Abstract
Objective
Both working memory (WM) (a brain system that provides temporary storage and manipulation of the information) and attention-deficit/hyperactivity disorder (ADHD) have been associated with educational deficits. Since WM deficits are prevalent in children with ADHD, the main aim of the present study was to examine whether educational deficits are driven by working memory deficits or driven by the effect of ADHD itself.
Method
Participants were referred youth with (N=276) and without (N=241) ADHD ascertained from pediatric and psychiatric sources. Assessment included measures of psychiatric, psychosocial, educational, and cognitive functioning. Education deficits were defined as grade retention or placement in special classes, and were assessed using interviews and written rating scales. Working memory was assessed using the WISC-R Freedom from Distractibility (FFD) factor based on digit span, arithmetic and coding.
Results
Significantly more youth with ADHD had WM deficits than controls (31.9% vs. 13.7%, p< 0.05). In ADHD children, WM deficits were significantly (p<0.01) associated with an increased risk for grade retention and placement in special classes as well as lower scores on reading and math achievement tests, relative to ADHD children without WM deficits. In contrast, no other differences were noted in other areas of functioning. Although WM deficits also had some adverse impact on educational and cognitive correlates in non ADHD controls, these differences failed to attain statistical significance.
Conclusion
WM deficits significantly and selectively increase the risk for academic deficits and cognitive dysfunction in children with ADHD beyond those conferred by ADHD. Screening for WM deficits may help identify children with ADHD at high risk for academic and cognitive dysfunction.
Keywords: attention deficit hyperactivity disorder, neuropsychology, executive function, working memory, children
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent, persistent, and impairing neurobiological disorder estimated to affect up to 7% of children (Willcutt, 2012) and 5% of adults worldwide (Kessler et al., 2006). Among the most prominent ADHD-associated adverse outcomes are educational deficits, which include academic under-attainment, increased needs for academic support, and high rates of placement in special classes (Biederman et al., 1999). Notably, both grade retention and dropping out of high school (Barbaresi, Katusic, Colligan, Weaver, & Jacobsen, 2007) are three times more prevalent among ADHD youth.
Nonetheless, ADHD is frequently associated with executive function deficits (EFDs) in general (Faraone et al., 2006), and working memory (WM) deficits in particular, which are also associated with academic dysfunction (Alloway, Elliott, & Place, 2010; Barkley & Murphy, 2010). This raises questions as to whether academic problems in ADHD are due to ADHD, WM deficits or both. Of the 13 controlled studies that addressed this issue (Alloway, Elliott, et al., 2010; Alloway, Gathercole, & Elliott, 2010; Bunford et al., 2015; Chiang & Gau, 2014; Coghill, Hayward, Rhodes, Grimmer, & Matthews, 2014; Gropper & Tannock, 2009; Kofler et al., 2011; Miller, Nevado-Montenegro, & Hinshaw, 2012; Nyman et al., 2010; Rennie, Beebe-Frankenberger, & Swanson, 2014; Rinsky & Hinshaw, 2011; Sjowall & Thorell, 2014; Tseng & Gau, 2013), 11 reported that WM deficits increase the risk for academic deficits beyond ADHD. However, with the exception of 2 studies that reported that WM deficits affect social skills (Kofler et al., 2011; Tseng & Gau, 2013), no study examined the question as to whether WM associated deficits are limited to these two areas or have more global negative effects in other areas of functioning.
Further, none of these studies examined the question as to whether WM deficits also adversely impact children without ADHD. Only one study (Etchepareborda & Abad-Mas, 2005) reported, in a non-ADHD sample, that impaired WM negatively influenced academic learning processes such as focusing attention, inhibition of irrelevant stimuli, recognition of priority patterns, ability to recognize hierarchies and the meaning of stimuli (analysis and synthesis), establishing an intention, and recognizing and selecting the goals that are best suited to solving a problem. Since these cognitive processes are critical for learning, their impairment can lead to educational dysfunction and low educational status. They can have a serious impact on educational success, and are worthy of further examination.
Further understanding as to whether WM deficits have a narrow or global effect on functioning and whether these effects differentially impact children with and without ADHD are areas of high clinical, educational, and public health relevance. Scientifically, children with ADHD and WM deficits may represent a distinct clinical entity informing genetic and neurobiological research. Since ADHD and WM clinically respond to different treatments and stimulant treatments may not affect executive function deficits (Biederman et al., 2008; Faraone, 2012; Swanson, Baler, & Volkow, 2011), such information can lead to the development of improved intervention approaches for affected children with one or both disorders. If WM deficits adversely impact the functioning of children outside the context of ADHD, it could lead to appropriate intervention strategies for children at risk for educational failure independently of a diagnosis of ADHD. This would support efforts to screen for WM deficits in children with academic struggles.
The main aim of the present study was to assess the clinical correlates of WM deficits in children with and without ADHD attending to the shortcomings of the literature. To this end, we compared the correlates of WM deficits using data from a large sample of children with and without ADHD of both sexes ascertained from psychiatric and pediatric sources stratified by the presence or absence of WM deficits. These children were comprehensively assessed in multiple, non-overlapping domains of functioning. Considering the critical importance of WM for optimal functioning, we hypothesized that WM deficits would be associated with impairments in multiple areas of functioning. We also hypothesized that WM deficits would have detrimental effects outside the context of ADHD. To the best of our knowledge, this is the most comprehensive study to date evaluating the scope of impact of deficits in WM in children with and without ADHD.
METHODS
Subjects
Detailed study methodology has been previously reported (J. Biederman et al., 2006; J Biederman et al., 2006). Briefly, participants were youth of both sexes derived from longitudinal, case-control family studies conducted at an academic hospital. These studies included participants aged 6-18 years with (N = 276) and without (N = 241) DSM-III-R ADHD.
ADHD cases were identified from consecutive referrals to a major academic medical center's pediatric psychopharmacology clinic. ADHD cases were also identified from consecutively ascertained pediatric clinic outpatients at a major Health Maintenance Organization (HMO). Healthy controls were ascertained from outpatients referred for routine physical examinations to pediatric medical clinics at each setting, and were identified from their computerized records as not having ADHD. In prior papers (Biederman et al., 1992; Biederman et al., 1999), we reported that the rates of other psychiatric disorders in the control sample are low and consistent with expectations from population studies.
Adoption, unavailable nuclear family, major sensorimotor handicaps, psychosis, autism, language barriers or an estimated IQ < 80 were exclusionary for both ADHD and control participants. Parents provided written informed consent and children and adolescents provided written assent. The institutional review board at the hospital approved this study.
Assessment Procedures
Psychiatric assessments relied on the Kiddie Schedule for Affective Disorders and Schizophrenia- Epidemiologic Version (K-SADS-E), (Orvaschel, 1994) conducted directly and individually with the mothers and the children. For children <12 years, interviews were conducted with their mothers (indirect interviews) only. A diagnosis was considered positive if it was endorsed in either interview. Interviews were administered by highly trained and supervised psychometricians who were blind to referral source or diagnostic status (ADHD or Control). Based on 500 assessments from interviews of children and adults, the median kappa coefficient of agreement between a psychometrician and an experienced clinician was 0.98.
The empirically derived Child Behavior Checklist (CBCL) (Achenbach & Rescorla, 2001) was used to asses other dimensions of psychopathology. Psychosocial functioning was assessed using the Social Adjustment Inventory for Children and Adolescents (SAICA) (John, Gammon, Prusoff, & Warner, 1987). Family functioning was assessed using the Moos Family Environment Scale (FES) (Moos & Moos, 1974). As a measure of overall lifetime functioning, we used the DSM-IV Global Assessment of Functioning (GAF) (American Psychiatric Association, 1994). Mothers provided information regarding their child's history of school problems (i.e., grade retention, special placements, and remedial assistance) and treatment history (i.e., counseling, medication, and hospitalization). Information on pregnancy and delivery complication was systematically obtained.
Intellectual functioning was assessed through the Vocabulary and Block Design subtests of the Wechsler Intelligence Scale for Children-Revised (WISC-R) (Wechsler, 1974). Using procedures suggested by Sattler (Sattler, 1988), we estimated Full Scale IQ from these two subtests using age-corrected scaled scores.
We used the Freedom from Distractibility (FFD) Factor from the WISC-R to assess WM. The initial conceptualization of WM in the revision of the Wechsler Intelligence Scale for Children–Revised was termed the Freedom From Distractibility (FFD) Factor and it was devised from a factor analysis of the Arithmetic, Digit Span, and Coding subtests of the Wechsler scale (Kaufman, 1979). Although the coding subtest was removed in the third edition of the Wechsler Intelligence Scale for Children (Wechsler, 1991), the term FFD Index was kept until the advent of the fourth edition (Wechsler, 2003) where it was replaced with that of “Working Memory (WM) Index” defined the same way (Kranzler, 1997). However, the significant correlation of 0.72 between the Freedom From Distractibility Factor and the Working Memory Index of the WISCIV renders the use of FFD Factor scores an appropriate proxy for WM Index. For simplicity of exposition, we will henceforth use the term Working Memory (WM). We also assessed reading and arithmetic achievement with subtests of the Wide Range Achievement Test-Revised (Jastak & Jastak, 1985).
We classified participants as having WM deficits using the following rules: 1) participants with a full scale IQ of 120 or less if their Freedom from Distractibility (FFD) score was 1 SD (15 points) lower than their full scale IQ. This method is based on the work of Biederman et al. (2004) indicating that individuals with scores in executive functioning 1 SD below the norm is indicative of poor academic outcomes; or 2) any participant with a FFD of ≤ 85. Based on the Wechsler Scales, a score of 85 is considered 1 SD below average falling at the 16th percentile; or 3) any participant with full IQ ≥120 with a FFD 1.5 SDs (22.5 points) below their full IQ. The predicted score method (vs. simple discrepancy) was employed so that individuals with high IQs would not be under-identified as not having working memory deficits. This method is often used in the identification of learning disability in high IQ individuals. We chose the FFD subscale because it has been considered by others to be a useful measure of WM (Bowden, Petrauskas, Bardenhagen, Meade, & Simpson, 2013).
Statistical Analysis
Because significant differences in cognition between children with ADHD and Controls have been well documented (Castellanos, Sonuga-Barke, Milham, & Tannock, 2006), our analytic approach focused on pairwise comparisons within the ADHD and Control groups stratified by the presence or absence of WM deficits. For continuous variables, pairwise t-tests were conducted. For categorical variables a chi-squared test was carried out; if the number of participants in any group was below 10 we used Fisher's exact test. All analyses were conducted using the R programming language (R Core Team, 2014). All tests were two tailed. Due to the many comparisons conducted, the alpha level was set at 1%.
RESULTS
Sociodemographic Characteristics
As shown in Table 1, there were no significant within group pairwise comparisons for age, sex, SES, or intactness of the family.
Table 1.
Socio-Demographic Characteristics of Sample
| ADHD |
Controls |
|||||
|---|---|---|---|---|---|---|
| ADHD + WM Deficit (N = 88) | ADHD (N = 188) | p | Controls + WM Deficit (N = 33) | Controls (N = 208) | p | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age | 11.2 (3.1) | 10.8 (3.2) | 0.26 | 12.2 (4) | 11.8 (3.2) | 0.50 |
| SES | 2 (1) | 1.8 (1) | 0.29 | 1.8 (0.9) | 1.6 (0.7)† | 0.12 |
| N (%) | N (%) | N (%) | N (%) | |||
| Male | 52 (59.1%) | 86 (45.7%) | 0.053 | 20 (60.6%) | 100 (48.1%) | 0.25 |
| Intact | 67 (76.1%) | 132 (70.2%) | 0.38 | 27 (81.8%) | 171 (82.2%) | 1 |
SES data is missing for 1 Control - WM Deficits subject
Abbreviations: ADHD = attention deficit hyperactivity disorder; WM = working memory; SES = socioeconomic status
As shown in Table 2, there was a significant difference between the two ADHD groups in mean number of symptoms of ADHD (p = 0.008). However, the observed effect size, though statistically significant, was small with the ADHD+WM group having on average 0.7 more symptoms. There were no meaningful differences between the two groups in the mean duration and age of onset of ADHD. Likewise, there were no meaningful differences between ADHD children with and without WM deficits in number of CBCL Total Problems, social functioning (SAICA) or GAF scores (Table 2). There was however, a significant difference in the CBCL school competence score between the two ADHD groups with and without WM deficits.
Table 2.
Functional Correlates
| ADHD + WM Deficit | ADHD | Controls + WM Deficit | Controls | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Domain Of Functioning | N | Group | N | Group | p | N | Group | N | Group | p |
| ADHD | ||||||||||
| Duration Of Illness (Mean±SD) | 88 | 7.7 (±3.4) | 188 | 7.2 (±4) | 0.30 | - | - | - | - | - |
| Age of Onset (Mean±SD) | 88 | 2.9 (±2.2) | 188 | 3.3 (±2.4) | 0.5 | - | - | - | - | - |
| Number of Symptoms (Mean±SD) | 88 | 10.9 (±2.2) | 188 | 11.6 (±1.9) | 0.008* | - | - | - | - | - |
| Severe (n (%)) | 87 | 36 (41.4%) | 188 | 79 (42%) | 0.5 | - | - | - | - | - |
| CBCL | ||||||||||
| Total Problems (Mean±SD) | 78 | 60.8 (±12) | 159 | 59.6 (±11.2) | 0.7 | 31 | 37.3 (±8.7) | 195 | 39.3 (±9.2) | 0.5 |
| Total Competence Scales (Mean±SD) | 65 | 39.5 (±12.9) | 133 | 44.3 (±12.6) | 0.03# | 26 | 53.8 (±10.1) | 173 | 53.9 (±13.2) | 1 |
| Social Competence (Mean±SD) | 69 | 41.4 (±9.5) | 139 | 44.2 (±8.3) | 0.02# | 29 | 51.5 (±4) | 172 | 50.5 (±5.9) | 0.7 |
| Activities Competence (Mean±SD) | 73 | 46.1 (±7.6) | 146 | 47 (±7) | 0.5 | 30 | 49.3 (±6.2) | 189 | 49.7 (±5.5) | 0.9 |
| School Competence (Mean±SD) | 74 | 35 (±8.3) | 149 | 39.9 (±8.5) | <0.001** | 30 | 49.1 (±9) | 188 | 50.5 (±5.7) | 0.6 |
| SAICA (Sum) (Mean±SD) | 84 | 22.1 (±4.9) | 179 | 21.1 (±5.6) | 0.2 | 32 | 16.8 (±4.2) | 201 | 17 (±4.8) | 1 |
| GAF (Mean±SD) | 84 | 50.4 (±8) | 179 | 51.7 (±7.8) | 0.7 | 32 | 67.5 (±9.4) | 201 | 68.7 (±8.3) | 0.8 |
Significance codes:
= Trend
= p <0.01
= p <0.001
Abbreviations: ADHD = attention deficit hyperactivity disorder; WM = working memory; CBCL = Child Behavior Checklist; SAICA = Social Adjustment Inventory for Children and Adolescents; GAF = Global Assessment of Functioning
The rate of WM deficits was significantly higher in children with ADHD compared to controls (32% vs. 14%, p < 0.001). Within group comparisons were made between ADHD children with (ADHD+WM Deficits: N=88) and without (ADHD: N=188) WM deficits and Controls with (Controls+WM Deficits: N=33) and without (Controls: N=208) WM Deficits.
As shown in Table 3, there were no significant differences between the two ADHD groups in psychiatric comorbidities. There were significance differences between the two groups in Learning Disabilities.
Table 3.
Psychiatric Comorbidities
| ADHD + WM Deficit | ADHD | Controls + WM Deficit | Controls | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comorbidity | N | Group | N | Group | p | N | Group | N | Group | p |
| Bipolar | 88 | 10 (11.4%) | 188 | 20 (10.6%) | 1 | 33 | 0 (0%) | 208 | 0 (0%) | 1 |
| Disruptive Behavior Disorders | 88 | 45 (51.1%) | 188 | 95 (50.5%) | 0.8 | 33 | 2 (6.1%) | 208 | 16 (7.7%) | 0.8 |
| Elimination Disorders | 88 | 27 (30.7%) | 188 | 58 (30.9%) | 0.9 | 33 | 5 (15.2%) | 208 | 21 (10.1%) | 0.7 |
| Language Disorders | 88 | 23 (26.1%) | 188 | 29 (15.4%) | 0.1 | 33 | 3 (9.1%) | 208 | 17 (8.2%) | 1 |
| Learning Disability | 87 | 38 (43.7%) | 183 | 23 (12.6%) | <.001** | 32 | 7 (21.9%) | 203 | 13 (6.4%) | 0.02# |
| Learning Disability - Arithmetic | 87 | 30 (34.5%) | 184 | 12 (6.5%) | <.001** | 32 | 5 (15.6%) | 205 | 10 (4.9%) | 0.07 |
| Learning Disability - Reading | 87 | 20 (23%) | 183 | 16 (8.7%) | 0.008* | 32 | 2 (6.2%) | 204 | 5 (2.5%) | 0.5 |
| Major Depression | 88 | 44 (50%) | 188 | 84 (44.7%) | 0.5 | 33 | 3 (9.1%) | 208 | 23 (11.1%) | 1 |
| Mood Disorders | 88 | 46 (52.3%) | 188 | 92 (48.9%) | 0.7 | 33 | 3 (9.1%) | 207 | 25 (12.1%) | 0.9 |
| Multiple (≥2) Anxiety Disorders | 88 | 21 (23.9%) | 187 | 48 (25.7%) | 0.9 | 33 | 3 (9.1%) | 208 | 7 (3.4%) | 0.3 |
| Psychosis | 88 | 0 (0%) | 188 | 1 (0.5%) | 1 | 33 | 0 (0%) | 208 | 1 (0.5%) | 1 |
| Substance Use Disorders | 88 | 3 (3.4%) | 188 | 8 (4.3%) | 0.9 | 33 | 2 (6.1%) | 207 | 5 (2.4%) | 0.5 |
Significance codes:
= Trend
= p <0.01
= p <0.001
Abbreviations: ADHD = attention deficit hyperactivity disorder; WM = working memory
Cognitive Outcomes
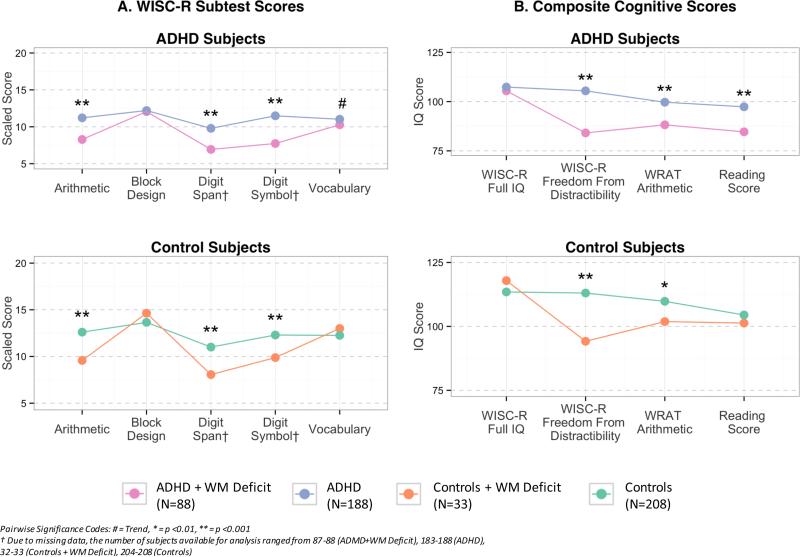
As shown in Figure 1, WISC-R subtest scores for Arithmetic, Digit Span, and Digit Symbol were significantly worse in both ADHD and Control participants with WM deficits than in those without WM deficits, as was expected based on the definition of WM (Figure 1A). Likewise, composite scores for both the ADHD and Control groups were significantly worse for those with WM deficits for WISC-R FFD and WRAT Arithmetic, but WRAT Reading was more impaired in ADHD+WM children only (Figure 2B).
Figure 1.
Cognitive Scores
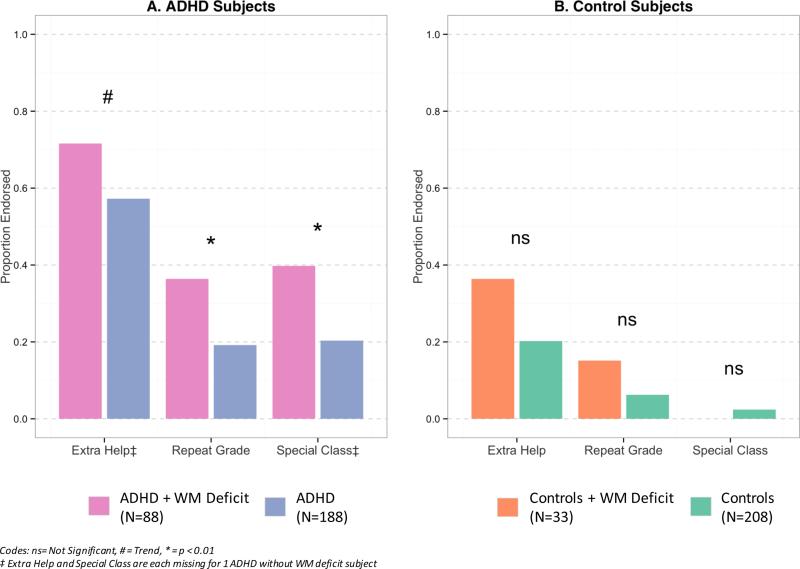
Figure 2.
School Deficits
School Outcomes
As shown in Figure 2A, the rates of grade retention and placement in special classes were significantly more prevalent in children with ADHD with WM deficits than in those without WM deficits. Although the need for extra help was significantly higher in ADHD children with WM deficits than in those without, this difference did not reach our a priori threshold for statistical significance (Figure 2A). While similar differences were also observed in Controls with WM deficits vs. Controls without WM deficits, these differences failed to reach our a priori level of statistical significance (Figure 2B).
DISCUSSION
In a large sample of comprehensively assessed youth of both sexes with and without ADHD ascertained from pediatric and psychiatric sources, we found that the presence of WM deficits in ADHD children significantly increased the risk for grade retention, placement in special classes and lower academic achievement in both reading and math. This was the case even after stringent statistical controls. These effects could not be accounted for by differences in the clinical features of ADHD or by patterns of comorbidity. In contrast, we did not find statistically significant evidence that WM deficits affected any other correlates. Results also showed that WM deficits did not have a statistically significant impact on children without ADHD. Taken together, these findings provide support for a selectively detrimental effect of WM deficits on academic functioning in youth with ADHD and suggest that their impact is particularly potent in the context of ADHD.
Our findings linking WM with compromised academic dysfunction in children with ADHD extend previous findings in the literature delineated in the introduction in several ways. While cognitive and academic deficits have consistently been associated with ADHD relative to non ADHD controls, we are now showing that these cognitive and academic deficits are much more compromised in children with ADHD and WM deficits compared to children with ADHD without such deficits. We also document that WM deficits associated with academic dysfunctions in children with ADHD were not due to psychiatric comorbidity or other confounders such as social class, age, sex, or IQ. Taken together, these results show that individuals with ADHD and comorbid WM deficits suffer from the detrimental synergism of the two conditions (ADHD and WM deficits) such that their academic performance is severely compromised when both are present.
Although the adverse educational and cognitive findings followed a similar pattern in non ADHD Controls, with the exception of arithmetic dysfunction, these findings failed to reach our a priori threshold for statistical significance. These results differed from findings reported by Etchepareborda and Abad-Mas (Etchepareborda & Abad-Mas, 2005) in a non ADHD sample, showing that impaired WM negatively influenced academic learning processes. Clearly, more work is needed to evaluate whether WM deficits may also lead to educational and cognitive morbidity outside the context of ADHD.
While our study further documents that WM deficits are over represented in children with ADHD, they also show that WM deficits are not universally associated with ADHD. This supports the hypothesis that WM deficits and ADHD represent separate (comorbid) conditions. Emerging preclinical, neuroimaging and pharmacological findings support the dissociation between ADHD and WM deficits. Preclinical studies showed that WM deficits are selectively linked to defects in the dopamine receptor D4 in a prenatal nicotine exposure (PNE) mouse model of ADHD (Lee et al., 2014). A recent neuroimaging study (Mattfeld et al., 2014) using resting state fMRI showed that compromised WM ability was associated with deficits in a unique circuit of the default mode network (DMN) connecting the medial prefrontal cortex (MPFC) with the dorsolateral prefrontal cortex (DLPC), whereas ADHD was linked to another node of the DMN connecting the posterior parietal cortex (PCC) to the MPFC. Additionally, while stimulant treatments for ADHD improve symptoms of ADHD proper, they have a limited impact on WM deficits (Faraone & Glatt, 2009). Taken together, these findings support the hypothesis that ADHD and WM deficits represent separate morbid clinical entities.
The selectivity of WM deficits on cognitive and academic dysfunction is supported by our findings that neither psychiatric comorbidity nor the social adjustment scale or GAF were significantly associated with WM deficits, regardless of ADHD status. However, our participants had not passed through the age of risk for the full unfolding of all detrimental effects that could be linked to WM deficits in non-academic domains. It is also possible that such broad deficits will not be manifested until adulthood since there is an abundance of parental involvement in decisions made during childhood and adolescence which might mask social, occupational, financial, or even global functional impairments. Thus, from this perspective, academic deficits could reasonably be the only domain within which WM deficits could cause problems for children. Long-term longitudinal studies are needed to confirm this hypothesis.
The cognitive and academic burdens associated with WM deficits in the context of ADHD have important clinical and scientific implications. Clinically, the presence of WM deficits significantly taxes the already compromised cognitive and academic performance of ADHD children beyond that conferred by ADHD itself. Considering that the presence of WM deficits can only be documented through cognitive testing, screening for such deficits can help identify a subgroup of ADHD children at very high risk for academic dysfunction. Since pharmacotherapy for ADHD has a limited impact on WM deficits, the identification of ADHD children with comorbid WM deficits can help implement appropriate educational intervention to help address them. Scientifically, children with ADHD and WM deficits may represent a meaningful subgroup of ADHD children with unique neurobiological underpinning worthy of further investigation.
Strengths of this study include its large sample size of well characterized and comprehensively assessed youth of both sexes, its ascertainment from psychiatric and pediatric sources, its accelerated design, the blindness of assessments, as well as its reliance on well-established objective psychometric tests to assess working memory.
However, our results should also be considered in light of some methodological limitations. While our study relied on the Freedom From Distractibility Factor from the WISC-R to assess Working Memory, which is not considered a true measure of WM, this index has documented very high correlation with WM index in the WISC-III revision (Wechsler, 1991). Because the sample of ADHD participants was referred and largely Caucasian, our findings may not generalize to non-referred samples and children from other ethnic groups. Although the data were collected in the 1990's, most of the core features of ADHD including symptom expression, neuropsychological underpinning and patterns of comorbidity have remained constant. This is consistent with the field trials of Lahey et al. (1994) in comparing DSM-III-R to DSM-IV and with the field trials of Matte et al. (2015) comparing DSM-IV to DSM-V. Another potential limitation stems from the lack of multiple sources. Teachers were initially asked to complete scales, but the number returned was too low for analysis. However, even without the teacher reports, information on objective education information including grade retention and placement in special classes was documented both in interview and on written rating scales. Finally, there were some missing data points for particular scales, including the CBCL.
Despite these considerations, our results show that significantly more youth with ADHD had WM deficits than controls without ADHD. Additionally, these deficits were selectively and significantly associated with increased risks for grade retention, placement in special classes and lower scores on reading and math achievement tests. Although to a lesser extent, WM deficits also had a detrimental impact on educational and cognitive correlates in non ADHD Controls. Screening for WM deficits may help identify children at high risk for educational impairments.
Acknowledgements
This work was supported bythe NIH under Grant R01MH050657 and Grant R01HD036317 to Dr. Biederman, and by the Pediatric Psychopharmacology Research Council Fund.
Disclosure Statement: Dr. Ronna Fried is currently receiving research support from the following sources: Lundbeck. In 2015, Dr. Fried received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. In previous years, Dr. Fried received research support from NIH and Shire.
In the past year, Dr. Faraone received income, travel expenses and/or research support from and/or has been on an Advisory Board for Pfizer, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma, Neurovance, Impax, NeuroLifeSciences and research support from the National Institutes of Health (NIH). With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by: Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk about Your Child's Mental Health, Oxford University Press: Schizophrenia: The Facts and Elsevier, ADHD: Non-Pharmacologic Treatments.
Dr. Joseph Biederman is currently receiving research support from the following sources: The Department of Defense, Food & Drug Administration, Ironshore, Lundbeck, Magceutics Inc., Merck, PamLab, Pfizer, Shire Pharmaceuticals Inc., SPRITES, Sunovion, Vaya Pharma/Enzymotec, and NIH. In 2015, Dr. Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He has a US Patent Application pending (Provisional Number #61/233,686) through MGH corporate licensing on a method to prevent stimulant abuse. In 2014, Dr. Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He received research support from AACAP, Alcobra, Forest Research Institute, and Shire Pharmaceuticals Inc. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH. In 2013, Dr. Biederman received an honorarium from the MGH Psychiatry Academy for a tuition-funded CME course. He received research support from APSARD, ElMindA, McNeil, and Shire. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Shire and Sunovion; these royalties were paid to the Department of Psychiatry at MGH. In 2012, Dr. Biederman received an honorarium from the MGH Psychiatry Academy and The Children's Hospital of Southwest Florida/Lee Memorial Health System for tuition-funded CME courses.
Footnotes
Affiliation where research was conducted.
Mr. Chan, Ms. Feinberg, Ms. Pope, and Ms. Woodworth do not have any financial relationships to disclose.
REFERENCES
- Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- Alloway TP, Elliott J, Place M. Investigating the relationship between attention and working memory in clinical and community samples. Child Neuropsychology. 2010;16(3):242–254. doi: 10.1080/09297040903559655. doi: 10.1080/09297040903559655. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, Elliott J. Examining the link between working memory behaviour and academic attainment in children with ADHD. Developmental Medicine & Child Neurology. 2010;52(7):632–636. doi: 10.1111/j.1469-8749.2009.03603.x. doi: 10.1111/j.1469-8749.2009.03603.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. Journal of Developmental & Behavioral Pediatrics. 2007;28(4):265–273. doi: 10.1097/DBP.0b013e31811ff87d. doi: 10.1097/DBP.0b013e31811ff87d. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR. Impairment in occupational functioning and adult ADHD: the predictive utility of executive function (EF) ratings versus EF tests. Archives of Clinical Neuropsychology. 2010;25(3):157–173. doi: 10.1093/arclin/acq014. doi: 10.1093/arclin/acq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, Tsuang MT. Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder. Patterns of comorbidity in probands and relatives in psychiatrically and pediatrically referred samples. Archives of General Psychiatry. 1992;49(9):728–738. doi: 10.1001/archpsyc.1992.01820090056010. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Mick E, Williamson S, Wilens TE, Spencer TJ, Zallen B. Clinical correlates of ADHD in females: findings from a large group of girls ascertained from pediatric and psychiatric referral sources. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(8):966–975. doi: 10.1097/00004583-199908000-00012. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux M, Mick E, Spencer T, Wilens T, Klein K, Faraone SV. Psychopathology in females with attention-deficit/hyperactivity disorder: A controlled, five-year prospective study. Biological Psychiatry. 2006;60(10):1098–1105. doi: 10.1016/j.biopsych.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, Faraone SV. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. Journal of Consulting and Clinical Psychology. 2004;72(5):757–766. doi: 10.1037/0022-006X.72.5.757. doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, Faraone SV. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychological Medicine. 2006;36(2):167–179. doi: 10.1017/S0033291705006410. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Biederman J, Seidman LJ, Petty CR, Fried R, Doyle AE, Cohen DR, Faraone SV. Effects of stimulant medication on neuropsychological functioning in young adults with attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry. 2008;69(7):1150–1156. doi: 10.4088/jcp.v69n0715. doi: ej07m03529 [pii] [DOI] [PubMed] [Google Scholar]
- Bowden SC, Petrauskas VM, Bardenhagen FJ, Meade CE, Simpson LC. Exploring the dimensionality of digit span. Assessment. 2013;20(2):188–198. doi: 10.1177/1073191112457016. doi: 10.1177/1073191112457016. [DOI] [PubMed] [Google Scholar]
- Bunford N, Brandt NE, Golden C, Dykstra JB, Suhr JA, Owens JS. Attention-deficit/hyperactivity disorder symptoms mediate the association between deficits in executive functioning and social impairment in children. Journal of Abnormal Child Psychology. 2015;43(1):133–147. doi: 10.1007/s10802-014-9902-9. doi: 10.1007/s10802-014-9902-9. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cognitive Science. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Chiang HL, Gau SS. Impact of executive functions on school and peer functions in youths with ADHD. Research in Developmental Disabilities. 2014;35(5):963–972. doi: 10.1016/j.ridd.2014.02.010. doi: 10.1016/j.ridd.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Coghill DR, Hayward D, Rhodes SM, Grimmer C, Matthews K. A longitudinal examination of neuropsychological and clinical functioning in boys with attention deficit hyperactivity disorder (ADHD): improvements in executive functioning do not explain clinical improvement. Psychological Medicine. 2014;44(5):1087–1099. doi: 10.1017/S0033291713001761. doi: 10.1017/S0033291713001761. [DOI] [PubMed] [Google Scholar]
- Etchepareborda MC, Abad-Mas L. [Working memory in basic learning processes.]. Revue Neurologique. 2005;40(Suppl 1):S79–83. [PubMed] [Google Scholar]
- Faraone SV. Understanding the effect size of lisdexamfetamine dimesylate for treating ADHD in children and adults. Journal of Attention Disorders. 2012;16(2):128–137. doi: 10.1177/1087054710379738. doi: 10.1177/1087054710379738. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Doyle A, Murray K, Petty C, Adamson JJ, Seidman L. Neuropsychological studies of late onset and subthreshold diagnoses of adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2006;60(10):1081–1087. doi: 10.1016/j.biopsych.2006.03.060. doi: 10.1016/j.biopsych.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. Journal of Clinical Psychiatry. 2009;71(6):754–763. doi: 10.4088/JCP.08m04902pur. doi: 10.4088/JCP.08m04902pur. [DOI] [PubMed] [Google Scholar]
- Gropper RJ, Tannock R. A pilot study of working memory and academic achievement in college students with ADHD. Journal of Attention Disorders. 2009;12(6):574–581. doi: 10.1177/1087054708320390. doi: 10.1177/1087054708320390. [DOI] [PubMed] [Google Scholar]
- Jastak JF, Jastak S. The Wide Range Achievement Test-Revised. Jastak Associates; Wilmington, Delaware: 1985. [Google Scholar]
- John K, Gammon GD, Prusoff BA, Warner V. The Social Adjustment Inventory for Children and Adolescents (SAICA): Testing of a new semistructured interview. Journal of the American Academy of Child & Adolescent Psychiatry. 1987;26(6):898–911. doi: 10.1097/00004583-198726060-00015. [DOI] [PubMed] [Google Scholar]
- Kaufman AS. Intelligent Testing with the WISC-R (Wiley Series on Personality Processes) John Wiley & Sons; New York: 1979. [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. The American Journal of Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS, Alderson RM. Working memory deficits and social problems in children with ADHD. Journal of Abnormal Child Psychology. 2011;39(6):805–817. doi: 10.1007/s10802-011-9492-8. doi: 10.1007/s10802-011-9492-8. [DOI] [PubMed] [Google Scholar]
- Kranzler JH. What does the WISC-III measure? Comments on the relationship between intelligence, working memory capacity, and information processing speed and efficiency. School Psychology Quarterly. 1997;12:110–116. [Google Scholar]
- Lahey B, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd G, Shaffer D. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. The American Journal of Psychiatry. 1994;151(11):1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- Lee K, Pineda N, Brune T, Patel K, Ganon N, Spencer T, Zhu J. Hyperactivity and working memory deficits induced by prenatal nicotine exposure are associated with dopamine D1 and D4 receptor dysfunction.. Paper presented at the The Society for Neuroscience Annual Meeting; Washington, D.C.. Nov 15-19, 2014. [Google Scholar]
- Matte B, Anselmi L, Salum GA, Kieling C, Goncalves H, Menezes A, Rohde LA. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychological Medicine. 2015;45(2):361–373. doi: 10.1017/S0033291714001470. doi: 10.1017/S0033291714001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattfeld AT, Gabrieli JD, Biederman J, Spencer T, Brown A, Kotte A, Whitfield- Gabrieli S. Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain. 2014;137(Pt 9):2423–2428. doi: 10.1093/brain/awu137. doi: 10.1093/brain/awu137. [DOI] [PubMed] [Google Scholar]
- Miller M, Nevado-Montenegro AJ, Hinshaw SP. Childhood executive function continues to predict outcomes in young adult females with and without childhood-diagnosed ADHD. Journal of Abnormal Child Psychology. 2012;40(5):657–668. doi: 10.1007/s10802-011-9599-y. doi: 10.1007/s10802-011-9599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Manual for the Family Environment Scale. Consulting Psychologists Press; Palo Alto, CA: 1974. [Google Scholar]
- Nyman A, Taskinen T, Gronroos M, Haataja L, Lahdetie J, Korhonen T. Elements of working memory as predictors of goal-setting skills in children with attention-deficit/ hyperactivity disorder. Journal of Learning Disabilities. 2010;43(6):553–562. doi: 10.1177/0022219410375001. doi: 10.1177/0022219410375001. [DOI] [PubMed] [Google Scholar]
- Orvaschel H. Schedule for Affective Disorders and Schizophrenia for School-Age Children Epidemiologic Version. 5th Edition ed. Nova Southeastern University, Center for Psychological Studies; Ft. Lauderdale: 1994. [Google Scholar]
- R Core Team [January 21, 2015];R: A language and environment for statistical computing. 2014 from http://www.r-project.org.
- Rennie B, Beebe-Frankenberger M, Swanson HL. A longitudinal study of neuropsychological functioning and academic achievement in children with and without signs of attention-deficit/hyperactivity disorder. Journal of Clinical and Experimental Neuropsychology. 2014;36(6):621–635. doi: 10.1080/13803395.2014.921284. doi: 10.1080/13803395.2014.921284. [DOI] [PubMed] [Google Scholar]
- Rinsky JR, Hinshaw SP. Linkages between childhood executive functioning and adolescent social functioning and psychopathology in girls with ADHD. Child Neuropsychology. 2011;17(4):368–390. doi: 10.1080/09297049.2010.544649. doi: 10.1080/09297049.2010.544649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler J. Psychological Assessment. Fourth ed. McGraw-Hill; New York: 1988. [Google Scholar]
- Sjowall D, Thorell LB. Functional impairments in attention deficit hyperactivity disorder: the mediating role of neuropsychological functioning. Developmental Neuropsychology. 2014;39(3):187–204. doi: 10.1080/87565641.2014.886691. doi: 10.1080/87565641.2014.886691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36(1):207–226. doi: 10.1038/npp.2010.160. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WL, Gau SS. Executive function as a mediator in the link between attention-deficit/hyperactivity disorder and social problems. Journal of Child Psychology and Psychiatry. 2013;54(9):996–1004. doi: 10.1111/jcpp.12072. doi: 10.1111/jcpp.12072. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children-Revised. The Psychological Corporation; New York: 1974. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children - Third Edition. The Psychological Corporation, Harcourt Brace Jovanovich, Inc; San Antonio: 1991. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Willcutt EG. The Prevalence of DSM-IV Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Neurotherapeutics. 2012;9(3):490–499. doi: 10.1007/s13311-012-0135-8. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]