Summary
Background
Bisphosphonates are thought to act through the osteoclast by changing bone microenvironment. Previous findings of adjuvant clodronate trials in different populations with operable breast cancer have been mixed. The National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol B-34 aims to ascertain whether oral clodronate can improve outcomes in women with primary breast cancer.
Methods
NSABP B-34 is a multicentre, randomised, double-blind, placebo-controlled study in 3323 women with stage 1–3 breast cancer. After surgery to remove the tumour, patients were stratified by age, axillary nodes, and oestrogen and progesterone receptor status and randomly assigned in a 1:1 ratio to either oral clodronate 1600 mg daily for 3 years (n=1662) or placebo (1661). The primary endpoint was disease-free survival, analysed by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT00009945.
Findings
Median follow-up was 90·7 months (IQR 82·7–100·0) and 3311 patients had data for this period. Disease-free survival did not differ between groups (286 events in the clodronate group vs 312 in the placebo group; hazard ratio 0·91, 95% CI 0·78–1·07; p=0·27). Moreover, no differences were recorded for overall survival (0·84, 0·67–1·05; p=0·13), recurrence-free interval (0·83, 0·67–1·04; p=0·10), or bone metastasis-free interval (0·77, 0·55–1·07; p=0·12). Non-bone metastasis-free interval was slightly increased with clodronate (0·74, 0·55–1·00; p=0·047). Analyses in women age 50 years or older on study entry showed benefits of clodronate for recurrence-free interval (0·75, 0·57–0·99; p=0·045), bone metastasis-free interval (0·62, 0·40–0·95; p=0·027), and non-bone metastasis-free interval (0·63, 0·43–0·91; p=0·014), but not for overall survival (0·80, 0·61–1·04, p=0·094). Adherence to treatment at 3 years was 56% for the clodronate group and 60% for the placebo group. Grade 3 or higher liver dysfunction was noted in 23 of 1612 patients in the clodronate group and 12 of 1623 patients in the placebo group; grade 3–4 diarrhoea was noted in 28 patients in the clodronate group and in ten in the placebo group. There was one possible case of osteonecrosis of the jaw in the clodronate group.
Interpretation
Findings of NSABP B-34 suggest that bisphosphonates might have anticancer benefits for older postmenopausal women. A meta-analysis of adjuvant bisphosphonate trials is suggested before recommendations for use in non-osteoporotic postmenopausal women with primary breast cancer are made.
Introduction
Systemic adjuvant treatment of operable breast cancer by chemotherapy, endocrine therapy, or both has led to improvements in disease-free and overall survival.1 The most frequent site of systemic recurrence of primary breast cancer is bone.2 Although endocrine therapy can be beneficial, bone as the first site of relapse might derive the least benefit from adjuvant chemotherapy.3 Anti-osteoporotic agents, particularly bisphosphonates,4 prevent development of bone metastases in animals,5,6 but difficulties arise when trying to show in these models either that other metastatic sites are affected beneficially by these agents or that doses currently used in clinical studies have an antitumour effect.7 In placebo-controlled trials of bisphosphonates, oral clodronate, oral ibandronate, intravenous pamidronate, and intravenous zoledronate reduce skeletal complications of women with metastatic breast cancer at rates depending on the population treated.8–10
Bisphosphonates inhibit osteoclast activity, thereby reducing the availability of bone resorption products. These products include growth factors such as transforming growth factor β, which are thought to contribute to enhanced proliferation of malignant cells within bone.11,12
Oral clodronate boosts bone mineral density in premenopausal and postmenopausal women with early breast cancer,13 reduces the incidence of bone metastases, and increases survival in women with operable breast cancer but who have malignant cells in bone marrow.14 In women with primary breast cancer, results have been mixed, with a large placebo-controlled study showing benefit15,16 but a smaller study suggesting that clodronate might be harmful.17 Findings of a clinical trial of intravenous zoledronate18 suggest that premenopausal women not receiving chemo- therapy who become postmenopausal with goserelin (plus either tamoxifen or anastrozole) have increased disease- free survival with intravenous zoledronate, with improvements in contralateral breast cancer and rates of locoregional and distant recurrence. By contrast, in a trial of intravenous zoledronate in women with node-positive primary breast cancer,19 the primary endpoint of disease- free survival was not met, but analyses of stratification variables suggested a survival benefit in women 60 years and older and in postmenopausal patients whose menopause took place 5 years or more before trial entry.
The conflicting results of initial clodronate studies prompted the National Surgical Adjuvant Breast and Bowel Project (NSABP) to undertake a clinical trial to ascertain whether oral clodronate with standard adjuvant treatment might reduce the incidence of metastases in patients with primary operable breast cancer. Here we present the definitive analysis of this study.
Methods
Study design
NSABP protocol B-34 is a randomised, double-blind, placebo-controlled, multicentre study undertaken at 162 centres in North America. We enrolled women with histologically confirmed operable breast cancer and no evidence of metastases. Every patient’s hormone receptor status (oestrogen [ER] and progesterone [PgR]) was required; testing for HER2 status was not routine in North America at the time this trial commenced accrual. Before random assignment, for every patient we took a full history and did a physical examination, complete blood count, renal and hepatic function assessments, and bone scans, with radiographs if indicated.
We excluded women with any relevant renal, hepatic, or non-malignant bone disease and if they had a previous history of malignant disease or bisphosphonate use. All patients had to be suitable physically to undergo 3 years of treatment with clodronate or placebo. This trial was approved by local human investigations committees or institutional review boards in accordance with assurances filed with and approved by the Department of Health and Human Services, in accordance with the Declaration of Helsinki. All patients provided written informed consent before study entry.
Randomisation and masking
After surgery to remove the tumour, we randomly assigned patients in a 1:1 ratio at the NSABP Biostatistical Center (Pittsburgh, PA, USA) to receive either adjuvant oral clodronate 1600 mg daily for 3 years or a matching placebo. All patients, clinicians who treated and assessed patients, and protocol doctors were masked to treatment group assignment. Stratified randomisation was done with a biased-coin minimisation approach to generate a treatment assignment on entry. We stratified patients (within every centre) by age (<50 and ≥50 years), number of positive axillary nodes (0, 1–3, and ≥4), and hormone receptor status (both ER and PgR negative, or one or both receptors positive). At relapse, study masking was maintained if the patient had no evidence of bone metastases.
Procedures
Timing of the assigned adjuvant treatment was dependent on the type of non-protocol-specified adjuvant treatment prescribed by the treating doctor (appendix, pp 1–2). Patients received appropriate local and systemic treatments at the investigator’s discretion. Local and regional treatments included mastectomy or lumpectomy plus radiotherapy. Use of chemotherapy was at the investigator’s discretion; if administered, chemotherapy was started after random assignment and concurrently with study drugs. Endocrine therapy was administered for 5 years with the choice of treatment at the investigator’s discretion. If bone metastases arose, we discontinued study drugs.
We assessed patients every 6 months, which included documentation of adverse events and laboratory tests, and continued this twice yearly assessment for 5 years, and annually thereafter. If clinical symptoms arose, further investigations were undertaken (apart from annual mammography and blood work). For example, patients with bone pain had diagnostic radionuclide scintigraphy and radiography; those with shortness of breath had chest radiography or a CT scan of the thorax.
The primary endpoint was disease-free survival, defined as time from random assignment to local, regional, or distant breast cancer recurrence, contralateral breast cancer, second primary malignant disease (other than squamous-cell or basal-cell carcinomas of skin, carcinoma-in-situ of cervix, or lobular carcinoma-in-situ of breast), or death from any cause before breast cancer recurrence. Secondary endpoints included overall survival (defined as time from random assignment to death from any cause), recurrence-free interval (defined as time from random assignment to local, regional, or distant breast cancer recurrence, not including contralateral breast cancer), bone metastasis-free interval (defined as time from random assignment to first diagnosis of skeletal metastases), and non-bone metastasis-free interval (defined as time from random assignment to development of any metastasis other than skeletal).
Statistical analysis
We did endpoint analyses according to assignment and included all patients with follow-up information. For women who withdrew consent for further follow-up after random assignment, we included events up to time-of-consent withdrawal. Protocol B-34 was powered to detect a 20·6% reduction in disease-free survival in the clodronate group compared with the placebo group. On the basis of previous reported rates of disease-free survival and a projection that about 75% of patients accrued to protocol B-34 would be node-negative, we estimated a 3·88% rate of disease-free survival in the placebo arm of the study. The disease-free survival comparison had 80% power, from an original failure risk reduction of 26% to a hypothesised reduction in risk of failure of 20·6%, after accounting for discontinuation of treatment. Assuming that a log-rank test would be used for treatment comparison and that a two-sided test at the α=0·05 level would be done, the number of disease-free survival events needed for adequate power would be 594 of a projected sample size of 3200 patients.
Interim endpoint analyses were presented to an external data monitoring committee after 172, 302, and 458 disease-free survival events were reported. With every interim analysis, the committee recommended continuation without modification. The 594 events needed to trigger the definitive analysis had occurred by March 31, 2011.
We used simple log-rank tests20 and Cox’s proportional-hazard models21 to make formal inferences about group comparisons of primary and secondary endpoints. Kaplan-Meier curves were used to quantify the values of time-to- event endpoints over time.22 In the Cox’s regression analyses, we made adjustments for stratification variables. For analyses of adherence, the proportions of patients on protocol therapy were presented over the 3-year period by a Kaplan-Meier approach in which an event was recorded at the time the patient ended treatment and a censor was recorded if the patient completed treatment per protocol criteria.
Inference about group comparisons used a Cox’s proportional-hazards model adjusting for stratification variables. For treatment comparisons, the placebo group was deemed the control group. Tests of the validity of the proportionality assumption were via the method proposed by Grambsch and Therneau.23 In addition to tests of treatment effects, we undertook analyses to ascertain if significant treatment by stratification variable interactions existed with respect to endpoints.24
We obtained p values for treatment comparisons of time to site of first treatment failure with the method by Fine and Gray.25 In the forest plots used to display subset analyses by each stratification variable, we adjusted treatment hazard ratios (HRs) for all other stratification variables. 95% CIs are reported, and all CIs and p values are two-sided, with an α-level for significance of 0·05. We did statistical analyses with SAS version 9.2 and R version 2.13.0. This trial is registered with ClinicalTrials. gov, number NCT00009945.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of this report, and had no access to the raw data. Federal resources were used to fund the independent data monitoring committee, which monitored the trial every 6 months. SJA, PZ, and JPC had access to all raw data. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
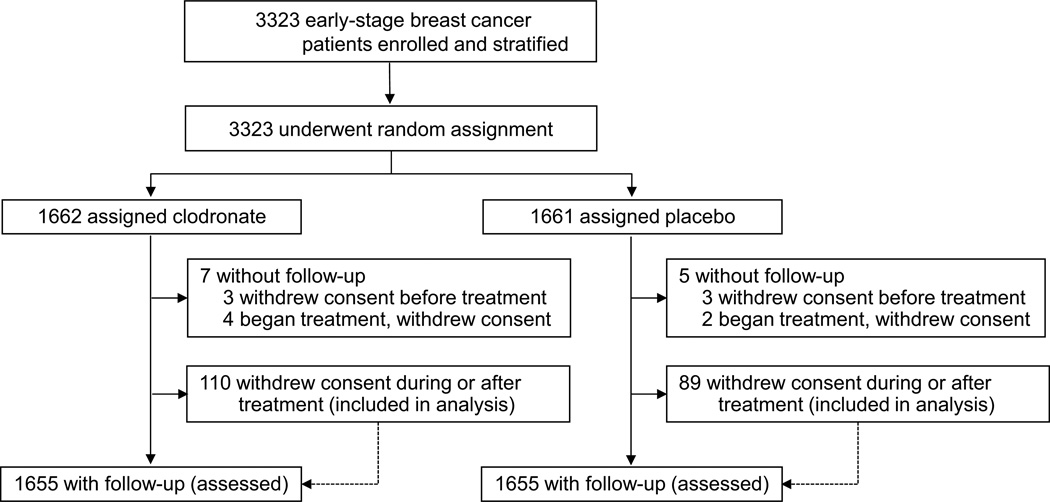
Between Jan 22, 2001, and March 31, 2004, 3323 women were enrolled into the study and underwent random assignment (figure 1). The distribution of patients across groups for important characteristics was satisfactory (table 1). About two-thirds of women were 50 years or older on entry, and three-quarters had negative axillary nodes and were ER-positive. Both chemotherapy and hormone treatment (mostly tamoxifen) were administered to 44% (728/1662) and 43% (720/1661) of patients receiving clodronate and placebo, respectively (table 1).
Figure 1. Trial profile.
Table 1.
Baseline and patients’ characteristics
| Characteristic | Placebo | Clodronate | ||
|---|---|---|---|---|
| Patients (n[%]) randomly assigned | 1661 | 1662 | ||
| Patients (n[%]) with follow-up data | 1656 | 1655 | ||
| Median (IRQ) follow-up (months)* | 91.5 (83.4–100.0) | 90.0 (82.3–100.0) | ||
| n | % | n | % | |
| Age at entry (years)† | ||||
| ≤49 | 589 | 35 | 594 | 36 |
| ≥50 | 1072 | 65 | 1068 | 64 |
| Ethnic origin | ||||
| White | 1375 | 83 | 1381 | 83 |
| Hispanic | 90 | 5 | 96 | 6 |
| Black | 126 | 8 | 117 | 7 |
| Pacific Islander | 9 | <1 | 4 | <1 |
| Asian | 43 | 3 | 48 | 3 |
| American Indian | 3 | <1 | 6 | <1 |
| Other | 10 | <1 | 8 | <1 |
| Unknown | 5 | <1 | 2 | <1 |
| Number of positive nodes† | ||||
| Negative | 1252 | 75 | 1258 | 76 |
| 1 – 3 | 295 | 18 | 296 | 18 |
| 4 or more | 114 | 7 | 108 | 6 |
| Hormone receptor status†‡ | ||||
| Both negative | 368 | 22 | 368 | 22 |
| Either or both positive | 1293 | 78 | 1294 | 78 |
| Adjuvant therapy | ||||
| Chemotherapy alone | 344 | 31 | 342 | 21 |
| Hormonal therapy alone | 518 | 21 | 512 | 31 |
| Both | 720 | 43 | 728 | 44 |
| None | 53 | 3 | 51 | 3 |
| Unknown | 26 | 2 | 29 | 2 |
| Pathologic Tumor Size | ||||
| ≤ 2.0 cm | 1119 | 67 | 1127 | 68 |
| 2.1 – 4 cm | 456 | 27 | 466 | 28 |
| > 4.1 cm | 81 | 5 | 64 | 4 |
| Unknown | 5 | <1 | 5 | <1 |
| Histologic grade | ||||
| Low | 374 | 23 | 377 | 23 |
| Intermediate | 665 | 40 | 667 | 40 |
| High | 589 | 35 | 575 | 35 |
| Unknown | 33 | 2 | 43 | 3 |
Values are based on all patients entered into the study, unless otherwise specified.
As of March 31, 2011 (based on 3004 patients reported to be alive at last follow-up).
As reported at time of random assignment.
Estrogen receptor and progesterone receptor.
A breakdown of adjuvant treatments by group can be found in the appendix (pp 1–2). As of March 31, 2011, median follow-up for surviving patients in both groups was 90·7 months (range 0·1–120·5, IQR 82·7–100·0).
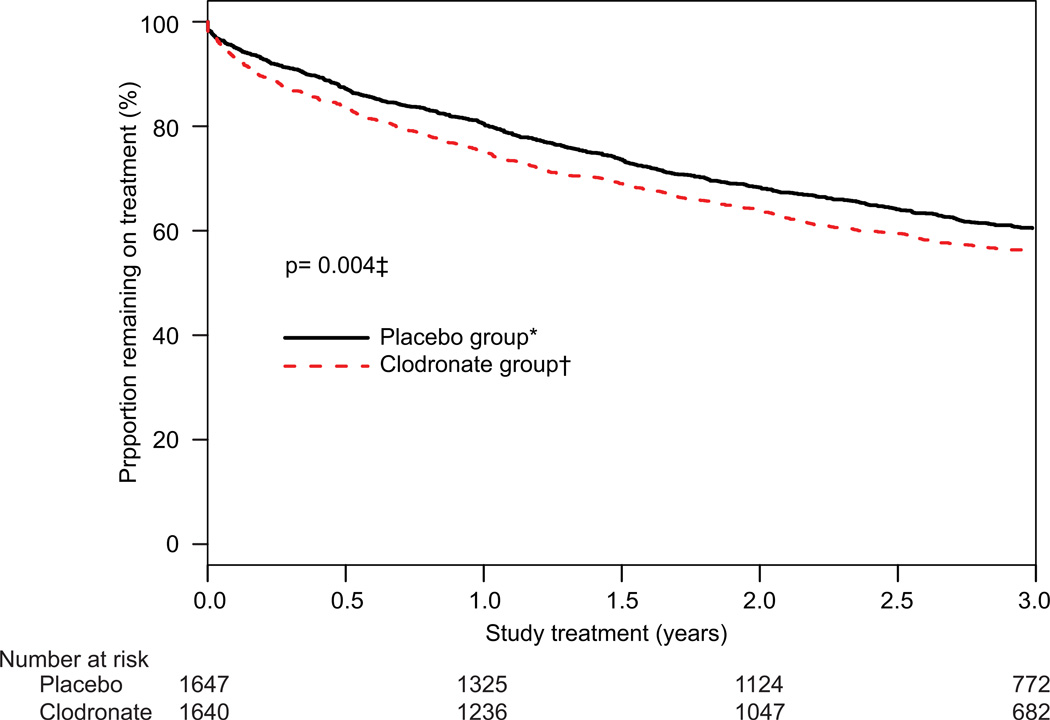
Adherence to treatment was not optimum. The main difference between clodronate and placebo groups in withdrawals from protocol treatment (possibly related to temporarily increased gastrointestinal side-effects) was noted in the first 6 months, when patients were receiving concomitant chemotherapy. By the end of the 3-year therapeutic period, 60% (992/1647) of women assigned placebo and 56% (919/1640) of those allocated clodronate remained on study drugs (figure 2).
Figure 2. Adherence to treatment.
*1647 with follow-up, whether or not they were on treatment (655 were off treatment). †1640 with follow-up, whether or not they were on treatment (721 were off treatment). ‡Adjusted for stratification variables.
Reported side-effects were low in both arms and were similar between treatments (table 2). One possible case of osteonecrosis of the jaw arose in a woman assigned clodronate who had a 1 mm area of exposed bone on the maxillary taurus, which has since healed. Of the 17 deaths (grade 5 toxic effects) noted in table 2, five of unknown cause were noted in the placebo group and one was recorded in the clodronate group.
Table 2.
Side-effects and toxic effects*
| Placebo (n=1623) | Clodronate (n=1612) | |||||
|---|---|---|---|---|---|---|
| Toxicity | Grade 3 n (%) |
Grade 4 n (%) |
Grade 5 n (%) |
Grade 3 n (%) |
Grade 4 n (%) |
Grade 5 n (%) |
| Overall | 223 (14) | 110 (7) | 12 (1) | 247 (15) | 85 (5) | 5 (<1) |
| Diarrhea | 10 (<1) | 0 | 0 | 27 (2) | 1 (<1) | 0 |
| ALT/AST | 10 (<1) | 2 (<1) | 0 | 21 (1) | 2 (<1) | 0 |
| Hypocalcemia | 2 (<1) | 0 | 0 | 1 (<1) | 0 | 0 |
| Creatinine | 0 | 0 | 0 | 3 (<1) | 1 (<1) | 0 |
| Thrombosis or embolism | 17 (1) | 13 (<1) | 0 | 13 (1) | 4 (<1) | 0 |
| Pancreatitis | 1 (<1) | 0 | 0 | 5 (<1) | 0 | 0 |
Based on the National Cancer Institute’s common toxicity criteria, version 4.0. ALT=alanine aminotransferase. AST=aspartate aminotransferase.
Information on toxic effects was available for 97% (3235/3323) of patients enrolled in the study.
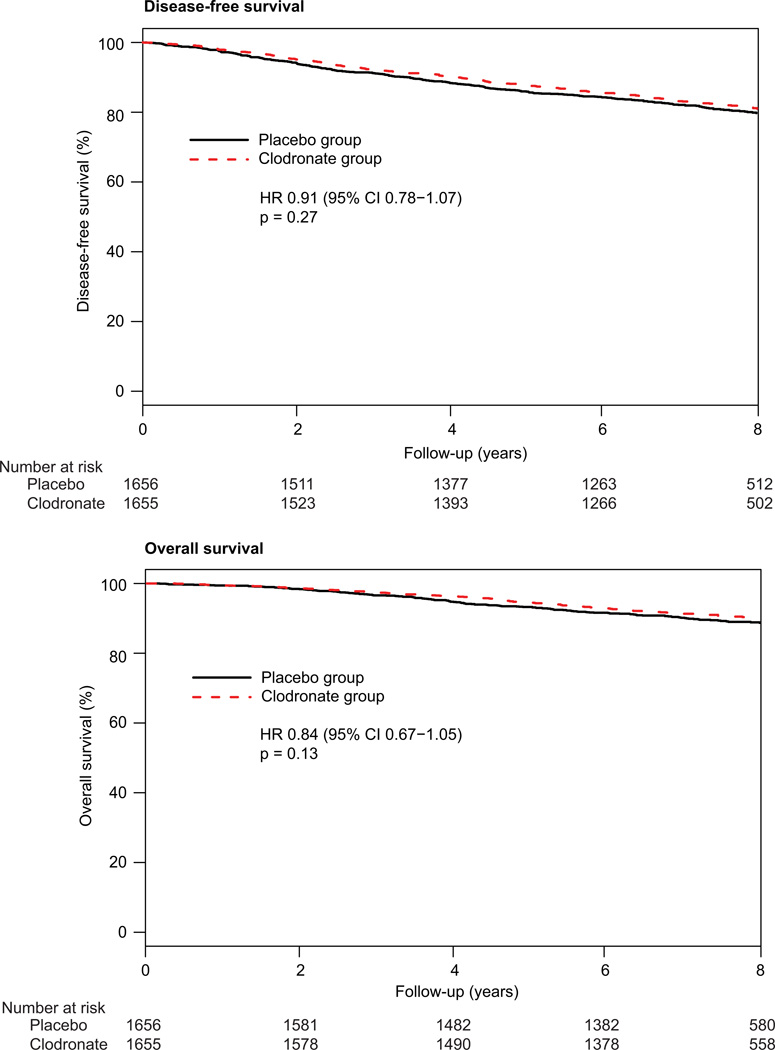
Disease-free survival did not differ between treatment groups (figure 3), and no differences between arms were recorded for overall survival, recurrence-free interval, or bone metastasis-free interval (table 3). Non- bone metastasis-free interval showed borderline significance in favour of clodronate (table 3). The frequency of second primary cancers was similar in each treatment group (114 events in the clodronate group vs 119 in the placebo group; HR 0·96, 95% CI 0·74–1·24; p=0·75). Furthermore, rates of local or regional recurrence, frequency of contralateral breast cancers, or second primary malignant disease did not differ between groups (table 4).
Figure 3. Disease-free and overall survival.
1656 patients in the placebo group and 1655 in the clodronate group. 312 events arose with placebo and 286 with clodronate. 167 patients died in the placebo group and 140 in the clodronate group.
Table 3.
Prespecified study endpoints
| Events (n) | Hazard ratio (95% CI) |
p* | ||
|---|---|---|---|---|
| Placebo | Clodronate | |||
| Disease-free survival | 312 | 286 | 0.91 (0.78 – 1.07) | 0.27 |
| Overall survival | 167 | 140 | 0.84 (0.67 – 1.05) | 0.13 |
| Recurrence-free interval | 177 | 148 | 0.83 (0.67 – 1.04) | 0.10 |
| Bone metastasis-free interval |
80 | 61 | 0.77 (0.55 – 1.07) | 0.12 |
| Non-bone metastasis-free survival |
105 | 78 | 0.74 (0.55 – 1.00) | 0.047 |
By log-rank test
Table 4.
Sites of first treatment failure
| Location of failure | Placebo (n=1656) |
Clodronate (n=1655) |
p* |
|---|---|---|---|
| n (%) | n (%) | ||
| Local | 44 (3) | 43 (3) | 0.92 |
| Regional | 9(<1) | 10 (<1) | 0.80 |
| Distant | 113 (7) | 90 (5) | 0.11 |
| Opposite breast | 37 (2) | 39 (2) | 0.78 |
| Second cancer, except opposite breast | 79 (5) | 70 (4) | 0.50 |
| Dead, no evidence of disease | 30 (2) | 34 (2) | 0.58 |
| Total alive, event free | 1344 (81) | 1369 (83) | – |
| Total events | 312 (19) | 286 (17) | 0.27 |
Difference in cumulative incidence of failure sites between groups.
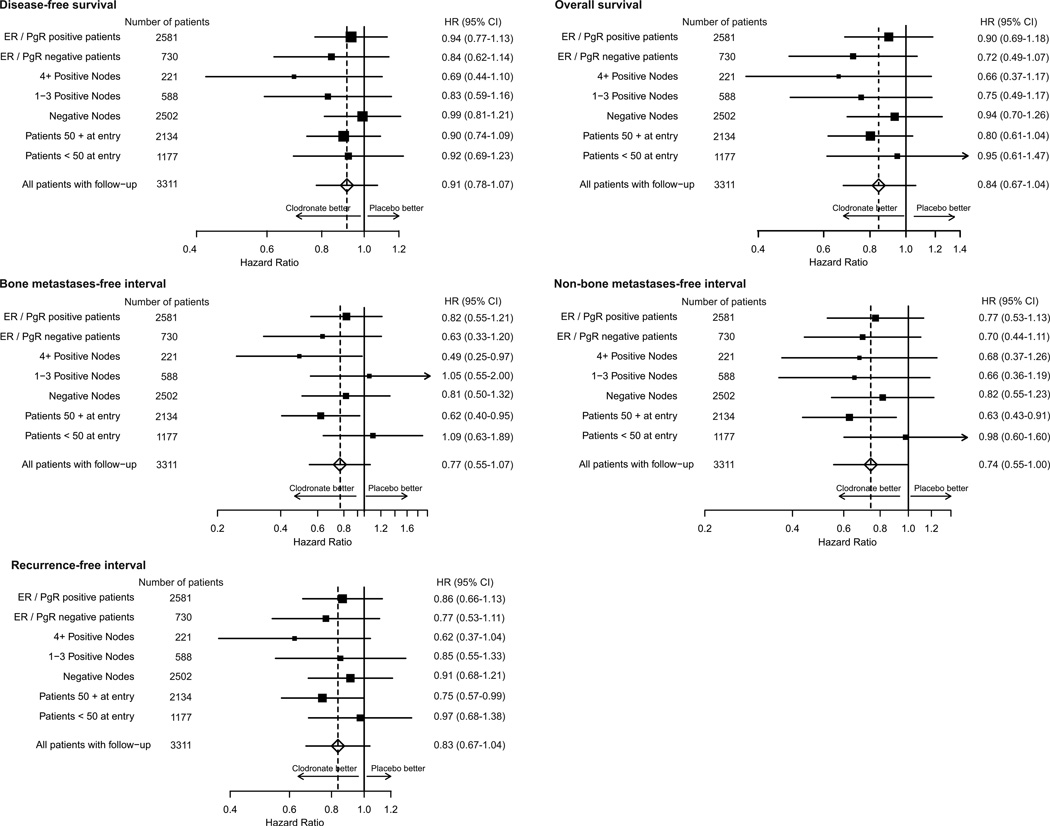
Figure 4 shows endpoint data stratified by age, axillary nodes, and hormone receptor status. With the exception of women who had negative axillary nodes, the clodronate group was favoured for all endpoints, but in all cases the CIs crossed unity. For recurrence-free interval, all stratification variables favoured clodronate. Women 50 years or older at study entry had a modest improvement in recurrence-free interval with clodronate (p=0·045).
Figure 4. Endpoint analysis.
Hazard ratios (HRs) stratified by age, axillary node status, and estrogen (ER) and progesterone (PgR) receptor status.
Formal analyses indicated no treatment-by-stratification variable interactions for disease-free survival or recurrence-free interval (figure 4). Significant improvements with clodronate were noted in bone metastasis- free interval (p=0·027) and non-bone metastasis-free interval (p=0·014) in women 50 years or older (figure 4). In this same group of patients, clodronate showed a borderline improvement in overall survival (101 deaths in the clodronate group vs 125 in the placebo group; HR 0·80, 95% CI 0·61–1·04; p=0·094). By comparison, there was little difference in overall survival for those younger than 50 years (42 deaths in the placebo group vs 39 in the clodronate group; HR 0·95, 95% CI 0·61–1·47; p=0·82).
A post-hoc analysis of treatment was done according to age (<50 years, 50–60 years, and ≥60 years; appendix, p 3–5). The effect of clodronate was progressively greater in older women with respect to bone metastasis-free interval and non-bone metastasis-free interval.
Discussion
In this analysis of data from NSABP protocol B-34, disease-free survival, overall survival, recurrence-free interval, and bone metastasis-free interval did not differ between clodronate and placebo groups over a 3-year treatment period. A borderline improvement in non-bone metastasis-free interval was noted with clodronate. In analyses of stratification subgroups, recurrence-free interval, bone metastasis-free interval, and non-bone metastasis-free interval were significantly better with clodronate in women 50 years and older.
About three-quarters of women enrolled in this trial had negative axillary nodes and, therefore, were a group with better prognosis and a lower recurrence rate than in most previously reported trials of adjuvant bisphosphonates.15,19 The proportion of node-negative patients had been projected accurately before the trial and was used for sample size calculations. Accordingly, on the basis of information from previous NSABP trials, we had estimated an annual recurrence rate of 3·88% in the control (placebo) group, but the event rate in the study was only 2·86%. Hence, because patients fared better than projected, the trial follow-up period was extended by 3 years so that the requisite number of events needed to power the trial could be recorded.
Adherence to oral study drug by the end of the 3-year treatment period was 60% for patients allocated placebo and 56% for those assigned clodronate. This proportion is less than optimum and has weakened our ability to ascertain the overall effect of clodronate. Clodronate (which has a known side-effect of diarrhoea) was started during the period of chemotherapy, and this timing led to an early non-compliance difference of about 4%. The gap between arms remained fairly constant during the follow-up period. The decision to use clodronate with chemotherapy was supported by findings in animals suggesting synergy between bisphosphonates and concomitant chemotherapy.6
Only a few side-effects were reported during follow- up. A slightly higher frequency of grade 3 diarrhoea was noted in the clodronate arm. Toxic effects, particularly the serious event of osteonecrosis of the jaw and renal complications, were very low. Prevalence of second primary malignant disease was similar in both groups
A limitation of our study was the fairly low number of events in this predominantly node-negative population. Furthermore, a potential effect of clodronate on disease-free survival might have been masked by the high rate of second cancers as a first event. Because of the older average age and early stage of patients enrolled in this study (compared with the general population of breast cancer patients and populations of comparable clinical trials), second primary malignant diseases were typically noted as first events (25%, 149/598) - - events for which clodronate had no observable effect. Use of disease-free survival as the primary endpoint in a trial of an adjuvant agent with a main presumed mode of action in bone can be defended because of the investigational nature of the trial. However, inclusion of an endpoint unlikely to be affected by clodronate, but which arises at a fairly high rate independent of the investigational agent, such as second primary malignant diseases, is likely to lower the ability to show a statistically clear benefit for breast cancer outcomes in patients for whom a real benefit could be present.
Assessment of the effect of clodronate within age subgroups suggested a beneficial reduction of distant metastatic events in women older than 50 years. For recurrence-free interval, which includes local and regional recurrences and for which no difference in frequency was noted between arms in the prespecified analysis, a benefit of clodronate was recorded. For bone metastasis-free interval and non-bone metastasis-free interval in women age 50 years and older, a clinically relevant effect of clodronate was indicated.
Had the age-related finding of apparent benefit been a finding unique to this trial, the scientific importance could be questioned. However, similar findings have been noted in other studies (panel). Powles and colleagues16 initially showed a bone metastasis-free interval and overall survival benefit in all patients on oral clodronate, but on subsequent review the benefit seemed to be confined to postmenopausal women. In a trial of patients with subclinical bone-marrow metastases, Diel and co-workers14 reported a disease-free survival benefit in older patients. The one negative adjuvant trial of oral clodronate,17 which was a small open- label study, was undertaken in a predominantly premenopausal population.
Using intravenous zoledronate in women with positive axillary nodes, Coleman and colleagues19 reported results similar to our findings, with no overall effect on disease-free survival in all patients but a benefit in those either older than 60 years or more than 5 years into menopause. In the ABCSG-12 trial,18 a disease-free survival benefit was noted for intravenous zoledronate in premenopausal women; these findings do not necessarily contradict our results, since patients in the ABCSG-12 trial received zoledronate after they had been converted to a postmenopausal state with goserelin. By contrast with our study, in these two trials, a reduction in local and regional recurrences in postmenopausal women was seen.
Younger patients seemed to derive no benefit from clodronate in our study, and the possibility of a harmful effect cannot be excluded. However, older women seemed to gain some beneficial effect with respect to delayed onset of bone metastases and non-bone metastases and the post-hoc composite of distant metastasis-free interval (HR 0·61, 95% CI 0·44–0·85; p=0·0031). In view of other trials with similar findings, our results suggest a beneficial effect of bisphosphonates at distant sites in older and postmenopausal women that is not seen in younger women. We cannot exclude, however, that factors other than menopausal status could lead to the apparently better outcomes for older women - - eg, ER status, type of endocrine treatment, stage of disease, or other unknown factors.
Our findings are difficult to explain with respect to the mechanisms of action of bisphosphonates at a cellular level. One hypothesis is that microenvironmental effects of these agents differ in premenopausal and postmenopausal women. Mechanisms of action of bisphosphonates at sites other than bone (which is the presumed primary site of activity of these drugs) could be important. Consistent reports of benefits of bisphosphonates on non-bone metastasis-free interval14,15,19 need explanation beyond that of a chance finding. Mechanisms of secondary spread from bone have been postulated. Bone is the most common site of distant recurrence and could act as a reservoir for subsequent seeding.
Menopausal status was not identified prospectively in this trial; a cutoff at age 50 years to demarcate premenopause and postmenopause is a surrogate used frequently for menopausal status. Low oestrogen states in early and late postmenopause are known to increase the activity of osteoclasts and to accelerate resorption of bone.26,27 Also, in older women, control of bone turnover differs from that in younger women. Ovarian-derived inhibins of the transforming growth factor β superfamily play a major part in control of bone turnover in the menopause transition.28 In later menopause, local autocrine or paracrine pathways have a more dominant role than in premenopause,29 and a bisphosphonate might exert a greater effect in this milieu. The intervention of a bisphosphonate in situations of augmented bone turnover could reduce the stimulatory effects of by-products of bone resorption on cancer-cell growth and proliferation to a clinically detectable beneficial level.
Findings of bisphosphonate studies, including NSABP protocol B-34, suggest a benefit in recurrence rates for postmenopausal women with breast cancer, but further studies (including meta-analyses) are needed before general application in the breast cancer population. Studies of bisphosphonates and other bone-active agents in older women with high-risk breast cancer seem justified.
Supplementary Material
Panel: Research in context.
Systematic review
In preparation for this study (in 2001), key journals in this subject area were searched by hand. References for pertinent results were tracked and retrieved for screening. We searched Medline from 1990 to 2001 with the following keywords: “breast cancer”, “bisphosphonates”, “adjuvant therapy”, and “clodronate.” Oral clodronate had been shown to improve bone mineral density in premenopausal and postmenopausal women with early breast cancer. In an early open-label study, this drug reduced the frequency of bone metastases and increased survival in women with operable breast cancer who had evidence of malignant cells in bone marrow. In women with primary breast cancer, two other trials have given conflicting results, with one large placebo-controlled study indicating benefit in overall survival and bone metastasis-free survival, whereas findings of a smaller open-label study suggested that clodronate might be harmful. These conflicting results prompted the NSABP to undertake a study over a longer period (3 years) of bisphosphonate as adjuvant therapy in women with stage 1–3 primary breast cancer.
Interpretation
Most patients in this trial were older women with stage 1 disease. The primary endpoint of disease-free survival was not met. The value of this endpoint was reduced owing to the high incidence of second primary malignant diseases as a first event. A secondary endpoint of non-bone metastasis-free survival was of borderline significance, and investigation of predetermined stratification variables showed a relation between age and reduced rates of osseous and extra-osseous distant recurrence. This effect has been recorded in other bisphosphonate trials. Design of future studies of bone-active agents should take this relation into consideration. Use of bisphosphonates or other bone-active agents as anticancer drugs in premenopausal women with primary breast cancer cannot be recommended. Their routine use as anticancer treatment for older women or those who are (naturally or artificially) postmenopausal needs further study and analysis.
Acknowledgments
We thank the members of the independent data monitoring committee, medical, nursing and data handling staff at the participating centres and all the patients who participated in NSABP B-34. Supported by NCI Department of Health and Human Services Public Health Service Grants: U10CA-12027, U10CA-69974, U10CA-37377, and U10CA-69651 (NSABP), with additional funding from Scherring AG; CA-021115-37 (CIF, ECOG); U10-CA095867 (TL, MBCOOP); and U10-44066-26 (AR, CHUM), and CA25224 (EAP, NCCTG/ACTION). The authors also wish to thank Barbara C. Good, PhD, Christine I. Rudock, and Wendy L. Rea (NSABP Division of Scientific Publications), Shelley K. Hando, RN, MSN, Radka Kerpedjieva, BS, Mary Jo Antonelli, MBA, and Darlene Kiniry, MS (NSABP Biostatistics).
Footnotes
See Online for appendix
Contributors
All authors approved the manuscript for submission. AHGP was the study chair, wrote first draft, contributed to follow-up, and was in charge of editing. SJA monitored the trial, performed all data analyses, originated figures and tables, and co-wrote the manuscript. CEG participated in the protocol meetings for the study, and served as Director of Medical Affairs during the conduct of the trial. AGHP, SJA, BCL, LF, CEG, SMS, JPC, and EPM, contributed to study design. AGHP, LF, KMK, LMW, CEG, and EPM accrued patients to the study. LF, KMK, AMB, TL, LB-D, JPC contributed to data collection. SJA, BCL, AMB, EAP, PZ, JPC contributed to data analysis. SJA, BCL, LF, JRG, EAP, PZ, CEG, SMS, EPM contributed to data interpretation. AGHP, SJA, BCL, JRG, EAP, CEG, SMS, EPM contributed to writing the manuscript. LF, LMW, AMB contributed to manuscript review.
Potential conflicts of interest
AGHP received honoraria from Bayer, Novartis, Amgen, and Roche Diagnostics.
SJA received travel costs for testimony about the trial to the US Food and Drug Administration in 2000 and 2004.
All other authors declare they have no conflicts of interest.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormone therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomized trials. Lancet. 2005;365:1687–1617. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Smith R, Wang J, Bryant J, Kay A, Paterson AG, Wolmark N. Primary breast cancer (PBC) as a risk factor for bone recurrence: NSABP experience. Proc Amer Soc Clin Oncol. 1999;18 (abstr 457) [Google Scholar]
- 3.Goldhirsch A, Gelber R, Price KN, et al. Effect of systemic adjuvant treatment on first sites of breast cancer relapse. Lancet. 1994;343:37781. doi: 10.1016/s0140-6736(94)91221-1. [DOI] [PubMed] [Google Scholar]
- 4.Fleisch H. Bisphosphonates. Pharmacology and use in the treatment of tumour-induced hypercalcaemic and metastatic bone disease. Drugs. 1991;42:91944. doi: 10.2165/00003495-199142060-00003. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki A, Boyce BF, Story B, et al. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res. 1995;55:355157. [PubMed] [Google Scholar]
- 6.Yoneda T, Michigami T, Yi B, Williams PJ, Niewolna M, Hiraga T. Actions of bisphosphonate on bone metastasis in animal models of breast carcinoma. Cancer. 2000;88(12 suppl):297988. doi: 10.1002/1097-0142(20000615)88:12+<2979::aid-cncr13>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Clézardin P, Ebetino FH, Fournier PG. Bisphosphonates and cancer-induced bone disease: Beyond their antiresorptive activity. Cancer Res. 2005;65:497174. doi: 10.1158/0008-5472.CAN-05-0264. [DOI] [PubMed] [Google Scholar]
- 8.Paterson AH, Powles TJ, Kanis JA, McCloskey E, Hanson J, Ashley S. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol. 1993;11:5965. doi: 10.1200/JCO.1993.11.1.59. [DOI] [PubMed] [Google Scholar]
- 9.Hortobagyi GN, Theriault RL, Porter L, et al. for the Protocol 19 Aredia Breast Cancer Study Group. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med. 1996;335:178591. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 10.Body JJ, Bartl R, Burckhardt P, et al. Current use of bisphosphonates in oncology. International Bone and Cancer Study Group. J Clin Oncol. 1998;16:389099. doi: 10.1200/JCO.1998.16.12.3890. [DOI] [PubMed] [Google Scholar]
- 11.Mundy GR, Yoneda T. Bisphosphonates as anticancer drugs. N Engl J Med. 1998;339:398–400. doi: 10.1056/NEJM199808063390609. [DOI] [PubMed] [Google Scholar]
- 12.Mundy GR. Metastasis to bone: Causes, consequences, and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 13.Powles TJ, McCloskey E, Paterson AH, et al. Oral clodronate and reduction on loss of bone mineral density in women with operable breast cancer. J Natl Cancer Inst. 1998;90:704–708. doi: 10.1093/jnci/90.9.704. [DOI] [PubMed] [Google Scholar]
- 14.Diel IJ, Solomeyer EF, Costa SD, et al. Reduction in new metastases in breast cancer with oral clodronate treatment. N Engl J Med. 1998;339:357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 15.Powles T, Paterson S, Kanis JA, et al. Randomised, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol. 2002;20:3219–3224. doi: 10.1200/JCO.2002.11.080. [DOI] [PubMed] [Google Scholar]
- 16.Powles TJ, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer. Breast Cancer Res. 2006;8:R13, 1–7. doi: 10.1186/bcr1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saarto T, Vehmanen L, Virkkrunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43:650–656. doi: 10.1080/02841860410032885. [DOI] [PubMed] [Google Scholar]
- 18.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 19.Coleman RE, Marshall H, Cameron D, et al. Breast cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 20.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A. 1972;135(part 2):185–198. [Google Scholar]
- 21.Cox D. Regression models and life tables (with discussion) J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assn. 1958;53:457–481. [Google Scholar]
- 23.Grambsch P, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 24.Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41(2):361–372. [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 26.Khosla S, Melton LJ, 3rd, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: A key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 27.Heshmati HM, Khosla S, Robins SP, O'Fallon WM, Melton LJ, III, Riggs BL. Role of low levels of endogenous estrogen in regulation of bone resorption in late postmenopausal women. J Bone Miner Res. 2002;17(1):172–178. doi: 10.1359/jbmr.2002.17.1.172. [DOI] [PubMed] [Google Scholar]
- 28.Gaddy-Kurten D, Coker JK, Abe E, Jilka RL, Manolagas SC. Inhibin suppresses and activin stimulates osteoblastogenesis and osteoclastogenesis in murine bone marrow cultures. Endocrinology. 2002;143:74–83. doi: 10.1210/endo.143.1.8580. [DOI] [PubMed] [Google Scholar]
- 29.Nicks KM, Fowler TW, Akel NS, Perrien DS, Suva LJ, Gaddy D. Bone turnover across the menopause transition: The role of gonadal inhibins. Ann NY Acad Sci. 2010;1192:153–160. doi: 10.1111/j.1749-6632.2009.05349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.