Abstract
Objective
Atherosclerosis is an inflammatory disease of the arterial wall. It is accompanied by an autoimmune response against ApoB100, the core protein of LDL, which manifests as CD4 T cell and antibody responses.
Approach and Results
To assess the role of the autoimmune response in atherosclerosis, the nature of the CD4 T cell response against ApoB100 was studied with and without vaccination with MHC-II restricted ApoB100 peptides. The immunological basis of autoimmunity in atherosclerosis is discussed in the framework of theories of adaptive immunity. Older vaccination approaches are also discussed. Vaccinating Apoe−/− mice with MHC-II restricted ApoB100 peptides reduces atheroma burden in the aorta by ~40%. The protective mechanism likely includes secretion of IL-10.
Conclusion
Protective autoimmunity limits atherosclerosis in mice and suggests potential for developing preventative and therapeutic vaccines for humans.
This article is based on the 2015 Russell Ross Memorial Lecturer in Vascular Biology, presented at the American Heart Association's Scientific Sessions Annual Conference, November 7-11, 2015, Orlando, FL. Atherosclerosis is an inflammatory disease of the arterial wall. This was first described by the Rudolf Virchow, the founder of cellular pathology, in 1858, who viewed atheroma as the result of chronic inflammatory disease of the arterial intima 1. Electron microscopic evidence of monocyte association with atherosclerotic lesions was provided by Ross Gerrity 2-4. Russell Ross, who originally had proposed the “response to injury model”, focused on endothelial cell damage and smooth muscle proliferation 5, later became a key proponent of the inflammatory nature of atherosclerosis 6, 7, and his work was instrumental in the widespread adoption of the inflammation hypothesis 8.
The role of low density lipoprotein (LDL)
The development of atherosclerosis requires levels of LDL cholesterol above those found in pre-agricultural societies9, although the clinical definition of “elevated” LDL cholesterol has changed over the years. The blood level of LDL cholesterol is the best known biomarker for atherosclerosis and its adverse events 10. LDL accumulates in lesions, can be oxidized (oxLDL) 11-13 and taken up by macrophages and dendritic cells 14, 15. However, this uptake of LDL is not per se pro-inflammatory. When human blood monocyte-derived macrophages are exposed to oxLDL in vitro, they express a gene expression pattern that is more similar to that of dendritic cells than to that of MCSF-driven macrophages 15. Indeed, dendritic cells in non-lymphoid tissues are mostly monocyte-derived whereas tissue macrophages are derived from embryonal precursor cells 16. Most macrophages in the healthy mouse aorta can self-renew and are also embryonal-derived 17, but monocyte-derived cells enter the arterial wall under pro-atherogenic conditions. The macrophage populations in atherosclerosis are heterogeneous 18, and their origin 19 is actively being discussed 20. When Ldlr−/− mice are challenged with a high fat diet, their peritoneal macrophages accumulate desmosterol and show an overall reduced level of inflammatory markers 21. Although LDL and its modified forms are unlikely to drive inflammation directly, vascular inflammation is widely recognized as a major contributor to the atherosclerotic process 7, 8, 22-24. Therefore, it becomes necessary to revisit the nature of the pro-inflammatory stimuli. Candidates include T cells 25, B cells 26 and direct activators of innate immune cells 27.
Immunological basis
An immune response is elicited when an antigen is recognized by T cells expressing a T cell receptor (TCR) that can bind the antigen with sufficient affinity. T cell-independent antigens, many of them carbohydrates or other non-peptide entities, will not be discussed here. A productive response requires co-stimulation by CD28 binding to CD80 or CD86 28 and/or by certain TNF/TNF receptor superfamily members 29. CD4 T cells recognize peptides presented by major histocompatibility complex (MHC)-II and CD8 T cells recognize peptides presented by MHC-I.
In 1961, Burnet proposed that T cells distinguish between self and non-self 30. However, this is not plausible for CD4 T cell responses because of the way positive and negative selection operate: If no peptide from self at the time of T cell development (childhood) is presented in the thymus, the corresponding T cell will simply not mature (dies by neglect). Many thymic-derived regulatory T cells (Tregs) recognize peptide antigens from the host-associated microbiome, suggesting that microbiome-derived antigens may also be involved in T cell development 31. Therefore, all T cells must recognize self (or microbiome-derived) peptides to develop. Negative selection eliminates T cells that bind the self-peptide/MHC complex with high affinity 32, 33. However, negative selection is not completely efficient 34, 35, and the natural repertoire, i.e. the number of antigen-specific T cells present without vaccination or infection, is only slightly lower for peptides similar to self peptides than for foreign peptides 36. The limited effectiveness of negative selection necessitates that self-tolerance is instead maintained by Tregs, whose TCRs bind self antigens presented by MHC-II with low to intermediate affinity 37. This view is strongly supported by the observation that interfering with antigen presentation in knockout mice does not result in immunodeficiency as expected, but instead results in rampant autoimmunity in mice in which dendritic cells are eliminated by diphtheria toxin expressed under the CD11c promoter 38. This means that dendritic cells (DCs) and other antigen-presenting cells are busy presenting self antigens to make Tregs to prevent autoimmunity. In fact, Tregs for organ-specific antigens are found in the draining lymph node of each organ 39. This concept is important for understanding how protective autoimmunity and vaccination against atherosclerosis work.
In 1989, the concept of self/non-self discrimination was modified because of findings that macrophages recognize non-self pathogen-associated molecular patterns (PAMPs) 40. According to this view, a productive immune response requires engagement of such patterns by Toll-like receptors (TLRs) and other PAMP-recognizing receptors 41. To be effective, vaccines require PAMP receptor stimulation, which is achieved by vaccinating with dead or attenuated infectious organisms that contain PAMPs, or by adding alum as an adjuvant, which stimulates inflammasome assembly and IL-1 production 42. Related to the PAMP model, the “danger” model 43 was proposed, which suggests that an immune response will be made against an antigen only when this antigen is seen in the context of danger. Indeed, vaccinating with an MHC-binding peptide alone, without adjuvant or other help, does not result in an effective immune response.
A more current concept is that of sequential immune responses44, recognizing that the primitive immune responses by parenchymal cells and by macrophages 45 were not superseded by the adaptive immune system, but are still very much active in all animals including humans. Some of the first animals, amoebas, are basically free-living macrophages 44-46. When multicellular animals evolved, cellular functions became compartmentalized and macrophages became specialized 47. Macrophages developed the ability to recruit and activate other newly appearing innate killer cells, such as neutrophils and innate lymphoid cells 48, 49. The adaptive immune system did not appear until fish species evolved 450-500 mya 46.
Various helper CD4 T cells
Depending on the costimulatory molecules, PAMP recognition, and cytokine environment, naïve CD4 T helper (Th) cells become polarized. Th1 develop in response to IL-12 and IFN-γ, express the defining transcription factor T-bet (Tbx21) and secrete the signature cytokine IFN-γ. Th2 develop in response to IL-4, IL-5 and IL-13, express the defining transcription factor GATA-3 (Gata3) and secrete the signature cytokines IL-4, IL-5 and IL-13. Regulatory T cells (Tregs) develop in response to TGF-β, express the defining transcription factor FOXP3 (Foxp3) and secrete the signature cytokine IL-10; natural Tregs develop in the thymus and induced Tregs develop in the periphery. Tr1 cells do not express FoxP3, but produce IL-10. Th17 cells develop in response to IL-6, TGF-β and IL-1, express the defining transcription factor ROR-γt (Rorc) and secrete the signature cytokines IL-17A, IL-17F and IL-21. Follicular helper T cells (TFH) develop in response to CD40 and ICOS ligand, express the defining transcription factor BCL6 (Bcl6) and secrete the signature cytokine IL-21. Undoubtedly, more T-helper subsets remain to be discovered.
In mouse experiments, it was established that it is the macrophage and dendritic cell M1/M2 polarization that shapes the immune response (Th1/Th2), not the other way around 45, 47. There is emerging evidence that “tolerogenic” DCs promote the differentiation of T cells to Tregs50 or, in the absence of retinoic acid, to Th17. A regulatory macrophage subset (Mregs) have formally been proposed based on in vitro experiments 51, supported by in vivo data in mice 52, 53. The antigen-presenting cell promoting TFH may be a germinal center B cells 54.
Taken together, the current view of adaptive immunity 44 suggests that antigens will elicit an immune response based on the context of macrophage and dendritic cell polarization in which they “see” the antigen. It follows that it should be possible to elicit a Treg or other anti-inflammatory T cell response (Tr1, mixed) by vaccination. The challenge is to determine the correct conditions for the desired response. One of the benefits of vaccination is that the resulting Tregs, just like other T cells, are antigen-specific and will secrete their immunosuppressive cytokines like TGF-β and IL-10 in response to TCR ligation. Unlike blocking inflammatory molecules or cytokines, vaccination for atherosclerosis is therefore unlikely to impair host defense.
Autoimmunity in Atherosclerosis
Both the innate 55-59 and the adaptive immune systems 25, 56, 60-63 play important roles in atherosclerosis. Early experiments in severe combined immunodeficiency (SCID) or recombinase activating gene (Rag) deficient mice indicated a role of the adaptive immune system62, 64-66. However, in these mice, B cells, CD4, CD8 and γδ T cells are all absent, making these results difficult to interpret because each of these lineages can have pro- and anti-atherosclerotic effects. Much stronger evidence for a role of T cells comes from various adoptive transfer experiments 67, 68, reviewed in 63, 69, 70.
Autoimmune diseases are characterized by antibodies and T cells recognizing self antigens. In most autoimmune diseases, this is believed to be associated with increased pathology. By contrast, clinical epidemiology suggests that autoantibodies are NEGATIVELY correlated with atherosclerotic disease burden and clinical events. This is best documented for autoantibodies to (modified) low density lipoprotein (LDL) 71, 72,73,36. In humans, IgM (and in some studies IgG) antibodies to oxidized (ox) LDL 73 negatively correlate with lesion burden 72, 74, reviewed in 25. Some studies suggest that much of the protective antibody activity resides in the IgM compartment 75. Statin treatment increases levels of oxidized phospholipids and IgM antibodies against these in patients with atherosclerosis. These IgMs are thought to be beneficial 76. Statins are thought to promote mobilization and clearance of oxidized phospholipids 77.However, opposite data (positive correlation between atherosclerosis and autoantibodies to modified LDL) was also reported 78. One report suggested that autoantibodies against a peptide found in ApoB100 are inversely related to atherosclerosis 79. Other studies found weak positive correlations between autoantibodies to modified LDL and cardiovascular disease 80 that were perhaps indirect 81. Many, but not all 26, studies suggest that antibodies to (modified) LDL can be protective in mice 78, 82-84. It is important to note that a positive correlation of antibodies and atherosclerosis does not necessarily mean the antibodies are proatherogenic. Recombinant antibodies to modified LDL were shown to induce regression of atherosclerosis in mouse models 85, 86, but interpretation of these data is limited by the fact that human, not mouse antibodies were infused. Adoptive transfer of IgM antibodies against phosphorylcholine reduced neointima formation in a vein graft model in mice 87, but effects on atherosclerosis were not reported. In a multicenter, randomized, double blind, placebo-controlled phase II study finished in July of 2012, the safety, tolerability, and activity of intravenous MLDL1278A, a monoclonal antibody to oxLDL, was evaluated using FDG-PET/CT imaging to assess vascular inflammation (ClinicalTrials.gov Identifier: NCT01258907). No study results were posted, and no further trials are listed. The contradictory nature of these findings illustrates that the field of vascular immunology is very much in flux.
Witztum and his group hypothesized that autoantibodies to oxLDL work by reducing the uptake of modified LDL into macrophages 88,89, and this has been proposed as an atheroprotective mechanism. Manipulations of B cells, which differentiate into antibody-secreting plasma cells, have shown both atheroprotective and pro-atherogenic roles of B cells. Splenectomy removes many B cells and exacerbates atherosclerosis 90, and mice lacking IL-5 show exacerbated atherosclerosis 90. Conversely, depleting B cells with an antibody to CD20 was atheroprotective 26.
Protective autoimmunity
The concept of protective autoimmunity was first proposed in 1981, when vaccination with attenuated encephalitogenic T cell clones showed protection from experimental autoimmune encephalitis (EAE), a rodent model of multiple sclerosis (MS) 91. Vaccination with peptides derived from the oligoclonal TCR sequences of encephalitogenic T cells was shown to deplete these T cells and curb EAE 92. A similar approach was later used in a mouse model of atherosclerosis 93. The term “protective autoimmunity” was coined by Michal Schwartz in 1999 94. Based on findings in models of traumatic brain injury 95-97, Schwartz proposed that vaccination with “safe” autologous peptide antigens would be protective under these conditions. Vaccinating mice with a homogenate of whole retinal proteins, interphotoreceptor retinoid-binding protein (IRBP) or S-antigen (retinal arrestin) was protective in a model of intraocular injection of aggregated β-amyloid or glutamate 98. For this protective autoimmunity to be effective, a Th1-like response must be induced and a Treg response is counterproductive 99. Neurodegeneration was augmented when Tregs were depleted and exacerbated when Tregs were injected into mice 98. This surprising insight has led to efforts to develop a vaccine for glaucoma 100. Encouraging results have been reported in rat models, but it not clear whether translational efforts were ever completed 101. One hypothesis is that activated T cells provide useful growth factors and cytokines for neuronal repair 102. So far, translational efforts have not been successful 103, 104. The concept of protective autoimmunity has not been explored in tissues and organs other than the central nervous system.
Vaccination against atherosclerosis
Many vaccination schemes against atherosclerosis have been proposed 105. Immunization of mice with modified LDL was shown to be protective, but the protection did not correlate with antibody titers, suggesting a possible T cell mechanism 106. Witztum's approach was to immunize rabbits with malondialdehyde (MDA)-LDL, which represents an LDL particle that contains MDA adducts of the apoB100 protein. LDL eluted from arteries of rabbits and humans contains LDL modified with MDA (and other) adducts. The modified LDL is taken up by macrophages via scavenger receptors 107. As mentioned above, autoantibodies to oxLDL were also found in plasma of people and animals with atherosclerosis 11-13. Vaccination with MDA-LDL resulted in reduced atherosclerosis 108. The mechanism was proposed to be antibody-based inhibition of uptake of modified LDL by macrophages 25, 73,88,89, but this was never rigorously tested. The various vaccination approaches are reviewed in 109, 110,70, 111.
Nilsson's group identified peptides from ApoB100 that were recognized by autoantibodies in human plasma 112, and immunization with these peptides conjugated to bovine serum albumin (BSA) reduced atherosclerosis in Apoe−/− mice 113. An intranasal vaccine was later developed based on one of these peptides known as p210 114. Animal experiments with intranasal and oral vaccines targeted to oxLDL 115, 116 or heat shock protein-60 117,118 have also been reported, but have not been developed clinically. The atheroprotection seen in these studies remains unexplained: First, p210 was conjugated to BSA, an allo-antigen, and the response to BSA alone was not tested. Second, because p210 does not bind I-Ab 63 and thus cannot elicit a p210-specific CD4 T cell response, its mechanism of action remains obscure.
By analyzing T cells from human atherosclerotic plaque, Hansson's group discovered that the immune response to oxLDL was (largely) MHC-II restricted 119. They later immunized human ApoB100 transgenic Ldlr−/− mice with human oxLDL and showed that all derived T cell clones recognized sequences found in native ApoB100 93, challenging the LDL oxidation hypothesis. The T cell clones were oligoclonal, and most expressed a single TCR β variable chain, TRBV31. Hansson hypothesized that the protective effect may be due to Tregs 120. Tregs were elevated in mice immunized with the p210 peptide of ApoB100 121, but antigen specificity of these Tregs was not tested. The hypothesis that Tregs are atheroprotective is indirectly supported by a recent study from Tabas’ group, who showed that the net effect of inhibiting Toll-like receptor (TLR) signaling in dendritic cells (DCs) was pro-atherogenic, presumably by preventing the formation of Tregs 122. In the natural course of atherosclerosis, the number of Tregs decreases 123 and that of effector T cells (Teff) increases over time 124. Statins, the most widely used class of drugs to prevent atherosclerosis, have been reported to increase Tregs 125-127. Some CD4 T cells in atherosclerotic plaques are known to express FoxP3, the defining transcription factor of regulatory T cells (Tregs) 128. Since Tregs have been shown to be atheroprotective 67, 121, 129, 130, it is reasonable to suspect that vaccines may work by increased Tregs in atherosclerotic lesions or other locations. However, no such effects have been reported to date.
There is very strong evidence that T-helper 1 CD4 T cells (Th1) are pro-atherogenic. This was shown by loss of function experiments: knockout mice lacking interferon-γ 131 or the Th1 transcription factor T-bet 132 or the co-stimulatory molecules CD80 and CD86 133. Adoptive transfer of Th1 cells exacerbated atherosclerosis Conversely, knocking out the co-inhibitory molecules PDL1 and PDL2 exacerbated atherosclerosis, and PDL1 and 2-deficient dendritic cells were more effective at promoting T cell proliferation in vitro 134. Similarly, ICOS-deficient mice had more atherosclerosis, suggesting that ICOS is needed for the expansion of Tregs128. Taken together, these data point to T cell-dependent pro- and anti-atherogenic mechanisms.
Vaccination with MHC-II-restricted ApoB100 peptides
Multiphoton imaging experiments showed that polyclonal CD62Llo CD44hi antigen-experienced CD4 T cells isolated from Apoe−/− mice interacted with antigen-presenting cells in the aortic wall identified by CD11c-YFP. In fact, the frequency and duration of these interactions was comparable to the frequency and duration of contacts between monoclonal CD4 T cells from TCR transgenic mice when antigenic peptide was added 135 (figure 1). This was specific, because the interaction was not seen when CD4 T cells were isolated from wild-type mice, or when naïve CD4 T cells from Apoe−/− mice were used. Remarkably, it was not necessary to add antigen. These experiments showed that the autoantigen and antigen-experience CD4 T cells were present in atherosclerotic mice. Since ApoB is a known autoantigen in atherosclerosis 111, 136, we next tested the ability of peptides from mouse Apo B in complete (1×) and incomplete (4×) Freund's adjuvant to prevent atherosclerosis in Apoe−/− mice, a widely used model of atherosclerosis. The peptides were selected for binding to mouse MHC-II (I-Ab, binding affinities ~10 nM). Vaccination with each of the peptides, but not irrelevant control peptides, reduced aortic en face lesion size by 35-60% 137. We saw no change in the number of FoxP3+CD25+ Tregs in lymph nodes or spleens, but significantly increased expression of IL-10 mRNA induced in aortas of immunized mice. The number of CD11c+CD103+RALDH+ dendritic cells (DCs), a phenotype that is consistent with DCs inducing peripheral tolerance, was expanded in aortas of immunized mice. We conclude that vaccination with MHC-II restricted peptides can protect from atherosclerosis in a relevant mouse model. The mechanism may involve IL-10, but the type and location of immune cells producing IL-10 remain to be discovered. It is also possible that other cytokines may contribute. As expected, the antibodies induced by vaccination recognize the antigenic peptide with perfect specificity. However, these antibodies do not recognize native LDL or modified LDL 137, suggesting that the antigenic peptides are not accessible to antibodies on the intact lipoprotein. This makes it unlikely that autoantibodies play a role in atheroprotection after peptide-based vaccination, but this has not been formally investigated yet. These efforts have encouraged studies to translate this mouse work aimed at developing an MHC-II-restricted peptide-based atheroprotective vaccine for humans.
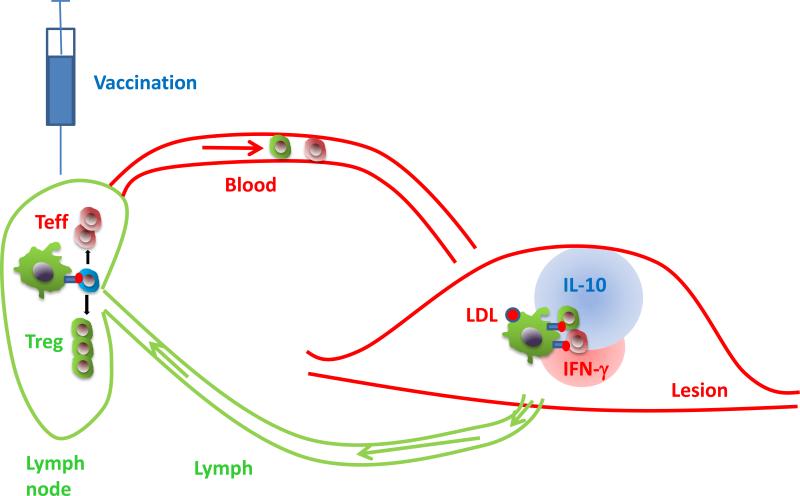
Figure. Protective autoimmunity in atherosclerosis.
Antigen-containing LDL (red ball) is taken up by DCs (green) in the atherosclerotic lesion, and ApoB100 peptides (red) are presented on MHC-II (blue). Antigen-laden DCs leave the vessel wall via lymphatics (green) and enter the draining lymph node, where they encounter naïve CD4 T cells (blue). If their TCR recognizes the MHC-II-bound ApoB100 peptide, the CD4 T cells expand, acquire homing receptors and become antigen-experienced effector T cells (red) and Tregs (green). This expansion can be boosted by vaccination (syringe). The antigen-experienced CD4 T cells home back to the atherosclerotic vessel wall and encounter the ApoB100 peptide bound to MHC-II again (recall response). This triggers secretion of anti-inflammatory cytokines like IL-10 (blue) and pro-inflammatory cytokines like IFN-γ (red).
Conclusion
The view of the role of the immune system in atherosclerosis has evolved over the last few decades. In immunocompetent animals and patients, a complex autoimmune response always accompanies the development of atherosclerosis. This response is fundamentally T cell-driven and can be pro- or anti-atherogenic. Circulating antibodies to LDL and other atherosclerosis antigens may be potential biomarkers, but evidence for the causal involvement of antibodies in atherosclerosis is scant. The field of vascular immunology is currently in flux and many of the classical paradigms of immunology and vascular biology are being challenged by new findings. Evolving new concepts may form an improved basis for the development of vaccination approaches aimed at curbing atherosclerosis.
Acknowledgments
Sources of funding
The original research underlying this mini-review was funded by the National Institutes of Health, HL115232, HL088093, HL121697, HL126543
References
- 1.Virchow R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI--Atheromatous affection of arteries. 1858. Nutr Rev. 1989;47:23–5. doi: 10.1111/j.1753-4887.1989.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 2.Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 3.Gerrity RG. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981;103:191–200. [PMC free article] [PubMed] [Google Scholar]
- 4.Gerrity RG, Naito HK. Ultrastructural identification of monocyte-derived foam cells in fatty streak lesions. Artery. 1980;8:208–214. [PubMed] [Google Scholar]
- 5.Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986;314:488–500. doi: 10.1056/NEJM198602203140806. %20. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 9.Rader DJ, Pure E. Lipoproteins, macrophage function, and atherosclerosis: beyond the foam cell? Cell Metab. 2005;1:223–230. doi: 10.1016/j.cmet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Colantonio LD, Bittner V, Reynolds K, Levitan EB, Rosenson RS, Banach M, Kent ST, Derose SF, Zhou H, Safford MM, Muntner P. Association of Serum Lipids and Coronary Heart Disease in Contemporary Observational Studies. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.011646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Itabe H, Ueda M. Measurement of plasma oxidized low-density lipoprotein and its clinical implications. J Atheroscler Thromb. 2007;14:1–11. doi: 10.5551/jat.14.1. [DOI] [PubMed] [Google Scholar]
- 14.Kruth HS, Jones NL, Huang W, Zhao B, Ishii I, Chang J, Combs CA, Malide D, Zhang WY. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2005;280:2352–2360. doi: 10.1074/jbc.M407167200. [DOI] [PubMed] [Google Scholar]
- 15.Cho H, Shashkin P, Gleissner C, Dunson D, Jain N, Lee J, Miller Y, Ley K. Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiological Genomics. 2007;29:149–160. doi: 10.1152/physiolgenomics.00051.2006. [DOI] [PubMed] [Google Scholar]
- 16.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 17.Ensan S, Li A, Besla R, Degousee N, Cosme J, Roufaiel M, Shikatani EA, El-Maklizi M, Williams JW, Robins L, Li C, Lewis B, Yun TJ, Lee JS, Wieghofer P, Khattar R, Farrokhi K, Byrne J, Ouzounian M, Zavitz CC, Levy GA, Bauer CM, Libby P, Husain M, Swirski FK, Cheong C, Prinz M, Hilgendorf I, Randolph GJ, Epelman S, Gramolini AO, Cybulsky MI, Rubin BB, Robbins CS. Self-renewing resident arterial macrophages arise from embryonic CX3CR1 precursors and circulating monocytes immediately after birth. Nat Immunol. 2015 doi: 10.1038/ni.3343. [DOI] [PubMed] [Google Scholar]
- 18.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van RN, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–37. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH, Jr., Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 23.Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114:55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 24.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van VE, Esposito B, Vilar J, Sirvent J, Van SJ, Tedgui A, Tedder TF, Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley K, Miller YI, Hedrick CC. Monocyte and Macrophage Dynamics during Atherogenesis. ATVB. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chattopadhyay K, Lazar-Molnar E, Yan Q, Rubinstein R, Zhan C, Vigdorovich V, Ramagopal UA, Bonanno J, Nathenson SG, Almo SC. Sequence, structure, function, immunity: structural genomics of costimulation. Immunol Rev. 2009;229:356–86. doi: 10.1111/j.1600-065X.2009.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnet FM. Immunological recognition of self. Science. 1961;133:307–11. doi: 10.1126/science.133.3449.307. [DOI] [PubMed] [Google Scholar]
- 31.Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P, Ignatowicz L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–62. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat Rev Immunol. 2014;14:377–91. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metzger TC, Anderson MS. Control of central and peripheral tolerance by Aire. Immunol Rev. 2011;241:89–103. doi: 10.1111/j.1600-065X.2011.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juang J, Ebert PJ, Feng D, Garcia KC, Krogsgaard M, Davis MM. Peptide-MHC heterodimers show that thymic positive selection requires a more restricted set of self-peptides than negative selection. J Exp Med. 2010;207:1223–1234. doi: 10.1084/jem.20092170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu W, Jiang N, Ebert PJ, Kidd BA, Muller S, Lund PJ, Juang J, Adachi K, Tse T, Birnbaum ME, Newell EW, Wilson DM, Grotenbreg GM, Valitutti S, Quake SR, Davis MM. Clonal Deletion Prunes but Does Not Eliminate Self-Specific alphabeta CD8(+) T Lymphocytes. Immunity. 2015;42:929–41. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson RW, Beisang D, Tubo NJ, Dileepan T, Wiesner DL, Nielsen K, Wuthrich M, Klein BS, Kotov DI, Spanier JA, Fife BT, Moon JJ, Jenkins MK. T Cell Receptor Cross-Reactivity between Similar Foreign and Self Peptides Influences Naive Cell Population Size and Autoimmunity. Immunity. 2014:10. doi: 10.1016/j.immuni.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wheeler KM, Samy ET, Tung KS. Cutting edge: normal regional lymph node enrichment of antigen-specific regulatory T cells with autoimmune disease-suppressive capacity. J Immunol. 2009;183:7635–7638. doi: 10.4049/jimmunol.0804251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janeway CA., Jr. The priming of helper T cells. Semin Immunol. 1989;1:13–20. [PubMed] [Google Scholar]
- 41.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 42.Eisenbarth SC, Williams A, Colegio OR, Meng H, Strowig T, Rongvaux A, Henao-Mejia J, Thaiss CA, Joly S, Gonzalez DG, Xu L, Zenewicz LA, Haberman AM, Elinav E, Kleinstein SH, Sutterwala FS, Flavell RA. NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature. 2012;484:510–513. doi: 10.1038/nature11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 44.Mills CD, Ley K, Buchmann K, Canton J. Sequential Immune Responses: The Weapons of Immunity. J Innate Immun. 2015 doi: 10.1159/000380910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mills CD, Ley K. M1 and M2 Macrophages: The Chicken and the Egg of Immunity. J Innate Immun. 2014 doi: 10.1159/000364945. DOI: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper EL. Evolution of immune systems from self/not self to danger to artificial immune systems (AIS). Phys Life Rev. 2010;7:55–78. doi: 10.1016/j.plrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 48.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 49.Gilbertson P, Wotherspoon J, Raison RL. Evolutionary development of lymphocyte heterogeneity: leucocyte subpopulations in the Pacific hagfish. Dev Comp Immunol. 1986;10:1–10. doi: 10.1016/0145-305x(86)90039-x. [DOI] [PubMed] [Google Scholar]
- 50.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 51.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 52.Cohen HB, Mosser DM. Extrinsic and intrinsic control of macrophage inflammatory responses. J Leukoc Biol. 2013;94:913–919. doi: 10.1189/jlb.0413236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleming BD, Mosser DM. Regulatory macrophages: setting the threshold for therapy. Eur J Immunol. 2011;41:2498–2502. doi: 10.1002/eji.201141717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. doi: 101146/annurev-immunol-031210-101400.:621-663. [DOI] [PubMed] [Google Scholar]
- 55.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 56.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annual Review of Immunology. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bjorkbacka H, Nilsson J. Innate immunity in atherosclerosis. J Innate Immun. 2010;2:305–306. doi: 10.1159/000314770. [DOI] [PubMed] [Google Scholar]
- 58.Nilsson J, Bjorkbacka H, Fredrikson GN. Apolipoprotein B100 autoimmunity and atherosclerosis - disease mechanisms and therapeutic potential. Curr Opin Lipidol. 2012;23:422–428. doi: 10.1097/MOL.0b013e328356ec7c. [DOI] [PubMed] [Google Scholar]
- 59.Hartvigsen K, Chou MY, Hansen LF, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. The role of innate immunity in atherogenesis. J Lipid Res. 2009;50(Suppl):S388–S393. doi: 10.1194/jlr.R800100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 62.Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, Bluestone J, Getz GS. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1011–1016. doi: 10.1161/01.atv.21.6.1011. [DOI] [PubMed] [Google Scholar]
- 63.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol. 2013;25:615–622. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dansky HM, Charlton SA, Harper MM, Smith JD. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1997;94:4642–4646. doi: 10.1073/pnas.94.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–259. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daugherty A, Pure E, Delfel-Butteiger D, Chen S, Leferovich J, Roselaar SE, Rader DJ. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E−/− mice. J Clin Invest. 1997;100:1575–1580. doi: 10.1172/JCI119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 68.Maganto-Garcia E, Bu DX, Tarrio ML, Alcaide P, Newton G, Griffin GK, Croce KJ, Luscinskas FW, Lichtman AH, Grabie N. Foxp3+-Inducible Regulatory T Cells Suppress Endothelial Activation and Leukocyte Recruitment. J Immunol. 2011 doi: 10.4049/jimmunol.1003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foks AC, Lichtman AH, Kuiper J. Treating Atherosclerosis With Regulatory T Cells. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.303568. %20. pii: ATVBAHA.114.303568.:ATVBAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamashita T, Sasaki N, Kasahara K, Hirata K. Anti-inflammatory and immune- modulatory therapies for preventing atherosclerotic cardiovascular disease. J Cardiol. 2015;66:1–8. doi: 10.1016/j.jjcc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 72.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Binder CJ, Shaw PX, Chang MK, Boullier A, Hartvigsen K, Horkko S, Miller YI, Woelkers DA, Corr M, Witztum JL. The role of natural antibodies in atherogenesis. J Lipid Res. 2005;46:1353–1363. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 74.Ravandi A, Boekholdt SM, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw KT, Tsimikas S. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J Lipid Res. 2011;52:1829–1836. doi: 10.1194/jlr.M015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fraley AE, Schwartz GG, Olsson AG, Kinlay S, Szarek M, Rifai N, Libby P, Ganz P, Witztum JL, Tsimikas S. Relationship of oxidized phospholipids and biomarkers of oxidized low-density lipoprotein with cardiovascular risk factors, inflammatory biomarkers, and effect of statin therapy in patients with acute coronary syndromes: Results from the MIRACL (Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering) trial. J Am Coll Cardiol. 2009;53:2186–2196. doi: 10.1016/j.jacc.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 77.Tsimikas S, Witztum JL, Miller ER, Sasiela WJ, Szarek M, Olsson AG, Schwartz GG. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110:1406–1412. doi: 10.1161/01.CIR.0000141728.23033.B5. [DOI] [PubMed] [Google Scholar]
- 78.Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:95–100. doi: 10.1161/01.atv.21.1.95. [DOI] [PubMed] [Google Scholar]
- 79.Sjogren P, Fredrikson GN, Samnegard A, Ericsson CG, Ohrvik J, Fisher RM, Nilsson J, Hamsten A. High plasma concentrations of autoantibodies against native peptide 210 of apoB-100 are related to less coronary atherosclerosis and lower risk of myocardial infarction. Eur Heart J. 2008;29:2218–26. doi: 10.1093/eurheartj/ehn336. [DOI] [PubMed] [Google Scholar]
- 80.Dotevall A, Hulthe J, Rosengren A, Wiklund O, Wilhelmsen L. Autoantibodies against oxidized low-density lipoprotein and C-reactive protein are associated with diabetes and myocardial infarction in women. Clin Sci (Lond) 2001;101:523–31. [PubMed] [Google Scholar]
- 81.Hulthe J, Wikstrand J, Fagerberg B. Relationship between C-reactive protein and intima-media thickness in the carotid and femoral arteries and to antibodies against oxidized low-density lipoprotein in healthy men: the Atherosclerosis and Insulin Resistance (AIR) study. Clin Sci (Lond) 2001;100:371–8. doi: 10.1042/cs1000371. [DOI] [PubMed] [Google Scholar]
- 82.Doran AC, Lipinski MJ, Oldham SN, Garmey JC, Campbell KA, Skaflen MD, Cutchins A, Lee DJ, Glover DK, Kelly KA, Galkina EV, Ley K, Witztum JL, Tsimikas S, Bender TP, McNamara CA. B-cell aortic homing and atheroprotection depend on Id3. Circ Res. 2012;110:e1–12. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston AT, Delignat S, Mandet C, Kohler HV, Kaveri SV, Nicoletti A. Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol. 2007;50:540–546. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 85.Schiopu A, Frendeus B, Jansson B, Soderberg I, Ljungcrantz I, Araya Z, Shah PK, Carlsson R, Nilsson J, Fredrikson GN. Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1(−/−)/low-density lipoprotein receptor(−/−) mice. J Am Coll Cardiol. 2007;50:2313–8. doi: 10.1016/j.jacc.2007.07.081. [DOI] [PubMed] [Google Scholar]
- 86.Schiopu A, Bengtsson J, Soderberg I, Janciauskiene S, Lindgren S, Ares MP, Shah PK, Carlsson R, Nilsson J, Fredrikson GN. Recombinant human antibodies against aldehyde-modified apolipoprotein B-100 peptide sequences inhibit atherosclerosis. Circulation. 2004;110:2047–52. doi: 10.1161/01.CIR.0000143162.56057.B5. [DOI] [PubMed] [Google Scholar]
- 87.Faria-Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, Cercek B, Shah PK. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006;189:83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 88.Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–28. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shaw PX, Horkko S, Tsimikas S, Chang MK, Palinski W, Silverman GJ, Chen PP, Witztum JL. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol. 2001;21:1333–1339. doi: 10.1161/hq0801.093587. [DOI] [PubMed] [Google Scholar]
- 90.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ben-Nun A, Wekerle H, Cohen IR. Vaccination against autoimmune encephalomyelitis with T-lymphocyte line cells reactive against myelin basic protein. Nature. 1981;292:60–1. doi: 10.1038/292060a0. [DOI] [PubMed] [Google Scholar]
- 92.Vandenbark AA, Hashim G, Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature. 1989;341:541–4. doi: 10.1038/341541a0. [DOI] [PubMed] [Google Scholar]
- 93.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 95.Schwartz M, Kipnis J. Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol Med. 2001;7:252–258. doi: 10.1016/s1471-4914(01)01993-1. [DOI] [PubMed] [Google Scholar]
- 96.Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen IR, Weiner H, Schwartz M. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Avidan H, Kipnis J, Butovsky O, Caspi RR, Schwartz M. Vaccination with autoantigen protects against aggregated beta-amyloid and glutamate toxicity by controlling microglia: effect of CD4+CD25+ T cells. Eur J Immunol. 2004;34:3434–3445. doi: 10.1002/eji.200424883. [DOI] [PubMed] [Google Scholar]
- 99.Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mizrahi T, Hauben E, Schwartz M. The tissue-specific self-pathogen is the protective self-antigen: the case of uveitis. J Immunol. 2002;169:5971–5977. doi: 10.4049/jimmunol.169.10.5971. [DOI] [PubMed] [Google Scholar]
- 101.Schwartz M. Vaccination for glaucoma: dream or reality? Brain Res Bull. 2004;62:481–484. doi: 10.1016/S0361-9230(03)00073-X. [DOI] [PubMed] [Google Scholar]
- 102.Barouch R, Schwartz M. Autoreactive T cells induce neurotrophin production by immune and neural cells in injured rat optic nerve: implications for protective autoimmunity. FASEB J. 2002;16:1304–1306. doi: 10.1096/fj.01-0467fje. [DOI] [PubMed] [Google Scholar]
- 103.Schwartz M, Raposo C. Protective Autoimmunity: A Unifying Model for the Immune Network Involved in CNS Repair. Neuroscientist. 2014;20:343–358. doi: 10.1177/1073858413516799. [DOI] [PubMed] [Google Scholar]
- 104.Schwartz M, Baruch K. Breaking peripheral immune tolerance to CNS antigens in neurodegenerative diseases: Boosting autoimmunity to fight-off chronic neuroinflammation. J Autoimmun. 2014;54C:8–14. doi: 10.1016/j.jaut.2014.08.002. doi: 10.1016/j.jaut.2014.08.002. Epub;%2014 Sep 8.:8-14. [DOI] [PubMed] [Google Scholar]
- 105.Hansson GK, Nilsson J. Vaccination against atherosclerosis? Induction of atheroprotective immunity. Semin Immunopathol. 2009;31:95–101. doi: 10.1007/s00281-009-0151-x. [DOI] [PubMed] [Google Scholar]
- 106.Freigang S, Horkko S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol. 1998;18:1972–1982. doi: 10.1161/01.atv.18.12.1972. [DOI] [PubMed] [Google Scholar]
- 107.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 108.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci U S A. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Binder CJ, Hartvigsen K, Witztum JL. Promise of immune modulation to inhibit atherogenesis. J Am Coll Cardiol. 2007;50:547–550. doi: 10.1016/j.jacc.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 110.Nilsson J, Wigren M, Shah PK. Vaccines against atherosclerosis. Expert Rev Vaccines. 2013;12:311–321. doi: 10.1586/erv.13.4. [DOI] [PubMed] [Google Scholar]
- 111.Kimura T, Tse K, Sette A, Ley K. Vaccination to modulate atherosclerosis. Autoimmunity. 2015;48:152–60. doi: 10.3109/08916934.2014.1003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fredrikson GN, Hedblad B, Berglund G, Alm R, Ares M, Cercek B, Chyu KY, Shah PK, Nilsson J. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:872–878. doi: 10.1161/01.ATV.0000067935.02679.B0. [DOI] [PubMed] [Google Scholar]
- 113.Fredrikson GN, Soderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23:879–884. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- 114.Klingenberg R, Lebens M, Hermansson A, Fredrikson GN, Strodthoff D, Rudling M, Ketelhuth DF, Gerdes N, Holmgren J, Nilsson J, Hansson GK. Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:946–952. doi: 10.1161/ATVBAHA.109.202671. [DOI] [PubMed] [Google Scholar]
- 115.van Puijvelde GH, Hauer AD, de VP, van den Heuvel R, van Herwijnen MJ, van der Zee R, van EW, van Berkel TJ, Kuiper J. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–1976. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- 116.Zhong Y, Wang X, Ji Q, Mao X, Tang H, Yi G, Meng K, Yang X, Zeng Q. CD4+LAP + and CD4 +CD25 +Foxp3 + regulatory T cells induced by nasal oxidized low-density lipoprotein suppress effector T cells response and attenuate atherosclerosis in ApoE−/− mice. J Clin Immunol. 2012;32:1104–1117. doi: 10.1007/s10875-012-9699-7. [DOI] [PubMed] [Google Scholar]
- 117.Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708–15. doi: 10.1161/01.cir.0000029750.99462.30. [DOI] [PubMed] [Google Scholar]
- 118.van Puijvelde GH, T. vE, van Wanrooij EJ, Habets KL, de VP, van der Zee R, van EW, van Berkel TJ, Kuiper J. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2677–2683. doi: 10.1161/ATVBAHA.107.151274. [DOI] [PubMed] [Google Scholar]
- 119.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ketelhuth DF, Hansson GK. Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb Haemost. 2011;106:779–786. doi: 10.1160/TH11-05-0321. [DOI] [PubMed] [Google Scholar]
- 121.Herbin O, Ait-Oufella H, Yu W, Fredrikson GN, Aubier B, Perez N, Barateau V, Nilsson J, Tedgui A, Mallat Z. Regulatory T-cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:605–612. doi: 10.1161/ATVBAHA.111.242800. [DOI] [PubMed] [Google Scholar]
- 122.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J Clin Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS One. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maganto-Garcia E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–195. doi: 10.1161/CIRCULATIONAHA.110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mausner-Fainberg K, Luboshits G, Mor A, Maysel-Auslender S, Rubinstein A, Keren G, George J. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis. 2008;197:829–839. doi: 10.1016/j.atherosclerosis.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 126.Zhang D, Wang S, Guan Y, Wang L, Xie W, Li N, Zhao P, Su G. Effect of oral atorvastatin on CD4+CD25+ regulatory T cells, FoxP3 expression, and prognosis in patients with ST-segment elevated myocardial infarction before primary percutaneous coronary intervention. J Cardiovasc Pharmacol. 2011;57:536–541. doi: 10.1097/FJC.0b013e318211d016. [DOI] [PubMed] [Google Scholar]
- 127.Meng X, Zhang K, Li J, Dong M, Yang J, An G, Qin W, Gao F, Zhang C, Zhang Y. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol Med. 2012;18:598–605. doi: 10.2119/molmed.2011.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 129.Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T-cell immunity in atherosclerosis. Trends Cardiovasc Med. 2007;17:113–118. doi: 10.1016/j.tcm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 130.Klingenberg R, Gerdes N, Badeau RM, Gistera A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Luscher TF, Jauhiainen M, Sparwasser T, Hansson GK. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 132.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH. B7-1/B7-2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low- density lipoprotein receptor-deficient mice. Circulation. 2004;109:2009–2015. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- 134.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina EV, Miller Y, Acton ST, Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. JCI. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–1091. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- 137.Tse K, Gonen A, Sidney J, Ouyang H, Witztum JL, Sette A, Tse H, Ley K. Atheroprotective Vaccination with MHC-II Restricted Peptides from ApoB-100. Frontiers in Immunology. 2013;4:493. doi: 10.3389/fimmu.2013.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]