Abstract
Rifaximin is a broad spectrum oral antibiotic with antimicrobial activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria. It is poorly absorbed and thus has a highly favorable safety profile. Rifaximin has been shown to be effective in the treatment of traveler’s diarrhea, functional bloating and irritable bowel syndrome, small bowel bacterial overgrowth and in the prevention of recurrent overt hepatic encephalopathy. In addition, there is emerging evidence for a possible beneficial effect of rifaximin in the treatment of uncomplicated diverticular disease and in the prevention of recurrent diverticulitis. The use of rifaximin is associated with a low incidence of development, or persistence of spontaneous bacterial mutants. Moreover, the development of important drug resistance among extra-intestinal flora during rifaximin therapy is unlikely because of minimal systemic absorption and limited cross-resistance of rifaximin with other antimicrobials. This review addresses the current and emerging role of rifaximin in the treatment of gastrointestinal and liver disorders.
Keywords: Irritable bowel syndrome, Inflammatory bowel disease, Hepatic encephalopathy, Bacterial overgrowth, Diverticular disease
Core tip: Rifaximin is a poorly absorbed oral antibiotic with highly favorable safety profile. Rifaximin is effective in the treatment of traveler’s diarrhea, functional bloating and irritable bowel syndrome, small bowel bacterial overgrowth and in the prevention of recurrent overt hepatic encephalopathy. There is emerging evidence for a possible beneficial effect of rifaximin in the treatment of other disorders including uncomplicated diverticular disease and in the prevention of recurrent diverticulitis. The use of rifaximin is associated with a low incidence of development of spontaneous bacterial mutants or drug resistance among extra-intestinal flora.
INTRODUCTION
Rifaximin is a poorly-absorbed broad spectrum oral antibiotic, first approved in Italy in 1987 and in the United States in 2004, and now approved in many European, African, and Asian countries for several indications[1,2]. Rifaximin is a rifamycin antimicrobial agent, a rifampin structural analog[3], that inhibits RNA synthesis by binding to the β-subunit of bacterial DNA-dependent RNA polymerase[4]. The addition of a pyridoimidazole ring to the rifampin molecule (Figure 1) makes rifaximin a largely water-insoluble, poorly-absorbable antibiotic[2] (< 0.4%)[5] and hence associated with few systemic adverse events and with a safety profile that is comparable to placebo[6].
Figure 1.
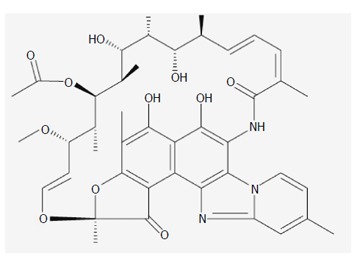
Chemical structure of rifaximin.
Although its poor gastrointestinal (GI) absorbability leads to low systemic blood levels, fecal concentrations remain elevated with unchanged drug[3,7]. Rifaximin does not cause drug-drug interaction and does not alter intestinal or hepatic cytochrome P3A activity[8].
Rifaximin has in vitro antimicrobial activity against Gram-positive and Gram-negative, aerobic and anaerobic flora[9]. The increased solubility of rifaximin in bile (an estimated 70- to 120-fold increase in solubility in vitro compared to aqueous solution)[3] leads to higher luminal concentrations and enhanced antimicrobial effects[10] against enteric bacteria, with possibly larger effects in the small intestine compared with the more aqueous colon[3] as well as low microbial resistance[11] with minimal effect on colonic microflora. In addition to its direct bactericidal effect, rifaximin has been shown to reduce bacterial virulence factors and morphology[12], the inflammatory response expected from virulent strains of enteroaggregative Escherichia coli (E. coli) (EAEC) and Shigella, bacterial epithelial attachment and plasmid transfer from donor to recipient strains by > 99% for bacteria resistant or susceptible to rifaximin[12], and to provide cytoprotection through altering cytokine expression and mucosal inflammation by activation of pregnane X receptor involved in detoxification and elimination of foreign chemicals and toxins in the gut in disease states[12,13].
CLINICAL EFFICACY
Irritable bowel syndrome
Irritable bowel syndrome (IBS) is a gastrointestinal syndrome characterized by chronic abdominal pain and altered bowel habits without any underlying organic pathology, diagnosed according to the revised ROME criteria[14]. IBS occurs in 5% of the general population[15] and is associated with a high socioeconomic burden by decreasing quality of life, work productivity[16-18], and increasing heath resource utilization[19,20]. Patients with IBS are subdivided on the basis of their bowel symptoms into 4 subgroups: IBS with constipation, IBS with diarrhea (IBS-D), alternating or mixed IBS, and un-subtyped IBS, with up to 40% suffering from the IBS-D form[21]. Though the pathophysiology of this entity remains incompletely defined, multiple factors including abnormal gastrointestinal motility[22-27], visceral hypersensitivity[28-40], food sensitivity[41-56], genetic[57-61] and psychosocial factors[62-70], intestinal inflammation[71-78], post infectious[79-83] and bacterial overgrowth are postulated to contribute to the disease. Furthermore, emerging data suggest that the fecal microbiota in individuals with IBS differ from healthy controls and varies with the predominant symptom[84-89].
IBS management has been primarily focused on symptomatic treatment until data revealed that patients suffering from IBS might have alterations in their gastrointestinal flora, manifested by excessive bacteria in the small bowel, known as bacterial overgrowth. As a result, the use of rifaximin as a potential therapeutic agent for the treatment of IBS has been increasingly investigated. In a randomized double-blind placebo-controlled trial by Sharara et al[90] in 124 patients with functional bloating, rifaximin 800 mg/d was found to be superior to placebo in providing global symptomatic relief (41.3% vs 22.9% respectively, P = 0.03). None of the study patients had an abnormal lactulose hydrogen breath test (LHBT) at baseline. However, hydrogen breath excretion dropped significantly among rifaximin responders and correlated with improvement in bloating and overall symptom scores. Another randomized placebo-controlled study evaluated the effect of rifaximin 1200 mg/d or placebo for 10 d in 87 patients with IBS[91]. By the end of the follow-up period of 10 wk, patients treated with rifaximin reported significantly greater global improvement compared with the placebo group (P = 0.02), with an average improvement of 36.4% in the rifaximin group compared to 21.0% in the placebo group[91]. In addition, there was a significant improvement in bloating with rifaximin while abdominal pain, diarrhea and constipation did not significantly change compared to placebo. The TARGET study group presented data from two identically designed Phase III double-blind, placebo-controlled clinical trials (TARGET 1 and TARGET 2) in non-constipated IBS patients showing that rifaximin 550 mg three times daily for 14 d relieved global IBS symptoms and bloating for at least two of the first four weeks of treatment, and improved daily assessments of IBS symptoms, bloating, abdominal pain, and stool consistency compared with placebo[92]. A recent phase 3 trial (TARGET 3) investigating treatment of symptom recurrence in patients with diarrhea predominant IBS (D-IBS) who had previously taken rifaximin demonstrated a significantly higher success rate of recurrence treatment when compared to placebo (33% vs 25%, P = 0.02)[93]. Rifaximin has since received recent FDA approval for use in adults with IBS-D.
Meyrat et al[94] investigated the prevalence of abnormal LHBT and the efficacy of rifaximin in patients suffering from IBS. Of the 150 patients enrolled, 71% had positive LHBT results and were treated with rifaximin 800 mg daily for two weeks. All symptoms under investigation (bloating, flatulence, diarrhea, and abdominal pain) as well as reduced overall well-being, significantly improved upon re-assessment after 1 mo and 3 mo of therapy initiation. A meta-analysis and systematic review by Menees et al[95] found that rifaximin is more effective than placebo for global IBS symptom improvement (OR = 1.57; 95%CI: 1.22-2.01) with a number needed to treat (NNT) of 10.2[95]. Rifaximin was also significantly more likely to improve bloating than placebo (OR = 1.55; 95%CI: 1.23-1.96). Although LHBT normalization has been correlated with clinical improvement[96], there are limited data investigating the changes in LHBT during and after treatment of IBS with rifaximin. A recent retrospective study investigated the association of rifaximin therapy and LHBT changes in non-constipated IBS patients who had normalized their LHBT values after treatment. Patients suffering from IBS symptoms had similar LHBT values prior to therapy and had received rifaximin 1200 mg daily for treatment periods of 4 wk, 8 wk or 12 wk. LHBT values were statistically significant in the three treatment groups, with higher values occurring in patients who received longer treatment periods. Symptomatic improvement to rifaximin was also demonstrated to occur prior to normalization of LHBT values and mostly occurring after 4 wk of treatment[97]. Although this study demonstrated that patients with higher LHBT values take longer time to normalize their values, LHBT gas levels were not associated global abdominal symptoms, findings that are contradictory to other studies[98-100]. In view of increasing evidence of the relationship of small intestinal bacterial overgrowth (SIBO) and IBS pathophysiology, identifying SIBO in IBS patients may prove beneficial for optimal treatment options. While LHBT is often used, data show that its diagnostic accuracy is inferior to glucose breath testing (GBT) (55.1% vs 71.7%), suggesting a need to switch to the more accurate GBT in future clinical trials assessing for bacterial overgrowth in IBS patients[101].
Traveler’s diarrhea
Traveler’s diarrhea (TD) is a common illness among travelers from resource-rich to resource-poor regions of the world. Diarrhea can be caused by a variety of bacterial, viral, and parasitic organisms, which are most often transmitted by contaminated food and water. Bacteria cause more than 90% of TD cases in most geographic areas, the most common organism being enterotoxigenic E. coli (ETEC)[102-104] accounting for 40% of cases, followed by enteroadhesive E. coli in 10%-20% of cases.
Rifaximin was tested in randomized controlled trials as treatment of TD and found to shorten the duration of the illness[105,106]. In a randomized controlled trial comparing 3 d of ciprofloxacin to rifaximin for TD, there was no significant difference in the 2 groups with respect to clinical improvement during the first 24 h, failure to respond to treatment, or microbiological cure. Compared to placebo, rifaximin resulted in significant reduction in the median time to last unformed stool over placebo (32.0 h vs 65.5 h respectively (P < 0.001) with no associated adverse events[107]. Rifaximin is routinely given for TD as 400mg twice a day for three days, and may be used as a self-treatment for TD as stated by the International Society of Travel Medicine[108]. With a lesser degree of efficacy, rifaximin can also be used for the prevention of TD[109]. Several trials have assessed the efficacy of rifaximin in preventing traveler’s diarrhea in different geographical areas using different dosing regimens. In a randomized-double blinded placebo controlled trial aimed at assessing the efficacy of TD prevention in a United States military airbase in Turkey, Armstrong et al[110] concluded that rifaximin 1100 mg once daily for two weeks was not different from placebo although the P value of 0.07 neared statistical significance. An estimated protective efficacy of 67% was demonstrated[110]. In 2011, Flores et al[111] similarly showed the lack of a statistically significant reduction in TD when 550 mg rifaximin was administered once daily for two weeks in Mexico. However, a noticeable reduction in mild diarrhea during the first week of therapy and comparable side effect profiles to 200 mg taken twice daily were demonstrated[111]. A placebo-controlled trial by Martinez-Sandoval et al[112] in United States travelers to Mexico found that rifaximin provided a 58% protection rate over placebo against TD. Fewer individuals on rifaximin developed all-cause TD compared to placebo (20% vs 48%, respectively; P < 0.0001) or required antibiotic therapy for TD (14% vs 32%, respectively; P = 0.003). Adverse events in the rifaximin group were fewer than placebo. A study by Zanger et al[109] in 2003 investigating the preventive efficacy of TD in individuals travelling to South and Southeast Asia showed that travelers consuming rifaximin 200 mg twice daily for a travel duration of 28 d had a reduced risk of TD during and post travel period when compared to placebo. A 48% protection was noted, lowering the incidence of TD from 1.99 (1.5-2.64) per 100 person-days to 1.04 (0.72-1.48) (incidence rate ratio of 0.52, 95%CI: 0.32-0.84 P = 0.005). However, similar incidences of TD between the rifaximin and placebo arms were noted 1 wk after return from travel[109]. Although this study demonstrated some degree of protective efficacy, it is speculated that the low 48% protective efficacy may be due to the low incidence of enteroinvasive pathogens and a 2-4 time higher Caympylobacter spp incidence causing TD in the South and Southeast Asia area[109].
Small intestinal bacterial overgrowth
Small intestinal bacterial overgrowth (SIBO) is characterized by an increase of overall bacterial burden and emergence of different species of enterobacteria, bacteroides, clostridia, and fusobacteria in the small intestine. Rifaximin has been shown to normalize the hydrogen breath test and improve symptoms of SIBO[113-115]. High doses of rifaximin (1200 or 1600 mg/d) lead to a significant improvement in terms of therapeutic efficacy and SIBO eradication without increasing the incidence of side effects[116,117]. A recent study by Zhang et al[118] on cirrhotic patients showed that rifaximin 200 mg TID for a week was effective in reducing SIBO events in parallel with minimal hepatic encephalopathy. Patient blood ammonium levels were also significantly reduced (56.1 to 39.1 μmol/L, P < 0.01) along with psychometric tests. A recent small open-label study on SIBO subjects with no IBS showed LHBT normalization in only 42.1% of participants and concluded that rifaximin was not effective in normalizing LHBT in that patient category[119].
Hepatic encephalopathy
Hepatic encephalopathy (HE) is a reversible syndrome of impaired brain function occurring in patients with advanced liver failure. Patients with overt hepatic encephalopathy have clinically apparent impairments in cognitive and neuromuscular function. The pathophysiology of HE is multifactorial but ammonia is the most implicated and best characterized neurotoxin that may precipitate HE. The gastrointestinal flora, mainly the urease-producing species, is an important source of ammonia, which enters the circulation via the portal vein. Normally, the liver clears most of the ammonia but in liver cirrhosis the impaired liver function leads to accumulation of ammonia in the circulation, which is also worsened by shunting of blood around the liver via dilated collaterals. Furthermore, muscle wasting may also contribute since muscle is an important site of extrahepatic ammonia removal.
Current AASLD/EASL guidelines continue to recommend lactulose as the first line therapy for OHE (Grade II-1, B1) and only suggest rifaximin as an add-on therapy for the prevention of OHE recurrence (Grade I-A, 1)[120] (see below). Data is insufficient regarding the use of rifaximin as a first line therapy for the treatment of OHE, and there are no recommendations regarding rifaximin use as a standalone therapeutic agent for the treatment of OHE[120]. Rifaximin may be used in combination with lactulose in patients with overt HE as the combined effect leads to reversal of the condition in 76% of patients vs 50.4% in those on lactulose alone[121]. Similar results were reached in another study[122].
Prevention of overt HE
A randomized, double blinded, placebo controlled trial in 299 patients with chronic liver disease in remission from recurrent HE compared 550 mg rifaximin twice daily to placebo over a period of 6 mo. Rifaximin significantly reduced the risk of OHE compared to placebo with a hazard ratio of 0.42 (95%CI:, 0.28-0.64, P < 0.001) and a relative risk reduction of 58% of breakthrough HE (22.1% in rifaximin users vs 45.9% in the placebo arm) with a NNT over 6 mo of 4. There was also a reduction in HE associated hospitalizations (13.6% vs 22.6% placebo) with a HR of 0.50 (95%CI:, 0.29-0.87, P = 0.01). It is important to note that more than 90% of patients in this study received concomitant lactulose, thus providing evidence of the superiority of rifaximin and lactulose vs lactulose monotherapy[123]. Rifaximin significantly improved health related quality of life (HRQOL) in patients with cirrhosis and recurrent HE[123,124].
The previous trial was followed by a 24 mo phase 3, open-label maintenance (OLM) study to assess the safety and rate of hospitalization with long-term rifaximin use[125]. Data were analyzed according to all patients who had received rifaximin in both studies (all-rifaximin arm n = 392), to patients who received placebo in the RCT (n = 82), patients who had been treated with rifaximin in the RCT (n = 70) and to patients who only participated in the OLM (n = 170). Patients receiving rifaximin demonstrated a lower rate of all-cause hospitalizations [0.45 events per person-exposure years [PYE)] compared to the placebo arm in the RCT (1.31 PYE). In addition, HE related hospitalizations in the all-rifaximin arm (0.21 PYE), OLM only-rifaximin arm (0.23 PYE) and RCT rifaximin arm (0.30 PYE) were similar and lower than the RCT placebo arm (0.72 PYE, P < 0.0001) Long-term rifaximin use (≥ 24 mo) was associated with a significant reduction in rates of hospitalization for any cause (0.45 events PYE with rifaximin vs 1.31 with placebo; P < 0.0001) and rates of HE-related hospitalizations (0.21 vs 0.72, P < 0.0001). All-rifaximin group had a lower AE rate (0.71 vs 2.76), lower drug-related AE rate (0.11 vs 0.74), lower severe AE rate (0.48 vs 1.37), and lower rate of discontinuations caused by AEs (0.25 vs 0.98) when compared to the RCT’s placebo arm. The all-rifaximin group was not associated with increased mortality rates (0.15) compared to RCT’s placebo group (0.24)[125]. Again, concomitant lactulose use was standard with 89.8% of the all-rifaximin group receiving lactulose and only 10.2% receiving only rifaximin. A recent analysis of the above placebo group (n = 82) was done to better clarify the impact of crossing over from placebo to rifaximin 550 mg twice daily on breakthrough HE and hospitalization rates[126]. Significantly lower rates of HE were noted in the first 6 mo of rifaximin use compared to placebo with an estimated 79% reduction in the risk of breakthrough HE episodes and a NNT of 3[126]. However, no statistically significant reduction was observed between the two groups in HE related or all-cause hospitalization. Most common adverse events (AE), severe AEs and infection-related AEs were also similar[126].
Minimal hepatic encephalopathy
Minimal hepatic encephalopathy is a subtle subclinical form of HE with only mild cognitive and psychomotor impairment[127] without disorientation, asterixis, or other signs and symptoms of overt hepatic encephalopathy[120]. Minimal hepatic encephalopathy (MHE) occurs in up to 50% of patients with chronic liver disease and may predict the future development of OHE[120]. There are no current gold standards for diagnosing MHE and data concerning the role of rifaximin in the treatment or prevention of MHE are limited. Current AASLD/EASL guidelines do not routinely recommend treatment of MHE or Covert Hepatic Encephalopathy (CHE) with exceptions made on a case by case basis using approved treatments for OHE (Grade II-2, B,1)[120]. A RCT by Sharma et al[127] investigated the prevalence of MHE and the effect of rifaximin along with other routinely used drugs in reversing MHE compared to placebo. One hundred twenty four patients were enrolled, 31 of which received rifaximin 400 mg three times daily for 2 mo and were compared to participants receiving L-ornithine-L-aspartate (LOLA), Cap Velgut, and placebo. Critical Flicker Frequency (CFF) improved significantly with rifaximin, LOLA, and Cap Velgut compared to placebo with no significant difference in the improvement effect between the 3 drugs. This study suggests a beneficial role of drug therapy, including rifaximin, for the improvement of MHE as measured by CCF and neuropsychometric tests; however more studies with larger sample sizes are required to corroborate these results[127]. The RiMINI trial is a recently initiated RCT aimed at investigating the efficacy of standalone rifaximin (550 mg twice daily) vs rifaximin + lactulose (30-60 mL) for 3 mo on the improvement of MHE through neuropsychometric and neurophysiological changes in 60 patients suffering from liver cirrhosis and MHE[128].
Diverticular disease
Diverticular disease of the colon is very common in Western countries and contributes significantly to healthcare costs. Its prevalence is age-dependent, increasing from less than 20% at age 40 to 60% by age 60[129,130]. Most patients remain asymptomatic during their whole life. Diverticulitis, defined as inflammation/infection of the colonic diverticula, occurs in around 10% to 25% of people with diverticula[131] although this number has been more recently challenged by retrospective and prospective studies suggesting an incidence closer to 4%-5%[132,133].
The pathophysiology of colonic diverticulosis and the mechanism(s) leading to diverticulitis is(are) not clearly defined. It has been suggested that colonic diverticular disease may be related in part to a colonic microflora disorder[134]. This is supported by limited data showing differences in microflora composition between high and low risk populations[135,136]. Few randomized controlled trials have assessed the efficacy of rifaximin in the treatment of symptomatic uncomplicated diverticular disease and in the prevention of recurrence of diverticulitis. A meta-analysis by Bianchi et al[137] examined 4 prospective randomized trials involving 1660 patients that examined the long-term efficacy administration of rifaximin plus fiber supplementation vs fiber supplementation alone on symptoms and complications in patients with symptomatic uncomplicated diverticular disease. The pooled rate difference for symptom relief was 29.0% (rifaximin vs control; 95%CI: 24.5%-33.6%; P < 0.0001; NNT = 3) and the pooled rate difference for complications was -1.7% in favor of rifaximin (95%CI: -3.2% to -0.1%; P = 0.03; NNT = 59). When considering only acute diverticulitis as complication, the pooled rate difference in the rifaximin group was -2% (95%CI: -3.4% to -0.6%; P = 0.0057; NNT = 50).
Recently, Lanas et al[138] conducted a multicenter randomized open controlled study in patients with a recent episode of colonic diverticulitis, currently in remission. Patients received 3.5 g of high-fiber supplementation twice daily with or without one week per month of rifaximin (400 mg twice daily) for 12 mo. The primary endpoint was recurrence of diverticulitis. The study was underpowered and was interrupted after enrolling 165 patients because of inability to meet the anticipated recruitment target. Recurrences occurred in 10.4% of patients given rifaximin plus fiber vs 19.3% of patients receiving fiber alone[138]. Logistic regression adjusted for confounders showed a significant treatment effect (OR = 3.20; 95%CI: 1.16-8.82; P = 0.025). Patients with diverticulitis diagnosed greater than one year before and receiving rifaximin had a lower incidence of recurrences. There is some evidence to suggest that the combination of rifaximin with mesalamine is superior to rifaximin alone for improving severity of symptoms, bowel habits, and preventing recurrent diverticulitis[139].
Inflammatory bowel disease
The pathogenesis of inflammatory bowel disease (IBD) remains poorly understood. It is hypothesized that the gut microflora plays a role in the initiation and/or perpetuation of the process[140,141]. Antibiotics have a well-established role in the treatment of septic complications of IBD. Their benefit in the primary treatment of IBD is not well elucidated, however they are still commonly used in practice. Several trials have been carried out with metronidazole, ciprofloxacin, clofazimine, and other combinations. They appear to be useful in the treatment of Crohn’s disease (CD)[142-144], ulcerative colitis (UC)[145] and pouchitis; however, prolonged use of these antibiotics is associated with various systemic side effects. Based on observational data, rifaximin was associated with some improvement in IBD. Rifaximin reduces development and to promote healing of colitis in mice by reducing bacterial translocation[146]. Rifaximin may improve the existing dysbiosis in patients with CD by modulating colonic microbiota and increasing Bifidobacteria and Faecalbacterium prausnitzii[147]. Rifaximin may also exert anti-inflammatory activities by increasing expression of pregnane-X-receptor and antagonizing the effects of tumor necrosis factor-alpha (TNF-α) on epithelial cells in vitro[148,149].
CD
Due to its antibacterial and anti-inflammatory properties, rifaximin was assessed in the treatment of active CD. In an open-label trial, 29 patients with active CD received 200 mg of rifaximin TID for 16 weeks. Rifaximin reduced the CD activity index (CDAI) score by more than 40% and induced remission in 59% of cases[150]. Rifaximin, as adjunctive therapy for CD, was also shown to induce remission in up to 70% of cases[151]. These observational data were followed by controlled studies to investigate the role rifaximin in IBD. In a multicenter placebo controlled trial, 83 patients with mild-to-moderate CD were randomized to receive rifaximin 800 mg BID or placebo for 12 wk. Rifaximin for 12 wk was superior to placebo and induced clinical remission in 52% of cases compared with 33% in the placebo group[152]. More recently, a multicenter randomized trial compared patients with moderately active CD who received extended intestinal release rifaximin (EIR) to those given placebo. By 12 weeks of treatment, 62% of patients who received 800 mg of rifaximin-EIR were in remission compared to 43% of patients who received placebo (P = 0.005). After the follow-up period of 12 wk, this difference was maintained (45% in the rifaximin-EIR and 29% in the placebo group, P = 0.02)[153]. Despite the above clinical evidence, the role of rifaximin in CD is unclear. It is important to note that most studies did not provide information on inflammatory markers such as CRP or fecal calprotectin, or on endoscopic mucosal healing.
UC
The efficacy of rifaximin was assessed in a small group of patients with mild-to-moderate clinical flare of UC who were intolerant to steroids. Following the addition of rifaximin at a dose of 400 mg twice a day for 4 wk, clinical remission was achieved in two thirds of cases[154]. Gionchetti et al[155] randomized 28 patients with steroid refractory UC to rifaximin 400 mg BID or placebo for 10 d. In the treatment group, 64.3% of patients had clinical improvement with significant reduction in stool frequency (P < 0.02), rectal bleeding (P < 0.05) and sigmoidoscopic score (P < 0.05) compared with placebo.
Restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) is the preferred surgical treatment for patients with medically refractory UC. The most common long-term complication after this type of surgery is pouchitis characterized by an increased number of loose bowel movements, urgency, and abdominal cramping. Patients often develop an antibiotic-dependent form of pouchitis requiring long-term antibiotic therapy for maintenance of remission. The role of rifaximin for maintenance therapy of antibiotic-dependent pouchitis was assessed in 51 patients after IPAA for ulcerative colitis. Patients received a 2-wk course of various antibiotics for induction of remission. Patients in remission then began maintenance therapy with rifaximin 200 mg/d (to 1800 mg/d) for up to 24 mo. At 3 mo, remission was maintained in 65% of patients; 79% of these patients were still in remission at 6 mo, 58% at 12 mo and 6% at 24 mo[156].
Primary prophylaxis of spontaneous bacterial peritonitis
Spontaneous bacterial peritonitis (SBP) is defined as an ascitic fluid infection without an evident intra-abdominal surgically-treatable source[157]. It is a complication of advanced liver disease occurring in as many as 25% of cirrhotic patients[158]. One of the early steps in the development of SBP is a disturbance in gut flora with overgrowth and translocation of a specific organism, most commonly E. coli[159,160]. Cirrhosis predisposes to the development of bacterial overgrowth possibly because of altered small intestinal motility[161] and increased intestinal permeability[162]. Rifaximin could theoretically prevent SBP in patients with liver cirrhosis by reducing gut bacteria. One retrospective study on 404 patients with liver cirrhosis and large ascites classified patients into two groups based on the use of rifaximin. Patients who were on any other antibiotic prophylaxis for SBP and those who had an episode of SBP prior to the use of rifaximin were excluded. The median follow-up time was 4.2 mo. During this time period, 89% of patients on rifaximin remained SBP-free compared with 68% of those not on rifaximin (P = 0.002)[163]. Similar results were obtained in patients with HE taking rifaximin over a 6-mo period where it resulted in a more effective prophylaxis of SBP compared to norfloxacin[164]. Nonetheless, in a recent prospective study on a heterogeneous population of 152 liver cirrhosis patients, rifaximin pre-treatment did not reduce the chance of occurrence of SBP[165]. Whether rifaximin is appropriate for long-term primary prophylaxis of SBP in cirrhotic patients with ascites remains unclear.
Rifaximin may exert a positive effect on portal hemodynamics in patients with liver cirrhosis and portal hypertension, by correcting bacterial translocation and endotoxemia. Vlachogiannakos et al[166] have shown that the use of rifaximin for 4 wk in patients with decompensated alcoholic liver disease (ALD) leads to a significant decrease in hepatic venous pressure gradient (HVPG) correlating with reduction of plasma endotoxin levels. The same group of investigators studied the effect of long-term rifaximin administration in decompensated ALD patients who had shown a hemodynamic response to rifaximin and compared them to matched controls. Patients who received rifaximin had a significant lower risk of developing variceal bleeding (35% vs 59.5%, P = 0.01), HE (31.5% vs 47%, P = 0.03), SBP (4.5% vs 46%, P = 0.03), and hepatorenal syndrome (4.5% vs 51%, P = 0.04) than controls[167]. The five-year cumulative probability of survival was significantly higher in patients receiving rifaximin than controls (61% vs 13.5%, P = 0.01).
Clostridium difficile infection
Clostridium difficile (C. difficile) infection (CDI) is one of the most common healthcare-associated infections and causes significant morbidity and mortality especially among elderly hospitalized patients[168]. C. difficile is the causative organism of antibiotic-associated pseudomembranous colitis. Metronidazole and vancomycin are currently the standard of care of CDI; their dose and route of administration depends on the severity of the disease[169]. Small clinical trials have tested rifaximin in the treatment of CDI. One randomized study OF 20 patients who received rifaximin 600 mg/d or vancomycin 1 g/d for 10 d demonstrated that rifaximin is as effective as vancomycin for resolving diarrhea[170]. In another open-label trial, rifaximin 1200 mg/d for 10 d demonstrated a favorable safety profile and was an effective initial therapy for CDI in hospitalized patients. Furthermore, the success rate in this study (86%) was similar to rates reported in studies of vancomycin and metronidazole[171].
Small case series have suggested that sequential therapy with vancomycin followed by rifaximin may be effective for the prevention of recurrence of CDI[172,173]. More recently, one retrospective analysis reviewed 32 cases of recurrent CDI treated with rifaximin, after a course of metronidazole or vancomycin. A 2-wk course of rifaximin (400 mg twice daily) was found to be beneficial for patients with recurrent CDI, with 17 of the 32 patients (53%) responding favorably[174]. Rifaximin may be effective in breaking the cycle of predictable recurrences following other regimens used to treat CDI[172]. It has an excellent safety profile and has not been shown to be associated with the emergence of resistant strains of C. difficile[175].
DEVELOPMENT OF RESISTANCE
In a placebo controlled trial evaluating the development of resistant coliform bacterial strains following short term rifaximin use (200 mg three times daily vs 600 mg three times daily vs placebo) for 3 d, there was no change in the minimum inhibitory concentration required to inhibit growth of 90% of organisms (MIC90) between pre (day 0) and post treatment (day 3 and 5) stool samples, indicating no significant changes in susceptibility in patients treated with rifaximin on a short term basis[176]. As member of the rifamycin family of drugs, chronic use of rifaximin may be associated with selection of highly resistant and stable bacterial mutants in the intestine, primarily due to genetic alteration in bacterial DNA dependent RNA polymerase. Rifaximin shows a lower degree of resistance compared to rifampin possibly because of high gut concentrations and extremely low systemic absorption[12]. High-dose exposure to rifaximin, 8 x the minimum inhibitory concentration (MIC) resulted in low incidence of spontaneous resistant mutations amongst the different strains of aerobic and anaerobic gram positive and gram negative bacteria, ranging from 1 × 10-9 to 1.7 × 10-7[177].
The effect of intermittent high-dose rifaximin (1800 mg daily in 3 treatment periods of 10 d, each followed by 25 d of washout) on enteric bacteria (enterococci, coliforms, lactobacilli, bifidobacteria, Bacteroides spp., and Clostridium perfringens) was studied in patients with ulcerative colitis[178]. After each washout period, concentrations of the bacteria tested returned to initial values, suggesting that the administration of high doses of rifaximin does not significantly modify the colonic microflora. Rifaximin-resistant isolates were found, mostly in Bifidobacteria and have documented rapid disappearance of bacteria resistant to rifaximin from the intestinal tract upon treatment washout[179].
Real life data are also available from studies on susceptibility alterations of bacterial isolates causing TD from different geographic locations and over time[180,181]. Bacterial isolates from individuals with TD while visiting India, Mexico, Jamaica or Kenya in 1997 were challenged against different antimicrobial agents, of which rifaximin demonstrated an intermediate activity with MIC50 of 16 μg/mL and MIC90 of 32 mg/L[180]. Around 10 years later, reevaluation of susceptibility changes in Mexico, India and Guatemala between 2006 and 2008 demonstrated no change in the MIC of isolates to rifaximin while other antimicrobial agents (e.g., fluoroquinolones, cephalosporins, azithromycin) had a significant increase in their MIC levels compared to bacterial isolates from a decade earlier[181].
Based on the above, rifaximin use appears to be associated with a low incidence of development or persistence of spontaneous bacterial mutants. Moreover, the development of important drug resistance among extra-intestinal flora during rifaximin therapy is unlikely because of minimal systemic absorption and limited cross-resistance of rifaximin with other antimicrobials.
CONCLUSION
Rifaximin is a broad spectrum poorly absorbed oral antibiotic with proven efficacy in a number of gastrointestinal and liver conditions (Table 1). In addition, there is emerging evidence for a possible beneficial role of rifaximin in other conditions such as diverticular disease, decompensated cirrhosis, inflammatory bowel disease and C. difficile infection. The extremely low systemic absorption, excellent safety profile, and limited cross-resistance are distinct advantages of this drug. Appropriate dosing for the proper indication is important to improve outcomes and limit potential for abuse and development of resistance.
Table 1.
Proposed treatment regimen(s) with rifaximin by indication
| Disorder | Evidence of beneficial effect | Recommended regimen | Ref. |
| IBS | Randomized controlled multicenter studies show improvement in global IBS symptoms | 400-550 mg three times daily for 2 wk May require intermittent retreatment | [87,88] |
| Treatment of TD | Randomized controlled trials show reduced duration of the illness | 200 mg three times daily for 3 d | [94,95] |
| Prevention of TD | Randomized controlled trials in patients traveling to south and southeast Asia; and Mexico | 200 mg twice daily or 600 mg daily while in high risk area | [97,98] |
| SIBO | Rifaximin normalizes the hydrogen breath test and improves symptoms | 400 mg three times daily for 2 wk May require retreatment | [99-101] |
| Hepatic encephalopathy | Randomized controlled trials, proved efficacy and safety | 550 mg 2 or 3 times daily chronically | [104-106] |
| Diverticular disease | Randomized controlled trials showed that rifaximin improves symptoms and prevents recurrence | 400 mg twice daily for 7 d every month | [110-115] |
| IBD and pouchitis | Observational data and small pilot studies Retrospective study | 400-800 mg twice daily for 12 wk May require retreatment or intermittent treatment 400 mg three times daily chronically | [125-129] [136] |
| SBP prophylaxis | |||
| Recurrent Clostridium difficile infection | Small case series and retrospective studies | 400 mg twice daily for 2 wk | [140-142] |
IBS: Irritable bowel syndrome; TD: Traveler’s diarrhea; SIBO: Small intestinal bacterial overgrowth; IBD: Inflammatory bowel disease; SBP: Spontaneous bacterial peritonitis.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Lebanon
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: None to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 19, 2016
First decision: May 27, 2016
Article in press: July 6, 2016
P- Reviewer: Caboclo GLF, Goetze TO, Perse M S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Scarpignato C, Pelosini I. Experimental and clinical pharmacology of rifaximin, a gastrointestinal selective antibiotic. Digestion. 2006;73 Suppl 1:13–27. doi: 10.1159/000089776. [DOI] [PubMed] [Google Scholar]
- 2.Baker DE. Rifaximin: a nonabsorbed oral antibiotic. Rev Gastroenterol Disord. 2005;5:19–30. [PubMed] [Google Scholar]
- 3.DuPont HL. Review article: the antimicrobial effects of rifaximin on the gut microbiota. Aliment Pharmacol Ther. 2016;43 Suppl 1:3–10. doi: 10.1111/apt.13434. [DOI] [PubMed] [Google Scholar]
- 4.Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy. 2005;51 Suppl 1:36–66. doi: 10.1159/000081990. [DOI] [PubMed] [Google Scholar]
- 5.Descombe JJ, Dubourg D, Picard M, Palazzini E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res. 1994;14:51–56. [PubMed] [Google Scholar]
- 6.Schoenfeld P, Pimentel M, Chang L, Lembo A, Chey WD, Yu J, Paterson C, Bortey E, Forbes WP. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment Pharmacol Ther. 2014;39:1161–1168. doi: 10.1111/apt.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang ZD, Ke S, Palazzini E, Riopel L, Dupont H. In vitro activity and fecal concentration of rifaximin after oral administration. Antimicrob Agents Chemother. 2000;44:2205–2206. doi: 10.1128/aac.44.8.2205-2206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trapnell CB, Connolly M, Pentikis H, Forbes WP, Bettenhausen DK. Absence of effect of oral rifaximin on the pharmacokinetics of ethinyl estradiol/norgestimate in healthy females. Ann Pharmacother. 2007;41:222–228. doi: 10.1345/aph.1H395. [DOI] [PubMed] [Google Scholar]
- 9.Robins GW, Wellington K. Rifaximin: a review of its use in the management of traveller’s diarrhoea. Drugs. 2005;65:1697–1713. doi: 10.2165/00003495-200565120-00011. [DOI] [PubMed] [Google Scholar]
- 10.Darkoh C, Lichtenberger LM, Ajami N, Dial EJ, Jiang ZD, DuPont HL. Bile acids improve the antimicrobial effect of rifaximin. Antimicrob Agents Chemother. 2010;54:3618–3624. doi: 10.1128/AAC.00161-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang ZD, DuPont HL. Rifaximin: in vitro and in vivo antibacterial activity--a review. Chemotherapy. 2005;51 Suppl 1:67–72. doi: 10.1159/000081991. [DOI] [PubMed] [Google Scholar]
- 12.DuPont HL. Biologic properties and clinical uses of rifaximin. Expert Opin Pharmacother. 2011;12:293–302. doi: 10.1517/14656566.2011.546347. [DOI] [PubMed] [Google Scholar]
- 13.Schrodt C, McHugh EE, Gawinowicz MA, Dupont HL, Brown EL. Rifaximin-mediated changes to the epithelial cell proteome: 2-D gel analysis. PLoS One. 2013;8:e68550. doi: 10.1371/journal.pone.0068550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 15.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 16.Simrén M, Abrahamsson H, Svedlund J, Björnsson ES. Quality of life in patients with irritable bowel syndrome seen in referral centers versus primary care: the impact of gender and predominant bowel pattern. Scand J Gastroenterol. 2001;36:545–552. doi: 10.1080/003655201750153476. [DOI] [PubMed] [Google Scholar]
- 17.Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, Groves D, Ofman JJ. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care. 2005;11:S17–S26. [PubMed] [Google Scholar]
- 18.Hungin AP, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21:1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 19.Longstreth GF. Irritable bowel syndrome: a multibillion-dollar problem. Gastroenterology. 1995;109:2029–2031. doi: 10.1016/0016-5085(95)90773-4. [DOI] [PubMed] [Google Scholar]
- 20.Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology. 1995;109:1736–1741. doi: 10.1016/0016-5085(95)90738-6. [DOI] [PubMed] [Google Scholar]
- 21.Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med. 2016;9:7–17. doi: 10.2147/IJGM.S93698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simrén M, Castedal M, Svedlund J, Abrahamsson H, Björnsson E. Abnormal propagation pattern of duodenal pressure waves in the irritable bowel syndrome (IBS) [correction of (IBD)] Dig Dis Sci. 2000;45:2151–2161. doi: 10.1023/a:1010770302403. [DOI] [PubMed] [Google Scholar]
- 23.Kumar D, Wingate DL. The irritable bowel syndrome: a paroxysmal motor disorder. Lancet. 1985;2:973–977. doi: 10.1016/s0140-6736(85)90525-2. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt T, Hackelsberger N, Widmer R, Meisel C, Pfeiffer A, Kaess H. Ambulatory 24-hour jejunal motility in diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol. 1996;31:581–589. doi: 10.3109/00365529609009131. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal A, Houghton LA, Reilly B, Morris J, Whorwell PJ. Bloating and distension in irritable bowel syndrome: the role of gastrointestinal transit. Am J Gastroenterol. 2009;104:1998–2004. doi: 10.1038/ajg.2009.251. [DOI] [PubMed] [Google Scholar]
- 26.Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499–1506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 27.Caldarella MP, Serra J, Azpiroz F, Malagelada JR. Prokinetic effects in patients with intestinal gas retention. Gastroenterology. 2002;122:1748–1755. doi: 10.1053/gast.2002.33658. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 29.Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 30.Zuo XL, Li YQ, Shi L, Lv GP, Kuang RG, Lu XF, Li JM, Desmond PV. Visceral hypersensitivity following cold water intake in subjects with irritable bowel syndrome. J Gastroenterol. 2006;41:311–317. doi: 10.1007/s00535-005-1766-x. [DOI] [PubMed] [Google Scholar]
- 31.Nozu T, Kudaira M, Kitamori S, Uehara A. Repetitive rectal painful distention induces rectal hypersensitivity in patients with irritable bowel syndrome. J Gastroenterol. 2006;41:217–222. doi: 10.1007/s00535-005-1748-z. [DOI] [PubMed] [Google Scholar]
- 32.Cook IJ, van Eeden A, Collins SM. Patients with irritable bowel syndrome have greater pain tolerance than normal subjects. Gastroenterology. 1987;93:727–733. doi: 10.1016/0016-5085(87)90434-3. [DOI] [PubMed] [Google Scholar]
- 33.Iovino P, Tremolaterra F, Consalvo D, Sabbatini F, Mazzacca G, Ciacci C. Perception of electrocutaneous stimuli in irritable bowel syndrome. Am J Gastroenterol. 2006;101:596–603. doi: 10.1111/j.1572-0241.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:3699–3704. doi: 10.3748/wjg.v13.i27.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawal A, Kern M, Sidhu H, Hofmann C, Shaker R. Novel evidence for hypersensitivity of visceral sensory neural circuitry in irritable bowel syndrome patients. Gastroenterology. 2006;130:26–33. doi: 10.1053/j.gastro.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Dorn SD, Palsson OS, Thiwan SI, Kanazawa M, Clark WC, van Tilburg MA, Drossman DA, Scarlett Y, Levy RL, Ringel Y, et al. Increased colonic pain sensitivity in irritable bowel syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. Gut. 2007;56:1202–1209. doi: 10.1136/gut.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houghton LA, Lea R, Agrawal A, Reilly B, Whorwell PJ. Relationship of abdominal bloating to distention in irritable bowel syndrome and effect of bowel habit. Gastroenterology. 2006;131:1003–1010. doi: 10.1053/j.gastro.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Lasser RB, Bond JH, Levitt MD. The role of intestinal gas in functional abdominal pain. N Engl J Med. 1975;293:524–526. doi: 10.1056/NEJM197509112931103. [DOI] [PubMed] [Google Scholar]
- 39.Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14–19. doi: 10.1136/gut.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serra J, Salvioli B, Azpiroz F, Malagelada JR. Lipid-induced intestinal gas retention in irritable bowel syndrome. Gastroenterology. 2002;123:700–706. doi: 10.1053/gast.2002.35394. [DOI] [PubMed] [Google Scholar]
- 41.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome-- etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60:667–672. doi: 10.1038/sj.ejcn.1602367. [DOI] [PubMed] [Google Scholar]
- 42.Simrén M, Månsson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, Björnsson ES. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 43.Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459–1464. doi: 10.1136/gut.2003.037697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zar S, Mincher L, Benson MJ, Kumar D. Food-specific IgG4 antibody-guided exclusion diet improves symptoms and rectal compliance in irritable bowel syndrome. Scand J Gastroenterol. 2005;40:800–807. doi: 10.1080/00365520510015593. [DOI] [PubMed] [Google Scholar]
- 45.Jun DW, Lee OY, Yoon HJ, Lee SH, Lee HL, Choi HS, Yoon BC, Lee MH, Lee DH, Cho SH. Food intolerance and skin prick test in treated and untreated irritable bowel syndrome. World J Gastroenterol. 2006;12:2382–2387. doi: 10.3748/wjg.v12.i15.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zwetchkenbaum J, Burakoff R. The irritable bowel syndrome and food hypersensitivity. Ann Allergy. 1988;61:47–49. [PubMed] [Google Scholar]
- 47.Gibson PR, Shepherd SJ. Personal view: food for thought--western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol Ther. 2005;21:1399–1409. doi: 10.1111/j.1365-2036.2005.02506.x. [DOI] [PubMed] [Google Scholar]
- 48.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 49.Choi YK, Johlin FC, Summers RW, Jackson M, Rao SS. Fructose intolerance: an under-recognized problem. Am J Gastroenterol. 2003;98:1348–1353. doi: 10.1111/j.1572-0241.2003.07476.x. [DOI] [PubMed] [Google Scholar]
- 50.Austin GL, Dalton CB, Hu Y, Morris CB, Hankins J, Weinland SR, Westman EC, Yancy WS, Drossman DA. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:706–708.e1. doi: 10.1016/j.cgh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelis GF, Vermeeren MA, Jansen W. Role of fructose-sorbitol malabsorption in the irritable bowel syndrome. Gastroenterology. 1990;99:1016–1020. doi: 10.1016/0016-5085(90)90621-7. [DOI] [PubMed] [Google Scholar]
- 52.Fernández-Bañares F, Esteve-Pardo M, de Leon R, Humbert P, Cabré E, Llovet JM, Gassull MA. Sugar malabsorption in functional bowel disease: clinical implications. Am J Gastroenterol. 1993;88:2044–2050. [PubMed] [Google Scholar]
- 53.Vesa TH, Seppo LM, Marteau PR, Sahi T, Korpela R. Role of irritable bowel syndrome in subjective lactose intolerance. Am J Clin Nutr. 1998;67:710–715. doi: 10.1093/ajcn/67.4.710. [DOI] [PubMed] [Google Scholar]
- 54.Sanders DS, Carter MJ, Hurlstone DP, Pearce A, Ward AM, McAlindon ME, Lobo AJ. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504–1508. doi: 10.1016/S0140-6736(01)06581-3. [DOI] [PubMed] [Google Scholar]
- 55.Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–1594. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, Carlson P, Lamsam J, Janzow D, Eckert D, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903–911.e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saito YA, Petersen GM, Locke GR, Talley NJ. The genetics of irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:1057–1065. doi: 10.1016/s1542-3565(05)00184-9. [DOI] [PubMed] [Google Scholar]
- 58.Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 59.Lembo A, Zaman M, Jones M, Talley NJ. Influence of genetics on irritable bowel syndrome, gastro-oesophageal reflux and dyspepsia: a twin study. Aliment Pharmacol Ther. 2007;25:1343–1350. doi: 10.1111/j.1365-2036.2007.03326.x. [DOI] [PubMed] [Google Scholar]
- 60.Yeo A, Boyd P, Lumsden S, Saunders T, Handley A, Stubbins M, Knaggs A, Asquith S, Taylor I, Bahari B, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–1458. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 62.Locke GR, Weaver AL, Melton LJ, Talley NJ. Psychosocial factors are linked to functional gastrointestinal disorders: a population based nested case-control study. Am J Gastroenterol. 2004;99:350–357. doi: 10.1111/j.1572-0241.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- 63.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solmaz M, Kavuk I, Sayar K. Psychological factors in the irritable bowel syndrome. Eur J Med Res. 2003;8:549–556. [PubMed] [Google Scholar]
- 65.Nicholl BI, Halder SL, Macfarlane GJ, Thompson DG, O’Brien S, Musleh M, McBeth J. Psychosocial risk markers for new onset irritable bowel syndrome--results of a large prospective population-based study. Pain. 2008;137:147–155. doi: 10.1016/j.pain.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drossman DA, Leserman J, Nachman G, Li ZM, Gluck H, Toomey TC, Mitchell CM. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–833. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 67.Talley NJ, Boyce PM, Jones M. Is the association between irritable bowel syndrome and abuse explained by neuroticism? A population based study. Gut. 1998;42:47–53. doi: 10.1136/gut.42.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koloski NA, Talley NJ, Boyce PM. A history of abuse in community subjects with irritable bowel syndrome and functional dyspepsia: the role of other psychosocial variables. Digestion. 2005;72:86–96. doi: 10.1159/000087722. [DOI] [PubMed] [Google Scholar]
- 69.Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 72.Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972–1979. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]
- 73.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 74.Guilarte M, Santos J, de Torres I, Alonso C, Vicario M, Ramos L, Martínez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 76.Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 77.Bueno L. Protease activated receptor 2: a new target for IBS treatment. Eur Rev Med Pharmacol Sci. 2008;12 Suppl 1:95–102. [PubMed] [Google Scholar]
- 78.Gecse K, Róka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztóczy A, Izbéki F, Fioramonti J, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 79.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM; Walkerton Health Study Investigators. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445–450; quiz 660. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 81.Zanini B, Ricci C, Bandera F, Caselani F, Magni A, Laronga AM, Lanzini A; San Felice del Benaco Study I. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol. 2012;107:891–899. doi: 10.1038/ajg.2012.102. [DOI] [PubMed] [Google Scholar]
- 82.Dizdar V, Gilja OH, Hausken T. Increased visceral sensitivity in Giardia-induced postinfectious irritable bowel syndrome and functional dyspepsia. Effect of the 5HT3-antagonist ondansetron. Neurogastroenterol Motil. 2007;19:977–982. doi: 10.1111/j.1365-2982.2007.00988.x. [DOI] [PubMed] [Google Scholar]
- 83.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 84.Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213–222. doi: 10.1016/j.femsim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 85.Codling C, O’Mahony L, Shanahan F, Quigley EM, Marchesi JR. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci. 2010;55:392–397. doi: 10.1007/s10620-009-0934-x. [DOI] [PubMed] [Google Scholar]
- 86.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shah ED, Basseri RJ, Chong K, Pimentel M. Abnormal breath testing in IBS: a meta-analysis. Dig Dis Sci. 2010;55:2441–2449. doi: 10.1007/s10620-010-1276-4. [DOI] [PubMed] [Google Scholar]
- 89.Ford AC, Spiegel BM, Talley NJ, Moayyedi P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279–1286. doi: 10.1016/j.cgh.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 90.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–333. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 91.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 92.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 93.Lembo A, Pimentel M, Rao SS. Efficacy and safety of repeat treatment with rifaximin for diarrhea-predominant irritable bowel syndrome (IBS-D): results of the TARGET 3 study. Presented at: American College of Gastroenterology (ACG); 2014. pp. Annual Scientific Meeting; October 17–22; Philadelphia, PA, 2014. [Google Scholar]
- 94.Meyrat P, Safroneeva E, Schoepfer AM. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment Pharmacol Ther. 2012;36:1084–1093. doi: 10.1111/apt.12087. [DOI] [PubMed] [Google Scholar]
- 95.Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28–35; quiz 36. doi: 10.1038/ajg.2011.355. [DOI] [PubMed] [Google Scholar]
- 96.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 97.Bae S, Lee KJ, Kim YS, Kim KN. Determination of rifaximin treatment period according to lactulose breath test values in nonconstipated irritable bowel syndrome subjects. J Korean Med Sci. 2015;30:757–762. doi: 10.3346/jkms.2015.30.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837–841. doi: 10.1111/j.1572-0241.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- 99.Davidson GP, Robb TA, Kirubakaran CP. Bacterial contamination of the small intestine as an important cause of chronic diarrhea and abdominal pain: diagnosis by breath hydrogen test. Pediatrics. 1984;74:229–235. [PubMed] [Google Scholar]
- 100.Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48:86–92. doi: 10.1023/a:1021738515885. [DOI] [PubMed] [Google Scholar]
- 101.Gupta A, Chey WD. Breath Testing for Small Intestinal Bacterial Overgrowth: A Means to Enrich Rifaximin Responders in IBS Patients? Am J Gastroenterol. 2016;111:305–306. doi: 10.1038/ajg.2016.32. [DOI] [PubMed] [Google Scholar]
- 102.DuPont HL, Capsuto EG. Persistent diarrhea in travelers. Clin Infect Dis. 1996;22:124–128. doi: 10.1093/clinids/22.1.124. [DOI] [PubMed] [Google Scholar]
- 103.DuPont HL, Ericsson CD. Prevention and treatment of traveler’s diarrhea. N Engl J Med. 1993;328:1821–1827. doi: 10.1056/NEJM199306243282507. [DOI] [PubMed] [Google Scholar]
- 104.Steffen R. Epidemiologic studies of travelers’ diarrhea, severe gastrointestinal infections, and cholera. Rev Infect Dis. 1986;8 Suppl 2:S122–S130. doi: 10.1093/clinids/8.supplement_2.s122. [DOI] [PubMed] [Google Scholar]
- 105.Taylor DN, Bourgeois AL, Ericsson CD, Steffen R, Jiang ZD, Halpern J, Haake R, Dupont HL. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg. 2006;74:1060–1066. [PubMed] [Google Scholar]
- 106.DuPont HL, Jiang ZD, Ericsson CD, Adachi JA, Mathewson JJ, DuPont MW, Palazzini E, Riopel LM, Ashley D, Martinez-Sandoval F. Rifaximin versus ciprofloxacin for the treatment of traveler’s diarrhea: a randomized, double-blind clinical trial. Clin Infect Dis. 2001;33:1807–1815. doi: 10.1086/323814. [DOI] [PubMed] [Google Scholar]
- 107.Dupont HL, Jiang ZD, Belkind-Gerson J, Okhuysen PC, Ericsson CD, Ke S, Huang DB, Dupont MW, Adachi JA, De La Cabada FJ, et al. Treatment of travelers’ diarrhea: randomized trial comparing rifaximin, rifaximin plus loperamide, and loperamide alone. Clin Gastroenterol Hepatol. 2007;5:451–456. doi: 10.1016/j.cgh.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 108.DuPont HL, Ericsson CD, Farthing MJ, Gorbach S, Pickering LK, Rombo L, Steffen R, Weinke T. Expert review of the evidence base for self-therapy of travelers’ diarrhea. J Travel Med. 2009;16:161–171. doi: 10.1111/j.1708-8305.2009.00300.x. [DOI] [PubMed] [Google Scholar]
- 109.Zanger P, Nurjadi D, Gabor J, Gaile M, Kremsner PG. Effectiveness of rifaximin in prevention of diarrhoea in individuals travelling to south and southeast Asia: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2013;13:946–954. doi: 10.1016/S1473-3099(13)70221-4. [DOI] [PubMed] [Google Scholar]
- 110.Armstrong AW, Ulukan S, Weiner M, Mostafa M, Shaheen H, Nakhla I, Tribble DR, Riddle MS. A randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of rifaximin for the prevention of travelers’ diarrhea in US military personnel deployed to Incirlik Air Base, Incirlik, Turkey. J Travel Med. 2010;17:392–394. doi: 10.1111/j.1708-8305.2010.00462.x. [DOI] [PubMed] [Google Scholar]
- 111.Flores J, Dupont HL, Jiang ZD, Okhuysen PC, Melendez-Romero JH, Gonzalez-Estrada A, Carrillo I, Paredes M. A randomized, double-blind, pilot study of rifaximin 550 mg versus placebo in the prevention of travelers’ diarrhea in Mexico during the dry season. J Travel Med. 2011;18:333–336. doi: 10.1111/j.1708-8305.2011.00549.x. [DOI] [PubMed] [Google Scholar]
- 112.Martinez-Sandoval F, Ericsson CD, Jiang ZD, Okhuysen PC, Romero JH, Hernandez N, Forbes WP, Shaw A, Bortey E, DuPont HL. Prevention of travelers’ diarrhea with rifaximin in US travelers to Mexico. J Travel Med. 2010;17:111–117. doi: 10.1111/j.1708-8305.2009.00385.x. [DOI] [PubMed] [Google Scholar]
- 113.Peralta S, Cottone C, Doveri T, Almasio PL, Craxi A. Small intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: experience with Rifaximin. World J Gastroenterol. 2009;15:2628–2631. doi: 10.3748/wjg.15.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cuoco L, Salvagnini M. Small intestine bacterial overgrowth in irritable bowel syndrome: a retrospective study with rifaximin. Minerva Gastroenterol Dietol. 2006;52:89–95. [PubMed] [Google Scholar]
- 115.Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Novi M, Sottili S, Vitale G, Cesario V, Serricchio M, Cammarota G, et al. Small intestinal bacterial overgrowth recurrence after antibiotic therapy. Am J Gastroenterol. 2008;103:2031–2035. doi: 10.1111/j.1572-0241.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- 116.Lauritano EC, Gabrielli M, Lupascu A, Santoliquido A, Nucera G, Scarpellini E, Vincenti F, Cammarota G, Flore R, Pola P, et al. Rifaximin dose-finding study for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;22:31–35. doi: 10.1111/j.1365-2036.2005.02516.x. [DOI] [PubMed] [Google Scholar]
- 117.Scarpellini E, Gabrielli M, Lauritano CE, Lupascu A, Merra G, Cammarota G, Cazzato IA, Gasbarrini G, Gasbarrini A. High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2007;25:781–786. doi: 10.1111/j.1365-2036.2007.03259.x. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y, Feng Y, Cao B, Tian Q. Effects of SIBO and rifaximin therapy on MHE caused by hepatic cirrhosis. Int J Clin Exp Med. 2015;8:2954–2957. [PMC free article] [PubMed] [Google Scholar]
- 119.Boltin D, Perets TT, Shporn E, Aizic S, Levy S, Niv Y, Dickman R. Rifaximin for small intestinal bacterial overgrowth in patients without irritable bowel syndrome. Ann Clin Microbiol Antimicrob. 2014;13:49. doi: 10.1186/s12941-014-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 121.Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458–1463. doi: 10.1038/ajg.2013.219. [DOI] [PubMed] [Google Scholar]
- 122.Kowdley KV, Burman BE. ACP Journal Club. Adding rifaximin to lactulose increased reversal and decreased mortality in hepatic encephalopathy. Ann Intern Med. 2013;159:JC8. doi: 10.7326/0003-4819-159-8-201310150-02008. [DOI] [PubMed] [Google Scholar]
- 123.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 124.Sanyal A, Younossi ZM, Bass NM, Mullen KD, Poordad F, Brown RS, Vemuru RP, Mazen Jamal M, Huang S, Merchant K, et al. Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy - a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34:853–861. doi: 10.1111/j.1365-2036.2011.04808.x. [DOI] [PubMed] [Google Scholar]
- 125.Mullen KD, Sanyal AJ, Bass NM, Poordad FF, Sheikh MY, Frederick RT, Bortey E, Forbes WP. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2014;12:1390–7.e2. doi: 10.1016/j.cgh.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 126.Bajaj JS, Barrett AC, Bortey E, Paterson C, Forbes WP. Prolonged remission from hepatic encephalopathy with rifaximin: results of a placebo crossover analysis. Aliment Pharmacol Ther. 2015;41:39–45. doi: 10.1111/apt.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharma K, Pant S, Misra S, Dwivedi M, Misra A, Narang S, Tewari R, Bhadoria AS. Effect of rifaximin, probiotics, and l-ornithine l-aspartate on minimal hepatic encephalopathy: a randomized controlled trial. Saudi J Gastroenterol. 2014;20:225–232. doi: 10.4103/1319-3767.136975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schulz C, Schütte K, Kropf S, Schmitt FC, Vasapolli R, Kliegis LM, Riegger A, Malfertheiner P. RiMINI - the influence of rifaximin on minimal hepatic encephalopathy (MHE) and on the intestinal microbiome in patients with liver cirrhosis: study protocol for a randomized controlled trial. Trials. 2016;17:111. doi: 10.1186/s13063-016-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Painter NS, Burkitt DP. Diverticular disease of the colon, a 20th century problem. Clin Gastroenterol. 1975;4:3–21. [PubMed] [Google Scholar]
- 130.Peery AF, Barrett PR, Park D, Rogers AJ, Galanko JA, Martin CF, Sandler RS. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142:266–72.e1. doi: 10.1053/j.gastro.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parks TG. Natural history of diverticular disease of the colon. Clin Gastroenterol. 1975;4:53–69. [PubMed] [Google Scholar]
- 132.Shahedi K, Fuller G, Bolus R, Cohen E, Vu M, Shah R, Agarwal N, Kaneshiro M, Atia M, Sheen V, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol. 2013;11:1609–1613. doi: 10.1016/j.cgh.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shayto R, Hanna K, Ziyadeh N, Chehab H, Chalhoub JM, Harb AH, Sarkis FS, Soweid A, Barada K, Mourad F, et al. The Natural History of Incidental Colonic Diverticulosis on Screening Colonoscopy: A Prospective Cohort Study. May 22-24; 2016; San Diego, CA. Presented at: Digestive Disease Week (DDW); 2016. p. Gastroenterology, 2016: 1411. [Google Scholar]
- 134.Commane DM, Arasaradnam RP, Mills S, Mathers JC, Bradburn M. Diet, ageing and genetic factors in the pathogenesis of diverticular disease. World J Gastroenterol. 2009;15:2479–2488. doi: 10.3748/wjg.15.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Finegold SM, Attebery HR, Sutter VL. Effect of diet on human fecal flora: comparison of Japanese and American diets. Am J Clin Nutr. 1974;27:1456–1469. doi: 10.1093/ajcn/27.12.1456. [DOI] [PubMed] [Google Scholar]
- 136.Segal I, Walker AR, Wadee A. Persistent low prevalence of Western digestive diseases in Africa: confounding aetiological factors. Gut. 2001;48:730–732. doi: 10.1136/gut.48.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bianchi M, Festa V, Moretti A, Ciaco A, Mangone M, Tornatore V, Dezi A, Luchetti R, De Pascalis B, Papi C, et al. Meta-analysis: long-term therapy with rifaximin in the management of uncomplicated diverticular disease. Aliment Pharmacol Ther. 2011;33:902–910. doi: 10.1111/j.1365-2036.2011.04606.x. [DOI] [PubMed] [Google Scholar]
- 138.Lanas A, Ponce J, Bignamini A, Mearin F. One year intermittent rifaximin plus fibre supplementation vs. fibre supplementation alone to prevent diverticulitis recurrence: a proof-of-concept study. Dig Liver Dis. 2013;45:104–109. doi: 10.1016/j.dld.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 139.Trivedi CD, Das KM. Emerging therapies for diverticular disease of the colon. J Clin Gastroenterol. 2008;42:1145–1151. doi: 10.1097/MCG.0b013e318188adc1. [DOI] [PubMed] [Google Scholar]
- 140.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 141.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 142.Feller M, Huwiler K, Schoepfer A, Shang A, Furrer H, Egger M. Long-term antibiotic treatment for Crohn’s disease: systematic review and meta-analysis of placebo-controlled trials. Clin Infect Dis. 2010;50:473–480. doi: 10.1086/649923. [DOI] [PubMed] [Google Scholar]
- 143.Nikfar S, Mirfazaelian H, Abdollahi M. Efficacy and tolerability of immunoregulators and antibiotics in fistulizing Crohn’s disease: a systematic review and meta-analysis of placebo-controlled trials. Curr Pharm Des. 2010;16:3684–3698. doi: 10.2174/138161210794079236. [DOI] [PubMed] [Google Scholar]
- 144.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. A meta-analysis of broad-spectrum antibiotic therapy in patients with active Crohn’s disease. Clin Ther. 2006;28:1983–1988. doi: 10.1016/j.clinthera.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 145.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. A meta-analysis of antibiotic therapy for active ulcerative colitis. Dig Dis Sci. 2007;52:2920–2925. doi: 10.1007/s10620-007-9760-1. [DOI] [PubMed] [Google Scholar]
- 146.Fiorucci S, Distrutti E, Mencarelli A, Barbanti M, Palazzini E, Morelli A. Inhibition of intestinal bacterial translocation with rifaximin modulates lamina propria monocytic cells reactivity and protects against inflammation in a rodent model of colitis. Digestion. 2002;66:246–256. doi: 10.1159/000068362. [DOI] [PubMed] [Google Scholar]
- 147.Maccaferri S, Vitali B, Klinder A, Kolida S, Ndagijimana M, Laghi L, Calanni F, Brigidi P, Gibson GR, Costabile A. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother. 2010;65:2556–2565. doi: 10.1093/jac/dkq345. [DOI] [PubMed] [Google Scholar]
- 148.Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, Krausz KW, Gonzalez FJ. Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther. 2010;335:32–41. doi: 10.1124/jpet.110.170225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mencarelli A, Migliorati M, Barbanti M, Cipriani S, Palladino G, Distrutti E, Renga B, Fiorucci S. Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol. 2010;80:1700–1707. doi: 10.1016/j.bcp.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 150.Shafran I, Johnson LK. An open-label evaluation of rifaximin in the treatment of active Crohn’s disease. Curr Med Res Opin. 2005;21:1165–1169. doi: 10.1185/030079905x53252. [DOI] [PubMed] [Google Scholar]
- 151.Shafran I, Burgunder P. Adjunctive antibiotic therapy with rifaximin may help reduce Crohn’s disease activity. Dig Dis Sci. 2010;55:1079–1084. doi: 10.1007/s10620-009-1111-y. [DOI] [PubMed] [Google Scholar]
- 152.Prantera C, Lochs H, Campieri M, Scribano ML, Sturniolo GC, Castiglione F, Cottone M. Antibiotic treatment of Crohn’s disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther. 2006;23:1117–1125. doi: 10.1111/j.1365-2036.2006.02879.x. [DOI] [PubMed] [Google Scholar]
- 153.Prantera C, Lochs H, Grimaldi M, Danese S, Scribano ML, Gionchetti P. Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn’s disease. Gastroenterology. 2012;142:473–481.e4. doi: 10.1053/j.gastro.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 154.Guslandi M, Petrone MC, Testoni PA. Rifaximin for active ulcerative colitis. Inflamm Bowel Dis. 2006;12:335. doi: 10.1097/01.MIB.0000215092.85116.6c. [DOI] [PubMed] [Google Scholar]
- 155.Gionchetti P, Rizzello F, Ferrieri A, Venturi A, Brignola C, Ferretti M, Peruzzo S, Miglioli M, Campieri M. Rifaximin in patients with moderate or severe ulcerative colitis refractory to steroid-treatment: a double-blind, placebo-controlled trial. Dig Dis Sci. 1999;44:1220–1221. doi: 10.1023/a:1026648812439. [DOI] [PubMed] [Google Scholar]
- 156.Shen B, Remzi FH, Lopez AR, Queener E. Rifaximin for maintenance therapy in antibiotic-dependent pouchitis. BMC Gastroenterol. 2008;8:26. doi: 10.1186/1471-230X-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Conn HO. Spontaneous peritonitis and bacteremia in laennec’s cirrhosis caused by enteric organisms. A RELATIVELY COMMON BUT RARELY RECOGNIZED SYNDROME. Ann Intern Med. 1964;60:568–580. doi: 10.7326/0003-4819-60-4-568. [DOI] [PubMed] [Google Scholar]
- 158.Navasa M, Follo A, Llovet JM, Clemente G, Vargas V, Rimola A, Marco F, Guarner C, Forné M, Planas R, et al. Randomized, comparative study of oral ofloxacin versus intravenous cefotaxime in spontaneous bacterial peritonitis. Gastroenterology. 1996;111:1011–1017. doi: 10.1016/s0016-5085(96)70069-0. [DOI] [PubMed] [Google Scholar]
- 159.Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997;26:1372–1378. doi: 10.1016/s0168-8278(97)80474-6. [DOI] [PubMed] [Google Scholar]
- 160.Runyon BA, Squier S, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol. 1994;21:792–796. doi: 10.1016/s0168-8278(94)80241-6. [DOI] [PubMed] [Google Scholar]
- 161.Madrid AM, Cumsille F, Defilippi C. Altered small bowel motility in patients with liver cirrhosis depends on severity of liver disease. Dig Dis Sci. 1997;42:738–742. doi: 10.1023/a:1018899611006. [DOI] [PubMed] [Google Scholar]
- 162.Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, Dal Lago A, Ojetti V, Ainora ME, Santoro M, et al. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323–327. doi: 10.1038/ajg.2009.558. [DOI] [PubMed] [Google Scholar]
- 163.Hanouneh MA, Hanouneh IA, Hashash JG, Law R, Esfeh JM, Lopez R, Hazratjee N, Smith T, Zein NN. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol. 2012;46:709–715. doi: 10.1097/MCG.0b013e3182506dbb. [DOI] [PubMed] [Google Scholar]
- 164.Mostafa T, Badra G, Abdallah M. The efficacy and the immunomodulatory effect of rifaximin in prophylaxis of spontaneous bacterial peritonitis in cirrhotic Egyptian patients. Turk J Gastroenterol. 2015;26:163–169. doi: 10.5152/tjg.2015.7782. [DOI] [PubMed] [Google Scholar]
- 165.Lutz P, Parcina M, Bekeredjian-Ding I, Nischalke HD, Nattermann J, Sauerbruch T, Hoerauf A, Strassburg CP, Spengler U. Impact of rifaximin on the frequency and characteristics of spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. PLoS One. 2014;9:e93909. doi: 10.1371/journal.pone.0093909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992–999. doi: 10.1111/j.1365-2036.2009.03958.x. [DOI] [PubMed] [Google Scholar]
- 167.Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450–455. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- 168.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 169.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–98; quiz 499. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 170.Boero M, Berti E, Morgando A, Verme G. Treatment for colitis caused by Clostridium difficile: results of a randomized open study of rifaximine vs. vancomycin. Microbiol Medica. 1990;5:74–77. [Google Scholar]
- 171.Rubin DT, Sohi S, Glathar M, Thomas T, Yadron N, Surma BL. Rifaximin Is Effective for the Treatment of Clostridium difficile-Associated Diarrhea: Results of an Open-Label Pilot Study. Gastroenterol Res Pract. 2011;2011:106978. doi: 10.1155/2011/106978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44:846–848. doi: 10.1086/511870. [DOI] [PubMed] [Google Scholar]
- 173.Garey KW, Ghantoji SS, Shah DN, Habib M, Arora V, Jiang ZD, DuPont HL. A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. J Antimicrob Chemother. 2011;66:2850–2855. doi: 10.1093/jac/dkr377. [DOI] [PubMed] [Google Scholar]
- 174.Mattila E, Arkkila P, Mattila PS, Tarkka E, Tissari P, Anttila VJ. Rifaximin in the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2013;37:122–128. doi: 10.1111/apt.12111. [DOI] [PubMed] [Google Scholar]
- 175.Huang JS, Jiang ZD, Garey KW, Lasco T, Dupont HL. Use of rifamycin drugs and development of infection by rifamycin-resistant strains of Clostridium difficile. Antimicrob Agents Chemother. 2013;57:2690–2693. doi: 10.1128/AAC.00548-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.DuPont HL, Jiang ZD. Influence of rifaximin treatment on the susceptibility of intestinal Gram-negative flora and enterococci. Clin Microbiol Infect. 2004;10:1009–1011. doi: 10.1111/j.1469-0691.2004.00997.x. [DOI] [PubMed] [Google Scholar]
- 177.Marchese A, Salerno A, Pesce A, Debbia EA, Schito GC. In vitro activity of rifaximin, metronidazole and vancomycin against Clostridium difficile and the rate of selection of spontaneously resistant mutants against representative anaerobic and aerobic bacteria, including ammonia-producing species. Chemotherapy. 2000;46:253–266. doi: 10.1159/000007297. [DOI] [PubMed] [Google Scholar]
- 178.Brigidi P, Swennen E, Rizzello F, Bozzolasco M, Matteuzzi D. Effects of rifaximin administration on the intestinal microbiota in patients with ulcerative colitis. J Chemother. 2002;14:290–295. doi: 10.1179/joc.2002.14.3.290. [DOI] [PubMed] [Google Scholar]
- 179.De Leo C, Eftimiadi C, Schito GC. Rapid disappearance from the intestinal tract of bacteria resistant to rifaximin. Drugs Exp Clin Res. 1986;12:979–981. [PubMed] [Google Scholar]
- 180.Gomi H, Jiang ZD, Adachi JA, Ashley D, Lowe B, Verenkar MP, Steffen R, DuPont HL. In vitro antimicrobial susceptibility testing of bacterial enteropathogens causing traveler’s diarrhea in four geographic regions. Antimicrob Agents Chemother. 2001;45:212–216. doi: 10.1128/AAC.45.1.212-216.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ouyang-Latimer J, Jafri S, VanTassel A, Jiang ZD, Gurleen K, Rodriguez S, Nandy RK, Ramamurthy T, Chatterjee S, McKenzie R, et al. In vitro antimicrobial susceptibility of bacterial enteropathogens isolated from international travelers to Mexico, Guatemala, and India from 2006 to 2008. Antimicrob Agents Chemother. 2011;55:874–878. doi: 10.1128/AAC.00739-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


