Abstract
Functionalization of nanoparticles with cationic moieties, such as polyethyleneimine (PEI), enhances binding to the cell membrane; however, it also disrupts the integrity of the cell’s plasma and vesicular membranes, leading to cell death. Primary fibroblasts were found to display high surface affinity for cationic iron oxide nanoparticles and greater sensitivity than their immortalized counterparts. Treatment of cells with cationic nanoparticles in the presence of incremental increases in serum led to a corresponding linear decrease in cell death. The surface potential of the nanoparticles also decreased linearly as serum increased and this was strongly and inversely correlated with cell death. While low doses of nanoparticles were rendered non-toxic in 25% serum, large doses overcame the toxic threshold. Serum did not reduce nanoparticle association with primary fibroblasts, indicating that the decrease in nanoparticle cytotoxicity was based on serum masking of the PEI surface, rather than decreased exposure. Primary endothelial cells were likewise more sensitive to the cytotoxic effects of cationic nanoparticles than their immortalized counterparts, and this held true for cellular responses to cationic microparticles despite the much lower toxicity of microparticles compared to nanoparticles.
INTRODUCTION
Engineered nanoparticles (NPs) for drug delivery encompass a multitude of classes, containing various subclasses and numerous modified versions. Each presentation of a NP has the potential for a unique cellular impact. Modifiable NP traits include size, shape, surface charge, chemical composition, and surface functionalization. As an example of biological influence, NP size and surface charge affect cellular uptake, with internalization of larger NPs (>500 nm) being dominated by cells with phagocytic potential,1 and engulfment of cationic NPs being favored over neutral and anionic NPs in specific cell populations.2,3
The surface charge of NPs alters biodistribution, cellular interactions and cytotoxicity, with highly cationic NPs being extremely cytotoxic. NP surface chemistry also influences particle interactions with plasma constituents, which further impacts effective mass transport and cellular interactions.4 Previously we demonstrated that oxidized, anionic silicon microparticles are avidly internalized by endothelial cells, however, in the presence of serum, uptake is inhibited.2,5 Macrophages, on the other hand, continue to internalize microparticles in the presence of serum, supporting a role for serum protein adsorption in modulating nanoparticle cellular interactions. Plasma constituents that functioned as endothelial dysopsonins and simultaneously as macrophage opsonins included immunoglobulins.
Candidates for trans-membrane delivery of therapeutics, such as nucleic acids, include polyethyleneimine (PEI).6,7 Unfortunately, concentrations of PEI necessary to achieve high levels of transfection efficiency cause a correlated increase in toxicity.8 PEI-induced cellular toxicity has been defined as a two-stage process, with the first stage taking place within 30 min of PEI uptake.9 Stage-one toxicity has been defined as necrosis that is based on compromise of cell membrane integrity mediated by PEI binding to plasma membrane proteoglycans.10 Stage two cell death is attributed to mitochondrial-mediated apoptosis. Free PEI inserts into the outer mitochondrial membrane, creating channels and leading to the release of cytochrome c, caspase 3 activation, loss of mitochondrial membrane potential, and production of reactive oxygen species.11 When PEI is bound to NPs, internalization leads to proton buffering, osmotic pressure and eventual lysis of lysosomal membranes, releasing hydrolytic enzymes and other lysosomal constituents into the cytoplasm.7
Beyond the influence of NP physico-chemical properties on cellular biocompatibility, cells are heterogeneous and respond to cellular insults with different levels of tolerance. As an example, primary and immortalized cells exhibit varying susceptibilities to toxic substances, due in part to changes in organelle content and changes in the expression profile of proteins.12,13 Understanding differentials in cellular responses and the influence of physiological factors that alter cellular responses to therapeutics and cytotoxic agents is essential to designing effective therapeutic platforms. In this study, the impact of NP surface potential, dose, time, cellular uptake, cell type, and the influence of serum masking of the NP surface on cellular biocompatibility are explored.
MATERIALS AND METHODS
Materials
Iron oxide NPs (IONP) were obtained from Ocean Nanotech (Springdale, AR, USA). PEI IONPs are coated with a branched PEI polymer with a molecular weight of 25 kDa. Three sample batches of 15 nm NPs were used, with increasing surface potential and amine density (designated PEI+, PEI++, and PEI+++). Silica microparticles (SiMPs), 1 μm in diameter, were obtained from Kisker Biotech (Steinfurt, Germany).
Particle Functionalization with PEI
SiMPs were oxidized using 10% HNO3 for 30 minutes, washed three times with isopropanol, and vacuum desiccated. PEI functionalization was performed using trimethoxysilylpropyl modified PEI (Gelest, Arlington, VA). Particles were either left unmodified or treated with 0.1%, 1% or 10% PEI silane in 95/5 (v/v) of isopropanol/water. Functionalization was conducted at 35 °C with 1300 rpm mixing for 2 hour. Particles were then washed three times with fresh isopropanol and vacuum desiccated overnight. The three classes of PEI-coated SiMPs are designated as SiMP-PEI+, SiMP-PEI++, and SiMP-PEI+++.
Evaluation of Particle Surface Potential and Amine Density
The surface potential and size of NPs was determined using a Malvern Zetasizer Nano ZS (Worcestershire, UK). Amine quantification was performed using a 4-nitrobenzaldehyde (NBA) hydrolysis assay. Particles were centrifuged and rinsed three times with coupling solution (0.8% glacial acetic acid in 99.2% ethanol). IONPs or SiMPs were then suspended in reaction solution (7 mg NBA in 10 ml coupling solution) and incubated for 3 hr at 50 °C with mixing at 900 RPM. After incubation, NPs were rinsed five times with ethanol and suspended in 1 ml hydrolysis solution (2.5 ml water, 2.5 ml ethanol, and 6.7 μl glacial acetic acid) for one hour incubation at 30 °C with 900 RPM mixing. Samples were centrifuged and the supernatant absorbance was measured at 268 nm and compared against an NBA standard curve.
Cell Culture
Cells were maintained at 37 °C with 5% CO2. Primary human dermal fibroblasts (ATCC, Manassas, VA, USA) were cultured in fibroblast basal medium supplemented with a low serum growth kit containing 2% serum (ATCC). L929 mouse fibroblasts (ATCC) were maintained in Minimum Essential Media supplemented with 10% horse serum and 1% penicillin-streptomycin. Human umbilical vein endothelial cells (HUVEC) and human microvascular endothelial cells (HMVEC) were maintained in EGM-2 and EGM media (2% serum; Lonza, Walkersville, MD), respectively.
LIVE/DEAD Assay
Cytotoxicity studies were performed using 0.5–7 μg/ml NPs as indicated in complete cell culture media for 24 hour or as indicated. Free PEI (25 kDa linear, Polysciences, Inc., Warrington, PA, USA) was used at 1–50 μg/ml for 24 hour Primary and immortalized cells were seeded in 6 well plates one day prior to NP treatment. During NP incubations, all cells were maintained in media containing 2% serum unless otherwise stated. Treated cells were harvested by trypsinization and cell pellets were resuspended in 500 μl PBS supplemented with 2% FBS. Cell viability was determined using a LIVE/DEAD® assay from Invitrogen (Grand Island, NY, USA) in which dead cells are detected based on uptake of ethidium homodimer-1 (2 μl of 2 mM stock) and live cells are detected based on esterase activity using calcein AM (1 μl of a 50 μM stock). Cells that are ethidium homodimer+/calcein AM− were designated as dead cells. Alternatively, cells were incubated with annexin V FITC and propidium iodide for 15 min at room temperature. Cells were analysed using a Becton Dickinson LSR-Fortessa cell analyser (San Jose, CA, USA).
Serum Titration
Primary or immortalized fibroblasts were maintained in 0%, 2%, 4%, 6%, 8%, 10% or 25% serum containing media. PEI+++ IONPs were added at a concentration of 0.5 or 1 μg/ml in 2 ml of media and incubated for 24 hour. Cell viability was assessed using the LIVE/DEAD staining assay and flow cytometry. To assess changes in PEI IONP zeta potential due to serum opsonization, PEI++ or PEI+++ IONPs were incubated with media containing a range of serum concentrations for either 15 min or 24 hours. NPs were pelleted and resuspended in phosphate buffer or water as indicated.
Prussian Blue Analysis of IONP Uptake by Cells
Primary fibroblasts and L929 cells were incubated with PEI+++ IONPs in various percentages of serum for 24 h then washed twice, released using trypsin and heated at 55 °C for 2 hour in 6N HCl. Iron was oxidized using 0.01% ammonium persulfate (10 min) and then 2% potassium ferrocyanide was added for 30 min and absorbance was measured at 690 nm using a Molecular Devices Spectramax 340 PC (Sunnyvale, CA, USA). For cell imaging, primary fibroblasts were seeded in 4-well chamber slides, treated with NPs in 2 or 25% serum, and then fixed with 4% paraformaldehyde and stained with Prussian blue for 30 min at 37 °C using a mixture of 2N HCl and 2% potassium ferrocyanide (1:2 parts). Images were acquired using a Nikon Eclipse equipped with a 60× differential interference contrast oil objective.
Fluorescent Microscopy
Cells were seeded in 4-well chamber glass slides at a density of 7 × 104 cells and allowed to adhere overnight. The next day, Mitotracker©Red CMXRos (Invitrogen) was added at a concentration of 15 nM in fresh media. Samples were rinsed with PBS after 45 min incubation at 37 °C, then fixed with 4% paraformaldehyde in PBS for 15 min, rinsed twice with PBS, and per-meabilized with 0.1% Triton-X in PBS for 5 min. Samples were then washed, incubated in approximately 165 nM phalloidin-Alexa Fluor 488 in 1% BSA-PBS for 20 min, rinsed with PBS and mounted using VectaShield with DAPI (Vector Laboratories, Burlingame, CA). Images were taken using a Nikon A1 confocal at 60× magnification and fluorescence was determined in the PE channel using a Becton Dickinson LSRFortessa cell analyser.
Real-Time Confocal Imaging
HMVECs were cultured in EGM (Lonza) in a 6-well cell culture plate until approximately 60% confluent. DRAQ7 (Abcam, Cambridge, MA) was added to each well at a concentration 1.8 nM and incubated for 5 minutes. The cells were then imaged using an Olympus IX81 inverted microscope at 37 °C in a climate controlled chamber with 5% CO2. Five fields per well were imaged at 10 min intervals for 2 hour. 10 μg/ml PEI+++ IONPs were added to the cells at time zero.
Transmission Electron Microscopy Imaging
HUVEC, HMVEC, L929, or human primary dermal fibroblasts were seeded in 6 well plates and incubated with 2.5 μg/ml PEI+ IONPs in 2 ml complete media for 6 hour or 2.5 μg/ml PEI++ IONPs for 24 hour. Samples were collected by trypsinization. Cells were pelleted and suspended in 5% BSA-PBS, pelleted again and the supernatant removed. Trump’s fixative™ (BBC Biochemical, Dallas, TX) was gently layered over the cell pellet and cells were centrifuged once more. The supernatant was kept on the cell pellet and samples were stored overnight at 4 °C. Samples were post fixed in 1% buffered osmium tetroxide, dehydrated in a graded series of ethanol, and embedded in resin. Thin sections were stained in 2% aqueous uranyl acetate and lead citrate. Samples were examined with a FEI Tecnai Spirit transmission electron microscope (Hillsboro, OR, USA).
Modeling
To illustrate the impact of NP contact with the cell membrane, and the influence of dose, a coarse-grained 2D model of a cell membrane interacting with NPs having 2 sizes was constructed. The membrane was modelled based on 2000 NP widths, 5 nm in diameter, with a charge of about 0.1e per NP. Membrane-adherent NPs were connected by a harmonic bond with a spring constant of 21 · 10−7 N/m and the membrane fixed at both ends. Simulations compared 100 and 200 NPs maintaining the cell:NP ratio at less than 10 and avoiding NP overcrowding. The NPs were arranged in a circle placed at the membrane and simulated for 11 ms using LAMMPS software.14 Stress and bond length analysis of NP interacting with the cell surface were analysed over the last 1 ms of the trajectory. All NP interactions with the cell membrane were based on Morse potential and electrostatic interactions. Stress in the membrane model was calculated as an ensemble average over all membrane bonds and various times over the last ms. Stresses in the local environment of the NPs were evaluated by averaging the stresses around 10 in the vicinity of the NPs.
Statistics
A 2-tailed, equal variance student’s t-test was used for comparisons between groups. Error bars represent standard deviations of n = 3 replicates.
RESULTS
Rapid Cell Death Induced by High Dose PEI IONPs
In this study we used three groups of PEI IONPs with variable amounts of surface PEI. IONP designations and their associated zeta potentials in phosphate buffer are as follows: PEI+ (+4.5 mV) < PEI++ (+7.69 mV) < PEI+++ (+10.3 mV). Using a 4-nitrobenzaldehyde (NBA) hydrolysis assay, IONPs contained 0.11 < 1.56 < 2.29 pmole PEI/μg Fe. As expected, increases in zeta potential corresponded to increases in PEI density on the IONP surface.
Cell death induced by high dose PEI+++ IONP treatment was monitored in real-time using live confocal imaging of immortalized HMVEC treated with 10 μg/ml IONPs in the presence of the nuclear dye DRAQ7, which penetrates into cells having a compromised cell membrane. Brightfield and fluorescent cell images are shown in Figure 1(a) for time 0, with representative fluorescent images from select time points. Compromise of the cell membrane was rapid, with 7% of the cells taking up DRAQ7 at 20 min, increasing to 47% and 67% at 30 and 40 min, respectively. Approximately 80% of the cells in the treated population were dead within one hour and 100% by two hour. While virtually no cell death was present in control cells, cell death across time was similar in 3 fields of view for cells treated with PEI+++ IONPs (Fig. 1(b)).
Figure 1.
Cytotoxicity of cationic NPs. (a) HMVEC were incubated with a high dose of PEI+++ IONPs (10 μg/ml) and loss of cell membrane integrity was measured as a function of fluorescent DRAQ7 uptake. A bright field microscopy image shows HMVEC at time 0, and fluorescent images show DRAQ7 nuclear labelling at select time points. (b) Average time-dependent loss of HMVEC membrane integrity in control and PEI+++ IONP-treated cells for 3 fields of view, with measurements taken every 10 min. (c) 2D model showing dose-dependent stress on a 10 μm section of cell membrane. (d) Percent primary [HUVEC and dermal fibroblast (FB)] and immortalized (HMVEC and L292) cell death, measured as a function of ethidium homodimer uptake, after 6 or 24 hour incubation with 7 μg/ml of PEI+ or PEI++ IONPs. (e) Fluorescence microscopy images of each cell type labelled with Alexa Fluor 488 phalloidin and Mitotracker® Red (bars 50 μm). (f) Cell death in primary dermal and immortalized L929 fibroblasts incubated with PEI IONPs at a concentration of 0.5 μg/ml for 6 or 24 hour (*p < 0.05 PEI++ vs PEI+++, and PEI++ 6 vs 24 hour; ***p < 0.001 PEI+++ 6 vs 24 hour). (g) Cell death following incubation of cells with free PEI (*p < 0.05; **p < 0.01; ***p < 0.001; compared to next lower dose).
A 2D model illustrating cationic NP-induced stress on the cell membrane is shown in Figure 1(c). A hypothetical 10 μm membrane section, approximately 5 nm in thickness, was exposed to NPs connected by harmonic potential simulated in dielectric media (~80) as water at room temperature. Approximately 200 and 100 15 nm-size NPs were simulated assuming the interactions of all NPs were attractive with the cell membrane. The stress increased to 122 and 150 · 1025 N · m−2 for 100 and 200 NPs, respectively. The propagation of stress away from NPs is shown in Figure 1(c), with strongly destabilized membrane zones appear in close vicinity of NPs. The model supports that membrane destabilization is high for cells with extensive membrane binding by NPs.
Cytotoxicity of Cationic NPs in Immortalized and Primary Cells as a Function of NP Surface Potential
Based on reported differences between primary and immortalized cells with respect to susceptibility to toxic substances, we examined cell death in primary fibroblasts and endothelial cells and their immortalized counterparts after 6 or 24 hour incubation with 7 μg/ml of PEI+ or PEI++ IONPs. While PEI++ IONPs were fatal to all cells at high dose, there were clear differences in cellular response to PEI+ IONPs (Fig. 1(d)). Immortalized cells, both endothelial (HMVEC) and fibroblast (L929), were less sensitive to the cationic IONPs (20–23% and 47–55% at 6 and 24 hour cell death, respectively) compared to primary HUVEC and dermal fibroblasts (80–90% and 78–79% cell death at 6 and 24 hours, respectively).
Confocal micrographs of the cell populations under study are displayed in Figure 1(e). The cellular cytoskeleton is displayed as green based on actin staining with phalloidin, mitochondria are labelled red using the cell-permeant MitoTracker® Red CMXRos (thiol-reactive chloromethyl) probes from Invitrogen, and nuclei are labelled blue with DAPI. In addition to clear differences in size between the chosen primary (large) and immortalized (small) cell populations, higher mitochondrial content was present in primary compared to their immortalized counterparts. Quantitative assessment using flow cytometry, showed a five-fold increase in the mitochondrial content of primary fibroblast cells compared to immortalized counterparts, and a two-fold increase for the endothelial cells (data not shown).
To more closely examine the differences between primary and immortalized cells with respect to susceptibility to PEI IONPs, primary dermal fibroblasts and immortalized L929 fibroblasts were treated with low doses of each version of PEI IONPs (0.5 μg Fe/ml; Fig. 1(f)). Treatment of fibroblasts with PEI IONPs in the presence of 2% serum demonstrated that positive surface potential was directly correlated with increases in cytotoxicity, with time dependent increases in cell death. Low density PEI+ IONPs were nontoxic to all cells at this dose.
However, treatment of primary fibroblasts with PEI++ IONPs resulted in 16% and 41% cell death for primary fibroblasts at 6 and 24 hour, respectively. PEI+++ IONPs induced 60% cell death at 24 hour, with cell death by both PEI++ and PEI+++ IONPs being significantly greater at 24 hour compared to 6 hour. Conversely, cell death observed in immortalized L929 fibroblasts was less than 10% at both 6 and 24 hour for all experimental conditions. Overall, immortalized fibroblasts were highly tolerant to PEI IONPs at low doses, while primary fibroblasts suffered high levels of cell death.
While PEI on the surface on NPs was extremely toxic to cells, free PEI required much greater doses to achieve the same level of cytotoxicity. As an example, at a dose of 0.5 μg/ml IONP, cells were exposed to a PEI dose equivalent of 0.02–0.03 μg/ml, with cell viability being compromised for primary cells at 6 and 24 hour. In contrast, both primary and immortalized cells tolerated free linear PEI at doses as high as 1 μg/ml. Doses of free PEI at 5 μg/ml or greater caused cell death in greater than 93% of primary fibroblasts, however, cell death in immortalized cells at this high dose were still only 13% after 24 hour of incubation (Fig. 1(g)).
Masking of the NP Surface with Serum
As NPs come into contact with serum, adsorption of proteins and lipids alter the NP surface. The schematic in Figure 2(a) shows a cationic NP in serum-free media, with incremental increases in serum there are increasing amounts of protein adsorbed to the particle surface (i.e., corona). DLS analysis supported high levels of NP aggregation in the presence of serum and increases in protein binding to the NP surface as the percentage of serum in the media is increased (Figs. 2(b),(c)). The average NP size increased from 51 nm to 101 and 110 nm for NPs treated with 2 and 25% serum, respectively. 77–81% of the NPs were aggregated at 2 and 25% serum. Transmission electron micrographs show control and serum treated-NPs. The IONP cores were highly homogeneous and adsorbed proteins were not detected on NPs treated with 25% serum (note: NPs were not stained with contrast agent; Fig. 2(c)).
Figure 2.
The NP corona/nimbus. (a) Schematic illustration of serum protein adsorption to cationic IONPs. As the concentration of serum proteins increases, there is a concomitant increase in protein binding to the NP surface and an associated increase in masking of the positive surface potential. (b) Dynamic light scattering (DLS) measurements of NP hydrodynamic diameters across the three NP populations (control, dark blue; 2%, pink; 25%, light blue). (c) DLS measurement for single particle populations. (d) Electron micrographs showing NP cores of control and serum-treated IONPs (bar 20 nm).
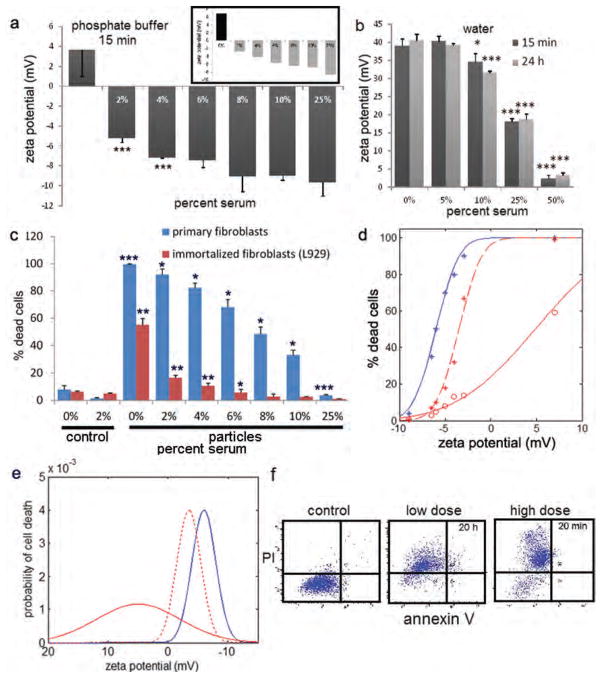
A striking inverse correlation was observed between serum content in cell culture media and IONP surface potential (Fig. 3(a)). PEI++ or PEI+++ (inset) IONPs were incubated with media containing incremental increases in serum for 24 hour and the zeta potential was determined in phosphate buffer. IONP surface potential decreased linearly from 6.8 mV in serum-free media to −8.7 mV in media containing 25% serum for PEI+++ IONPs, and ranged from 3.6 to −9.7 mV for serum-treated PEI++ IONPs. A similar trend in surface potential was seen when NPs were incubated with serum in water, with no difference is zeta potential for NPs incubated for 15 min compared to those incubated for 24 hour, indicating rapid protein binding to the NP surface (Fig. 3(b)).
Figure 3.
Serum masking of the cationic surface. (a) Zeta potential analysis of PEI++ IONPs in phosphate buffer following 24 hour incubation in serum-free (0%) media or media containing variable percentages of serum (***p < 0.005). The bordered inset in the upper right is a similar experiment using of PEI+++ IONPs. (b) Zeta potential analysis of PEI+++ IONPs (in water) following incubation of NPs in water containing increasing concentrations of serum for either 15 min or 24 hour. (c) Cell death in primary and immortalized fibroblasts incubated with 0–25% serum-containing media for 24 hour in the presence of PEI+++ IONPs at a concentration of 1 μg/ml. (d) Cell death presented as a function of zeta potential. Dose response curve showing cell death as a function of IONP zeta potential (primary: blue line: 1.0 μg Fe/ml; immortalized cells: solid red line: 1.0 μg Fe/ml; dotted red line: 2.5 μg Fe/ml). (e) Profile of biological susceptibility as a function of zeta potential in primary (blue line; 0.5 μg/ml) and immortalized cells at low (0.5 μg/ml; solid red line) and medium doses (1 μg/ml; dotted red line). (F) Flow cytometric dot blots showing annexin V and propidium iodide profiles of HMVEC treated with low (5 μg/ml) or high (100 μg/ml) dose PEI++ IONPs for 20 hours or 20 min in media containing 2% serum.
PEI+++ IONPs were incubated with primary and immortalized fibroblasts in the presence of 0–25% serum for 24 hour at 1 μg/ml (Fig. 3(c)). Cell death, which was 100% for primary fibroblasts incubated with PEI+++ IONPs in serum-free media, decreased linearly, declining to levels similar to no NP treatment controls when incubated with cells in the presence of 25% serum. Immortalized fibroblasts, which were more tolerant to PEI IONPs, exhibited a similar but more rapid decrease in cytotoxicity in the presence of serum, with cell death similar to no treatment controls in the presence of 6% serum. Serum masking of the positive surface potential was strongly anti-correlated with IONP-induced cell death (0.97 Pearson correlation).
A detailed analysis of the effect of NP surface potential (and thereby surface masking) on primary and immortalized cell populations was conducted using the serum-exposed NPs detailed above. Cell death curves as a function of zeta potential for a dose of 1 μg/ml (solid lines) PEI+++ IONPs are displayed in Figure 3(d). An increased dose of 2.5 μg/ml was required to a produce dose-response curve for immortalized fibroblasts similar to that of primary cells. The plots are cumulative distribution functions. Their profile is determined by the probability distribution function of biological susceptibility to the PEI+++ IONPs, where the integral of heterogeneity gives the dose-response cumulative distribution function.
The cell death was used to determine the population distribution with respect to probability of cell death as a function of zeta potential (Fig. 3(e)). The probability distribution function were modelled using Gaussian curves, with the variables of mean and standard deviation optimized such that their cumulative integrals matched the experimental cumulative distribution functions in Figure 3(d).
Incubating IONP-treated cells (HMVEC) with propidium iodide further confirmed that rapid cell death caused by high dose (100 μg/ml) or cell death following extended exposure to relatively low dose (5 μg/ml) PEI++ IONPs in the presence of low levels of serum (2%) was due to necrosis. In contrast to what others have reported for free PEI, apoptosis did not play a role in cell death based on exposure to PEI presented by IONPs as indicated by a lack of annexin V on the cell surface (Fig. 3(f)).
Cellular Interactions with PEI IONPs
In order to investigate the cause of differential NP toxicity in the selected cell populations, we examined cellular morphology and association with cationic NPs 6 hour after addition of NPs to cells using transmission electron microscopy (Fig. 4(a)). Treatment of cells with PEI+ (low cationic) IONPs caused primary fibroblasts to become somewhat vacuolated, and while there was evidence of NP uptake by all cell types, the primary fibroblasts displayed NP binding across the cell membrane. Magnification of the boxed regions in Figure 4(a) revealed vesicular localization of internalized PEI+ IONPs. The impact of serum on NP internalization was examined in primary and immortalized fibroblasts (Fig. 4(b), left) and endothelial cells (Fig. 5). Cells were treated with 2 μg/ml of PEI+++ IONPs for 24 hour following by Prussian blue labelling of cell-associated iron. While NP uptake was comparatively low in immortalized fibroblasts, in the population with the largest amount of viable cells (i.e., 25% serum group) there was virtually no detectible iron. For primary cells, there was an increase in iron detection in cells treated with increasing amounts of serum. While the increase most likely reflects the increase in viable cell number, it clearly illustrates that while adsorption by serum proteins masked the cationic surface charge and reduced cytotoxicity, it did not impair association of IONPs with cells. Mimicking the impact of serum on free IONPs, IONPs were predominately present as aggregates both inside the cells and on the cell membrane.
Figure 4.
Cellular uptake and response to PEI IONPs. (a) Primary and immortalized fibroblasts were incubated with 2.5 μg/ml PEI+ IONPs for 6 hour. Transmission electron micrographs show treated cells at low (2500×; left column) and high (75,000×; right most images) magnification, emphasizing IONP uptake and cellular location. The boxed insets show the succeeding amplified regions. (b) Cellular association with IONPs after 24 hour incubation as determined by Prussian Blue analysis (left). Intracellular vesicles containing IONPs in treated primary dermal fibroblast 24 hour after introduction of 2 μg/ml PEI+++ in 2% serum and Prussian blue staining (several IONP clusters are highlighted by arrows). (c) Primary dermal fibroblasts treated with 2.5 μg/ml PEI++ IONPs for 24 hour. Transmission electron micrographs show high vacuolization, loss of membrane integrity, IONP binding to the plasma membrane and protein-rich cell-free clusters of IONPs (white arrows).
Figure 5.
Cellular uptake of PEI IONPs by endothelial cells. (A) Primary and immortalized endothelial cells were incubated with 2.5 μg/ml PEI+ IONPs for 6 hr. Transmission electron micrographs show control cells in the left column and treated cells to the right at two magnification levels, emphasizing cellular morphology, NP uptake, and intracellular location. The inset in the bottom right image designates a cluster of IONPs in proximity to a mitochondrion.
The membrane-bound clusters of intracellular IONPs were seen as punctate Prussian blue entities in DIC micrographs of primary dermal fibroblasts treated with PEI++ (mid-level cationic) IONPs in the presence of 2% serum (Fig. 4(b), right).
Treatment of primary dermal fibroblasts with mid-level cationic (PEI++) IONPs for 24 hour caused cells to acquire heavy vacuolization throughout the cell and complete loss of cell structure (TEM images; Fig. 4(c)). A dense layer of IONPs, similar to that seen at 6 hour after treated with PEI+ IONPs, was present on the cell surface (Fig. 4(c); right). Protein-associated (electron dense) IONP clusters/aggregates can be seen outside the cell (white arrows). In these images, the presence of membranes surrounding the IONP clusters, supporting release of the IONP loaded endolysosomes, is unclear, however, previous work supports the release of membrane-bound vesicles laden with IONPs.15). Evidence of cellular uptake and encapsulation of PEI IONPs in vesicles was present for both primary and immortalized endothelial cells (Fig. 5).
Differential Cytotoxicity in Primary and Immortalized Cells is Mirrored in Microparticles
In order to determine if primary and immortalized cells also display differential toxicity to cationic microparticles, as SiMPs were coated with PEI and introduced to cells. SiMPs were oxidized then coated with PEI using 0.1, 1, and 10% trimethoxysilylpropyl. The resulting three particle samples, SiMP-PEI+ (+10.3 mV), SiMP-PEI++ (+12.7 mV), and SiMP-PEI+++ (+10.7 mV) had similar zeta potenitals, and displayed relatively low (0.02 and 0.04 pmole PEI/μg silica for SiMP-PEI+ and SiMP-PEI++, respectively) or medium (0.18 pmole PEI/μg silica for SiMP-PEI+++) PEI densities. Similar zeta potentials likely indicate that complete surface silylization was achieved at the lowest silane concentration, with more extensive polymer coating present at higher concentrations. Treatment of primary HUVECs and immortalized HMVECs with PEI SiMPs at a concentration of 7 μg/ml resulted in a familiar tolerance to cytotoxicity for immortalized cells to cationic particles compared to primary cells, with cytotoxicity being much less for microparticles compared to that induced by nanoparticles. Cell death in primary endothelial cells (HUVEC) was 14–17%, compared to control levels of 7% (p < 0.005; Fig. 6(a)). Immortalized endothelial cells, on the other hand, were completely resistant to PEI SiMPs in the presence of 2% serum.
Figure 6.
Cellular uptake and response to SiMPs. (a) Primary (HUVEC) and immortalized (HMVEC) endothelial cells were treated with either control or PEI-modified silica microparticles (SiMPs) at a concentration of 7 μg/ml for 24 hour. Cell death is shown as a function of ethidium homondimer-1 uptake using flow cytometry (n = 3, p < 0.05). (b) Scanning electron micrograph (SEM) of IONPs (IO) and SiMPs (Si). (c), (d) SEM images of immortalized L929 fibroblasts incubated with PEI SiMPs for 45 min in the presence of 10% serum. The cell membrane is pseudo-colored in green while the SiMPs are displayed in yellow.
Contrast in IONP and SiMP size is displayed in the scanning electron micrograph in Figure 6(b). Cellular uptake of the PEI SiMPs in the presence of serum was confirmed using electron microscopy. All cell types were incubated with PEI SiMPs for 45 min. Early cellular uptake was characterized by pseudopodia wrapping around the microparticles (Fig. 6(c)), with subsequent complete membrane coverage shown in Figure 6(d).
DISCUSSION
Cellular responses to NPs are influenced heavily by the outer shell of the NP, with the surface chemistry and geometry of the NP dictating interactions with surrounding biomolecules. In physiological fluids, this includes proteins and lipids present in the microenvironment that adhere to the NP surface. Adsorption of serum components to the NP surface is mediated heavily by electrostatic interactions.16 For example, electronegative proteins neutralize or reverse the positive surface charge of cationic NPs5 and simultaneously provide anchors for binding surface receptors on specific cell populations, thereby influencing uptake. As stated previously, we reported that serum exposure to ‘originally anionic,’ but not ‘originally cationic,’ silicon and polystyrene microparticles inhibits cellular uptake of the microparticles by endothelial cells.2,5 This effect is greater in the presence of fresh plasma compared to heat-inactivated serum. Thus the composition of the biological fluid dictates what proteins or other entities are available to adhere to NP surface.5,17 As constituents in the environment change, the outer NP shell changes based on high affinity proteins replacing low affinity proteins.18 In addition to impacting cellular interactions, adsorbed proteins mask the original NP surface. In this study, serum proteins masked the cationic PEI surface of IONPs in a dose-dependent manner, with greater masking leading to concurrent incremental decreases in cationic IONP cytotoxicity.
For PEI-coated IONPs, amine density on the IONP surface was directly correlated with cytotoxicity, with low density amines being relatively benign in all cell types tested. Similar zeta potentials for IONPs treated with serum for 15 min and 24 hour indicated that 15 min was sufficient for formation of a fairly stable NP shell in the unchanging microenvironment. At all serum levels, extensive NP aggregation existed.
Primary dermal fibroblasts, which were more sensitive to cationic IONPs than their immortalized counterparts, showed high levels of IONP binding to the plasma membrane at all serum levels. The high density of bound PEI IONPs led to loss of membrane integrity, cellular structure, and abundant cell death. Tolerance of immortalized L929 fibroblasts to PEI IONPs was attributed in part to lower levels of association with the IONPs compared to primary dermal fibroblasts. However, the probability of cell death for the immortalized cells could be made to mirror that of primary cells by increasing the dose of PEI IONPs. Greater tolerance to cationic particles by immortalized cells compared to primary cells was also found for endothelial cells in response to both IONPs and PEI-coated microparticles, however, overall toxicity was much lower for microparticles compared to nanoparticles, despite similar zeta potentials.
These findings support differential cellular responses to IONPs based on cell type and IONP surface potential. They clearly emphasize the relevance of blood components to masking of the NP surface and creation of a unique protein shell that influences the biological impact of the NP, further emphasizing the strong influence of the microenvironment to cellular responses to NPs.
Acknowledgments
The authors thank Jim Barrish and Deborah Townley of the Texas Children’s Hospital Electron Microscopy Core and the Baylor College of Medicine Integrated Microscopy Core, respectively, for cell processing and TEM imaging, with funding for the latter provided by the NIH (HD007495, DK56338, and CA125123), the Dan L. Duncan Cancer Center, and the John S. Dunn Gulf Coast Consortium for Chemical Genomics. We acknowledge use of the Houston Methodist Research Institute Flow Cytometry and Microscopy Cores. We thank Scott Holmes, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, for artwork presented in Figure 2(a), and we acknowledge use of the RSCB protein database.19 We also thank Sarah Suki and Vazrik Keshishian for technical support. This research was supported by the National Institute of Health Grant RC2GM092599 and U54CA143837.
References
- 1.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 2.Serda RE, Gu J, Bhavane RC, Liu X, Chiappini C, Decuzzi P, Ferrari M. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials. 2009;30:2440. doi: 10.1016/j.biomaterials.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Krasnici S, Werner A, Eichhorn ME, Schmitt-Sody M, Pahernik SA, Sauer B, Schulze B, Teifel M, Michaelis U, Naujoks K, Dellian M. Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels. International Journal of Cancer Journal International Du Cancer. 2003;105:561. doi: 10.1002/ijc.11108. [DOI] [PubMed] [Google Scholar]
- 4.Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, Torchilin VP, Munn LL. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002;62:6831. [PubMed] [Google Scholar]
- 5.Serda RE, Blanco E, Mack A, Stafford SJ, Amra S, Li Q, van de Ven A, Tanaka T, Torchilin VP, Wiktorowicz JE, Ferrari M. Proteomic analysis of serum opsonins impacting biodistribution and cellular association of porous silicon microparticles. Molecular Imaging. 2011;10:43. [PMC free article] [PubMed] [Google Scholar]
- 6.Kopatz I, Remy JS, Behr JP. A model for non-viral gene delivery: Through syndecan adhesion molecules and powered by actin. The Journal of Gene Medicine. 2004;6:769. doi: 10.1002/jgm.558. [DOI] [PubMed] [Google Scholar]
- 7.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. The Journal of Gene Medicine. 2005;7:657. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 8.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7297. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Molecular Therapy. 2005;11:990. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Mislick KA, Baldeschwieler JD. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12349. doi: 10.1073/pnas.93.22.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bortner CD, Cidlowski JA. Caspase independent/dependent regulation of K(+), cell shrinkage, and mitochondrial membrane potential during lymphocyte apoptosis. The Journal of Biological Chemistry. 1999;274:21953. doi: 10.1074/jbc.274.31.21953. [DOI] [PubMed] [Google Scholar]
- 12.Krebs FC, Miller SR, Catalone BJ, Fichorova R, Anderson D, Malamud D, Howett MK, Wigdahl B. Comparative in vitro sensitivities of human immune cell lines, vaginal and cervical epithelial cell lines, and primary cells to candidate microbicides nonoxynol 9, C31G, and sodium dodecyl sulfate. Antimicrobial Agents and Chemotherapy. 2002;46:2292. doi: 10.1128/AAC.46.7.2292-2298.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan C, Kumar C, Bohl S, Klingmueller U, Mann M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol Cell Proteomics. 2009;8:443. doi: 10.1074/mcp.M800258-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plimpton S. Fast parallel algorithms for short-range molecular dynamics. Journal of Computational Physics. 1995;117:1. [Google Scholar]
- 15.Ferrati S, McConnell KI, Mack AC, Sirisaengtaksin N, Diaz R, Bean AJ, Ferrari M, Serda RE. Cellular communication via nanoparticle-transporting biovesicles. Nanomedicine (Lond) 2013 doi: 10.2217/nnm.13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arsianti M, Lim M, Marquis CP, Amal R. Assembly of polyethylenimine-based magnetic iron oxide vectors: Insights into gene delivery. Langmuir: The ACS Journal of Surfaces and Colloids. 2010;26:7314. doi: 10.1021/la9041919. [DOI] [PubMed] [Google Scholar]
- 17.Lesniak A, Campbell A, Monopoli MP, Lynch I, Salvati A, Dawson KA. Serum heat inactivation affects protein corona composition and nanoparticle uptake. Biomaterials. 2010;31:9511. doi: 10.1016/j.biomaterials.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 18.Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Bombelli FB, Dawson KA. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. 2011;133:2525. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 19.Walter MR, Cook WJ, Ealick SE, Nagabhushan TL, Trotta PP, Bugg CE. Three-dimensional structure of recombinant human granulocyte-macrophage colony-stimulating factor. J Mol Biol. 1992;224:1075. doi: 10.1016/0022-2836(92)90470-5. [DOI] [PubMed] [Google Scholar]