Abstract
After a Turkish scientist took Nobel Prize due to his contributions to understand clock genes, melatonin, closely related to these genes, may begin to shine. Melatonin, a hormone secreted from the pineal gland at night, plays roles in regulating sleep-wake cycle, pubertal development and seasonal adaptation. Melatonin has antinociceptive, antidepressant, anxiolytic, antineophobic, locomotor activity-regulating, neuroprotective, anti-inflammatory, pain-modulating, blood pressure-reducing, retinal, vascular, anti-tumor and antioxidant effects. It is related with memory, ovarian physiology, and osteoblast differentiation. Pathologies associated with an increase or decrease in melatonin levels are summarized in the review. Melatonin affects by four mechanisms: 1) Binding to melatonin receptors in plasma membrane, 2) Binding to intracellular proteins such as calmoduline, 3) Binding to Orphan nuclear receptors, and 4) Antioxidant effect. Receptors associated with melatonin are as follows: 1) Melatonin receptor type 1a: MT1 (on cell membrane), 2) Melatonin receptor type 1b: MT2 (on cell membrane), 3) Melatonin receptor type 1c (found in fish, amphibians and birds), 4) Quinone reductase 2 enzyme (MT3 receptor, a detoxification enzyme), 5) RZR/RORα: Retinoid-related Orphan nuclear hormone receptor (with this receptor, melatonin binds to the transcription factors in nucleus), and 6) GPR50: X-linked Melatonin-related Orphan receptor (it is effective in binding of melatonin to MT1). Melatonin agonists such as ramelteon, agomelatine, circadin, TIK-301 and tasimelteon are introduced and side effects will be discussed. In conclusion, melatonin and related drugs is a new and promising era for medicine. Melatonin receptors and melatonin drugs will take attention with greater interest day by day in the future.
Keywords: Melatonin, receptors, agonists, pineal gland
Öz
Saat genlerini anlamamıza olan katkısından dolayı bir Türk bilim adamı Nobel ödülünü aldıktan sonra, bu genlerle yakın ilişkili olan melatoninin önemi arttı. Melatonin, pineal bezden gece salınan bir hormondur, uyku/uyanıklık döngüsünde, pubertal gelişimde ve mevsimsel adaptasyonda rol alır. Melatoninin antinosiseptif, antidepresan, anksiyolitik, antineofobik, locomotor aktiviteyi düzenleyici, nöroprotektif, antiinflamatuvar, ağrı düzenleyici, kan basıncını düşürücü, retinal, vasküler, tümör baskılayıcı ve antioksidan etkileri vardır. Hafıza, over fizyolojisi ve osteoblast diferansiasyonuyla ilişkilidir. Melatonin seviyelerinde artış veya azalışla seyreden patolojiler derlemede özetlenmiştir. Melatonin 4 mekanizmayla etkir: 1) Plazma membranında melatonin reseptörlerine bağlanma, 2) Kalmodulin gibi hücre içi reseptörlere bağlanma, 3) Yetim nükleer reseptörlere bağlanma, 4) Antioksidan etkiler. Melatoninle ilişkili reseptörler şunlardır: 1) Melatonin reseptör tip1a: MT1 (hücre zarında), 2) Melatonin reseptör tip1b: MT2 (hücre zarında), 3) Melatonin reseptör tip1c (balık, amfibiler ve kuşlarda bulunur), 4) Kuinon redüktaz-2 enzimi (MT3 reseptörü, bir detoksifikasyon enzimi), 5) RZR/RORα: Retinoid-ilişkili Yetim çekirdek hormon reseptörü (bu reseptörle melatonin çekirdekte transkripsiyon faktörlerine bağlanır), 6) GPR50: X-geçişli Melatonin-ilişkili yetim reseptör (melatoninin MT1’e bağlanmasında etkilidir). Ramelteon, agomelatin, sirkadin, TIK-301 and tasimelteon gibi Melatonin agonistleri tanıtılacak ve yan etkileri karşılaştırılacaktır. Sonuçta, melatonin ve ilişkili ilaçlar tıpta yeni ve umut vaat eden bir alandır. Melatonin reseptörleri ve ilaçları gelecekte de artan oranda ilgi çekmeye devam edecektir.
Melatonin and Associated Pathologies
Melatonin is a hormone secreted from the pineal gland at night. Its peak levels in the dark are associated with age as well as various illnesses (Figure 1). Melatonin plays roles in regulating sleep-wake cycle, pubertal development and seasonal adaptation [1]. Melatonin is related with memory, and its associations with control of body posture and balance have been shown [1]. Melatonin regulates memory formation by directly affecting hippocampal neurons [2]. Melatonin has antinociceptive, antidepressant, anxiolytic, antineophobic (being afraid of new things) and locomotor activity-regulating effects [3]. There are neuroprotective, anti-inflammatory, pain-modulating, blood pressure-reducing, retinal, vascular, seasonal reproductive, ovarian physiology, osteoblast differentiation, anti-tumor and antioxidant effects of melatonin [2, 4].
Figure 1.
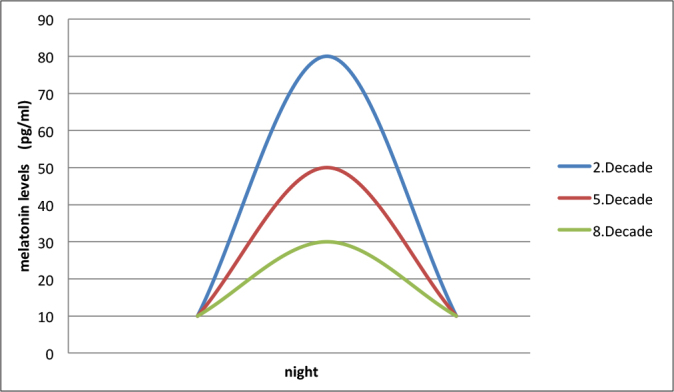
Peak melatonin levels at night tend to decrease with advanced age in human.
Dopaminergic system is important for behavior and rewarding and also in cases of drug addiction such as cocaine [3]. Melatonin inhibits dopamine release [3]. The increase in melatonin receptor-related cAMP in the mesolimbic dopaminergic system shows that the effect of melatonin may be present in regulation of addictive behavior [1]. It corrects the behavior disorders related to dopamine addiction and alleviates the findings of cocaine abstinence [3]. Other effects of melatonin are inhibition of dopamine release in hypothalamus and retina [1]. The anti-excitatory effects of melatonin are probably secondary to its antioxidant effect [5]. When it is administered in pharmacological doses in children, it leads to reduction in severity and frequency of epileptiform activity [6]. It shows an effect contrary to glutamate, which is an excitatory neurotransmitter, i.e. it is an inhibitor [3].
High melatonin level is associated with exercise-related menstrual disorders, oligospermia and delayed puberty [7]. Melatonin regulates the secretion of gonadotrophin-releasing hormone (GnRH) from the hypothalamic neurons [8]. GnRH controls the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Melatonin stimulates the secretion of progesterone from granulosa cells [8]. Melatonin also suppresses the expression of estrogen receptor and estrogen activation [9].
Neurological disorders which have been reported to be improved by administration of melatonin are as follows: Parkinsonism [10], Alzheimer’s disease [11], brain edema and traumatic brain injury [12], alcoholism [13], depression [14], cerebral ischemia [15], hyperhomocysteinuria [16], glioma [17] and phenylketonuria [18]. Alzheimer’s disease is an age-related progressive neurodegenerative disorder, characterized with loss of cognitive functions, dementia and other neurobiological findings [2, 19]. Melatonin was shown to enhance antifibrillogenic (inhibiting amyloidosis) effects [20].
The disorders in which variations in production of endogenous melatonin production were shown are as follows: Sleep disorders, Alzheimer’s disease, Parkinson’s disease, glaucoma, depression, breast cancer, prostate cancer, hepatoma, melanoma, congestive heart failure, cardiac syndrome X and sepsis [1, 21]. Melatonin production is reduced with aging, various cancers, Alzheimer’s disease, senile dementia, pineal calcification [22], cardiovascular disorders and hypothalamic hamartoma or craniopharyngioma which lead to precocious puberty in youngsters [1, 23]. Other disorders in which melatonin was shown to be reduced are stress, pain, endocrine and metabolic disorders, particularly DM type 2 and acute intermittent porphyria [24].
Intravenous administration of melatonin increases peripheral blood circulation [25]. Melatonin directly leads to vasoconstriction in cerebral arteries [8]. Daytime melatonin administration lowers the body temperature due to vasodilation in distal parts of the body [1, 26, 27]. The effects of melatonin on blood vessels seem to be related to its noradrenergic effects and/or its effects on NO [21]. Vasodilator and peripheral resistance-reducing effect may be related to NO potentialisation, anti-noradrenergic mechanism or inhibition of vasopressin release [21]. Activation of MT1 receptors leads to vasoconstriction and activation of MT2 receptors leads to vasodilation [1].
The Mechanism of Effect of Melatonin
Melatonin shows its affects by four mechanisms in mammals:
Binding to melatonin receptors in plasma membrane
Binding to intracellular proteins such as calmoduline
Binding to Orphan nuclear receptors
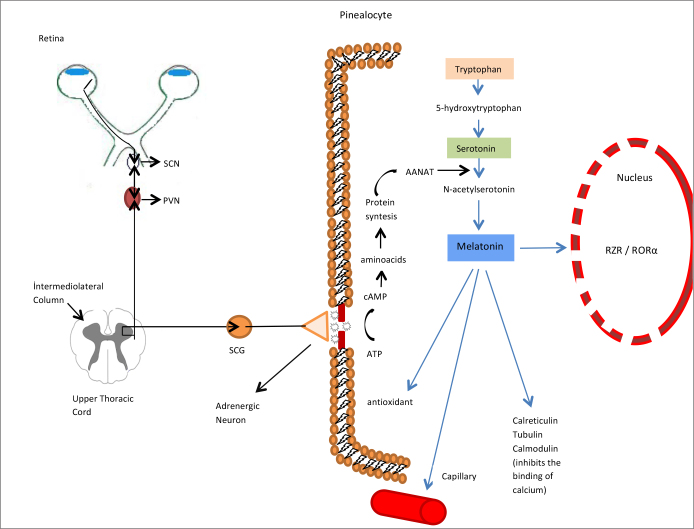
Figure 2.
The neurologic pathway from the eyes through the pineal gland.
After adrenergic stimuli, intracellular synthesis of melatonin in the pinealocyte and its effects are summarized.
SCN: suprachiasmatic nucleus; PVN: paraventricular nucleus of the hypothalamus; SCG: superior cervical ganglion; RZR/RORα: Retinoid-related Orphan nuclear hormone receptor.
Melatonin interacts with intracellular proteins named calmoduline, calreticulin and tubulin [1]. Calmoduline is an intracellular secondary messenger. Melatonin directly antagonizes binding of calcium to calmoduline [1, 21] (Figure 2). The anti-proliferative effect in cancer may be related to this. Retinoid-related Orphan nuclear hormone receptor family (RZR/ROR) is responsible for the immunomodulatory effects of melatonin. IL-2 and IL-6 are produced in mononuclear cells by this mechanism [21] (Figure 2).
Melatonin receptors
The locations that melatonin receptors are found in the body are as follows: brain, retina, cardiovascular system, cardiac ventricular wall, aorta, coronary and cerebral arteries, liver and gallbladder, duodenal enterocytes, colon, cecum and appendix vermiformis, skin, parotid gland, exocrine pancreas, kidney, cells of immune system, platelets, brown and white adipocytes, epithelial cells of prostate and breast, ovary/granulosa cells, myometrium, placenta and fetal kidney [3, 24]. In gastrointestinal system, melatonin receptors are found most commonly in jejunal and colonic mucosa [1].
There are three different membrane receptors and one nuclear receptor:
-
Melatonin receptor type 1a: Mel 1a, ML1a, ML1, MT1, MTNR1A
It is encoded in human chromosome #4 and consists of 351 amino acids [4]. MT1 receptor constitutes adenylate cyclase inhibition by binding to various G-proteins [2]. MT1 receptors are commonly found in human skin [1]. During aging process and Alzheimer’s disease, the expression of MT1 receptor in suprachiasmatic nucleus (SCN) and cortex decreases [1]. MT1 receptors reduce the neuronal discharge rate in SCN and suppress prolactin secretion [8].
-
Melatonin receptor type 1b: Mel 1b, ML1b, MT2, MTNR1B
It is encoded in human chromosome #11 and consists of 363 amino acids [4]. MT2 receptor creates adenylate cyclase inhibition by binding to various G-proteins. Additionally, it inhibits the soluble guanylyl cyclase pathway [2]. Through melatonin receptor activation, adenylate cyclase inhibition occurs and the production of cyclic AMP (cAMP) is reduced [28, 29].
In the skin, MT2 receptors are located within normal and malign melanocytes and eccrine sweat glands [1]. MT2 receptors inhibit GABA-A receptor-related functions in the hippocampus in rats [8].
In Alzheimer’s disease, MT2 receptor expression is reduced. MT2 receptors are involved in antidepressant activity [24]. MT2 receptors contribute to the pathophysiology and pharmacology of sleep disorders, anxiety, depression, Alzheimer’s disease and pain [2]. MT2 receptors may be the new target for development of hypnotic agents [2]. MT2 receptors are responsible for anxiolytic effects of melatonin. Pharmacological studies have revealed that MT2 receptors regulate sleep, particularly NREMS [2]. MT2 receptor ligands have more powerful hypnotic properties when compared to non-selective MT1/ MT2 ligands [2].
Mel1c, MTNR1C: It is not present in humans. It is found in fish, amphibians and birds [4]. In chicken, the rhythm of MTNR1C receptor is the opposite of MT1 and MT2. Its level is highest at daytime and lowest at nighttime [4, 30].
MT3, ML2= NQO2= Quinone reductase 2 enzyme= QR2. This enzyme belongs to the reductase group, which are involved in prevention from oxidative stress by inhibiting the electron transfer reactions of quinones [1]. This enzyme (or MT3 receptor) is located in liver, kidney, heart, lung, intestine, muscle and brown fat tissue. It is a detoxification enzyme [21]. There is evidence for its involvement in regulation of intra-ocular pressure [21].
-
RZR/RORα: Retinoid-related Orphan nuclear hormone receptor
With this receptor, melatonin binds to the transcription factors in nucleus which belong to retinoic acid receptor super-family. The following are described for retinoic acid receptor super-family variants RORα (retinoic acid receptor-related Orphan receptor-α; human gene ID: 6095): RORα isoform a (aka RORα1), RORα isoform b (aka RORα2) and RORα isoform d (also known as RZRα), and the product of another gene, RORβ (aka RZRβ; human gene ID: 6096) [1].
GPR50: H9, ML1X: Melatonin-related Orphan receptor. ‘X linked Orphan G-protein coupled’ (It is an X-linked inherited receptor, binding to G-protein. It is the orthologue of MEL1c, which is found in non-mammalian living creatures [31]. Its gene is located on the X chromosome (Xq28) and consists of 618 amino acids [4]. It is present in all mammalians including humans. It does not have the characteristics of binding to melatonin [21]; however, it is effective in binding of melatonin to MT1 [32]. GPR50 is not present in birds and fish [4, 33]. It is located in the brain and periphery. Its natural ligand has not been defined yet. It was reported that a deletion mutant in GPR50 might have been associated with bipolar disorder and major depression [34]. GPR50 has no affinity to melatonin; however, when it dimerizes with MT1, it inhibits the melatonin signal [29, 35]. GPR50 has other functions apart from melatonin [4]. GPR50 interacts [4] with neurite outgrow inhibitor (NOGO-A) [4, 36] and TIP60 (glucocorticoid receptor signal coactivator and histone acetyltransferase) [4, 37].
After MT1 and MT2 receptors adhere to the cell surface, they create their effects through G-protein. Activation of MT1 receptor leads to inhibition of cAMP formation which was stimulated by forkolin, together with inhibition of Protein kinase A (PKA) [21]. Similarly, activation of MT2 receptor leads to inhibition of cAMP formation, which was stimulated by forkolin [21]. Additionally, it inhibits formation of cGMP [21]. While membrane receptors are basically located in the central nervous system, RZR/RORα is located at both periphery and the brain [9]. Membrane receptors and their specific agonists are associated with circadian rhythm, whereas RZR/ RORα seems to be responsible for immunomodulation at the periphery, cellular growth and differentiation of bone [9]. Activation of Protein kinase C-α is a critical step in the formation of melatonin effect [1].
Development of pharmacological agents, which are effective on MT receptors, may be associated with antihypertensive, anti-cancer or immunostimulant effects or they may facilitate falling asleep [21]. In addition to its anti-inflammatory effect, its immunostimulant effect is an undesired situation in autoimmune disorders and melatonergic drugs may be contraindicated in such patients [24]. For example, melatonin aggravates the symptoms of rheumatoid arthritis by stimulating proinflammatory cytokines [38].
Melatonin Agonist Pharmaceuticals
For people having difficulties in falling asleep, short-acting drugs are sufficient. Melatonin reduces the sleep latency, even small doses such as 0.1–0.3 mg/day are sufficient for this purpose [39]. All synthetic melatonergic drugs can provide this effect.
Ramelteon (Rozerem©, Takeda, Japan; TAK-375): It is a non-selective (MT1/MT2) melatonin receptor agonist. In US, it received approval from FDA in 2005 for treatment of insomnia in individuals having difficulty in falling asleep [40]. Ramelteon is rapidly absorbed from gastrointestinal system with a rate of 84% and its half-life in blood circulation is 1–2 hours [41]. Among melatonergic agonist drugs, Ramelteon is the one having relatively higher affinity to both receptor subtypes [24]. In elderly individuals having primary chronic insomnia, the effect of Ramelteon in maintaining sleep has been found to be highly variable [42]. Ramelteon is metabolized basically by CYP1A2, CYP2C9 and CYP3A4 [43].
Agomelatine (Valdoxan©, Servier, France; S20098): In November 2008, it received approval from EMA (European Medicines Agency) in Europe for treatment of major depression in adults [2, 24, 44]. It is a non-selective (MT1/MT2) melatonin receptor agonist + serotonergic 5-HT2c antagonist. It is the first reported melatonergic drug having anxiolytic and antidepressant effects [1]. In major depression, it is used with a daily dose of 25 mg. In seasonal affective disorder, it can be effective in very small doses (0.225–0.3 mg/day), without affecting sleep [45]. The half-life of agomelatine is 1–2 hours [24]. Inhibition of 5HT2c is held responsible for its direct antidepressant effect [46]. The advantage of agomelatin is not its better antidepressant effect, but its improving effect on sleep together with its antidepressant effects. Indeed, conventional antidepressants often trigger sleep disorders [24]. Agomelatine is metabolized basically by CYP1A1, CYP1A2 and CYP2C9 [43].
-
Melatonin controlled release tablets (prolonged release melatonin) (Circadin©, Neurim, Israel and UK): It received approval from EMA (European Medicines Agency) for insomnia treatment in patients over 55 years of age in Europe [24]. Its dose is 2 mg/day. In a study conducted on Amyotrophic lateral sclerosis patients, even doses of 300 mg/day for up to 2 years was found to be safe [47].
The affinity of natural melatonin hormone is higher to MT1 than MT2. This situation is valid for ramelteon and agomelatine, also [24]. The circulatory half-life of melatonin is very short, being 20–30 minutes in average with a maximum of 45 minutes [48]. As a solution for this problem, controlled release tablets of the hormone or synthetic products with longer half-lives were developed.
TIK-301: It is a melatonergic agonist and serotonergic antagonist drug. It is a more powerful antagonist of serotonergic 5-HT2c and 5-HT2b receptors when compared to agomelatine [49]. Theoretically, it has an antidepressant effect. Its half-life is one hour [50]. It received approval from FDA for using in sleep disorders of visually impaired individuals [24].
Tasimelteon (Hetlioz, Vanda Pharmaceuticals, USA): It is approved by FDA in January, 2014 for the treatment of non-24-hour sleep–wake disorder. Later in July 2015, it was approved in Europe for the treatment of non-24-hour sleep-wake rhythm disorder in totally blind adults. Its half-life is approximately 2 hours. Its sleep-initiating and antidepressant effects are on trial [24]. It is basically metabolized by CYP1A1, CYP1A2, CYP2D6 and CYP2C9 [43].
Liver failure, renal failure, alcohol addiction and high lipid levels are contraindications for melatonin agonists [51].
The adverse effects of melatonin agonists are as follows: nausea, headache and elevations in some liver parameters, rebound insomnia, withdrawal symptoms when used for 6 to 12 months or addiction [24]. There is a risk for hepatotoxicity [52]. In experimental animal studies, it is carcinogenic in very high doses [52].
In a recent systematic review, the most common side effects related to melatonin agonists have been reported as headache, somnolence, palpitations and abdominal pain [53]. Rarely reported side effects are nasopharyngitis, arthralgia, tachycardia, dizziness, nausea, vomiting, nightmares, difficulties in swallowing and breathing, hypnotic activity, feeling of heaviness in the head, heartburn, belching, arm and leg swelling, sweating, hot flushes, exanthema, sleep problems, depression and sleep-walking [53]. A brief summary of altered melatonin metabolism on different systems and pathologies are demonstrated in Table 1.
Table 1.
Altered melatonin metabolism on different systems and pathologies
| System or Mechanism | Effect |
|---|---|
| Sleep Modulator | Treatment of Jet Lag, Treatment of Phase Shift |
| Psychiatric | Anti-depressant, Anxiolytic, Antineophobic, Drug addiction treatment |
| Central Nervous System | Neuroprotective, Anti-inflammatory, Pain-modulating, Regulating memory formation, Brain edema treatment, Antiepileptic in children |
| Endocrin System | Seasonal reproductive, Ovarian physiology, Regulating reproductive hormon release, Osteoblast differentiation, Type 2 DM |
| Autoimmune Diseases | Multiple Sclerosis, Type 1 DM, Inflammatory Bowel Disease, SLE in females, Rheumatoid arthritis, Autoimmune hepatitis |
| Cardiovascular System | Antihypertensive, Cardiac Syndrome X |
| Locomotor System | Antinociceptive, Locomotor activity-regulating |
| Oncology | Anti-tumor |
| Other | Antioxidant, Retinal, Hepatoma, Pineal calcification, Sepsis |
Important Melatonin Antagonist Pharmaceuticals
Conclusion
Melatonin and related drugs are a new and promising era for medicine. Melatonin is highly associated with circadian rhythm and circadian clock genes. The first Turkish scientist and biochemist Aziz Sancar was honored by Nobel Prize due to his contributions to understand the mechanism in this field. Hypothalamic suprachiasmatic nucleus expresses various clock genes in a rhythmic fashion. For example expressions in diurnal variations in per1, a clock gene, depends on pineal gland and melatonin secretion [55]. Melatonin receptors and melatonin drugs will take attention with greater interest day by day in the future.
Acknowledgments
We wish to thank to Dr. Sibel Guclu for her contributions to drawing the images.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.E.; Design - M.E., H.A.; Supervision - M.E., Z.H., A.H.; Resources - L.O., M.H.; Materials - L.O., M.H.; Data Collection and/or Processing - M.E., Z.H.; Analysis and/or Interpretation - M.E., Z.H., A.H.; Literature Search - H.O., L.O., M.Y.; Writing Manuscript - M.E., H.O., L.O., M.Y.; Critical Review - M.E., Z.H., A.H.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Pandi-Perumal SR, Trakht I, Srinivasan V, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335–53. doi: 10.1016/j.pneurobio.2008.04.001. http://dx.doi.org/10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Comai S, Gobbi G. Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psychiatry Neurosci. 2014;39:6–21. doi: 10.1503/jpn.130009. http://dx.doi.org/10.1503/jpn.130009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uz T, Arslan AD, Kurtuncu M, et al. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res. 2005;136:45–53. doi: 10.1016/j.molbrainres.2005.01.002. http://dx.doi.org/10.1016/j.molbrainres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Li DY, Smith DG, Hardeland R, et al. Melatonin receptor genes in vertebrates. Int J Mol Sci. 2013;14:11208–23. doi: 10.3390/ijms140611208. http://dx.doi.org/10.3390/ijms140611208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bondy SC, Sharman EH. Melatonin and the aging brain. Neurochem Int. 2007;50:571–80. doi: 10.1016/j.neuint.2006.12.014. http://dx.doi.org/10.1016/j.neuint.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Fauteck J, Schmidt H, Lerchl A, et al. Melatonin in epilepsy: first results of replacement therapy and first clinical results. Biol Signals Recept. 1999;8:105–10. doi: 10.1159/000014577. http://dx.doi.org/10.1159/000014577. [DOI] [PubMed] [Google Scholar]
- 7.Genell H. Melatonin and the pineal gland. Journal of Neuroscience Nursing. 2002;34:74–8. http://dx.doi.org/10.1097/01376517-200204000-00006. [Google Scholar]
- 8.Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci. 2003;8:d1093–1108. doi: 10.2741/1089. http://dx.doi.org/10.2741/1089 [DOI] [PubMed] [Google Scholar]
- 9.Carlberg C. Gene regulation by melatonin. Ann N Y Acad Sci. 2000;917:387–96. doi: 10.1111/j.1749-6632.2000.tb05403.x. http://dx.doi.org/10.1111/j.1749-6632.2000.tb05403.x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas B, Mohanakumar KP. Melatonin protects against oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the mouse nigrostriatum. J Pineal Res. 2004;36:25–32. doi: 10.1046/j.1600-079x.2003.00096.x. http://dx.doi.org/10.1046/j.1600-079X.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan V, Pandi-Perumal SR, Cardinali DP, Poeggeler B, Hardeland R. Melatonin in Alzheimer’s disease and other neurodegenerative disorders. Behav Brain Funct. 2006;2:15. doi: 10.1186/1744-9081-2-15. http://dx.doi.org/10.1186/1744-9081-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehghan F, Khaksari Hadad M, Asadikram G, Najafipour H, Shahrokhi N. Effect of melatonin on intracranial pressure and brain edema following traumatic brain injury: role of oxidative stresses. Arch Med Res. 2013;44:251–8. doi: 10.1016/j.arcmed.2013.04.002. http://dx.doi.org/10.1016/j.arcmed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Baydas G, Yasar A, Tuzcu M. Comparison of the impact of melatonin on chronic ethanol-induced learning and memory impairment between young and aged rats. J Pineal Res. 2005;39:346–52. doi: 10.1111/j.1600-079X.2005.00257.x. http://dx.doi.org/10.1111/j.1600-079X.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 14.Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S, Nelson RJ. Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull. 2006;68:425–9. doi: 10.1016/j.brainresbull.2005.09.016. http://dx.doi.org/10.1016/j.brainresbull.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Lee EJ, Lee MY, Chen HY, et al. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J Pineal Res. 2005;38:42–52. doi: 10.1111/j.1600-079X.2004.00173.x. http://dx.doi.org/10.1111/j.1600-079X.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 16.Baydas G, Ozer M, Yasar A, Tuzcu M, Koz ST. Melatonin improves learning and memory performances impaired by hyperhomocysteinemia in rats. Brain Res. 2005;1046(1–2):187–94. doi: 10.1016/j.brainres.2005.04.011. http://dx.doi.org/10.1016/j.brainres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Martin V, Herrera F, Carrera-Gonzalez P, et al. Intracellular signaling pathways involved in the cell growth inhibition of glioma cells by melatonin. Cancer Res. 2006;66:1081–8. doi: 10.1158/0008-5472.CAN-05-2354. http://dx.doi.org/10.1158/0008-5472.CAN-05-2354. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Cruz F, Osuna C, Guerrero JM. Mitochondrial damage induced by fetal hyperphenylalaninemia in the rat brain and liver: its prevention by melatonin, Vitamin E, and Vitamin C. Neurosci Lett. 2006;392:1–4. doi: 10.1016/j.neulet.2005.02.073. http://dx.doi.org/10.1016/j.neulet.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 19.Di Carlo M, Giacomazza D, San Biagio PL. Alzheimer’s disease: biological aspects, therapeutic perspectives and diagnostic tools. J Phys Condens Matter. 2012;24:244102. doi: 10.1088/0953-8984/24/24/244102. http://dx.doi.org/10.1088/0953-8984/24/24/244102 [DOI] [PubMed] [Google Scholar]
- 20.Poeggeler B, Miravalle L, Zagorski MG, et al. Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Abeta peptide. Biochemistry. 2001;40:14995–5001. doi: 10.1021/bi0114269. http://dx.doi.org/10.1021/bi0114269. [DOI] [PubMed] [Google Scholar]
- 21.Ekmekcioglu C. Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother. 2006;60:97–108. doi: 10.1016/j.biopha.2006.01.002. http://dx.doi.org/10.1016/j.biopha.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Kunz D, Schmitz S, Mahlberg R, et al. A new concept for melatonin deficit: on pineal calcification and melatonin excretion. Neuropsychopharmacology. 1999;21:765–72. doi: 10.1016/S0893-133X(99)00069-X. http://dx.doi.org/10.1016/S0893-133X(99)00069-X. [DOI] [PubMed] [Google Scholar]
- 23.Lipton J, Megerian JT, Kothare SV, et al. Melatonin deficiency and disrupted circadian rhythms in pediatric survivors of craniopharyngioma. Neurology. 2009;73:323–5. doi: 10.1212/WNL.0b013e3181af78a5. http://dx.doi.org/10.1212/WNL.0b013e3181af78a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardeland R. Melatonin in aging and disease -multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012;3:194–225. [PMC free article] [PubMed] [Google Scholar]
- 25.van der Helm-van Mil AH, van Someren EJ, van den Boom R, et al. No influence of melatonin on cerebral blood flow in humans. J Clin Endocrinol Metab. 2003;88:5989–94. doi: 10.1210/jc.2003-031107. http://dx.doi.org/10.1210/jc.2003-031107. [DOI] [PubMed] [Google Scholar]
- 26.Krauchi K, Cajochen C, Wirz-Justice A. A relationship between heat loss and sleepiness: effects of postural change and melatonin administration. J Appl Physiol (1985) 1997;83:134–9. doi: 10.1152/jappl.1997.83.1.134. [DOI] [PubMed] [Google Scholar]
- 27.van den Heuvel CJ, Kennaway DJ, Dawson D. Thermoregulatory and soporific effects of very low dose melatonin injection. Am J Physiol. 1999;276:E249–54. doi: 10.1152/ajpendo.1999.276.2.E249. [DOI] [PubMed] [Google Scholar]
- 28.Chaste P, Clement N, Botros HG, et al. Genetic variations of the melatonin pathway in patients with attention-deficit and hyper-activity disorders. J Pineal Res. 2011;51:394–9. doi: 10.1111/j.1600-079X.2011.00902.x. http://dx.doi.org/10.1111/j.1600-079X.2011.00902.x. [DOI] [PubMed] [Google Scholar]
- 29.Levoye A, Dam J, Ayoub MA, et al. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006;25:3012–23. doi: 10.1038/sj.emboj.7601193. http://dx.doi.org/10.1038/sj.emboj.7601193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rada JA, Wiechmann AF. Melatonin receptors in chick ocular tissues: implications for a role of melatonin in ocular growth regulation. Invest Ophthalmol Vis Sci. 2006;47:25–33. doi: 10.1167/iovs.05-0195. http://dx.doi.org/10.1167/iovs.05-0195. [DOI] [PubMed] [Google Scholar]
- 31.Dufourny L, Levasseur A, Migaud M, et al. GPR50 is the mammalian ortholog of Mel1c: evidence of rapid evolution in mammals. BMC Evol Biol. 2008;8:105. doi: 10.1186/1471-2148-8-105. http://dx.doi.org/10.1186/1471-2148-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsch-Rodriguez E, Imbesi M, Manev R, Uz T, Manev H. The pattern of melatonin receptor expression in the brain may influence antidepressant treatment. Med Hypotheses. 2007;69:120–4. doi: 10.1016/j.mehy.2006.11.012. http://dx.doi.org/10.1016/j.mehy.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gubitz AK, Reppert SM. Assignment of the melatonin-related receptor to human chromosome X (GPR50) and mouse chromosome X (Gpr50) Genomics. 1999;55:248–51. doi: 10.1006/geno.1998.5661. http://dx.doi.org/10.1006/geno.1998.5661. [DOI] [PubMed] [Google Scholar]
- 34.Thomson PA, Wray NR, Thomson AM, et al. Sex-specific association between bipolar affective disorder in women and GPR50, an X-linked orphan G protein-coupled receptor. Mol Psychiatry. 2005;10:470–8. doi: 10.1038/sj.mp.4001593. http://dx.doi.org/10.1038/sj.mp.4001593. [DOI] [PubMed] [Google Scholar]
- 35.Chaste P, Clement N, Mercati O, et al. Identification of pathway-biased and deleterious melatonin receptor mutants in autism spectrum disorders and in the general population. PLoS One. 2010;5:e11495. doi: 10.1371/journal.pone.0011495. http://dx.doi.org/10.1371/journal.pone.0011495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunewald E, Kinnell HL, Porteous DJ, Thomson PA. GPR50 interacts with neuronal NOGO-A and affects neurite outgrowth. Mol Cell Neurosci. 2009;42:363–71. doi: 10.1016/j.mcn.2009.08.007. http://dx.doi.org/10.1016/j.mcn.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Hand LE, Meng QJ, Loudon AS, Bechtold DA. GPR50 interacts with TIP60 to modulate glucocorticoid receptor signalling. PLoS One. 2011;6:e23725. doi: 10.1371/journal.pone.0023725. http://dx.doi.org/10.1371/journal.pone.0023725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forrest CM, Mackay GM, Stoy N, Stone TW, Darlington LG. Inflammatory status and kynurenine metabolism in rheumatoid arthritis treated with melatonin. Br J Clin Pharmacol. 2007;64:517–26. doi: 10.1111/j.1365-2125.2007.02911.x. http://dx.doi.org/10.1111/j.1365-2125.2007.02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandi-Perumal SR, Srinivasan V, Poeggeler B, Hardeland R, Cardinali DP. Drug Insight: the use of melatonergic agonists for the treatment of insomnia-focus on ramelteon. Nat Clin Pract Neurol. 2007;3:221–8. doi: 10.1038/ncpneuro0467. http://dx.doi.org/10.1038/ncpneuro0467. [DOI] [PubMed] [Google Scholar]
- 40.McGechan A, Wellington K. Ramelteon. CNS Drugs. 2005;19:1057–1065. doi: 10.2165/00023210-200519120-00007. discussion 1066-1057. [DOI] [PubMed] [Google Scholar]
- 41.Karim A, Tolbert D, Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high-affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia. J Clin Pharmacol. 2006;46:140–8. doi: 10.1177/0091270005283461. http://dx.doi.org/10.1177/0091270005283461. [DOI] [PubMed] [Google Scholar]
- 42.Pandi-Perumal SR, Spence DW, Verster JC, et al. Pharmacotherapy of insomnia with ramelteon: safety, efficacy and clinical applications. J Cent Nerv Syst Dis. 2011;3:51–65. doi: 10.4137/JCNSD.S1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardeland R. New approaches in the management of insomnia: weighing the advantages of prolonged-release melatonin and synthetic melatoninergic agonists. Neuropsychiatr Dis Treat. 2009;5:341–54. doi: 10.2147/ndt.s4234. http://dx.doi.org/10.2147/NDT.S4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bodinat C, Guardiola-Lemaitre B, Mocaer E, Renard P, Munoz C, Millan MJ. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nature reviews Drug discovery. 2010;9:628–42. doi: 10.1038/nrd3140. http://dx.doi.org/10.1038/nrd3274. [DOI] [PubMed] [Google Scholar]
- 45.Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–9. doi: 10.1073/pnas.0602425103. http://dx.doi.org/10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millan MJ, Gobert A, Lejeune F, et al. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther. 2003;306:954–64. doi: 10.1124/jpet.103.051797. http://dx.doi.org/10.1124/jpet.103.051797. [DOI] [PubMed] [Google Scholar]
- 47.Weishaupt JH, Bartels C, Polking E, et al. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J Pineal Res. 2006;41:313–23. doi: 10.1111/j.1600-079X.2006.00377.x. http://dx.doi.org/10.1111/j.1600-079X.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 48.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. http://dx.doi.org/10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Landolt HP, Wehrle R. Antagonism of serotonergic 5-HT2A/2C receptors: mutual improvement of sleep, cognition and mood? Eur J Neurosci. 2009;29:1795–809. doi: 10.1111/j.1460-9568.2009.06718.x. http://dx.doi.org/10.1111/j.1460-9568.2009.06718.x. [DOI] [PubMed] [Google Scholar]
- 50.Mulchahey JJ, Goldwater DR, Zemlan FP. A single blind, placebo controlled, across groups dose escalation study of the safety, tolerability, pharmacokinetics and pharmacodynamics of the melatonin analog beta-methyl-6-chloromelatonin. Life Sci. 2004;75:1843–56. doi: 10.1016/j.lfs.2004.03.023. http://dx.doi.org/10.1016/j.lfs.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Hardeland R, Poeggeler B, Srinivasan V, Trakht I, Pandi-Perumal SR, Cardinali DP. Melatonergic drugs in clinical practice. Arzneimittelforschung. 2008;58:1–10. doi: 10.1055/s-0031-1296459. [DOI] [PubMed] [Google Scholar]
- 52.Agomelatine: new drug Adverse effects and no proven efficacy. Prescrire Int. 2009;18:241–45. [PubMed] [Google Scholar]
- 53.Costello RB, Lentino CV, Boyd CC, et al. The effectiveness of melatonin for promoting healthy sleep: a rapid evidence assessment of the literature. Nutrition journal. 2014;13:106. doi: 10.1186/1475-2891-13-106. http://dx.doi.org/10.1186/1475-2891-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferry G, Hecht S, Berger S, et al. Old and new inhibitors of quinone reductase 2. Chem Biol Interact. 2010;186:103–9. doi: 10.1016/j.cbi.2010.04.006. http://dx.doi.org/10.1016/j.cbi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Manev H, Uz T. Clock genes: influencing and being influenced by psychoactive drugs. Trends Pharmacol Sci. 2006;27:186–9. doi: 10.1016/j.tips.2006.02.003. http://dx.doi.org/10.1016/j.tips.2006.02.003 [DOI] [PubMed] [Google Scholar]