated chemical space.
Scheme 1.
Strategies to Access Fluorinated Biaryls
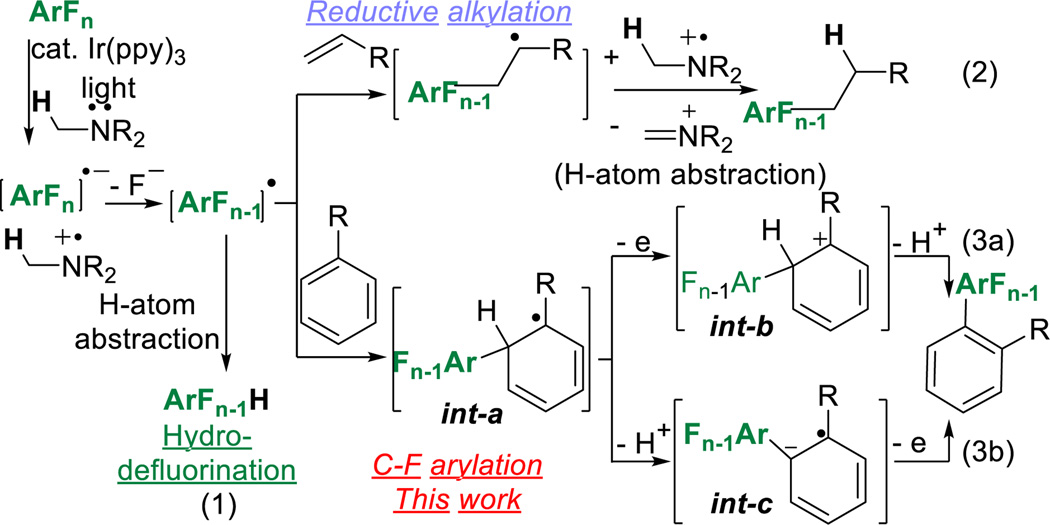
While the synthetic advantages of the dual C–F, C–H biaryl formation are manifold, achieving such a goal would require solutions for the aforementioned issues. Photocatalysis has been shown to be capable of reductive cleavage of Ar-X groups, i.e. Ar-X to Ar-H.33–37 In 2014,38 we showed that catalytic amounts of fac-Ir(ppy)3 in the presence of light and an amine reductant resulted in a highly efficient hydrodefluorination (HDF) (eqn 1, Scheme 2) with excellent regioselectivity and functional group tolerance, suggesting that it might provide a novel route for direct C–F functionalization. Indeed in 2015,39 we showed that the same reactive intermediate could be intercepted with an alkene (eqn 2) which then underwent a subsequent reduction of the presumed alkyl radical. We were curious to know whether the incipient radical-formed as a result of C–C bond formation, could be oxidized rather than reduced leading to an alkene.39 Given their propensity to regain aromaticity after temporary loss, arenes were a logical place to start, since we could use rearomatization to our advantage to help facilitate the oxidation (eqn 3).
Scheme 2.
Photocatalytic C-F Functionalization
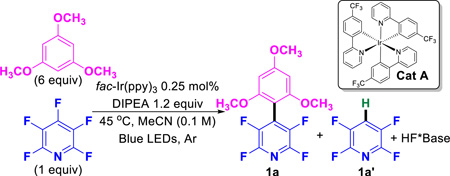
From the outset,40 several obvious challenges associated with the desired reaction were apparent (eqn 3, Scheme 2); the need to achieve an oxidation of int-a under reducing conditions,41 the regioselectivity of the C–C coupling event with respect to the aryl–H, and competing HDF. Oxidation of int-a could occur prior to deprotonation (3a) or afterwards (3b). The dominant path may depend the nature of both the arene partners. Nonetheless, we began our investigation with conditions that had facilitated the HDF38 using pentafluoropyridine with the addition of 6 equivalents of trimethoxybenzene which removed the possibility of regioisomers (Entry 1, Table 1). We were pleased to see that the desired C–C coupled product was indeed formed as the major product in reasonable yields along with a significant amount of the HDF product. By optimization we hoped to increase the yield and simultaneously decrease the equivalents of the arene-H coupling partner. A survey of solvents (Entries 2–4) showed that both DMF and DMSO provided the desired product, albeit in lower yields than MeCN, while DCM and THF resulted in low yields of only undesired products. Next we screened photocatalysts for the ability to influence the product ratio and yield (Entry 5),42 while a subtle improvement in product ratio was observed, the reaction was noticeably slower. Next, we evaluated both the structure of the amine43 as well as the loading of the amine (Entries 6–10) as reaction parameters. While Et3N worked reasonably well, it led to products stemming from N-perfluoarylation44 byproducts which were substantially decreased by use of more sterically cumbersome diisopropyl ethyl amine (DIPEA). When the amount of amine was varied, two key features were revealed (Entries 8–10). First, despite the fact that both a reduction and oxidation occur in the same reaction which begs the question whether the amine might just serve as an initiator. While the amine does demonstrate superstoichiometric activity (entry 8), the reaction becomes inefficient. Secondly, too much amine results in increased amounts of HDF product, 1a’. Previously, we have shown that the use of less soluble amines could retard the rate of photocatalytic reduction.45 However, in this case, less soluble iPr2Nn-hept (Entry 10, 0.15 M, max conc. in MeCN vs 1.3 M for DIPEA, both at rt) dramatically slowed the rate of the reaction. Next, we looked at the effect of reducing the amount of trimethoxybenzene, in hopes that we could approach near stoichiometric ratios (Entries 11–12). As expected, diminishing the amount of arene-H led to increased amounts of HDF-product, 1a’. Next, we investigated the effect of temperature. We were pleased to see that dropping the temperature led to an improved 1a:1a’ ratio (Entries 12 vs. 13). Finally, we suspected that the amine was serving two roles, both as a reductant that initiates the C–F fragmentation as well as a base to scavenge the HF which is formed in the reaction. In order to address this, we added KHCO3 (Entry 14) which allowed us to simultaneously lower the loading of both the amine and trimethoxybenzene and achieve the best ratio of 1a:1a’. Finally, controls were run (entries 15–18) which indicated the necessity of light, catalyst, and amine as well as the sensitivity towards air.
Table 1.
Optimization of Reaction Conditions.
 | ||||
|---|---|---|---|---|
| entry | modification | 1a:1a' | conversion to 1aa |
time, h |
| 1 | none | 5:1 | 47/73% | 3/19b |
| 2 | DMF instead of MeCN | 1:2 | 22/31% | 3/19b |
| 3 | DMSO instead of MeCN | 3:1 | 58/58% | 3/19b |
| 4 | DCM, THF instead of MeCN | nac | nac | 3/19 |
| 5 | Cat A instead of Ir(ppy)3 | 6:1 | 10/52% | 4/15b |
| 6 | Et3N instead of DIPEA | 4:1 | 47/66% | 5/19b |
| 7 | without DIPEA | na | <5% | 2/17 |
| 8 | 0.5 equiv of DIPEA | 6:1 | 22/79% | 2/17b |
| 9 | 3.0 equiv of DIPEA | 3:1 | 40/61% | 2/17b |
| 10 | iPr2Nn-hept instead of DIPEA | <5% | 21 | |
| 11 | 1.2 equiv. of arene-H | 2:1 | 57% | 17b |
| 12 | 3.0 equiv. of arene-H | 3:5:1 | 66% | 17b |
| 13 | 0 °C with 3.0 equiv arene-H | 6:1 | 75% | 19b |
| 14 | 0 °C with 2.4 equiv arene-H, 0.4 equiv. DIPEA and 1.2 equiv. KHCO3 |
13:1 | 71% | 24b |
| 15 | same as 14 with no DIPEA | na | 1% | 24 |
| 16 | same as 14 with no fac-Ir(ppy)3 | na | not detected | 24 |
| 17 | in air | na | 11% | 19 |
| 18 | in dark | na | not detected | 19 |
Determined by 19F NMR
Reaction complete.
<20% conv. to undesired products.
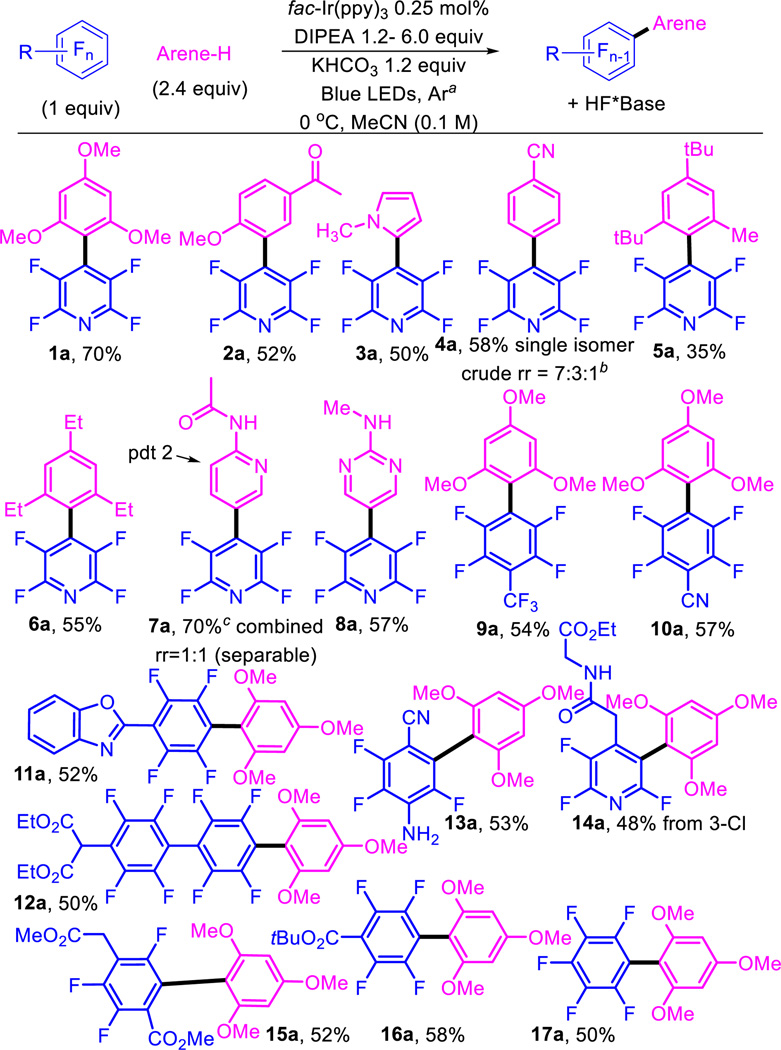
Using conditions from entry 14 we evaluated the scope of the reaction (Table 2). We were pleased to see that good to modest yields could be obtained with electron rich arenes with a variety of perfluoroarenes (1a, 9a–17a) as well as acetophenones (2a), pyrroles (3a), and nitriles (4a). The ability of the perfluoroaryl radical to form highly congested C–C bonds (5a and 6a) is noteworthy. The Minisci reaction is commonly employed to functionalize basic heterocycles.46 Interestingly, we observe anti-Minisci selectivity (7a and 8a), likely owing to the fact that basic heterocycle is not protonated under these conditions as is typical in the Minisci reaction. Substrates 11a and 12a suggest that the method can be used to access extended aryl systems and that even moderately acidic protons are tolerated under the reaction conditions. 13a indicates that the second site of photocatalytic C–F functionalization occurs ortho to the nitrile. 14a shows the preference for the 3-Cl fragmentation compared to the fluorines and allows access to complimentary regioisomers. Comparison of 15a and 16a demonstrate that both the 2- and 4- positions are accessible on the perfluorobenzoate system.38,39,47 Hexafluorobenzene, devoid of electron withdrawing functional groups, which would facilitate reduction, also undergoes arylation (17a). In general, the scope is broad in terms of fluoroarene while the arene-H benefits from substitution or polarizing functional groups.
Table 2.
Scope of the photocatalytic C-F arylation (limiting perfluoroarene)
The reaction was degassed by bubbling argon for 10 min.
Yield is for isomer shown. The minor regioisomers (meta and ortho) were not separated, assigned, or counted for yield.
Yield is the sum of isomers which were separated.
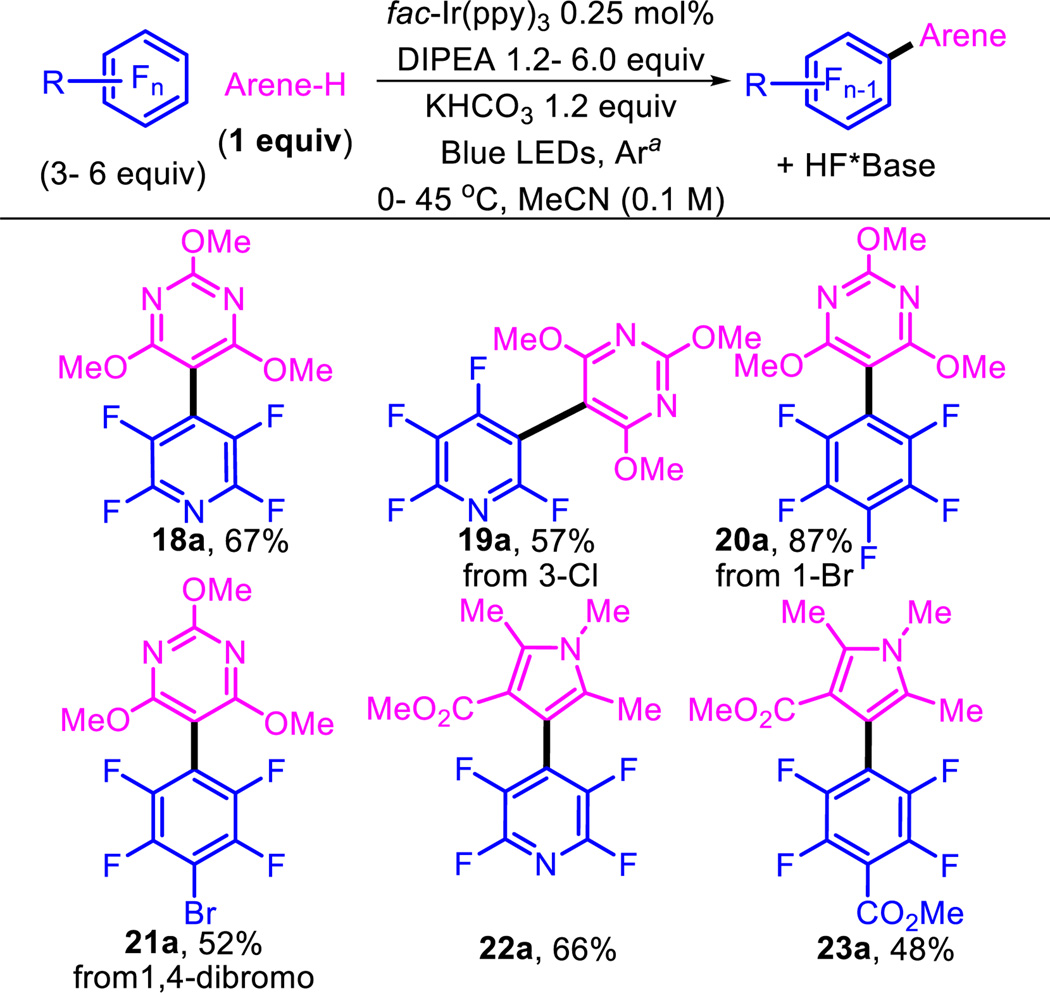
Next, we investigated the scenario in which the arene-H would be the more valuable coupling partner by switching the limiting reagent to the arene-H and using the perfluoroarene in excess (Table 3). We were pleased to see that the reaction worked under these conditions and allowed the formation of fully substituted biaryls. Trisubstituted pyrimidines underwent addition to give the C–H coupled product (18a–21a). Tetra-substituted pyrroles also coupled with the expected C–F selectivity (22a and 23a) to give biologically relevant pentasubstituted pyrroles (i.e. see Lipitor®), suggesting it may be possible to use this method to accomplish late-stage modifications of biologically active molecules. conditions. 19a–21a in which substitution of C–Cl or C–Br bonds occurs indicate a halogen-fragmentation pattern consistent with that of radical anion fragmentation observed by Bunnett48,49 and Rossi,50 (i.e. I>Br>Cl>F). This selectivity is both an inherent limitation as well as an opportunity to build complimentary regioisomers. Importantly, use of the fluorinated arene in excess protects the bromine of product 21a from subsequent reduction of the C–Br, providing opportunity for further elaboration by other methods. In general, the biggest limitation of the method is the use of arene-H’s that are either highly substituted or polarized as this reduces the number of isomers that are formed.
Table 3.
Scope of the photocatalytic arylation (limiting arene-H)
The reaction was degassed by bubbling argon for 10 min.
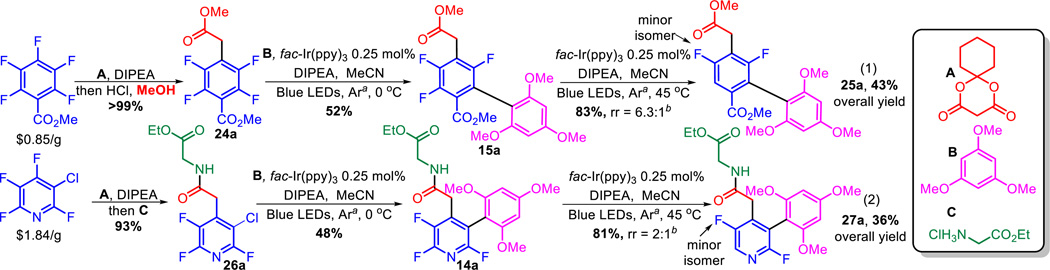
In addition to the esoteric reasons to develop C–F functionalization chemistry, it also has the potential to be the most rapid and versatile method for accessing multifluorinated arenes which would be useful in discovery chemistry efforts involving fluorinated aromatics.51–54 Specifically, it starts with readily available perfluorinated arenes which have all of the difficult to install fluorines in the desired positions.
The challenge then becomes selectively functionalizing the unwanted fluorines to build the desired molecule. To demonstrate this concept and its feasibility, we started with commercially available perfluoroarenes and subjected them to a series of both photocatalytic and SNAr functionalizations. This allowed us to rapidly obtain multifluorinated benzoates and pyridines that would be otherwise very difficult to synthesize by any method, thus allowing access to new fluorinated chemical space (Scheme 3). Addition of a Meldrum’s acid derivative followed by decomposition in the presences of a nucleophile gave near quantitative yields of the product of α-perfluoroarylation (24a and 26a).55 Photocatalytic arylation then desymmetrizes the molecules (15a and 14a). The total fluorine content can then be reduced to generate hard to access difluorinated arenes (25a) and heteroarenes (27a). The regiochemistry of the HDF appears to be dominated by the electronics, i.e. proximity to the electron withdrawing functional group, but other competing influences lead to formation of regioisomers. On radical anions of benzene systems, halogen fragmentation is preferred at the position opposite of a substituent, which we believe gives rise to the minor isomer of 25a. Hydrogen bonding may be an even stronger influence on the selectivity as the amide is essential to obtaining the minor regioisomer of 27a. We have previously observed similar switches of selectivity with NHAc perfluoroarenes.38 Understanding the subtle and potential exploitable phenomena concerning C–F selectivity is the topic of an ongoing investigation in our group.
Scheme 3.
The Utility of C–F functionalization to Access Complex Multifluorinated Biaryls
a. The reaction was degassed by bubbling argon for 10 min. b. Yields correspond to isolated product. Regioisomeric ratio (rr) was determined by 1H NMR of the isolated products.
In conclusion, we have generated and utilized the perfluoroaryl radical-generated by photocatalyst fac-Ir(ppy)3, blue light, and an amine, to form a new C–C bond via dual C–F, C–H functionalization. This allows access a wide array of multifluorinated biaryls. From a synthetic perspective, this reaction has the potential for significant impact given the single step coupling of such broadly accessible starting materials, the mild conditions, and functional group tolerance of the method. From a mechanistic perspective, we have shown that the perfluoroaryl radical is capable of adding to pi-systems of a wide range of arenes, including some that give anti-Minisci selectivity, followed by oxidation and rearomatization. Given these initial findings, we expect that this chemistry will facilitate investigation into new fluorinated chemical space and therefore enable a number of research efforts.
Supplementary Material
Acknowledgments
The research results discussed in this publication were made possible in total or in part by funding through the award for project number HR-14-072, from the Oklahoma Center for the Advancement of Science and Technology. We gratefully acknowledge NIH NIGMS (GM115697) for financial support of this work and thank JID for help in editing the manuscript.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Complete experimental procedures, additional optimization experiments, and product characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare a competing financial interest in that they hold a patent, United States Serial No.: 62/043,650 concerning the structure and method for the Meldrum’s acid adducts.
REFERENCES
- 1.Zahn A, Brotschi C, Leumann C. J. Chem. Eur. J. 2005;11:2125. doi: 10.1002/chem.200401128. [DOI] [PubMed] [Google Scholar]
- 2.Purser S, Moore PR, Swallow S, Gouverneur V. Chem. Soc. Rev. 2008;37:320. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]
- 3.Selby TP, Bereznak JF, Bisaha JJ, Ding AX, Gopalsamuthiram V, Hanagan MA, Long JK, Taggi AE. Substituted azoles as fungicides and their preparation. USA: E. I. du Pont de Nemours and Company; [Google Scholar]
- 4.Gregory V, Taggi AE. Preparation of fungicidal imidazole derivatives, their mixtures with other fungicides, and use for controlling plant diseases caused by fungal plant pathogens. USA: E. I. Du Pont de Nemours and Company; [Google Scholar]
- 5.Weck M, Dunn AR, Matsumoto K, Coates GW, Lobkovsky EB, Grubbs RH. Angew. Chem. Int. Ed. 1999;38:2741. doi: 10.1002/(sici)1521-3773(19990917)38:18<2741::aid-anie2741>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Nitschke JR, Tilley TD. J. Am. Chem. Soc. 2001;123:10183. doi: 10.1021/ja011018s. [DOI] [PubMed] [Google Scholar]
- 7.Hwang D-H, Song SY, Ahn T, Chu HY, Do L-M, Kim SH, Shim H-K, Zyung T. Synth. Met. 2000;111–112:485. [Google Scholar]
- 8.Tsuzuki T, Shirasawa N, Suzuki T, Tokito S. Adv. Mater. 2003;15:1455. [Google Scholar]
- 9.Kitamura T, Wada Y, Yanagida S. J. Fluorine Chem. 2000;105:305. [Google Scholar]
- 10.Sakamoto Y, Suzuki T, Miura A, Fujikawa H, Tokito S, Taga Y. J. Am. Chem. Soc. 2000;122:1832. [Google Scholar]
- 11.Babudri F, Farinola GM, Naso F, Ragni R. Chem. Commun. 2007:1003. doi: 10.1039/b611336b. [DOI] [PubMed] [Google Scholar]
- 12.Korenaga T, Kosaki T, Fukumura R, Ema T, Sakai T. Org. Lett. 2005;7:4915. doi: 10.1021/ol051866i. [DOI] [PubMed] [Google Scholar]
- 13.Wei Y, Kan J, Wang M, Su W, Hong M. Org. Lett. 2009;11:3346. doi: 10.1021/ol901200g. [DOI] [PubMed] [Google Scholar]
- 14.Kinzel T, Zhang Y, Buchwald SL. J. Am. Chem. Soc. 2010;132:14073. doi: 10.1021/ja1073799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frohn H-J, Adonin NY, Bardin VV, Starichenko VF. J. Fluorine Chem. 2002;117:115. [Google Scholar]
- 16.Coe PL, Pearl GM. J. Organomet. Chem. 1971;31:55. [Google Scholar]
- 17.DePasquale RJ, Tamborski C. J. Org. Chem. 1969;34:1736. [Google Scholar]
- 18.Shang R, Fu Y, Wang Y, Xu Q, Yu H-Z, Liu L. Angew. Chem. Int. Ed. 2009;48:9350. doi: 10.1002/anie.200904916. [DOI] [PubMed] [Google Scholar]
- 19.Shang R, Xu Q, Jiang Y-Y, Wang Y, Liu L. Org. Lett. 2010;12:1000. doi: 10.1021/ol100008q. [DOI] [PubMed] [Google Scholar]
- 20.Lindner S, Bräse S, Masters K-S. J. Fluorine Chem. 2015;179:102. [Google Scholar]
- 21.Meyer AU, Slanina T, Yao C-J, König B. ACS Catal. 2016;6:369. [Google Scholar]
- 22.Wei Y, Su W. J. Am. Chem. Soc. 2010;132:16377. doi: 10.1021/ja109383e. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Liu J, Sun C-L, Li B-J, Shi Z-J. Org. Lett. 2011;13:276. doi: 10.1021/ol102688e. [DOI] [PubMed] [Google Scholar]
- 24.Lentz D, Braun T, Kuehnel MF. Angew. Chem. Int. Ed. Engl. 2013;52:3328. doi: 10.1002/anie.201205260. [DOI] [PubMed] [Google Scholar]
- 25.Ghorbani-Vaghei R, Shahbazi H, Veisi H. Tetrahedron Lett. 2012;53:2325. [Google Scholar]
- 26.Prakash GKS, Mathew T, Hoole D, Esteves PM, Wang Q, Rasul G, Olah GA. J. Am. Chem. Soc. 2004;126:15770. doi: 10.1021/ja0465247. [DOI] [PubMed] [Google Scholar]
- 27.Manolikakes G, Schade MA, Hernandez CM, Mayr H, Knochel P. Org. Lett. 2008;10:2765. doi: 10.1021/ol8009013. [DOI] [PubMed] [Google Scholar]
- 28.Burton DJ, Jairaj V. J. Fluorine Chem. 2004;125:673. [Google Scholar]
- 29.Sun AD, Love JA. Org. Lett. 2011;13:2750. doi: 10.1021/ol200860t. [DOI] [PubMed] [Google Scholar]
- 30.Yoshikai N, Mashima H, Nakamura E. J. Am. Chem. Soc. 2005;127:17978. doi: 10.1021/ja056327n. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura Y, Yoshikai N, Ilies L, Nakamura E. Org. Lett. 2012;14:3316. doi: 10.1021/ol301195x. [DOI] [PubMed] [Google Scholar]
- 32.Saijo H, Sakaguchi H, Ohashi M, Ogoshi S. Organometallics. 2014;33:3669. [Google Scholar]
- 33.Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hager D, MacMillan DWC. J. Am. Chem. Soc. 2014;136:16986. doi: 10.1021/ja5102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNally A, Prier CK, MacMillan DWC. Science. 2011;334:1114. doi: 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayanam JMR, Stephenson CRJ. Chem Soc Rev. 2011;40:102. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen JD, D'Amato EM, Narayanam JMR, Stephenson CRJ. Nature Chemistry. 2012;4:854. doi: 10.1038/nchem.1452. [DOI] [PubMed] [Google Scholar]
- 38.Senaweera SM, Singh A, Weaver JD. J. Am. Chem. Soc. 2014;136:3002. doi: 10.1021/ja500031m. [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Kubik JJ, Weaver JD. Chem. Sci. 2015;6:7206. doi: 10.1039/c5sc03013g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The first step of the reaction is transfer of an electron to the perfluoroarene. Energetically speaking both reductive and oxidative quenching are nearly equally endothermic (0.17-0.16 V vs. SCE) in the case of pentafluoropyridine, DIPEA, and Ir(ppy)3. Fluoride extrusion is likely an irreversible step that could serve to drive the reaction. With other, less reducible perfluoroarenes reductive quenching is likely operative. See reference 39 for more discussion.
- 41.Both the reduced Ir(ppy)3− species (−2.245 V vs. SCE) and the photoexcited Ir(ppy)3* (−2.012 V vs. SCE) are strongly reducing. Oxidative quenching would produce an Ir(ppy)3+ (0.738 V vs. SCE) that could most likely oxidize int-A. However, given the low concentration of both species, this would be a statistically unlikely event.
- 42.More photocatalysts were screened but none gave better results, see SI for details.
- 43.More amines were screened but none gave better results than DIPEA, see SI for details.
- 44.The 19F NMR shifts are consistent with other N-substituted tetrafluoropyridines observed in our lab which have been observed to undergo N-arylation followed by dealkylation. No further attempts were made to characterize this product.
- 45.Arora A, Teegardin KA, Weaver JD. Org. Lett. 2015;17:3722. doi: 10.1021/acs.orglett.5b01711. [DOI] [PubMed] [Google Scholar]
- 46.Minisci F. Heterocycles. 1989;28:489. [Google Scholar]
- 47.Senaweera SM, Singh A, Weaver JD. J. Am. Chem. Soc. 2014;136:3002. doi: 10.1021/ja500031m. [DOI] [PubMed] [Google Scholar]
- 48.Bunnett JF. Acc. Chem. Res. 1978;11:413. [Google Scholar]
- 49.Bunnett JF, Zahler RE. Chem. Rev. 1951;49:273. [Google Scholar]
- 50.Rossi RA. Acc. Chem. Res. 1982;15:164. [Google Scholar]
- 51.Ahrens T, Kohlmann J, Ahrens M, Braun T. Chem. Rev. 2015;115:931. doi: 10.1021/cr500257c. [DOI] [PubMed] [Google Scholar]
- 52.Kiplinger JL, Richmond TG. Chem. Commun. 1996:1115. [Google Scholar]
- 53.Kiplinger JL, Richmond TG, Osterberg CE. Chem. Rev. 1994;94:373. [Google Scholar]
- 54.Amii H, Uneyama K. Chem. Rev. 2009;109:2119. doi: 10.1021/cr800388c. [DOI] [PubMed] [Google Scholar]
- 55.Senaweera S, Weaver JD. J. Org. Chem. 2014;79:10466. doi: 10.1021/jo502075p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.