Abstract
Cyanobacteria associated with biological soil crusts (BSCs) have important attributes, such as nitrogen fixation and soil stabilisation. However, research on these organisms has been minimal, and their diversity and distribution throughout temperate Europe is currently unknown. The SCIN (Soil Crust International) project is a multidisciplinary research initiative that aims to achieve improved understanding of the BSCs of Europe, one facet being an investigation into the cyanobacterial communities of BSCs across a latitudinal gradient. Cyanobacteria assemblages were analysed by both morphological and molecular analysis. Two treatments were applied prior to DNA extraction, continued sample wetting and a dry sample process, and 16S ribosomal RNA (rRNA) amplicons were processed by Illumina MiSeq sequencing. The results reveal high and variable cyanobacterial diversity with each site showing a unique assemblage. Many common cyanobacterial genera, for example Nostoc and Microcoleus, were found in all sites but the abundances of different genera varied considerably. The polyphasic approach was found to be essential in recording the presence of important cyanobacteria that a single method itself did not highlight. The wet and dry treatments showed some differences in diversity, but mainly in abundance, this may suggest how cyanobacterial composition of BSCs changes with seasonal variability.
Keywords: cyanobacteria diversity, biological soil crust (BSC), Europe, Illumina MiSeq, 16S rRNA, morphology
Investigating the diversity and community composition of cyanobacteria associated with biological soil crusts in climatically different regions of Europe.
INTRODUCTION
Biological soil crust (BSC) organisms live within and on the surface of soil forming a crust from a few millimetres to centimetres thick (Belnap and Lange 2003). BSCs are formed by an intimate association between their many constituents such as soil particles, (cyano)-bacteria, microalgae, microfungi, bryophytes, liverworts and lichens in various compositions. BSCs are especially important in arid, semi-arid, polar and alpine environments (Büdel and Veste 2008), where climatic conditions can diminish vascular plant development. Consequently, BSC ecosystems are dominant in many regions worldwide and their composition and functions should be understood before assessing key current questions such as global nutrient cycles and possible climate change scenarios.
Elbert et al. (2012) theorised that cryptogamic covers, of which BSCs are a major proportion, are responsible for 7% of total terrestrial vegetation carbon (C) input and ca. 45% of the total amount of biologically fixed nitrogen (N). N-fixation capabilities have been shown to vary across different types of BSC. Later successional stages, colonised by Nostoc and Scytonema species, have much higher rates than earlier successional stages, dominated by Microcoleus (Belnap 2002, 2003; Housman et al. 2006), which often dominates in initial successional stages of BSCs in warm climates (Belnap 2003; Garcia-Pichel et al. 2013; Büdel et al. 2014). Cyanobacteria (especially the filamentous types) aggregate soils by exuding mucilaginous substances. Lange et al. (1992) described BSC cyanobacteria diversity of the Negev Desert in Israel, where the BSCs contribute significantly to the stabilisation of sand dunes. For instance, Microcoleus sociatus (=Trichocoleus sociatus) sheaths were found attached to quartz soil particles. The contribution of BSC cyanobacteria to N cycles and soil stabilisation highlight the importance of understanding their composition and functioning in BSCs worldwide. In addition, it has recently been suggested that climate change is likely to affect N-fixing microorganisms, especially cyanobacteria (Singh et al. 2010; Santos et al. 2014).
Nostoc sp. are widely found in all types of BSC, usually located on the surface (Belnap and Lange 2003) and are particularly evident and often visible when water availability increases. In most cases, BSCs are also dominated by filamentous cyanobacteria. Microcoleus spp., Phormidium spp., Plectonema spp., Schizothrix spp., Tolypothrix spp. and Scytonema spp. are the most common genera found in both hot and cold deserts worldwide (Johansen 1993). However, research on cyanobacterial diversity of BSCs has mainly focused on arid and semi-arid regions, such as the Negev Desert, Israel (Lange et al. 1992; Hagemann et al. 2015), the Colorado plateau and Sonoran Desert (Garcia-Pichel, López-Corté and Nübel 2001; Yeager et al. 2004; Nagy, Garcia-Pichel and Pérez 2005; Steven et al. 2013), the Desert in China (Zhang et al. 2011), Adam and Muscat in Oman (Abed et al. 2010) and the western cold deserts of the Himalayas (Janatková et al. 2013; Čapková et al. 2016). In addition, the terrestrial cyanobacterial diversity in Europe within the Arctic Circle has been investigated to some extent. Cyanobacterial dominance in terms of biomass and productivity (Vincent et al. 2000) and their being primary colonisers of moraines in the wake of receding glaciers (Solheim, Endal and Vigstad 1996) make them essential components of Arctic ecology. Pushkareva and Elster (2013) describe diversity of cyanobacteria and microalgae within BSCs from Petunia Bay, Svalbard. They classified them into three types: black-brown, brown and grey-brown, dependent on the diversity of cyanobacteria and microalgae identified. The dominant genera were found to be Gloeocapsa, Nostoc, Microcoleus, Scytonema and Chroococcus. They found that altitude does not affect the diversity of cyanobacteria and microalgae, but reported that abundance increases with altitude. Other studies, including Komárek et al. (2012, 2015) and Pushkareva et al. (2015), describe diversity of cyanobacteria in many habitats in Svalbard including tundra and soils. However, studies on European BSC cyanobacteria outside Arctic areas have seen little attention and BSCs themselves throughout Europe have only been recently highlighted as important ecosystems (Büdel et al. 2014).
In this contribution, we present an insight into the cyanobacterial communities of BSCs across a latitudinal gradient of Western Europe. Cyanobacterial diversity and relative abundances were investigated using a polyphasic approach to provide an enhanced view into the variability found within cyanobacterial community assemblages associated with European BSCs.
Investigation sites
The following presents a brief introduction to the four sites. The climate information was obtained from the installed climate stations and covers a period of 2 year (2012–2014), summer months were considered as April to September and winter October to March. For detailed information of all sites, see Büdel et al. (2014).
Nature Reserve Gynge Alvar, Öland, Sweden. The site (56°32′N, 16°28′E) is situated in Mörbylånga community, Resmo parish, about 20 m above sea level (a.s.l.), on the island of Öland, Sweden. Öland has a maritime climate, but is situated in a rain shadow and with 500 mm/year, has the lowest mean precipitation of any Swedish province. The mean temperature in summer is 13.5°C, and in winter 3°C with an annual mean of 8°C. The Alvar regions are usually seen as semi-natural open areas on limestone pavement which have existed since the last glaciation (ca. 11 000 years before present), containing both relicts from postglacial arctic conditions and from later steppe-like conditions in warm periods. These areas were thus originally open and dependent on grazing from larger herbivores to remain so. Human settlers have continued to impact the area through grazing activities and firewood collection. It is clear that at least those areas with somewhat thicker soils will become overgrown by shrubs if grazing stops. The Alvar areas, therefore, result from a combination of naturally thin soils on limestone pavement bedrock, grazing by larger mammals and continuous human impact for thousands of years.
Nature Reserve ‘Ruine Homburg’, Gössenheim, northern Bavaria, Germany. The site is situated at 50°01′N and 9°48′E in an area with Triassic shell limestone (Muschelkalk) as bedrock. The elevation is 295 m a.s.l. The climate is warm temperate with mean summer temperatures of 16°C, winter of 4°C and annually 9.5°C. Annual precipitation is 600 mm. The nearby castle was founded in 1080 and is the reason that the landscape has remained open and thus prevented higher plants from outcompeting the BSCs.
Hochtor, near the Großglockner High Alpine Road, Hohe Tauern National Park, Austria. The site is situated in the high mountains of Hohe Tauern at 47°05′N and 12°51′E. The area is part of the upper Schieferhülle (Tauern window); in the stricter sense it belongs to the Seidlwinkl Triassic, which mostly consists of lime marble, dolomite and Rauwacke. The elevation ranges from 2500 m to 2600 m a.s.l. The climate is alpine; mean summer air temperature is 2°C, winter is –4°C and the annual mean is –1°C. On average, there are 250 frost days, 150 to 200 ice days and 80 to 90 frost alternation days each year. Mean annual precipitation is between 1750 and 2000 mm, with more than 70% as snow. Snow cover lasts for 270 to 300 days. Except very rare grazing by cattle, no land use has been noticed.
Tabernas field site, north of Almeria, Spain. The site (37°00′N, 2°26′W) is located in the Tabernas basin 250 m a.s.l., surrounded by the Betic Cordilleras and subsequently filled by Serravallian—early Messinian continental and marine sediments. The parent material is a gypsum-calcareous mudrock mainly composed by silt-size (>60%) siliceous and gypsum-calcareous particles. The climate of the area is semi-arid warm Mediterranean, with a mean annual precipitation of 220 mm (with 37% of interannual variation and 76%–215% of monthly variation). The number of days with rain each year varies from 25 to 55 (average 37). Mean annual temperature is 18.5°C, with a summer mean of 23°C and winter of 13.5°C. Potential evapotranspiration is around five to seven times higher than annual precipitation. The average annual insolation is more than 3000 h/year. Land use has probably been minimal during the last 60 years, and certainly it has been very light during the last 23 years. The area has been protected since 1989 as ‘Paraje Natural’.
METHODS
At each of the four sites, vegetation coverage was determined from a homogenous area of 100 × 100 m, which had been identified as representative of the BSC coverage, using the point intercept method (Levy and Madden 1933). Different BSC types were classified as green algal crust, cyanobacterial crust, chlorolichen, cyanolichen or moss from 150 random subplots of 25 × 25 cm (see Büdel et al. 2014). Twenty individual BSC samples were taken at regular intervals throughout the vegetation survey for cyanobacterial morphological and molecular investigations. All samples were collected in 2012 (Spain: February, Sweden: May, Germany: June, Austria: July). The samples were extracted from the surrounding BSC by removing the top 1–3 cm, depending on BSC thickness, from the soil by pressing a sterile 94 mm diameter Petri dish into the crust, excess soil was removed with the Petri-dish lid. Samples were left to air dry in the field immediately after collection, for 2–3 days, until lack of any condensation formation was recognised. Samples were transported in a sealed dry state and preserved at –20°C upon return until further processing.
Direct determination of field samples
The samples were pre-cultivated in solid and liquid BG-11 media for cyanobacteria (ATCC Medium 616) and cultured in a culture cabinet at 16°C –18°C under a light/dark regime of 12:12 h at a light intensity of ca. 20–50 μmol photons m−2 s−1 described in Langhans, Storm and Schwabe (2009). Cyanobacterial populations were studied by light microscopy using AxioVision software (Carl Zeiss, Jena, Germany) and appropriate taxonomic keys (Geitler 1932; Komárek and Anagnostidis 1998, 2005).
Culture-independent approach using next-generation sequencing
16S PCR amplification and Illumina MiSeq sequencing were used to assess the diversity and community composition of cyanobacteria. Three samples from each site were randomly selected and prepared for molecular analysis. Each sample was split into two, one of which was subjected to a dry treatment where samples were air-dried for 1 day before DNA extraction. The other to a wet treatment where samples were saturated three times a week with H2O and stored in the above-mentioned culture cabinet, under identical conditions, for 2 weeks before DNA extraction. This was intended to increase the likelihood of sampling the entire cyanobacterial diversity found within the samples. DNA was extracted using the PowerSoil DNA Isolation Kit (MOBIO, Carlsbad, CA) according to the protocol. The samples were difficult to lyse; therefore, an additional incubation step was included; the PowerBead tubes were placed in a heat block at 65°C for 30 min followed by 3 × 30 s of bead beating.
Fragments of the 16S rRNA gene were amplified by PCR using the cyanobacterial specific primers, CYA359F and an equimolar mixture of CYA781Ra and CYA781Rb (Nübel, Garcia-Pichel and Muyzer 1997). For each 50 μl PCR reaction, Hotstart-Taq Plus DNA Polymerase Kit (Qiagen, Hilden, Germany) was used containing 1× of 10× PCR Buffer, 200 μM dNTP mix (10 mM of each), 0.4 μM primers (CYA359F and CYA781R) (Eurofins MWG, Ebersberg, Germany), 2.5 units HotStartTaq Plus DNA Polymerase and 100 ng total genomic DNA. The PCR cycling parameters were as follows: initial denaturation for 5 min at 95°C; 35 cycles of denaturing at 95°C for 1 min; annealing at 60°C for 1 min; extension at 72°C for 1 min and the final extension at 72°C for 10 min. Amplicons of the 16S rRNA gene were processed by SeqIT Kaiserslautern using MiSeq sequencer (Illumina) read length 2 × 250 bp for obtaining sequences.
Quality control checks were performed on the raw sequence data using FastQC v. 0.10.1. Files were merged, with overlapping paired ends, using FLASH v. 1.2.8 (Fast Length Adjustment of Short Reads). Sequence data were processed using QIIME v. 1.6.0. (Caporaso et al. 2010) and the QIIME workflow described in Navas-Molina et al. (2013). For the QIIME quality-filtering process, the default parameters were used, including the operational taxonomic unit (OTU) picking closed-reference pipeline and the identification of chimeric sequences by UCHIME (Edgar et al. 2011). Sequences were clustered at 97% sequence identity and the taxonomy of the representatives from each OTU was assigned using blast+ (Camacho et al. 2009) against the Greengenes database (DeSantis et al. 2006). Sequences were taxonomically classified down to the genus level and OTUs identified other than cyanobacteria were discarded. Samples were rarefied to 10 000 sequences per sample. This method determined the taxonomic composition and genetic variation of the cyanobacteria within the BSCs. Non-metric multidimensional scaling was used to compare sample distances and visualise clustering for the Bray–Curtis dissimilarity using the R phyloseq package (McMurdie and Holmes 2013, version 1.8.2) in R v.3.1.0 (R Core Team 2013). Non-parametric analyses for multivariate data (adonis) were performed using the R vegan package (Oksanen et al.2013, version 2.0-10). Rarefaction curves were built for alpha diversity measures Chao 1, observed OTUs and Shannon indices calculated by QIIME which suggested that the sequencing effort was sufficient for representing and comparing the cyanobacterial communities. The lowest taxonomic unit was included where possible, and manual taxonomic classification was carried out in order to follow the most recent classification system of cyanobacteria by Komárek et al. (2014). The exception being Phormidiaceae, which is no longer recognised as a family, but can be included in either the Microcoleaceae or Oscillatoriaceae. Data were compiled for graphical construction in Excel and SigmaPlot v. 10.0. Two-way ANOVA analyses (Statistica v. 10, Stat soft), with Levene's tests for data normality and Fisher LSD post-hoc tests, were performed, on each taxa individually and on the taxa grouped into orders (Nostocales, Oscillatoriales, Synechococcales, Chroococcales), to test for the effects of site and treatment. Data were log transformed after deviations from normality were discovered; in some cases, this rectified the problem. However, for many taxa normality could not be assumed and therefore constituted a deviation from a required ANOVA assumption. However, the interaction between variables was of interest. An ANOVA is considered robust enough if the P values are highly significant and can be confirmed by a non-parametric equivalent, such as a Kruskal–Wallis test (Fry 1993; Zar 1996). In cases where log transformation, highly significant P values nor Kruskal–Wallis tests supported the data the taxa were omitted from the statistical analysis. See Table S1 in the supplementary material for results of Levene's and Kruskal Wallis tests that lend support to the ANOVA tests demonstrated in the results.
Fastq files containing the raw data from this study were submitted to the NCBI sequence read archive (www.ncbi.nlm.nih.gov/sra) and can be accessed by the accession number PRJNA325717.
RESULTS
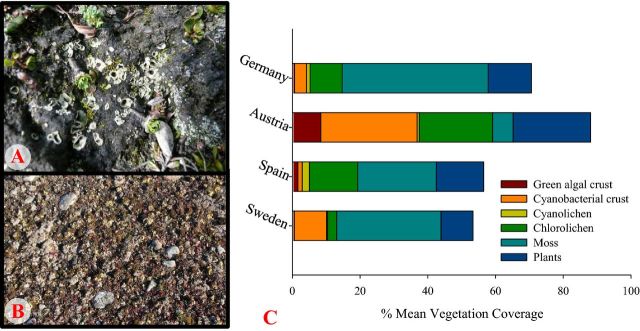
The BSC coverage was different between the four sites (Fig. 1A and B). The mean percentage cover of BSCs in all sites clearly expresses the dominance of cyanobacteria in Austria compared to the other sites (Fig. 1C).
Figure 1.
Examples of BSC coverage: (A) Hochtor, dominated by cyanobacterial crust with chlorolichens. (B) Öland, dominated by moss and chlorolichens. (C) Represents the total mean BSC and higher plant coverage of the four SCIN sites, the unrepresented coverage includes stones, gravel, bare ground and litter.
Overall 19 different genera commonly associated with BSC were identified morphologically, of which Nostoc, Oscillatoria, Pseudanabaena, Phormidium and Microcoleus were found from all sites (Table 1 and Fig. 2). Figure 2a shows a cross-section of cyanobacterial crust taken from Austria in which the structure is well represented. It can be seen that Nostoc, Gloeocapsa, green algae and black yeasts contribute to a dense and well-defined BSC in the top millimetres of soil. Several cyanobacteria genera identified form mucilage bound colonies which bind soil particles and have a stabilising effect on the stratum (Fig. 2b, d, g and h).
Table 1.
Cyanobacteria genera identified through morphological investigation from the BSC of the four SCIN sites.
| Germany | Austria | Spain | Sweden | |
|---|---|---|---|---|
| Pseudanabaena | X | X | X | X |
| Calothrix | X | X | ||
| Chroococcus | X | X | ||
| Chroococcidiopsis | X | X | ||
| Fischerella | X | |||
| Gloeocapsa | X | X | X | |
| Leptolyngbya | X | X | ||
| Lyngbya | X | |||
| Microcoleus | X | X | X | X |
| Aphanothece | X | X | X | |
| Nostoc | X | X | X | X |
| Oscillatoria | X | X | X | X |
| Phormidium | X | X | X | X |
| Plectonema | X | X | ||
| Schizothrix | X | |||
| Scytonema | X | |||
| Stigonema | X | X | ||
| Synechocystis | X | X | ||
| Tolypothrix | X | |||
| Total | 9 | 11 | 14 | 11 |
Figure 2.
(a) BSC crust profile, Nostoc, Gloeocapsa, green algae, black yeasts, Austria (b); Microcoleus, Spain (c); Nostoc, Austria (d); Chroococcus, Germany (e); Nostoc, Austria (f); Gloeocapsa, Austria (g); Microcoleus in mucilage, Austria (h); Gloeocapsa, Sweden (i); Pseudanabaena, Spain. Scale bar = 20 μm.
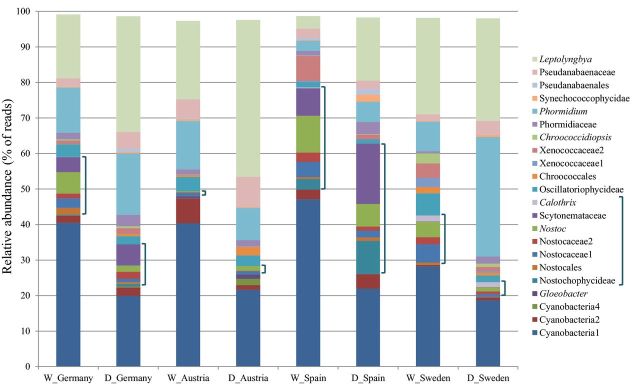
The total number of taxa identified through molecular investigation from all sites was 53, of which 20 were found to be significantly different based on site, 8 based on treatment and 7 on the interaction between site and treatment (Table S2, supplementary material). All sites except Spain included taxa that were, in this study, unique to the locality. Austria with five unshared taxa (Table 2), one of which was identified as Gloeobacter, was the most unique. A total of 27 of the identified taxa were found in all sites, Austria and Sweden had the most in common (Table 2), including Stigonema, Chroococcaceae and Oscillatoria taxa that were undetected in Germany and Spain samples. The diversity of taxa with more than 1% relative abundance in at least one site demonstrates that site variation is substantial, but all are dominated by Leptolynbya, Phormidium and a cyanobacteria taxon that cannot be further identified through the utilised databases (Fig. 3). The Nostocales, the only order found to be significantly different based on site (P < 0.001), were by far more relatively abundant in Spain than the other sites. More than 30% of the taxa were identified as such, compared to less than 3% in Austria, which was instead dominated by large percentages of Synechococcales (wet:31%, dry:54%) and Oscillatoriales (wet:19%, dry:14%). Scytonemaceae has a high relative abundance in Spain (wet:8%, dry:17%) compared to the other sites and was not identified in Austria, through either methodologies. Although the sequence assignment could not identify this taxon further than family, Scytonema is the only genus within the family that is commonly associated with BSC, the morphological investigation also corroborates the identification of this genus and therefore further discussion will refer to Scytonema in this instance. The relative abundance data (Fig. 3) shows influence from the DNA extraction pre-treatment. It can be seen that certain cyanobacterial taxa have a propensity towards amplification or reduction when the wet experimental method was utilised. Significant differences (Table S2 supplementary material) were observed between many taxa as a result of the preparatory method, but not to the same magnitude as the disparity due to site. Some taxa were only identified in either the wet or dry treatments. However, due to their very low abundances and infrequent occurrences this cannot be seen on the relative abundance graph (Fig. 3). Examples of taxa which were only identified in specific sites wet-treated samples include Tolypothrix and Rivulariaceae from Germany and Spain. Interestingly, the two taxa were present in the samples taken from the site in Sweden when prepared via both methods. Calothrix was identified in the wet Germany and Spain, dry Austria and both Sweden treatments (see Table S2 supplementary material). In Spain, two Scytonema taxa and a Nostocophycideae taxon showed significantly higher abundances in the dry treatment, compared to Xenococcaceae taxa which had higher when wet treated (significant differences observed in Fisher LSD post-hoc tests, Table S2 supplementary material). Leptolyngbya was found to be dominant in all sites and especially in the dry samples. This genus appeared to be adversely affected by the wetting treatment, but was only found to be significantly different between the wet and dry treatments of Spain. Many taxa's relative abundance increased after the 2 weeks wetting regime, in detriment to other taxa. In Sweden, the wet treatments caused Chroococcidiopsis and Xenococcaceae taxa to increase significantly, whereas in Austria two Chroococcales taxa significantly decreased with wetting. Phormidium was inconsistent across the sites, generally decreasing in wet treatments, most evident in samples from Sweden, but in samples taken from Austria wetting created an increase (Fig. 3); however, Phormidium was not found to generate statistically significant results. Consequently, many taxa expressed notable differences between treatments and these changes were site specific. Spain and Sweden both had six taxa, two shared, which showed significant differences based on treatment alone. Germany and Austria had only two and three, none shared, respectively. For the complete breakdown of results based on site and treatment, see Table S2 in the supplementary material.
Table 2.
Number of cyanobacterial taxa identified through molecular investigation from, and shared between, the four SCIN sites.
| Taxa shared between sites | |
|---|---|
| Sweden only | 2 |
| Spain only | 0 |
| Germany only | 2 |
| Austria only | 5 |
| Spain + Austria | 0 |
| Spain + Sweden | 0 |
| Spain + Germany | 0 |
| Austria + Sweden | 5 |
| Austria + Germany | 1 |
| Sweden + Germany | 1 |
| Sweden + Germany + Austria | 4 |
| Sweden + Germany + Spain | 5 |
| Germany + Austria + Spain | 1 |
| Sweden + Spain + Austria | 0 |
| All sites | 27 |
| Total number of taxa | 53 |
Figure 3.
Relative abundance of taxa identified with >1% abundance in at least one site from dry and wet treated samples. Includes >95% of total sequences identified in all samples. D = dry treatment, W = wet treatment.
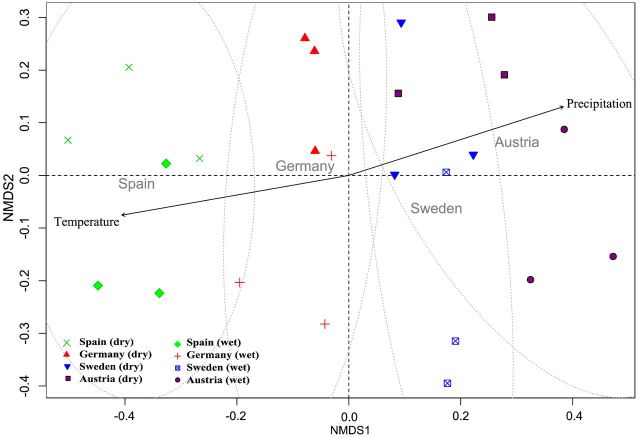
The beta diversity analysis (Fig. 4) illustrates the similarity within and between the sites and suggests high diversity between the sites (R2 = 0.301, P < 0.001, adonis). Austria and Sweden have overlapping similarity, as also seen by the shared taxa (Table 2), and Spain and Austria are the least similar to each other. Furthermore, the differences in community composition were associated with the wet/dry treatments (R2 = 0.500, P < 0.001, adonis).
Figure 4.
Non-metric multidimensional scaling comparing sample distances and visualising clustering for Bray–Curtis dissimilarity indices.
The alpha diversity analysis suggests that all sites and treatments are significantly different to each other. Except for Spain, the wet treatment generally increases diversity and the variation found between samples from one site is large, with Sweden usually found to be the most diverse site in both treatments (Fig. 5).
Figure 5.
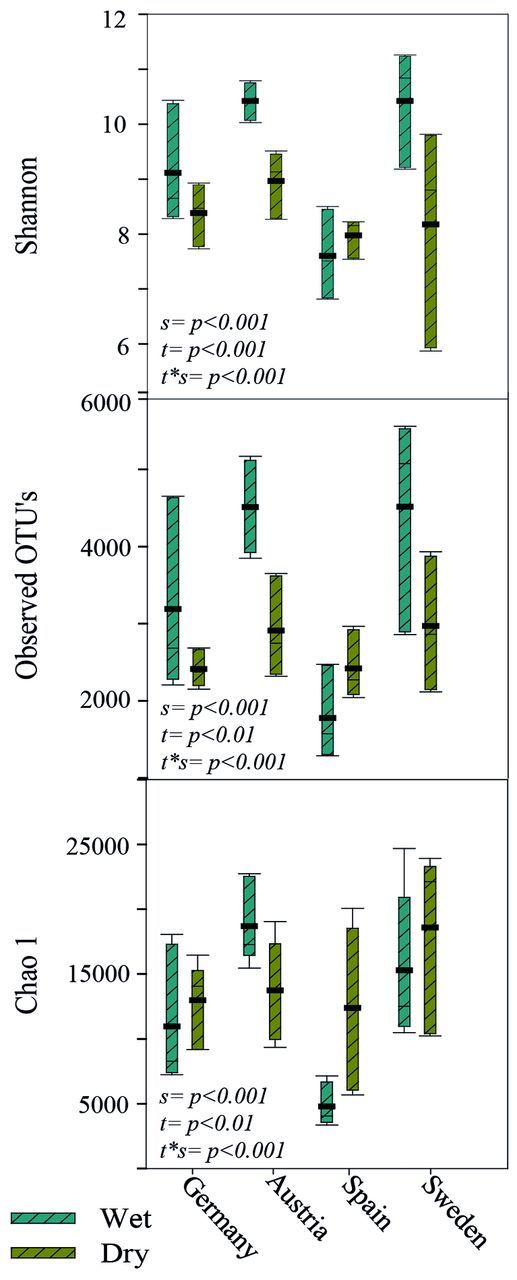
Cyanobacterial richness and diversity estimates, based on 10 000 sequences per sample, calculated for the different sites. ▄ = mean. P values represent a two-way ANOVA analysis and significant differences based on: s = site, t = treatment and t*s = treatment*site.
DISCUSSION
Cyanobacteria play similar functional roles in different BSC ecosystems but their community composition can be very variable. Understanding the various assemblages of the different organisms is essential when addressing important questions regarding global nutrient cycles and climate change scenarios. On a European scale, the knowledge that different areas are home to unique BSC-dominated habitats lends support to conservation and restoration strategies.
BSC composition has been shown to be variable throughout Europe (Büdel et al. 2014) and this study highlights that the cyanobacterial community compositions are also unique to their respective sites. Many similarities and differences are observable throughout the sites as well as with BSC systems described in the literature. In contrast to studies focusing on only arid sites (Büdel et al.2009; Garcia-Pichel et al.2013; Hagemann et al.2015), Microcoleus species were not found to dominate, but rather contribute to the BSC community. Scytonema often found to be a dominant genus in arid sites (e.g. Kumar and Adhikary 2015), is in accordance with the genus being dominant in Spain. Compared to Austria, the high altitude site, where Scytonema was not identified, concurs with studies of the Himalayas (Janatková et al. 2013; Čapková et al. 2016). Pseudanabaenaceae taxa are known from hot (Dojani et al.2014) and cold (Komárek et al. 2012) desert BSCs, but have received little discussion; they were however found to be significant constituents of all sites cyanobacterial composition in this study. The Nostocales are dominant in BSCs worldwide and this study is no exception. However, the relative abundance varies greatly between sites and is the dominant order in Spain alone. The ability to fix nitrogen whenever liquid water is available would appear to be a more important attribute in arid areas. Nevertheless, during the morphological investigation it was observed that the incidence of Nostocales within the BSC of Austria is high, compared to the lowest relative abundance of all sites. Thus, cautioning against an assumption that relative abundance data necessarily reflects absolute abundance.
Gloeobacter was identified by 16S rRNA gene sequencing in this study but has not previously been typically associated with BSCs. Gloeobacter like sequences were described from Ellesmere Island in the High Arctic, from mats of Ward Hunt Lake (Jungblut, Lovejoy and Vincent 2010; Lionard et al. 2012), and Mareš et al. (2013) suggested that the genus may be commonly distributed in wet-rock habitats worldwide. The two known species are Gloeobacter violaceus, isolated from rock in Switzerland (Rippka, Waterbury and Cohen-Bazire 1974) and G. kilaueensis, identified from an epilithic biofilm in a lava cave, Hawaii (Saw et al. 2013). Couradeau et al. (2016) included Gloeobacterales in their community composition of BSCs from Moab, Utah, and Pushkareva et al. (2015) identified taxa that were assigned to the order Gloeobacterales, but with little conviction, in BSCs of Svalbard. These appear to be the first references of possible Gloeobacter species associated with BSCs. Although this study has similar limitations, due to the identification based on sequence identity and databases, to that of the Pushkareva et al. (2015) study, the finding of taxa assigned to this order in arid, arctic and alpine BSCs may suggest that Gloeobacter is in fact present in these ecosystems.
The wet and dry treatments may suggest that cyanobacteria relative abundance show seasonal fluctuations which are variable by site. Nostoc blooms are a common sight across Europe with increased rainfall, and the soil crust diazotrophic community was shown to fluctuate with rainfall patterns in the Colorado Plateau (Yeager et al. 2012). However, the site-specific changes in abundance that these results suggest have, to our knowledge, not been previously documented. Scytonema and Nostoc are known for their desiccation resistance due to multiple survival mechanisms (Tomaselli and Giovannetti 1993). Therefore, these genera being dominant in the semi-arid Spain site, especially in the dry samples, may be expected. In this case, it appears that other taxa recover from the effects of desiccation quicker, being able to increase in abundance in detriment to the dry adapted taxa. This may be supported by the lack of Scytonema, and the low relative abundance of all the Nostocales, in Austria, which was the wettest site included in this study. This emphasises the importance of Scytonema, and the Nostocales in general, in arid BSC systems, but less so in wet, alpine sites, where BSC formation is mostly due to environmental factors such as altitude and low temperatures, rather than water limitation. The methodology utilised here only compared dry samples to a 2-week wetting regime, with all samples cultivated at the same temperature and light values, regardless of their source climate. In addition, samples were collected from each site within a 6-month period in 2012, and consequently, comparisons based on season, either within one site or between sites, cannot be made. However, the speed in which the cyanobacteria community responds to the wet treatment is noteworthy due to the environmental implications. Changing climate patterns or even short-term phenomena could have a huge influence on the cyanobacteria community structure. However, this was a very small scale experiment, without seasonal comparisons; in order to investigate the influence of a changing climate, further experiments are required.
Most cyanobacterial studies concentrate either on phenotypic diversity using morphological identification or genotypic diversity using various PCR techniques. Morphological identifications utilising culture-based methods are not without their disadvantages; fast-growing strains suppress the growth of others and those that are not cultivatable will be missed completely. The disadvantage of molecular methods is that they may introduce biases which miscalculate diversity. DNA-extraction methods may produce contrasting results (Ferrera et al. 2010), choice of primer pair and sequencing errors may also contribute. Combining both methodologies allows the disadvantages of both to be mitigated. The morphological analysis has the advantage of identifying cyanobacteria to the genus or even the species level. This is often not possible through molecular analysis due to the incomplete databases available for sequence comparison and the relatively low numbers of base pairs of the 16S rRNA gene utilised. The identification of cyanobacterial taxa that are extremely unlikely is also a consequence of the molecular analyses. cf. Dolichospermum and cf. Acaryochloris were found to be the closest match for some sequences and therefore the taxa were assigned these identities. However, these are planktonic and symbionts of marine invertebrates, respectively. cf. Arthrospira is also not a genus that would usually be associated with BSCs; however, it was identified from the coastal Sweden site only and therefore, may have been blown in from the sea. In addition, a large number of sequences were assigned to a single taxon that could not be identified further than cyanobacteria; this taxon, named cyanobacteria 1, incorporates a large percentage of the relative abundance in all sites (Fig. 3). The molecular analysis certainly gives an enhanced view of the total diversity, and the many taxa that were identified with very low relative abundances would have been extremely difficult to pick out through microscopy. Nevertheless, the morphological investigation is essential in the corroboration of identification, assessing the structure and function of cyanobacteria within a BSC and exploring the incidence of cyanobacteria within the different crusts. Utilising both methods has in this instance shown the shortfalls of relying on a single methodological approach. Combining approaches will continue to provide the most reliable datasets until cyanobacteria taxonomy, and studies into their functioning within ecosystems have seen far more attention.
Supplementary Material
Acknowledgments
We would like to thank Dr. Owain Williams for his many useful comments and language editing.
FUNDING
This research was funded by the program, with the national funders (DFG) (grant number ), Austrian Science Fund (FWF), a part of the 2010–2011 BiodivERsA joint call.
Conflict of interest. None declared.
REFERENCES
- Abed RMM, Kharusi SA, Schramm A, et al. Bacterial diversity, pigments and nitrogen fixation of biological desert crusts from the Sultanate of Oman. FEMS Microbiol Ecol. 2010;72:418–28. doi: 10.1111/j.1574-6941.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- Belnap J. Nitrogen fixation in biological soil crusts from southeast Utah, USA. Biol Fert Soils. 2002;35:128–35. [Google Scholar]
- Belnap J. The world at your feet: desert biological soil crusts. Front Ecol Environ. 2003;1:181–9. [Google Scholar]
- Belnap J, Lange OL. Biological Soil Crusts: Structure, Function and Management. Berlin: Springer; 2003. Ecological Studies 150. [Google Scholar]
- Büdel B, Colesie C, Green TGA, et al. Improved appreciation of the functioning and importance of biological soil crusts in Europe: the Soil Crust International Project (SCIN) Biodivers Conserv. 2014;23:1639–58. doi: 10.1007/s10531-014-0645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büdel B, Darienko T, Deutschewitz K, et al. Southern African biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microb Ecol. 2009;57:229–47. doi: 10.1007/s00248-008-9449-9. [DOI] [PubMed] [Google Scholar]
- Büdel B, Veste M. Biological crusts. In: Breckle S, et al., editors. Sand Dune Ecosystems in the Negev-Desert. Berlin: Springer; 2008. pp. 149–55. Ecological Studies 200. [Google Scholar]
- Camacho CG, Coulouris V, Avagyan N, et al. BLAST+: architecture and applications: BMC. Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čapková K, Hauer T, Řeháková K, et al. Some Like it High! Phylogenetic Diversity of High-Elevation Cyanobacterial Community from Biological Soil Crusts of Western Himalaya. Microb Ecol. 2016;71:113–23. doi: 10.1007/s00248-015-0694-4. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couradeau E, Karaoz U, Lim HC, et al. Bacteria increase arid-land soil surface temperature through the production of sunscreens. Nat Commun. 2016;7:10373. doi: 10.1038/ncomms10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis TZP, Hugenholtz N, Larsen M, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microb. 2006;72:5069–72. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dojani S, Kauff F, Weber B, et al. Genotypic and phenotypic diversity of cyanobacteria in biological soil crusts of the Succulent Karoo and Nama Karoo of southern Africa. Microb Ecol. 2014;67:286–301. doi: 10.1007/s00248-013-0301-5. [DOI] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27::2194–200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert W, Weber B, Burrows S, et al. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosci. 2012;5:459–62. [Google Scholar]
- Ferrera I, Massana R, Balagué V, et al. Evaluation of DNA extraction methods from complex phototrophic biofilms. Biofouling. 2010;26:349–57. doi: 10.1080/08927011003605870. [DOI] [PubMed] [Google Scholar]
- Fry JC. Biological Data Analysis: A Practical Approach. Oxford: Oxford University Press; 1993. p. 418. [Google Scholar]
- Garcia-Pichel F, López-Corté A, Nübel U. Phylogenetic and morphological diversity of Cyanobacteria in soil desert crusts from the Colorado Plateau. Appl Environ Microb. 2001;67:1902–10. doi: 10.1128/AEM.67.4.1902-1910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pichel F, Loza V, Marusenko Y, et al. Temperature drives the continental-scale distribution of key microbes in topsoil communities. Science. 2013;340:1574–7. doi: 10.1126/science.1236404. [DOI] [PubMed] [Google Scholar]
- Geitler L. Cyanophyceae Von Europa Unter Berücksichtigung Der Anderen Kontinente. Leipzig: Akademische Verlagsgesellschaft; 1932. [Google Scholar]
- Hagemann M, Henneberg M, Felde VJMNL, et al. Cyanobacterial diversity in biological soil crusts along a precipitation gradient, northwest Negev desert, Israel. Microb Ecol. 2015;70:219–30. doi: 10.1007/s00248-014-0533-z. [DOI] [PubMed] [Google Scholar]
- Housman DC, Powers HH, Collins AD, et al. Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. J Arid Environ. 2006;66:620–34. [Google Scholar]
- Janatková K, Rehakova K, Dolezal J, et al. Community structure of soil phototrophs along environmental gradients in arid Himalaya. Environ Microbiol. 2013;15:2505–16. doi: 10.1111/1462-2920.12132. [DOI] [PubMed] [Google Scholar]
- Johansen JR. Cryptogamic crusts of semiarid and arid lands of North America. J Phycol. 1993;29:140–7. [Google Scholar]
- Jungblut AD, Lovejoy C, Vincent WF. Global distribution of cyanobacterial ecotypes in the cold biosphere. ISME J. 2010;4:191–202. doi: 10.1038/ismej.2009.113. [DOI] [PubMed] [Google Scholar]
- Komárek J, Anagnostidis K. Cyanoprokaryota 1. Teil: Chroococcales. Jena: Gustav Fischer; 1998. [Google Scholar]
- Komárek J, Anagnostidis K. Cyanoprokaryota 2. Teil: Oscillatoriales. München: Elsevier; 2005. [Google Scholar]
- Komárek J, Kováčik L, Elster J, et al. Cyanobacterial diversity of Petuniabukta, Billefjorden, central Spitsbergen. Pol Polar Res. 2012;33:347–68. [Google Scholar]
- Komárek J, Kaŝtovský J, Mareŝ J, et al. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) using a polyphasic approach. Preslia. 2014;86:295–335. [Google Scholar]
- Komárek J, Pessi IS, Wilmotte A, et al. Cyanobacterial community composition in Arctic soil crusts at different stages of development. FEMS Microbiol Ecol. 2015;91:fiv143. doi: 10.1093/femsec/fiv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Adhikary SP. Diversity, molecular phylogeny, and metabolic activity of cyanobacteria in biological soil crusts from Santiniketan (India) J Appl Phycol. 2015;27:339–49. [Google Scholar]
- Lange OL, Kidron GJ, Büdel B, et al. Taxonomic composition and photosynthetic characteristics of the “biological soil crusts” covering sand dunes in the western Negev Desert. Funct Ecol. 1992;6:519–27. [Google Scholar]
- Langhans TM, Storm C, Schwabe A. Community assembly of biological soil crusts of different successional stages in a temperate sand ecosystem, as assessed by direct determination and enrichment techniques. Microb Ecol. 2009;58:394–407. doi: 10.1007/s00248-009-9532-x. [DOI] [PubMed] [Google Scholar]
- Levy EB, Madden EA. The point method of pasture analysis. New Zeal J Agr. 1933;46:267–79. [Google Scholar]
- Lionard M, Péquin B, Lovejoy C, et al. Benthic cyanobacterial mats in the high Arctic: multi-layer structure and fluorescence responses to osmotic stress. Front Microbiol. 2012;3:140. doi: 10.3389/fmicb.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareš J, Hrouzek P, Kaňa R, et al. The primitive thylakoid-less cyanobacterium gloeobacter is a common rock-dwelling organism. PLoS One. 2013;8:e66323. doi: 10.1371/journal.pone.0066323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy ML, Garcia-Pichel F, Pérez A. The prokaryotic diversity of biological soil crusts in the Sonoran Desert (Organ Pipe Cactus National Monument, AZ) FEMS Microbiol Ecol. 2005;54:233–45. doi: 10.1016/j.femsec.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Navas-Molina JA, Peralta-Sánchez JM, González A, et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013;531:371–444. doi: 10.1016/B978-0-12-407863-5.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nübel U, Garcia-Pichel F, Muyzer G. PCR primers to amplify 16S rRNA genes from Cyanobacteria. Appl Environ Microb. 1997;63:3327–32. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen JF, Blanchet G, Kindt R, et al. vegan: Community Ecology Package. R package 2.0-10. 2013. http://CRAN.R-project.org/package=vegan.
- Pushkareva E, Elster J. Biodiversity and ecological classification of cryptogamic soil crusts in the vicinity of Petunia Bay, Svalbard. Czech Polar Rep. 2013;3:7–18. [Google Scholar]
- Pushkareva E, Pessi IS, Wilmotte A, et al. Cyanobacterial community composition in Arctic soil crusts at different stages of development. FEMS Microb Ecol. 2015;91:fiv143. doi: 10.1093/femsec/fiv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna: Austria: R Foundation for Statistical Computing; 2013. http://www.R-project.org. [Google Scholar]
- Rippka R, Waterbury J, Cohen-Bazire G. A cyanobacterium which lacks thylakoids. Arch Microbiol. 1974;100:419–36. [Google Scholar]
- Santos HF, Carmo FL, Duarte G, et al. Climate change affects key nitrogen- fixing bacterial populations on coral reefs. ISME J. 2014;8:2272–9. doi: 10.1038/ismej.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw JH, Schatz M, Brown MV, et al. Cultivation and complete genome sequencing of Gloeobacter kilaueensis sp. nov., from a Lava Cave in Kilauea Caldera, Hawaii. PLoS One. 2013;8:e76376. doi: 10.1371/journal.pone.0076376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BK, Bardgett RD, Smith P, et al. Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat Rev Microbiol. 2010;8:779–90. doi: 10.1038/nrmicro2439. [DOI] [PubMed] [Google Scholar]
- Solheim B, Endal A, Vigstad H. Nitrogen fixation in Arctic vegetation and soils from Svalbard, Norway. Polar Biol. 1996;16:35–40. [Google Scholar]
- Steven B, Gallegos-Graves LV, Belnap J, et al. Dryland soil microbial communities display spatial biogeographic patterns associated with soil depth and soil parent material. FEMS Microbiol Ecol. 2013;86:1–13. doi: 10.1111/1574-6941.12143. [DOI] [PubMed] [Google Scholar]
- Tomaselli L, Giovannetti L. Survival of diazotrophic cyanobacteria in soil. World J Microb Biot. 1993;9:113–6. doi: 10.1007/BF00656530. [DOI] [PubMed] [Google Scholar]
- Vincent WF, Gibson JAE, Pienitz R, et al. Ice shelf microbial ecosystems in the high Arctic and implications for life on snowball earth. Naturwissenschaften. 2000;87:137–41. doi: 10.1007/s001140050692. [DOI] [PubMed] [Google Scholar]
- Yeager CM, Kornosky JL, Housman DC, et al. Diazotrophic community structure and function in two successional stages of biological soil crusts from the Colorado Plateau and Chihuahuan Desert. Appl Environ Microb. 2004;70:973–83. doi: 10.1128/AEM.70.2.973-983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager CM, Kuske CR, Carney TD, et al. Response of biological soil crust diazotrophs to season, altered summer precipitation and year-round increased temperature in an arid grassland of the Colorado Plateau, USA. Front Microbiol. 2012;3:358. doi: 10.3389/fmicb.2012.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 3rd edn. UpperSaddleRiver, NJ: Prentice-Hall; 1996. p. 662. [Google Scholar]
- Zhang B, Zhang Y, Downing A, et al. Distribution and composition of cyanobacteria and micro-algae associated with biological soil crusts in the Gurbantunggut Desert, China. Arid Land Res Manag. 2011;25:275–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.