Abstract
Introduction
For the past three decades, carbapenems played a central role in our antibiotic armamentarium, trusted to effectively treat infections caused by drug-resistant bacteria. The utility of this class of antibiotics has been compromised by the emergence of resistance especially among Enterobacteriaceae.
Areas covered
We review the current mainstays of pharmacotherapy against infections caused by carbapenem-resistant Enterobacteriaceae (CRE) including tigecycline, aminoglycosides, and rediscovered 'old' antibiotics such as fosfomycin and polymyxins, and discuss their efficacy and potential toxicity. We also summarize the clinical experience treating CRE infections with antibiotic combination therapy. Finally, we review ceftazidime/avibactam and imipenem/relebactam, a new generation of beta-lactamase inhibitors, which may offer alternatives to treat CRE infections. We critically evaluate the published literature, identify relevant clinical trials and review documents submitted to the United States Food and Drug Administration.
Expert Opinion
It is essential to define the molecular mechanisms of resistance and to apply insights about pharmacodynamic and pharmacokinetic properties of antibiotics, in order to maximize the impact of old and new therapeutic approaches against infections caused by CRE. A concerted effort is needed to carry out high-quality clinical trials that: i) establish the superiority of combination therapy vs. monotherapy; ii) confirm the role of novel beta-lactam/beta-lactamase inhibitor combinations as therapy against KPC- and OXA-48 producing Enterobacteriaceae; and, iii) evaluate new antibiotics active against CRE as they are introduced into the clinic.
1. Introduction
The bright, rich, purple pigmentation of the mycelium of a streptomycete isolated from a soil sample in New Jersey, US, may not have been initially recognized as a sign of extraordinary therapeutic potential. Nevertheless, taxonomists acknowledged its bold, orchid color and named the organism Streptomyces cattleya [1]. By 1976, further investigation of the ability of culture broths of S. cattleya to inhibit peptidoglycan synthesis led to the discovery of thienamycin, the precursor of carbapenems [2]. Thienamycin differentiated itself from other natural antibiotics because it displayed an unusually broad spectrum of activity, encompassing most Gram-positive and Gram-negative bacteria, including Enterobacteriaceae and remarkably, Pseudomonas aeruginosa [3]. Furthermore, the activity of thienamycin was not inactivated by beta-lactamases that conferred resistance to penicillins and cephalosporins [4]. Thus began a promising new era in beta-lactam chemotherapy.
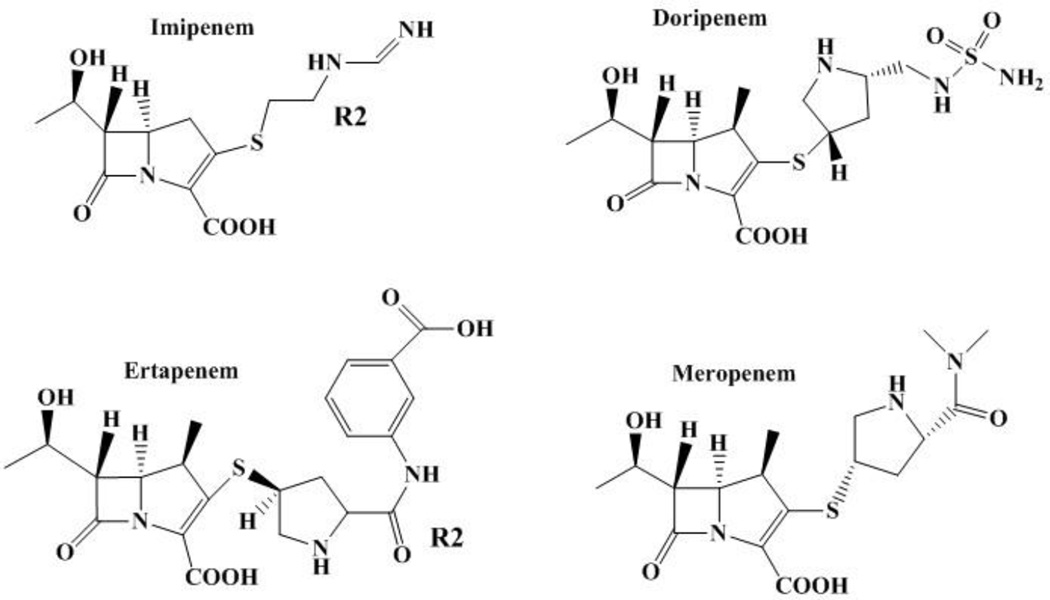
The chemical instability and limited solubility of thienamycin did not permit its therapeutic development, but appreciation of its unique affinity to penicillin-binding proteins (PBPs) and inhibition of beta-lactamases fostered interest in thienamycin derivatives [5]. Major advancements resulted from the conception of penems by Professor R. B. Woodward, a legendary organic chemist, and from the total synthesis of thienamycin in the hands of B. G. Christensen [6,7]. In 1985, imipenem became the first commercially available carbapenem [8]. This eerily coincided with the initial emergence of class A extended-spectrum beta-lactamases (ESBLs) of the SHV and TEM families among Enterobacteriaceae. The unique properties of imipenem, the first commercially available carbapenem, led to therapeutic success against infections caused by ESBL-producing Enterobacteriaceae [9]. For the past three decades, imipenem and the other carbapenems (meropenem, ertapenem and doripenem) played a central role in our armamentarium, trusted to effectively treat severe infections caused by suspected drug-resistant bacteria in very ill patients (Figure 1). Regrettably, the utility of this class of antibiotics is now severely compromised by the emergence of resistance to carbapenems especially in Enterobacteriaceae.
Figure 1.
Structure of commercially available carbapenems.
2. Impact of Carbapenem-resistant Enterobacteriaceae
Carbapenem-resistant Enterobacteriaceae (CRE) are designated by the United States Centers for Diseases Control and Prevention (CDC) as a “nightmare bacteria” (http://www.cdc.gov/media/releases/2013/p0305_deadly_bacteria.html), an example of antibiotic resistance threat with potentially the greatest impact on human health. Although Enterobacteriaceae account for 27% of health care associated infections in the US, CRE remain relatively uncommon in most American hospitals [10]. The number of US facilities reporting CRE, however, is rising steadily and include 4% of acute hospitals and 18% of long term acute care facilities [11].
A salient feature of CRE infections is their association with poor clinical outcomes; Patel et al., for instance, observed lethality rates of 40 –50% in patients with bloodstream infection caused by carbapenem-resistant Klebsiella pneumoniae [12]. To place this in perspective, the WHO estimates that the death rate from Ebola virus disease is 50–60% (WHO estimates http://www.who.int/mediacentre/factsheets/fs103/en/). This realization is staggering and indicates that the emergence of CRE, just like Ebola, is a problem of global concern. CRE affect the most vulnerable patients, the elderly, immunosuppressed, chronically and critically ill. Most CRE infections are linked with substantial healthcare exposures [11,13] and transmission of CRE associated with post-acute care facilities plays a central role in the epidemiology of CRE [14]. One of the best-known hospital outbreaks of CRE occurred at the US National Institutes of Health Clinical Center, involving patients with primary immunodeficiency, solid tumors, lymphoma, and organ and bone marrow transplants [15]. The devastating impact of CRE in patients with severe illness is also illustrated by the outcomes of CRE bloodstream infections in a cohort of patients with neutropenia and hematologic malignancy: initial empirical therapy was ineffective in 89% of cases, and mortality reached 69% [16]. Other studies have established an association between mortality and patient’s underlying severity of illness and the delayed administration of appropriate antimicrobial therapy in CRE infections [17,18]. These findings underscore the therapeutic challenge posed by CRE, especially given the multidrug resistant phenotype of CRE and the limitations of the few remaining antibiotic options.
3. Genetic background and context of Carbapenem-Resistant Enterobacteriaceae
Although the term “CRE” is useful as descriptor of our current epidemic, the considerable diversity of bacteria expressing that phenotype and the complex mechanisms that determine carbapenem resistance are notable. Enterobacteriaceae are a very large family, even when restricted to genera that cause infections in humans. K. pneumoniae and Escherichia coli, the most commonly species found in the clinic, have distinct virulence and epidemiologic profiles and important strain-dependent variations [19,20]. Carbapenem-resistant Enterobacter may affect the elderly, [21] it appears children and particularly neonates in intensive care units are susceptible to Enterobacter spp.; Serratia may occupy a similar epidemiologic niche [22,23].
The chief determinants of carbapemem-resistance in Enterobacteriaceae are beta-lactamases that hydrolyze carbapenems, or carbapenemases. Among these, KPC (standing for Klebsiella pneumoniae carbapenemase), a class A serine beta-lactamase, is the most prevalent mechanism responsible for the CRE phenotype in the United States [24]. Although a carbapenemase, the hydrolytic activity of this beta-lactamase against carbapenems is relatively modest; rather, KPC is distinguished by its incredible substrate versatility (i.e., penems, cephems, carbapenems, cephalosporins including cephamycins and advanced generation cephalosporins) and its ability to hydrolyze the commercially available beta-lactamase inhibitors (i.e., sulbactam, tazobactam, clavulanic acid) [25]. Interestingly, the unique ability to inactivate both clavulanic acid and imipenem indicate a very important evolutionary link. It has been hypothesized that unraveling the chemistry that drives the hydrolysis of the commercially available inhibitors and carbapenems may offer new chemical insights to inhibiting these enzymes.
Another class A carbapenemase, SME, is restricted to the chromosome of Serratia marcescens and does not hydrolyze ceftazidime [26]. Metallo-beta-lactamases (MBLs), or class B beta-lactamases, such as NDM (New Delhi metallo-beta-lactamase), VIM (Verona integron mediated metallo-beta-lactamases) and IMP (initially found in imipenem-resistant S. marcescens) are potent carbapenemases and cephalosporinases, yet they do not hydrolyze the monobactam aztreonam [27]. The class D enzyme OXA-48 has emerged as a difficult to detect mechanism of carbapenem resistance, because of its relative inactivity against cephalosporins [28]. Table 1 summarizes mechanisms of resistance conferring the CRE phenotype, and their distribution by species. Changes in the net penetration of carbapenems into the bacterial cell, mediated by alterations in outer membrane porin proteins (e. g., OmpK35, OmpK36) and, in some instances, up-regulation of efflux pumps (e. g., AcrAB-TolC) are increasingly recognized as contributors to carbapenem resistance when combined with the production of beta-lactamases with cephalosporinase activity (e. g., DHA, ACT, CMY, SHV-5, CTX-M-15), eventhough these enzymes lack intrinsic carbapenemase activity [29–32].
Table 1.
Beta-lactamases that confer a carbapenem-resistance phenotype, their classification, common genetic platform and distribution among Enterobacteriaceae.
| Enzyme/Ambler classification | Common genetic platform | Species distribution in Enterobacteriaceae |
|---|---|---|
| KPC (Klebsiella
pneumoniae carbapenemase)/class A |
K. pneumoniae sequence type 258 and ST11 inter alia, transposon Tn4401x |
Klebsiella pneumoniae, Escherichia coli, Enterobacter sp.; diverse Enterobacteriaceae |
| NDM (New Delhi metallo-beta- lactamase)/class B |
Various plasmid types |
K. pneumoniae and E. coli predominantly; diverse Enterobacteriaceae |
| OXA-48 (oxacillinase)/class D | Incl/M-type plasmid |
K. pneumoniae predominantly, diverse Enterobacteriaceae |
| VIM (Verona integron-encoded metallo-beta-lactamase)/class B |
Gene cassettes in class 1 integrons |
K. pneumoniae |
| IMP/class B | Gene cassettes in class 1 integrons |
K. pneumoniae |
| SME/class A | Chromosome | Serratia marcescens |
| DHA-1/class C, in combination with OmpK35/36 loss |
Plasmid | K. pneumoniae |
| ACT/class C, in combination with OmpK35/36 loss |
Plasmid | K. pneumoniae |
| CTX-M-15/class A, in combination with OmpK35/36 loss |
Plasmid | K. pneumoniae |
| SHV-5/class A, in combination with OmpK35/36 loss |
Plasmid | K. pneumoniae |
| GES-5/class A, in combination with OmpK35/36 loss |
Self-conjugative plasmid | K. pneumoniae |
4. Geographic diversity among Carbapenem-resistant Enterobacteriaceae
There are remarkable geographic variations in the molecular epidemiology of CRE. In the United States, attention has focused on K. pneumoniae harboring KPC-2 and KPC-3 and belonging to sequence type (ST) 258, which in some regions comprise approximately 80% of CRE. Two different clades are identified within K. pneumoniae ST258; these two clades are distinguished by genetic variations in their capsular polysaccharide (cps) region [33–36]. Fortunately, CRE containing MBLs are uncommon in North America and, with few exceptions, are usually imported [37]. Although K. pneumoniae ST258 remains an important and successful global strain, geographic differences need to be noted. For instance in Colombia, South America, after the early spread of KPC in K. pneumoniae, nationwide studies demonstrated diversity of Enterobacteriaceae harboring KPC, and even coexistence of VIM and KPC within K. pneumoniae strains of various sequence types [38–40]. In Israel, KPC-producing K. pneumoniae ST258 remains the predominant clone of CRE in acute and post-acute-care hospitals [41,42]. In neighboring Lebanon, in contrast, CRE is mostly represented by OXA-48 producing K. pneumoniae, also endemic in Turkey and North Africa [43–45], and present in South Africa [46]. In Greece and Italy there are various degrees of coexistence between KPC and VIM producing K. pneumoniae, even within the same strains [47]. These generalizations should not obscure the fact that “all epidemiology is local”: a contemporary survey of CRE in Spanish hospital found that 75% were Klebsiella spp., but Enterobacter spp. (14%), and S. marcescens (11%) were also frequent. The MBL VIM-1 was the most frequent carbapenemase (82%), followed by KPC-2 (15%), while IMP occurred only sporadically. Nine K. pneumoniae STs were identified, but ST11 (associated with VIM) and ST101 (associated with KPC) were dominant; nationwide surveys, however, have identified OXA-48 as the predominant mechanism of CRE in Spain [48,49].
The global impact of CRE is illustrated by the rapid international dissemination of NDM-containing Enterobacteriaceae. The first NDM harboring isolate was identified in 2009 from a Swedish patient who returned from the Indian sub-continent. Subsequently, NDM-harboring bacteria were commonly found in healthcare institutions and in environmental sources throughout that region, and are now detected around the world [50]. Initially, identification of NDM-producing Enterobacteriaceae isolates in the US typically involved patients who recently traveled to India or Pakistan [51]. However, exposure to duodenoscopes with apparent bacterial contamination was associated with transmission of NDM-1 producing E. coli at a hospital in Chicago [52]. A dramatic example of the potential for widespread geographic and interspecies dissemination of NDM, given its association with plasmids, is provided by a case of Salmonella enterica serovar Corvallis originating in Asia and found in a migratory bird in Germany [53]. NDM and OXA-48 predominate among CRE from Saudi Arabia and the Gulf States, a hub of international travel half way between the Mediterranean and India [54]. China, where NDM has also been found, displays an overall low prevalence of CRE but with significant carbapenemase diversity, including IMP and KPC, the latter associated with K. pneumoniae ST11 [55,56].
5. Available options to treat Carbapenem-resistant Enterobacteriaceae
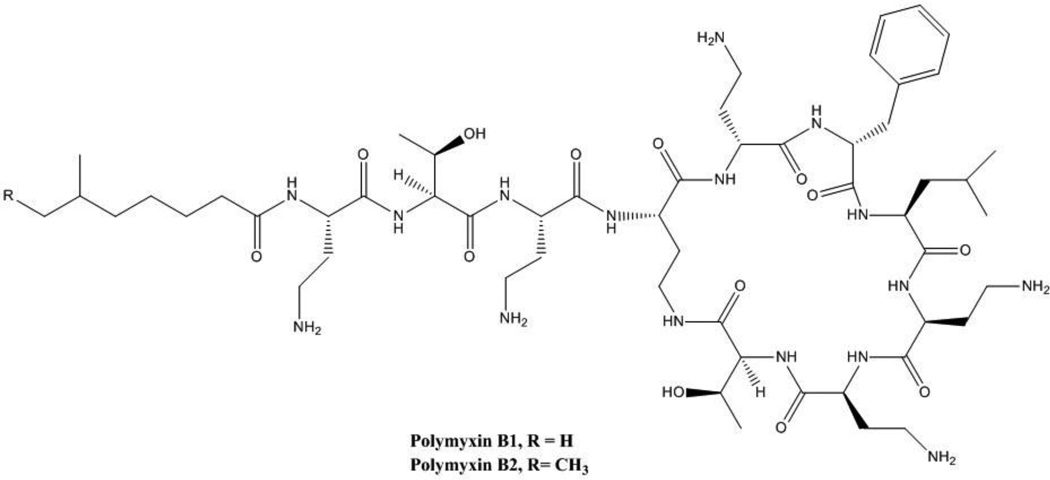
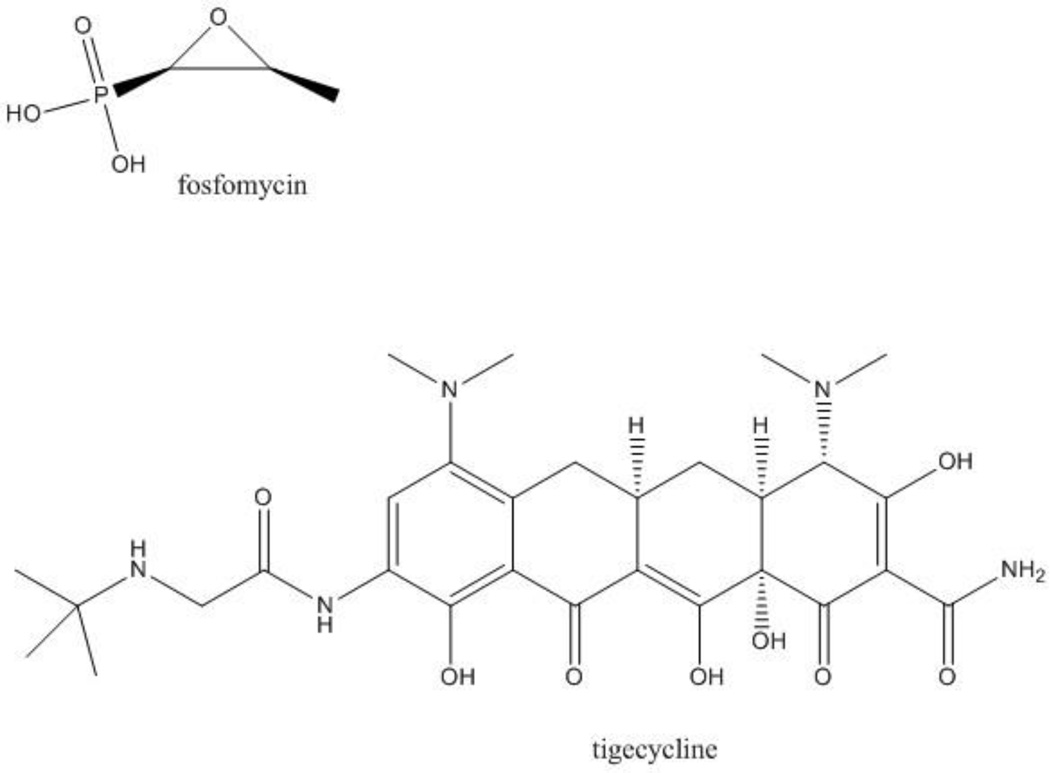
CRE are usually resistant to all commercially available beta-lactams, including carbapenems, penicillins, cephalosporins and combinations with beta-lactamase inhibitors. An important exceptions exists: Enterobacteriaceae that produce MBLs may be susceptible to aztreonam. This, however, occurs only in the minority of cases; for instance, 33/37 isolates of NDM-1 producing Enterobacteriaceae from the United Kingdom and India demonstrated resistance because of the coexistence of ESBLs or other cephalosporinases that hydrolyze aztreonam [57]. Susceptibility to fluoroquinolones is rare among CRE, while susceptibility to sulfas and aminoglycosides may be unpredictable and can vary according to geographic location and strain type: in a strain of KPC-producing K. pneumoniae ST258 from Northeast Ohio, for instance, trimethoprim/sulfamethoxazole susceptibility was 31%, gentamicin susceptibility was 39%, and amikacin susceptibility was 76% [33]. It should be noted that these observations pertain to a single report and that the numbers are likely to differ when other collections of strains are considered. Additionally, there is increasing prevalence of 16S rRNA methylases conferring resistance to all aminoglycosides, especially associated with NDM-producing CRE [50]. Antibiotics with reliable activity against CRE (>85% of strains susceptible) typically include tigecycline, polymyxin B and polymyxin E (or colistin), and fosfomycin (Figures 2 and 3). There are, however, important concerns regarding the limited efficacy of these options because of their pharmacologic charateristics, and reports of increasing resistance, toxicity and adverse events.
Figure 2.
Structure of polymyxin.
Figure 3.
Structure of fosfomycin and tigecycline.
5. 1 Polymyxins
Polymyxins are a class of antimicrobials derived from the fermentation products of the Gram-positive Bacillus polymyxa. These cationic antimicrobial polypeptides have a common chemical scaffold, sharing a chain of amino acids and a fatty acid [58] (Figure 2). Polymyxin B features a phenylalanine in place of the D-leucine present in polymyxin E (colistin), a drug derived from B. polymyxa v. colistinus discovered in Japan. Colistin is administered as colistimethate sodium (CMS), which is a prodrug of colistin hydrolyzed in serum.
Polymyxins are believed to bind to negatively charged phosphate moieties in the lipid A fraction of lipopolysaccharide (LPS) present in the outer membrane of Gram-negative bacteria; the disruption of cell membranes results in loss of intracellular products, thus achieving bactericidal activity. Polymyxin B was introduced into the clinic in the United States in the mid-1950s, and colistin towards the end of that decade. Therefore, polymyxins were not subject to the scrutiny of contemporary drug safety trials and gaps remain in what is known about these antibiotics. The initial clinical enthusiasm with polymyxins in the 1960s, was motivated by their broad spectrum of antimicrobial activity including P. aeruginosa and most Enterobacteriaceae, but was soon tempered by their nephrotoxic and neurotoxic effects [59]. With the advent of aminoglycosides and cephalosporins, agents that were safer, polymyxins took a secondary place in the antibiotic armamentarium. For decades the use of polymyxins was confined to inhalation therapy in patients with cystic fibrosis, as a component of gastrointestinal tract decontamination regimens, and as topical antibiotic therapy. Polymyxins were “rediscovered” in the late 1990s, initially to address the therapeutic challenge posed by carbapenem-resistant Acinetobacter baumannii (CRAB) and P. aeruginosa. The first clinical reports from the “modern era” supported the clinical effectiveness of polymyxins but reignited concerns for nephrotoxicity and, perhaps to a lesser extent, neurotoxicity associated with these drugs [60]. One of the first published cases of CRE infection successfully treated with a polymyxin originated from Greece and occurred in a critically ill man with bloodstream infection due to K. pneumoniae probably harboring a MBL [61]. Since then, there has been a sustained effort to better understand these drugs [62,63].
In 1963 Dr. Robert Petersdorf, one of the pioneers of infectious diseases in the United States, wrote about colistin (polymyxin E): “Despite the accumulation of considerable clinical experience with this agent, it is still subject to a good deal of controversy. Three topics are at issue: 1) is colistin actually superior to polymyxin B with respect to antibacterial activity, therapeutic efficacy, and relative lack of toxicity?; 2) what is the toxic potential of colistin?; is it, in fact, as nontoxic to the kidneys as claimed in some enthusiastic early reports?; 3) should this agent become the drug of choice in Gram-negative infections, particularly before sensitivity [sic] tests are available?” [64].
These questions retain their relevance 50 years later. Following the spirit of that early reappraisal, experts have convened to discuss unresolved issues pertaining to the clinical use of polymyxins, such as: 1) dosing; 2) pharmacokinetics in critically ill patients; 3) variability of preparations; 4) susceptibility testing and breakpoints; 5) superiority of colistin vs. polymyxin B for treatment of UTI, but inferiority in other infections; 6) increased nephrotoxicity of colistin relative to that of polymyxin B; 7) benefit of combining nebulized and intravenous preparations for the treatment of pneumonia; 8) mechanisms of resistance to colistin; and 9) combination with other antibiotics to optimize outcomes [65]. In these last regards, some certainties are emerging. Dosing schemes that are commonly recommended (up to 300 mg of colistin base activity per day) do not appear to result in sufficient initial concentrations of colistin to achieve bacterial killing, especially among patients with preserved renal function [66]. Furthermore, caution is required with colistin monotherapy due to suboptimal exposure in subjects with normal kidney function, and the potential for the emergence of resistance [67]. Based on the current understanding of PK/PD parameters, colistin demonstrates a very narrow therapeutic window, where an average concentration at steady state of 2 µg/ml is needed to achieve therapeutic targets but where a concentration of approximately 2.5 µg/ml results in renal toxicity [68]. Issues such as the importance of a loading dose to more rapidly achieve concentrations that correlate with therapeutic targets, and the acute toxic effects of aggressive dosing remain areas of active investigation [69]. Polymyxins are perhaps most effective as part of a “highly active” combination of antibiotics. However, the precise components and effectiveness of such combination regimens still require clarity.
The use of colistin is associated with a high rate of nephrotoxicity that limits treatment. The actual incidence of nephrotoxicity associated with polymyxins varies from study to study, due to differences in the definition of kidney injury, dosing regimens, concomitant risks for kidney injury, and the population sampled. It appears, however, that nephrotoxicity occurs more frequently with colistin than polymyxin B [70]. Strategies that limit the nephrotoxicity of colistin are urgently needed in the clinic. Investigations in rats suggested a protective effect of ascorbic acid against colistin-induced nephrotoxicity and tubular apoptosis [71]. Nevertheless, a preliminary randomized controlled trial in humans failed to reduced kidney injury with the co-administration of ascorbic acid and colistin [72]. Similarly, a protective effect of melatonin against colistin-induced nephrotoxicity is suggested by animal studies, but this intervention has not been tested in humans [73].
The continued utility of polymyxins for the treatment of CRE infections is undermined by the emergence of resistance. Outbreaks of colistin resistant CRE have occurred in various parts of the globe [74–77]. In an endemic setting, a survey from a tertiary center in Spain reported an increase in colistin resistance from 13.5% to 31.7% among K. pneumoniae isolates from 2010–2012 [49]. In Italy, colistin resistance among KPC producing K. pneumoniae blood isolates increased more than threefold in the past 5 years; 30-day mortality of colistin-resistant KPC-producing K. pneumoniae bloodstream infection was as high as 51%, significantly higher than for drug susceptible K. pneumoniae [78]. Several strategies are employed by bacteria to evade polymyxins, and the mechanisms that lead to resistance in Enterobacteriaceae are complex and not fully characterized. Modifications in the lipid A component of LPS through substitution of phosphate groups in lipid A with 4-amino-4-deoxy-L-arabinose (L-Ara4N) play a central role in the colistin-resistant phenotype. In particular, modification of lipid A with L-Ara4N is accomplished by activation of the two-component systems phoP/phoQ and pmrA/pmrB in response to low magnesium concentrations and other environmental stimuli, including polymyxins themselves. Overexpression of the outer membrane protein OprH, also contributes to colistin resistance, as well as the capsule polysaccharide of K. pneumoniae and efflux pumps. [79].
Among K. pneumoniae, alterations in the mgrB gene are an important mechanism for acquired resistance to colistin by removing a negative feedback on the PhoP/PhoQ regulatory system [80,81]. This is the mechanism behind the epidemic dissemination of colistin-resistant CRE in Italian hospitals, and has been found in global surveys that include isolates from Europe, Asia, Africa and the Americas [80]. The in vivo evolution of colistin resistance can occur in association with low dose exposures, through mutations in PmrB [82]. A combination of genome sequencing and transcriptional profiling by RNA-seq analysis reveals the coexistence of multiple pathways to polymyxin resistance that may vary according to strain type, including mutations in the gene for a putative novel two-component regulatory system (TCRS) designated crrAB, in addition to mutations in genes for the known two-component systems phoP/phoQ and pmrA/pmrB [83]. A deeper understanding of the complexity and redundancy of mechanisms of resistance to this class of drugs will require a “systems” approach, and is needed in order to better delineate the therapeutic limitations and virtues of polymyxins. Development of polymyxin resistance has been associated in some species with fitness cost and decreased virulence, especially in the case of A. baumannii and Salmonella [79]. The applicability of this finding to K. pneumoniae is uncertain and should not be over interpreted as a “beneficial consequence of resistance”, given the epidemiologic success of colistin resistant K. pneumoniae [76]. On the contrary, lipid A modifications in K. pneumoniae that result in resistance to polymyxins may pose increased virulence due to cross-resistance to the hosts’ antimicrobial-peptides [84].
5.2 Fosfomycin
Fosfomycin is a cell wall active antibiotic, with a unique epoxide moiety, notable for its structural simplicity and low molecular weight (Figure 3). Discovered in 1969, the commercial development of fosfomycin was the joint effort of American and Spanish scientists and members of the same team who would later developed thienamycin [85,86]. Indeed, fosfomycin is referred to as the “Spanish antibiotic”, for it was a soil sample from Alicante that contained Streptomyces fradie, the natural source of fosfomycin [87].
Penetration of fosfomycin into the bacterial cell wall is achieved through the transport systems utilized by alpha-glycerol-phospate and glucose-6-phosphate. Fosfomycin inhibits the first step in the synthesis of peptidoglycan by blocking the formation of N-acetylmuramic acid through competitive inhibition of phosphoenol pyruvate synthetase. Fosfomycin is therefore active in the log-phase of bacterial growth, and is bactericidal with a broad spectrum of activity that includes Gram-positive (e.g. Enterococccus faecalis) and Gram-negative bacteria (e.g. E. coli) [87]. Importantly, fosfomycin activity has been documented against approximately 80% of CRE, especially KPC-producing K. pneumoniae, even in strains that display decreased susceptibility to colistin and tigecycline [88,89]. As a result of its mechanism of action and safety profile, fosfomycin seems well predisposed for synergistic combination therapy, including regimens containing beta-lactams and aminoglycosides.
The pharmacokinetic profile of fosfomycin is quite unique: fosfomycin tromethamine is >50% bioavailable after oral administration, has a prolonged half-life, is largely excreted unchanged into the urine, and achieves high penetration into the genitourinary system including the prostate [90]. A regimen of 3 gm of fosfomycin tromethamine administered orally every 72 hours has demonstrated utility in the treatment of lower UTI caused by CRE. This use differs from the single 3 gm dose recommended the package insert approved by the United States Food and Drug Administration (FDA) (http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050717s005lbl.pdf); indeed, optimal dosing of this drug remains unclear. Underlying immunosuppression and other patient associated factors may contribute to therapeutic failure [91]. With the intravenous (iv) formulation, not approved for use in the United States, high serum concentrations can be maintained by administering up to 4 gm of fosfomycin every 6 hours. There is adequate penetration into various tissues, including lung, central nervous system and bone. Clinical series from Japan, Spain and other European countries where iv fosfomycin is available, indicate an overall success rate > 80% in various infections caused by Gram negative bacteria different from CRE, such as bloodstream infection, endocarditis, meningitis, endophtalmitis, pneumonia, diabetic foot infections, peritonitis, gastroenteritis, among others. Of note, these uncontrolled observations include frequent combination therapy with penicillins, cephalosporins, aminoglycosides and fluoroquinolones. Fosfomycin is well tolerated, with nausea, vomiting and diarrhea, or skin rashes, occurring in up to 5% of patients [92]. With regards to the use of fosfomycin to treat infections caused by CRE, reports from Greece indicate favorable clinical and microbiological outcomes in a majority (55%) of patients. Of note, successful outcomes occurred in 60% of cases caused by CRE resistant to colistin, and resistance to fosfomycin during treatment developed in three cases. As will be disccused, fosfomycin was used in combination with other agents without exception [93].
Resistance to fosfomycin can result from mutations in the chromosomally encoded transport systems (GlpT and UhpT) that lead to inability to incorporate the antibiotic into the bacterial cell. Despite the frequent occurrence of these mutations in E. coli studied in vitro, they exert a high fitness cost and fosfomycin resistance remains rare in clinical isolates.[87] This is in contrast to other Enterobacteriaceae such as Klebsiella and Enterobacter sp., where resistance to fosfomycin in clinical isolates reaches 20%, and monotherapy with fosfomycin may readily select for resistance [94]. Fosfomycin resistance in Enterobacteriaceae is also mediated through plasmid-born genes coding for fosfomycin-inactivating enzymes, such as fosA. Here, fosfomycin binds to glutathione through fosfomycin-glutathione S transferase, which results in opening of the epoxide ring [95]. Dissemination of a clone carrying both blaKPC-2 and fosA3 in the same plasmid accounts for the high rate (60%) of fosfomycin resistance observed in carbapenem-resistant K. pneumoniae in China [96].
5. 3 Tigecycline
Tigecycline is a bacteriostatic antimicrobial agent related to tetracyclines that interferes with protein synthesis by binding to the 30S ribosomal subunit (Figure 3). The pharmocokinetic and pharmacodynamic (PK/PD) parameters and the clinical utility of this glycylcycline have been evaluated in complicated skin and skin structure infections (cSSSI), community acquired pneumonia (CAP), and complicated intra-abdominal infections (cIAI) [97]. Tigecycline has a large volume of distribution (7 to 10 L/Kg), a long elimination half-life of approximately 40 hours, and a low total clearance (0.2–0.3 L/h/Kg) [98,99]; it is mainly excreted into feces via bile, and very little tigecycline is excreted in the urine. Peak serum concentrations of tigecycline only reach 0.60 µg/ml after a 100 mg infusion. The PD index that correlates with activity, however, is the area under the concentration curve (AUC)/MIC. Analyses indicate that attaining a target AUC/MIC > 12.5 correlates with cure in cSSSI, which suggests clinical breakpoints of 0.25–0.5 µg/ml [100]. In contrast, a worldwide collection of CRE producing KPC and MBLs had an MIC90 of 1 µg/ml [101]. In addition to PK/PD considerations, there are published reports of a higher risk of death among patients receiving tigecycline compared to other antibacterial drugs [102]. The greatest increase in mortality was observed in patients with ventilator-associated pneumonia (VAP). A review undertaken by the FDA suggested that the increased mortality associated with tigecycline may be explained by a combination of factors, including the progression of disease, the bacteriostatic nature of tigecycline, as well as suboptimal dosing [102].
Review of the clinical experience indicates that tigecycline is used primarily for the treatment of cSSSI and cIAI when caused by CRE, among other MDR pathogens [103]. Nevertheless, tigecycline may still play an important role in the treatment of carbapenem-resistant K. pneumoniae bloodstream infection, especially as part of combination regimens including colistin and carbapenems [104–106]. Recent evidence suggests that the utility of tigecycline against Gram negative bacterial infections may be improved by increasing the dose. In a retrospective study of critically ill patients most of whom had VAP caused by MDR bacteria including XDR A. baumannii, but also CRE, standard tigecycline dosing (50 mg every 12 hours) was compared to high dose tigecycline (100 mg every 12 hours) [107]. The administration of high doses of tigecycline appeared to be well tolerated and was an independent predictor of cure in patients with VAP, supporting the theory that suboptimal dosing in this group of patients could contribute to the increased mortality associated with tigecycline. Although tigecycline achieves low penetration in the urine, some have advocated its use in high doses for the treatment of urinary tract infection (UTI) [108]. In practice, the use of tigecycline to treat CRE (especially KPC-producing K. pneumoniae) UTI is common, but may be associated with the development of resistance [109,110]. Mutations in the gene ramA are associated with decreased tigecycline susceptibility due to its role in the overexpression of the AcrAB-TolC multidrug efflux pump in Enterobacteriaceae [111]. Variants resistant to tigecycline can exhibit a multidrug resistance phenotype due to increased transcription of the marA, rarA, acrAB, and oqxAB genes [112]. Recently, a mutation conferring resistance to tigecycline was located in the S10 ribosomal protein, in close proximity to the tigecycline target site in the 30S ribosomal subunit [113].
5.4 Aminoglycosides
Aminoglycosides have been part of our antibiotic armamentarium since the inception of streptomycin in the 1940s. While a decline in aminoglycoside use in recent decades is likely due to the availability of less nephrotoxic and ototoxic alternatives [114], the relatively low rate of aminoglycoside resistance among some of the emergent XDR Gram negative bacteria, such as is the case with CRE, fuel a renewed interest in their use.
When active in vitro, Aminoglycosides are a viable option for monotherapy and combination therapy against CRE, notwithstanding the limitations imposed by their toxicity profile. In a retrospective cohort study of CRE bacteriuria, the rate of microbiologic clearance was significantly higher using aminoglycosides (88%) compared to using either polymixin B (64%) or tigecycline (43%) [115]. Unfortunately, susceptibility of CRE to currently available aminoglycosides is unpredictable. Multiple studies have evaluated the in vitro activity of aminoglycosides against CRE isolates and rates of non-susceptibility are non-negligible, ranging from 35% to 62.7% for gentamicin, 16% to 82.3% for amikacin and as much as 61% to 98% for tobramycin [116–118]. These variations may be strain dependent.
A novel option among parental aminoglycoside formulations is plazomicin, a next-generation aminoglycoside (ACHN-490) that is currently in clinical development. Plazomicin is currently the subject of a Phase 3, multicenter, randomized, open-label superiority study comparing it to colistin in combination with either meropenem or tigecycline for the treatment of bloodstream infections and pneumonia caused by CRE, evaluating all-cause mortality at 28 days (ClinicalTrials.gov identifier: NCT01970371). Plazomicin is a sisomicin synthetic derivative active against both gram-negative and gram-positive bacteria that evades formerly identified aminoglycoside-modifying enzymes; however, plazomicin is not active against isolates harboring ribosomal methyltransferases [117,119]. Unfortunately, the latter mechanism is associated with NDM-producing CRE, limiting the effectiveness of plazomicin in that context [50]. Plazomicin is active (MIC ≤ 2 µg/ml) against CRE isolates with mechanisms different from NDM [116–118]. In contrast, CRE isolates harboring NDM are resistant to plazomicin (MIC ≥ 64 µg/ml) and to all other aminoglycosides [117].
Their potential synergistic activity in combination with beta-lactams is one appealing feature of aminoglycosides. Synergy is thought to result both from enhanced intracellular uptake of aminoglycosides caused by the increased permeability of bacteria when exposed to cell wall synthesis inhibitors such as beta-lactams, and from reduction in carbapenemase production resulting from the inhibition of protein synthesis by the aminoglycoside [120]. Combination antibiotic regimens that include aminoglycosides, among others, is supported as an effective approach to treat infections caused by CRE by a growing body of observational evidence.
6. Combination therapy for Carbapenem-resistant Enterobacteriaceae
Evidence gleaned from the review of cases and the experience of individual centers published during the last few years, suggest that the treatment of CRE with combination antimicrobial therapy might offer a survival benefit when compared to monotherapy [18,45,94,104,105,121–123]. The conclusions drawn from these studies need to be tempered since most are retrospective or nonrandomized prospective cohorts of clinically heterogeneous populations. Despite these limitations, the currently available evidence seems to support the use of combination antibiotic therapy as an effective approach to treat serious infections caused by CRE. This approach will need to be reassessed after the adoption of new antibiotics against CRE, and in light of highly anticipated randomized studies looking at the matter in specific patient populations [123–126]. The use of combination therapy is also motivated by the decreasing activity of currently available options, i.e., colistin, tigecycline and fosfomycin, and their apparent selection of resistance when used as monotherapy against CRE [94,106].
6.1 In vitro synergy studies as a guide to therapy?
In vitro synergy of various combinations has been demonstrated in several studies using time-kill methods. Unfortunately, the number of such studies remains small and limited in scope, with data on activity against NDM-type MBLs or OXA-48 not yet fully available. However, they offer a “proof of concept” for the use of combination therapy against CRE and give some insight regarding which combinations might be considered in the clinical setting [106]. A systematic review and meta-analysis of in vitro interactions of any of the carbapenems with colistin or polymyxin B against carbapenem-resistant, colistin-susceptible K. pneumoniae detected an overall synergy rate of 55% [127]. A study of KPC-producing K. pneumoniae ST258, 75% (9/12) of which were resistant to colistin, synergy occurred in more isolates treated with colistin-doripenem-ertapenem (8/12) than with colistin-ertapenem (5/12) or with colistin-doripenem (6/12). Another study of KPC-producing K. pneumoniae confirmed the synergy between doripenem and colistin, occurring in 60 and 67% of colistin and pan-drug resistant isolates, respectively [128]. Other in vitro data suggest synergy when rifampin, doxycycline and tigecycline are added to polymyxin B for polymyxin B-resistant carbapenemase-producing K. pneumoniae [129]. Interestingly, synergy with the triple combination occurred in isolates with high porin expression but not in those with low expression [130].
Even double carbapenem therapy has been investigated: Bulik and Nicolaou showed that ertapenem and doripenem together have enhanced efficacy compared to either agent alone against infections caused by KPC-producing K. pneumoniae, using in vitro chemostat and in vivo murine thigh infection models. The success of double carbapenem regimens may be because ertapenem is “trapped” by KPC more readily and acts as an “inhibitor” due to its low turnover, which frees doripenem to act on penicillin binding proteins [131]. Another synergistic combination that is recently gaining attention is that of fosfomycin with carbapenems. Synergy of fosfomycin with imipenem, meropenem and doripenem was shown in approximately 70% of CRE [132,133]. Synergy was less significant albeit still present when fosfomycin was combined with colistin, netilmicin and tigecycline, while the combination of fosfomycin and gentamicin resulted in indifference.
6.2 Clinical Experience
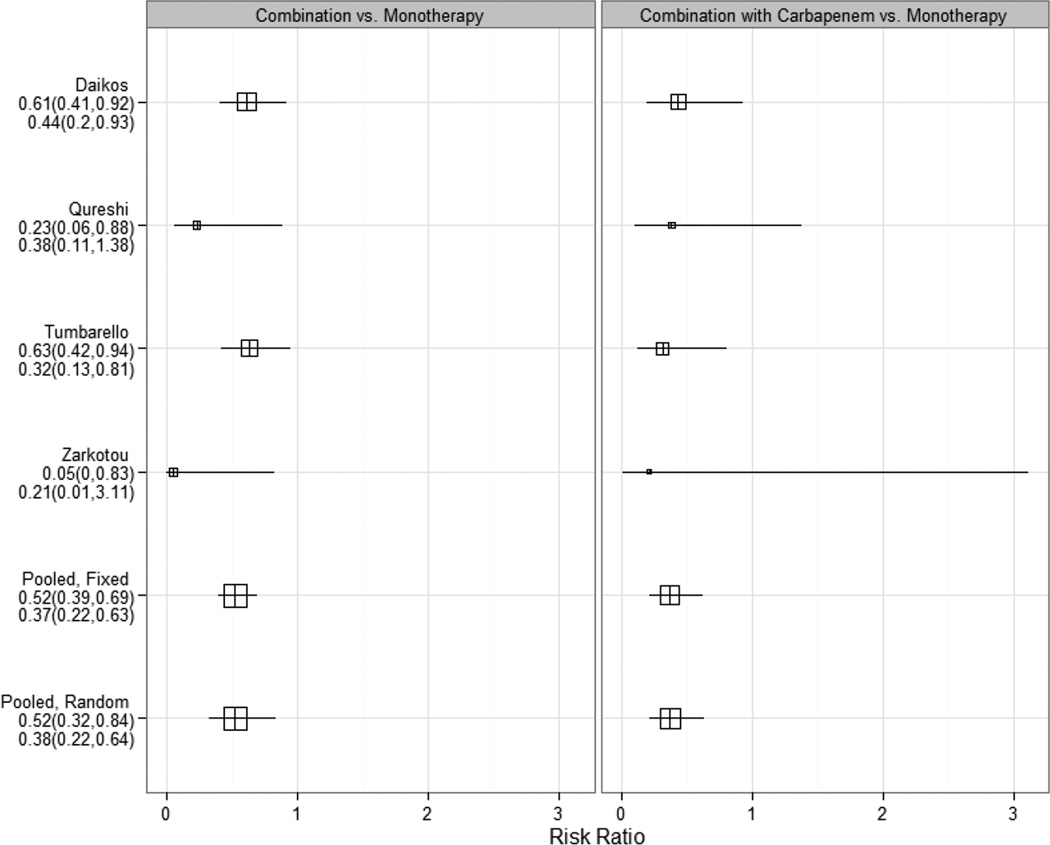
The number of reports that compare monotherapy against the combination of antibiotics for the treatment of infections caused by CRE is limited. When selectively comparing studies reporting on several hundred patients with CRE bloodstream infections who received appropriate antimicrobial therapy, a significant survival benefit (mortality risk reduction of approximately 50%) occurred in patients treated with combination antimicrobial therapy when compared with monotherapy [18,104,105,122,134] (Table 2 and Figure 4). The majority of these studies report on KPC-producing K. pneumoniae only (although some include KPC and VIM producers). Frequently, carbapenem-containing combinations were used in addition to colistin or polymyxin B and/or tigecycline. Although it might seem counter-intuitive, these studies suggest an additional benefit of including a carbapenem in combination regimens. This appears to be true especially in the context of strains with low MICs against carbapenems. An earlier systematic review of the available evidence by Tzouvelekis et al. identified a total of 301 patients with KPC and MBL-producing K. pneumoniae, 244 of who had bloodstream infection, and highlighted that the lowest failure rate (8.3%) was observed in the group treated with a carbapenem-containing antimicrobial combination. Monotherapy with either tigecycline or colistin resulted in failure rates similar to that of inappropriate therapy (defined as no drug to which the infecting organism was classified as susceptible in vitro). Of note, the efficacy of carbapenem monotherapy in 50 CRE infected patients from 15 studies was inversely proportional to the MIC for the carbapenem: clinical success ranged from 25% for infections caused by isolates with MIC >8 µg/ml, to 66.7%, 71.4% and 72.3% for infections caused by isolates with MICs of 8, 4 and 2 µg/ml or less, respectively.[106] Similarly, among 234 patients (132 with VIM-producing-K. pneumoniae; 102 with KPC-producing K. pneumoniae) included in nine studies published from Greece (2004–2011), the success rate of combination therapy was significantly higher than that of monotherapy (odds ratio 2.41; 95% confidence interval (CI) 1.2–4.7). Furthermore, carbapenem-containing regimens were associated with 6.7% failure rate vs. 26.9% for carbapenem-sparing regimens [94]. In a cohort of 205 patients with KPC and VIM-producing K. pneumoniae from Greece, 103 were treated with combination therapy (31/103 carbapenem-containing regimen), and 72 received monotherapy. Mortality was significantly higher in the monotherapy group (44.4% vs. 27.2%, p=0.018) and combination therapy was an independent predictor of survival (hazard ratio of death for monotherapy vs. combination therapy, 2.08; 95%CI 1.23–3.51). The lowest mortality rate was seen in those patients treated with a carbapenem-containing combination regimen (19.3% vs. 30.6% for carbapenem-sparing regimen). As in other reports, mortality increased from 19.3% for patients with an isolate that demonstrated a carbapenem MIC ≤ 8 µg/ml, to 35.5% for those with isolates with MIC > 8 µg/ml[122]. Other cohorts also show a survival benefit when a carbapenem is added to the combination regimen [46,104,105,134]. Finally, in a large Italian cohort of 661 patients with various types of infecton caused by KPC-producing K. pneumoniae described by Tumbarello et al., combination therapy with at least two active drugs was associated with significantly lower 14-day mortality. Significantly higher survival rates were observed when that combination included meropenem, provided the isolate had a meropenem MIC ≤ 8 µg/ml [134].
Table 2.
Outcomes of combination therapy and monotherapy for bloodstream infections caused by carbapenem-resistant Enterobacteriaceae.
| Organisms | Source of bloodstream infection (% of total infection) |
Population characteristics |
Reference, design | Combination therapy | Monotherapy | ||||
|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | No. of patients |
Survival rate (%) |
Antibiotic | No. of patients |
Survival rate (%) |
||||
| KPC and VIM-1- producing K. pneumoniae |
BSI – sources : lung (21), abdomen (14), line related (10.7), UTI (9.3), SSSI (2.9), CNS (1.5), unknown (40.5) |
56.6% ICU patients |
Daikos et al., MC retrospective cohort; 2009– 2010, Greece |
Carb-Tige-AG or Col Carb-Tige Carb-AG Carb-Col Tige-AG-Col Tige-AG Tige-Col AG-Col Other |
11 4 9 7 11 20 21 17 3 |
100 50 89 57.2 73 55 76.2 70.6 100 |
Tige Col AG Carba Other |
27 22 9 12 2 |
59.3 45.5 77.8 41.7 100 |
| KPC-producing K. pneumoniae |
BSI – sources : lung (24.4), line related (31.7), UTI (17.1), primary (14.6) |
53.7% ICU patients |
Qureshi et al., SC retrospective cohort; 2005– 2009, USA |
Col-Carb Col-Tige Col-FQ Tige-Carb Tige-AG Carb-FQ Azt-FQ Cfpm-Gen |
5 1 1 3 2 1 1 1 |
80 100 100 100 100 0 100 100 |
Col Tige Carb Gen A-S P-T |
7 5 4 1 1 1 |
43 20 50 100 100 0 |
| KPC-producing K. pneumoniae |
BSI – sources : LRTI, line related, UTI, other, and unknown |
42.4% ICU patients |
Tumbarello et al., MC retrospective cohort; 2010– 2011, Italy |
Tige-Col Tige-Gen Col-Gen Tige-Col-Carb Tige-Gen-Carb Col-Gen-Carb Other |
23 12 7 16 6 1 14 |
70 50 43 87 83 0 57 |
Tige Col Gen |
19 22 5 |
47 50 20 |
| KPC-producing K. pneumoniae |
BSI – sources : primary (43.4), line related (22.6) |
71.7% ICU patients |
Zarkotou et al., SC prospective cohort; 2008– 2010, Greece |
Tige-Col Tige-Gen Tige-Col-Carb Tige-Col-Gen Tige-Carb Tige-Amk Col-Gen Carb-Gen |
9 3 2 1 1 1 2 1 |
100 100 100 100 100 100 100 100 |
Col Tige Gen Carb |
7 5 2 1 |
43 60 100 0 |
KPC, Klebsiella pneumoniae carbapenemase; SC, single center; MC, multicenter; BSI, bloodstream infection; UTI, urinary tract infection; LRTI, lower RTI; SSSI, skin and skin structure infection; CNS, central nervous system; ICU, intensive care unit; Carb, carbapenem; Col, colistin; Tige, tigecycline; Gen, gentamicin; Amk, amikacin; A-S, ampicillin-sulbactam; AG, aminoglycoside; Cfpm, cefepime; P-T, piperacillin-tazobactam.
Figure 4.
Comparison of risk ratio of mortality of patients with bloodstream infection caused by carbapenem-resistant Klebsiella pneumoniae treated with combination antibiotic therapy (with and without a carbapanem) versus monotherapy, determined and pooled from four retrospective observational studies (Daikos et al. [122], Qureshi et al. [104], Tumbarello et al. [105], Zarkotou et al. [18]). The size of each square indicates the relative size of each study; horizontal lines denote 95% confidence intervals. Results less than 1 indicate lower mortality with combination therapy. Pooled numbers are Mantel-Haenszel risk ratio estimates.
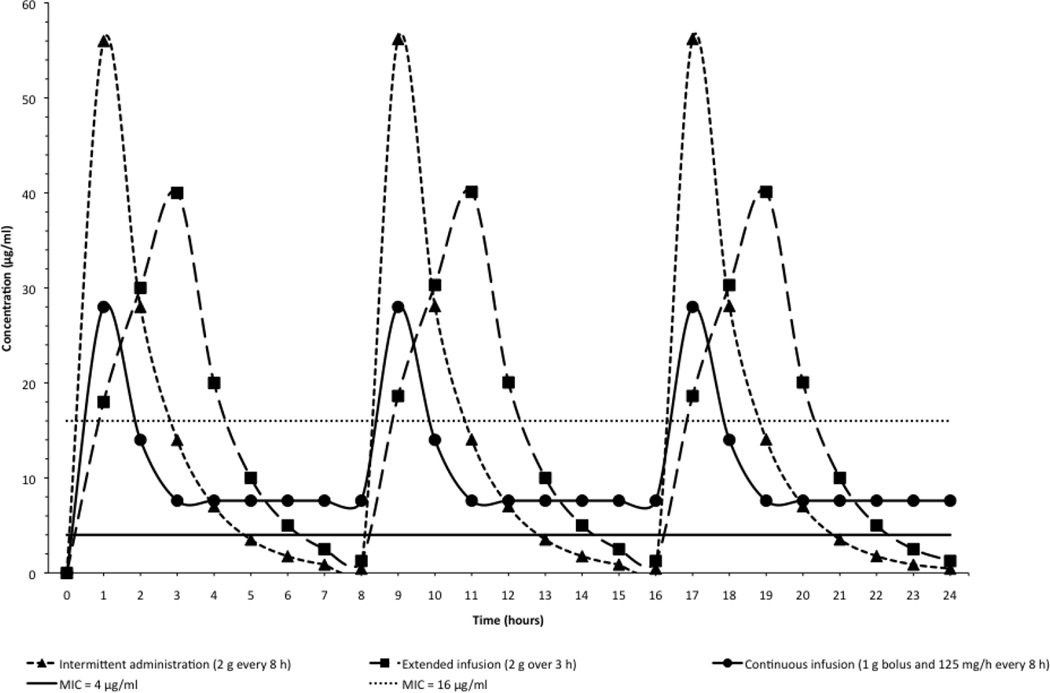
Carbapenems display bactericidal activity when free drug concentrations remain above the MIC for 40 to 50% of the time between dosing intervals, and the probability of attaining that parameter is reduced with increasing MICs or insufficient dosing [106]. As such, the administration of high dose carbapenems by prolonged infusion offers theoretical advantages when compared to standard dosing via short infusion. Consistent with the above considerations, the delivery of carbapenems by prolonged infusion (extended or continuous), in combination with other antimicrobials, is therefore reasonable for such infections when the carbapenem MIC is <16 µg/ml [106,124] (Figure 5). Indeed, a recent systematic review observed a lower mortality among patients receiving prolonged infusion of carbapenems [135], and a randomized controlled trial demonstrated that the continuous administration of beta-lactam antibiotics in general achieve higher plasma antibiotic concentrations than intermittent administration and improve the outcome of patients in intensive care [136]. Therapeutic drug monitoring (TMD) is another approach to optimize β-lactam exposure that is the focus of ongoing investigation. Clinical experience with TDM remains scarce, but it is most likely to benefit critically ill patients with severe infections, where physiologic derangements inherent to their disease status render pharmacologic parameters unpredictable. [137]. There is still a lot to be learned about the implementation and clinical impact of TDM; in a survey that included specialists from 328 hospitals in 53 countries, therapeutic drug monitoring of carbapenems was used by only 2% of the respondents [138].
Figure 5.
Concentration of meropenem (in µg/ml) over time (in hours) when administered as an intermittent (triangle), extended (square), or continuous (circle) infusion (derived from Nicolau [166], Dandekar et al. [167] and Krueger et al.[168]. Continuous line represents MIC = 4 µg/ml, and dotted line MIC = 16 µg/ml. Note that only an extended infusion may result in sufficiently elevated serum meropenem concentrations when isolates approach MIC of 16 µg/ml. Antibiotics that permeabilize the bacterial cell membrane (e.g. polymyxins), interfere with cell wall synthesis (e.g. fosfomycin), or inhibit protein synthesis (e.g. aminoglycosides or tigecycline) may decrease the MIC sufficiently so that it is exceeded when a carbapenem is co-admininstered as a prolonged (continuous or extended) infusion, thereby achieving satisfactory microbiological and clinical outcomes.
Clinical data on fosfomycin-containing combinations as an emerging therapeutic option against CRE is limited. Only two studies to date have evaluated the efficacy of fosfomycin in that context. An observational prospective cohort of patients with carbapenemase-producing K. pneumoniae (n=41) and carbapenemase-producing P. aeruginosa (n=17) treated with fosfomycin combined with either colistin or tigecycline showed a successful outcome in 54.2% of patients and a 28-day mortality of 37.5% [93]. Michalopoulos et al. prospectively followed 11 ICU patients with carbapenemase-producing K. pneumoniae treated with intravenous fosfomycin combined with colistin in 6 patients, gentamicin in 3 and piperacillin/tazobactam in 1; all-cause hospital mortality, the primary endpoint, was 18.2% (2/11 patients) [139]. More evidence is needed to determine whether fosfomycin-containing combinations are a worthwhile addition to combination regimens for the treatment of infections caused by CRE.
Notwithstanding the observations noted above, a recent systematic review of antibiotic treatment of infections caused by CRE failed to confirm a possible survival advantage of combination therapy. Falagas et al. assembled twenty nonrandomized studies consisting of 692 patients with CRE, mostly Klebsiella spp. They observed mortality rates of up to 50% for tigecycline-gentamicin combinations, up to 64% for tigecycline-colistin, and up to 67% for carbapenem-colistin [126]. The clinical, therapeutic and molecular heterogeneity of the infections described in these studies, as well as their small size and issues inherent to their design (nonrandomized, mostly retrospective cohorts and use of older resistance breakpoints), were recognized as important limitations that precluded solid conclusions in comparing combination therapy with monotherapy. The potentially detrimental impact of combination therapy on the total use of antibiotics, especially the increased use of carbapenems, must be acknowledged. All these reasons underscore the urgent need for high-quality clinical data derived from the ongoing randomized studies investigating the outcome of combination antimicrobial therapy vs. single agent use in treating infections caused by CRE. And yet questions will remain, as future treatment paradigms for infections caused by CRE will also have to incorporate newly licensed antibiotics and agents that are still in development.
7. Novel therapeutic agents against Carbapenem-resistant Enterobacteriaceae: a new generation of beta-lactam/beta-lactamase inhibitor combinations
Historically, combination with beta-lactamase inhibitors has been a fruitful strategy to expand the activity of beta-lactams against Enterobacteriaceae. The first generation of beta-lactamase inhibitors developed in the 1980s and 1990s were effective against the class A SHV and TEM enzymes, and included sulfone beta-lactams such as: 1) sulbactam, paired with ampicillin or cefoperazone; 2) tazobactam, partnered with piperacillin or now with the novel cephalosporin ceftolozane; and 3) clavulanic acid, combined with ticarcillin or amoxicillin. The role of these combinations in our antibiotic armamentarium is constrained by the contemporary emergence of beta-lactamases resistant to those inhibitors, such as the class A KPCs, the class B MBLs, as well as the class C cephalosporinases [140]. Of note, although the combination of the novel cephalosporin ceftolozane with tazobactam has promising in vitro activity against some ESBL-producing Enterobacteriaceae, it is not active against CRE.
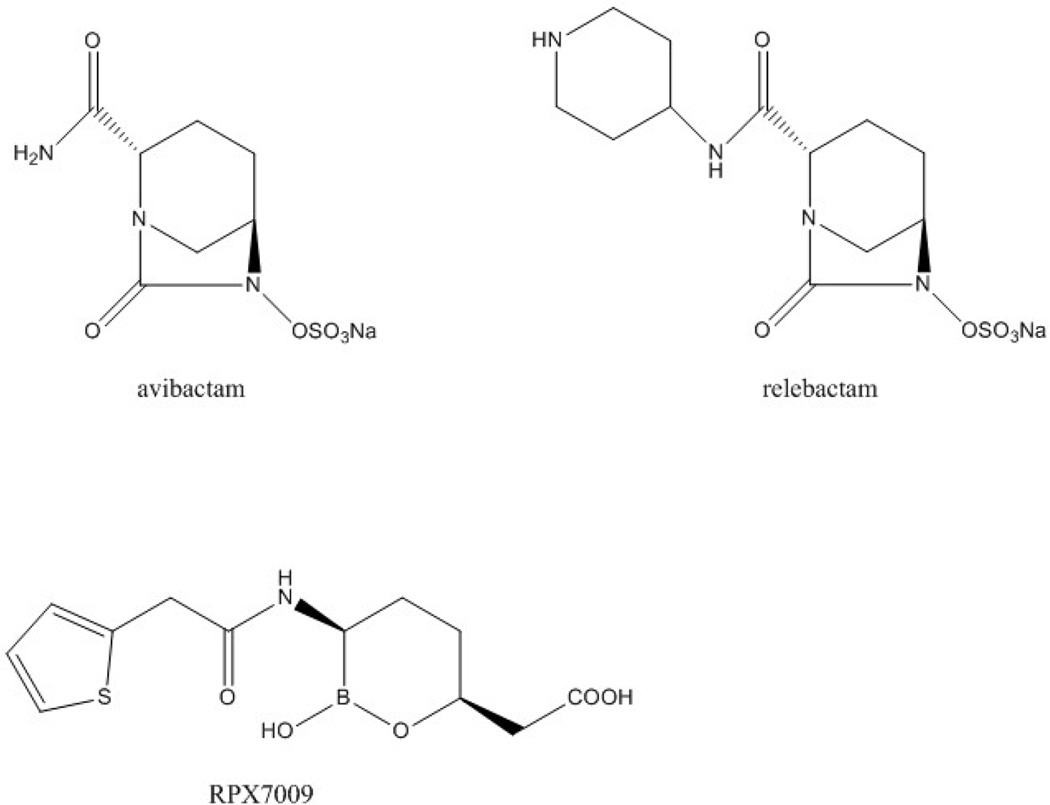
A new generation of inhibitors may yet rescue the combination of beta-lactams with beta-lactamase inhibitors as an effective strategy to overcome the therapeutic challenge posed by CRE [141]. Two very similar non-beta-lactam beta-lactamase inhibitors belonging to the group of diazabicyclooctanes (DBOs) are entering the clinic: avibactam (formerly known as NXL104), and relebactam (previously designated as MK-7655) (Figure 6). DBOs are able to inactivate many of the class C and class A beta-lactamases, including KPCs, through covalent carbamylation of the serine residue in the active site of the enzyme. Interestingly, in the case of avibactam, the inhibitor is regenerated at the time of deacylation, although slow hydrolysis has been observed after its interaction with KPC. DBOs do not inhibit class B MBLs, but they slowly inhibit OXA-48, a class D enzyme. Regarding the inactivation of OXA-48, access of the inhibitor to the serine residue in the active site is constrained and regeneration of DBO is extremely slow, resulting in inhibition of the enzyme for the effective life of the bacterial cell [142].
Figure 6.
Structure of avibactam and relebactam (diazabicyclooctane beta-lactamase inhibitors) and of RPX7009 (oxaboronate beta-lactamase inhibitor).
Another category of inhibitors different from DBOs is that of boronic based beta-lactamase inhibitors. Among these, RPX7009 (Figure 6) is undergoing further clinical development and demonstrates inhibition against serine carbapenemases (class A), including ESBLs and KPC enzymes; MBLs and the class D enzyme OXA-48, however, are resistant to inhibition by this compound [141].
7.1 In vitro studies
Avibactam has been paired with ceftazidime, a long established extended-spectrum cephalosporin with antipseudomonal activity. Ceftazidime-avibactam has been tested against various arrays of CRE with well-characterized mechanisms of beta-lactam resistance. For organisms which demonstrated carbapenem resistance (meropenem MIC ≥ 2 µg/ml) due to the combination of class A ESBLs and class C cephalosporinases with porin losses, ceftazidime-avibactam combination brought the ceftazidime MIC from > 256 to < 1 µg/ml) [143]. KPC-2 and KPC-3 harboring K. pneumoniae ST258 demonstrated inhibition with ceftazidime-avibactam at a concentration < 1 µg/ml [144]. Similarly, 25 K. pneumoniae producing OXA-48 from Turkey, with various other beta-lactamases in the background (SHV, TEM and CTX-M-type), were tested against ceftazidime-avibactam. Isolates were resistant to imipenem (MIC90 ≥ 4 µg/ml) and ceftazidime (MIC90 ≥ 512 µg/ml); avibactam reduced the MIC90 values of ceftazidime to 0.5 µg/ml [145]. SME-producing Serratia is also susceptible to ceftazidime-avibactam, both because of inhibition of SME by avibactam and inability of that enzyme to hydrolyze ceftazidime [143]. Comparable results have been observed with avibactam paired with ceftaroline, a cephalosporin with activity against methicillin-resistant Staphylococcus aureus, and with aztreonam [146].
A large-scale evaluation of ceftazidime-avibactam incorporating several thousand clinical isolates collected from 73 medical centers throughout the US demonstrated that 99.7% of all Enterobacteriaceae strains and 95.7% of 115 meropenem-nonsuscpeptible K. pneumoniae were inhibited by ceftazidime-avibactam, with MICs ≤ 2 µg/ml [147]. Similar results were obtained from a multi-hospital survey in China and from the examination of a global collection of Enterobacteriaceae [148,149].
Relebactam (MK-7655), paired 4:1 with imipenem/cilastatin, has also been evaluated in microbiological studies. Approximately 10,000 isolates of Enterobacteriaceae were collected from 45 countries, as part of the SMART study: out of 394 isolates non-susceptible to imipenem, 251 (64%) were rendered susceptible by the addition of relebactam [150]. CRE were studied under the effect of imipenem and relebactam combination, with checkerboard MICs determined by agar dilution. As predicted, combination with relebactam did not potentiate the activity of imipenem against CRE producing VIM, IMP and NDM MBLs. Isolates known to produce KPC carbapenemases and isolates that produced class A ESBLs or class C cephalosporinases in conjunction with porin changes were inhibited at 0.12–1 µg/ml of imipenem with the addition of 4 µg/ml of relebactam. Interestingly, growth inhibition of CRE strains producing OXA-48 was not consistent, even with high concentrations of relebactam [151].
As noted, DBOs in combination with cephalosporins or carbapenems do not demonstrate activity against organisms harboring MBLs, since neither avibactam nor relebactam inhibit this class of enzymes. In contrast, when aztreonam was combined with avibactam and tested against K. pneumoniae harboring NDM-1 and the ESBLs SHV-12 and CTX-M-3, there was a 128-fold reduction in the MIC. The explanation is that aztreonam, as a monobactam, is impervious to hydrolysis by NDM-1, but is compromised by ESBLs; restitution of susceptibility by avibactam, therefore, is achieved by inhibition of the ESBLs in the background and not by inhibition of NDM. Similar observations were made for IMP containing Enterobacteriaceae that coproduced ESBLs and class B cephalosporinases, and for VIM containing isolates [143,149].
7.2 In vivo studies
Studies in various animal models have evaluated the combination of ceftazidime with avibactam. A murine lethal septicemia model using two KPC-producing K. pneumoniae representative of the predominant strains in the United States, revealed that the co-administration of ceftazidime and avibactam reduced the median effective doses for 50% of the animals (ED50) for each of the tested strains from 1,578 and 709 mg/kg, to 15.1 and 3.8 mg/kg, respectively. Using the same strains, a neutropenic thigh infection model with ceftazidime-avibactam, demonstrated bactericidal activity with doses of ≥256:64 mg/kg and ≥128:32 mg/kg, respectively. Paradoxically, KPC producing K. pneumonia isolates with higher resistance in vitro demonstrated decreased lethality in vivo, suggesting that virulence factors independent of resistance have an impact on the outcome of CRE infection [152].
Other studies using the neutropenic thigh infection model and immunocompetent thigh infection model tested a wider array of CRE (including Klebsiella, Proteus, Enterobacter and Serratia spp.) Observations derived from these mouse models indicate that enough potency is achieved by dosing ceftazidime at 2 g combined with 0.5 g of avibactam as a 2 hour infusion every 8 hours. In the neutropenic model, this translated to reliable efficacy with ceftazidime-avibactam at MICs ≤ 16 µg/ml, which allows free concentration of the drug to exceed the MIC sufficient time to achieve bactericidal activity (fT > MIC ≥ 62%). As expected, the overall efficacy of ceftazidime-avibactam was enhanced in the immunocompetent model [153].
Observations from in vivo models also provide important insights into the efficacy of ceftazidime-avibactam against OXA-48-producing Enterobacteriaceae. In murine neutropenic and immunocompetent thigh infection models, ceftazidime resulted in reliable efficacy when MICs ≤ 1 µg/ml (corresponding to 100% fT > MIC) [154], consistent with limited hydrolysis of extended-spectrum cephalosporins by OXA-48 [155]. Of note, isolates co-producing OXA-48 and ESBLs have elevated ceftazidime MICs (≥ 64 µg/ml); the addition of avibactam to ceftazidime inhibit ESBLs and restore activity of ceftazidime against OXA-48 and ESBL producing Enterobacteriaceae.
7.3 Clinical development
Phase 1 studies in healthy subjects have assessed the pharmacokinetic profile of ceftazidime and avibactam administered in combination, revealing that both drugs retain their elimination half-lives (approximately 2 hours); dosage needs to be adjusted in renally impaired patients (http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM425459.pdf). Similar studies after a single infusion of imipenem-relebactam in combination, demonstrate that the half-life of relebactam is about 1.5 hours, and remains unaffected by the co-administration of the two drugs [156,157].
The efficacy, safety and tolerability of ceftazidime/avibactam plus metronidazole was compared against meropenem in the treatment of cIAIs in hospitalized adults, in the context of a phase 2 randomized controlled clinical trial [158] (ClinicalTrials.gov identifier: NCT00752219). Patients enrolled were adults who required surgical intervention and antibiotics for the following categories of cIAI: cholecystitis with gangrenous rupture or progression, diverticulitis or appendicitis with perforation or abscess, acute gastric or duodenal perforation, traumatic enteric perforation, secondary peritonitis, or intra-abdominal/intraperitoneal abscess. A total of 204 patients were randomized in a 1:1 ratio to receive ceftazidime/avibactam plus metronidazole in the treatment arm, or meropenem as the comparator. Ceftazidime-avibactam combination was given as 2000 mg of ceftazidime and 500 mg of avibactam iv every 8 hours, with the addition of an iv infusion of 500 mg of metronidazole given over 1 hour every 8 hours to provide coverage for anaerobic pathogens. Patients in the comparator group received meropenem at 1 gm iv every 8 hours. Identification and antimicrobial susceptibility testing was available for 153 isolates from 127 patients. E. coli was the most common organism (105 isolates); K. pneumnoniae (17 isolates), Enterobacter spp. (5 isolates) and K. oxytoca were much less frequent. Among Enterobacteriaceae, only 3 isolates demonstrated non-susceptibility to ceftazidime (MIC >8 µg/ml), and there was no mention of whether they were CRE; other ceftazidime non-susceptible pathogens were Gram-negative non-lactose fermenters. Overall, favorable outcomes were observed with both treatment regimens, including patients with isolates that were not susceptible to ceftazidime at baseline. In total, 25 of 26 patients (96%) in the ceftazidime/avibactam plus metronidazole group and 16 of 17 patients (94%) in the meropenem group who had ceftazidime-non-susceptible pathogens in vitro demonstrated a favorable microbiological outcome. The clinical response in microbiologically evaluable patients at the test-of-cure visit 2 weeks after the last dose of study therapy (the primary endpoint) was 91.2% (62/68) in the treatment group and 93.4% (71/76) in the comparator group. In terms of adverse events, the rate was similar between both arms (64.4% for ceftazidime-avibactam plus metronidazole; 57.8% for meropenem). There were more cases of nausea and vomiting and abdominal pain in the ceftazidime/avibactam plus metronidazole group (perhaps attributable to the latter agent), and more cases of liver enzyme elevations in the meropenem group. This study is to be followed by a phase 3 randomized, multicenter study that plans to enroll >400 patients to compare ceftazidime-avibactam plus metronidazole, versus meropenem, in the treatment of cIAI in hospitalized adults (NCT01726023). A similarly designed phase 3 study focused on nosocomial pneumonia and ventilator associated pneumonia is also underway, aiming to recruit approximately 500 patients, and comparing 2000 mg of ceftazidime and 500 mg of avibactam iv every 8 hours in the treatment arm, with meropenem 1 gm every 8 hours in the comparator arm (NCT01808092).
The efficacy and safety of ceftazidime-avibactam for the treatment of complicated urinary tract infection (cUTI) was assessed in a prospective phase 2, randomized, clinical trial (NCT00690378) [159]. Patients aged between 18 and 90 years with acute pyelonephritis or other cUTI were randomized 1:1 to receive intravenous ceftazidime 500 mg and avibactam 125 mg every 8 hours or imipenem 500 mg every 6 hours. The primary efficacy objective was a favorable microbiological response at the test-of-cure visit 5–9 days later. Overall, 135 patients received treatment and 62 could be evaluated at the end of therapy. Similar to previous studies, the most common pathogen was E. coli. At the test of cure visit, favorable microbiological response was noted in 70.4% of patients receiving ceftazidime–avibactam and 71.4% of those treated with imipenem. In patients with ceftazidime-resistant pathogens, response was observed in 6/7 (85.7%) receiving ceftazidime–avibactam. Adverse events were observed in 67.6% of patients receiving ceftazidime-avibactam and in 76.1% of those receiving imipenem [159]. A phase 3 study enrrolled approximately 500 hospitalized patients with cUTI, including pyelonephritis and compared doripenem given at 500 mg every 8 hours against ceftazidime-avibactam, administered at the higher dose of 2000 mg of ceftazidime with 500 mg of avibactam every 8 hours (NCT01599806). Ceftazidime/avibactam received approval by the FDA for the treatment of cUTI, and the registration trials included the successful treatment with ceftazidime/avibactam of one patient with cUTI caused by carbapenem-resistant K. pneumoniae, with meropenem MIC > 8 µg/ml, where carbapenem resistance was proven to be due to KPC. (http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM425459.pdf.)
Regarding imipenem-relebactam, a phase 2 randomized controlled trial that compared the safety, tolerability, and efficacy of adding 125 mg or 250 mg of relebactam to 500 mg of imipenem vs. imipenem alone in patients with cIAI was completed; the results are not published at this time (NCT01506271). A similar phase 2 study is in progress to compare imipenem-relebactam with imipenem alone in adults with complicated cUTI (NCT0150563).
The boronic-based beta-lactamase inhibitor RPX7009, an oxaboronate, is being developed in combination with carbapenems, initially biapenem and more recently meropenem, under the name Carbavance. The initial version of Carbavance (biapenem/RPX7009) completed a phase 1 clinical trial (NCT01772836). Phase 3 randomized, open-label studies are underway comparing Carbavance (as meropenem 2 g plus RPX7009 2 g administered IV every 8 hours) with best available therapy against cUTI, HAP/VAP and bloodstream infection caused by CRE (NCT02168946), and comparing Carbavance with piperacillin-tazobactam in patients with cUTI (NCT02166476).
8. Conclusion
Infections caused by CRE represent a threat to hospitalized patients worldwide. The current mainstays of therapy against CRE are polymyxins, tigecycline, fosfomycin and aminoglycosides. There is considerable observational data suggesting that patients benefit when these agents are used in combination with carbapenems, and high-quality prospective randomized controlled studies evaluating combination therapy vs. monotherapy against CRE are ongoing. Efforts in the development of new antibiotic agents against CRE have culminated in the development of novel DBO beta-lactamase inhibitors, such as relebactam and avibactam. Combinations of cephalosporins and carbapenems with these inhibitor demonstrate in vitro activity against most CRE, with the exception of strains harboring MBLs, and perform well in animal models. Ceftazidime/avibactam has been approved for the treatment of cIAI and cUTI, based on trials that did not include CRE. There is considerable optimism, however, regarding the addition of DBO inhibitiors to our armamentarium against CRE. Therefore, a careful analysis of the clinicial experience with ceftazidime/avibactam will be key to define the place of these types of compounds in treatment schemes against CRE.
9. Expert Opinion
The clinical experience treating infections caused by CRE comprises a decade, at most. In that period of time, also coinciding with the emergence of other extensively drug resistant (XDR) pathogens such as A. baumannii and P. aeruginosa, previous assumptions about antibiotic pharmacotherapy for infections caused by Enterobacteriaceae have been challenged. In certain locales, clinicians have come to accept that a single antibiotic is not likely anymore to effectively treat most bacteria responsible for severe infectious syndromes in the “empiric” period. Similarly, it is understood that “optimizing” antibiotic therapy comes now at a higher “cost”, measured in toxicity and adverse effects. In these respects, carbapenems remain irreplaceable, notwithstanding the utility derived from the use of tigecycline and polymyxins.
The novel beta-lactam/beta-lactamase inhibitor combinations, such as those containing avibactam and relebactam, are promising to be the best options for the treatment of CRE where carbapenem resistance is mediated by KPC or OXA-48. We recognize, however, that these drugs do not show efficacy against MBLs and are not to be considered “silver bullets” for the ongoing crisis in the therapy of XDR Gram-negative bacteria. Although new beta-lactam/beta-lactamase inhibitor combinations have been subjected to a rigorous program of development, their “real life” efficacy against CRE remains unknown because clinical trials did not focus on the treatment of infections caused by such pathogens.
The rise of CRE has also challenged the notion that monotherapy is the best treatment for infections caused by Enterobacteriaceae. Seeking better outcomes for their patients, clinicians have incorporated lessons learned from pharmacokinetic and pharmacodynamic modeling and attempted various forms of combination therapy. We recognize the potential for both selection bias (healthier patients may be perceived to tolerate additional drugs) and survivor treatment selection bias (patients may not live long enough to receive additional antibiotics), among other limitations in the current body of observational data [160]. Nevertheless, we are not agnostic about the potential benefits of combination therapy and continuous administration of beta-lactams in patients with CRE bloodstream infection. Our view of this is synthesized in Figure 5, which illustrates how these approaches may result in effective treatment of some CRE. The question should be clarified by rigorously designed studies, especially the two randomized controlled trials comparing polymyxins as monotherapy versus polymyxins plus carbapenems for the treatment of XDR Gram-negative infections, including CRE, that are being conducted in Europe and the US. In the future, it will be very interesting to investigate the performance of the newly developed beta-lactam/carbapenemase inhibitors in combination with other agents, such as fosfomycin.
Aside from ongoing questions about the superiority of combination therapy to treat infections caused by CRE and other XDR Gram-negative pathogens, it is unclear whether antibiotics administered in combination help prevent or rather propitiate further development of multidrug resistance. There is an unresolved debate between two competing hypotheses: 1) antibiotics that act in synergy may prevent the emergence of resistant strains; vs. 2) additional antibiotics may provide greater selective pressure towards the development of resistance. Further, it should be recognized that the various paradigms of combination therapy employed in the clinic (HIV, tuberculosis, cancer) incorporate more than two drugs, whereas combination therapy to treat CRE and other XDR Gram-negative organisms usually consists of two antibiotics. Earlier models incorporating synergistic agents against Staphylococcus aureus suggested that combination therapy favored the evolution of resistance [161]. Other adaptive evolution experiments carried in S. aureus and E. coli, however, appear to suggest that some drug combinations indeed limit the evolution of resistance by inducing ‘collateral susceptibility’, but that there are variations in these responses according to the mechanisms of resistance to the drugs included in the combination [162,163]. Similarly, there may be an effect on selection of resistance when antibiotics are applied in sequence (as it often occurs in clinical practice). Computational models indicate that in the majority of cases (approximately 70%) sequential treatments with 2–4 beta-lactam antibiotics increase the likelihood of resistance emerging in E. coli. The corollary is that it is possible to design a treatment sequence that avoids the emergence of resistance [164].
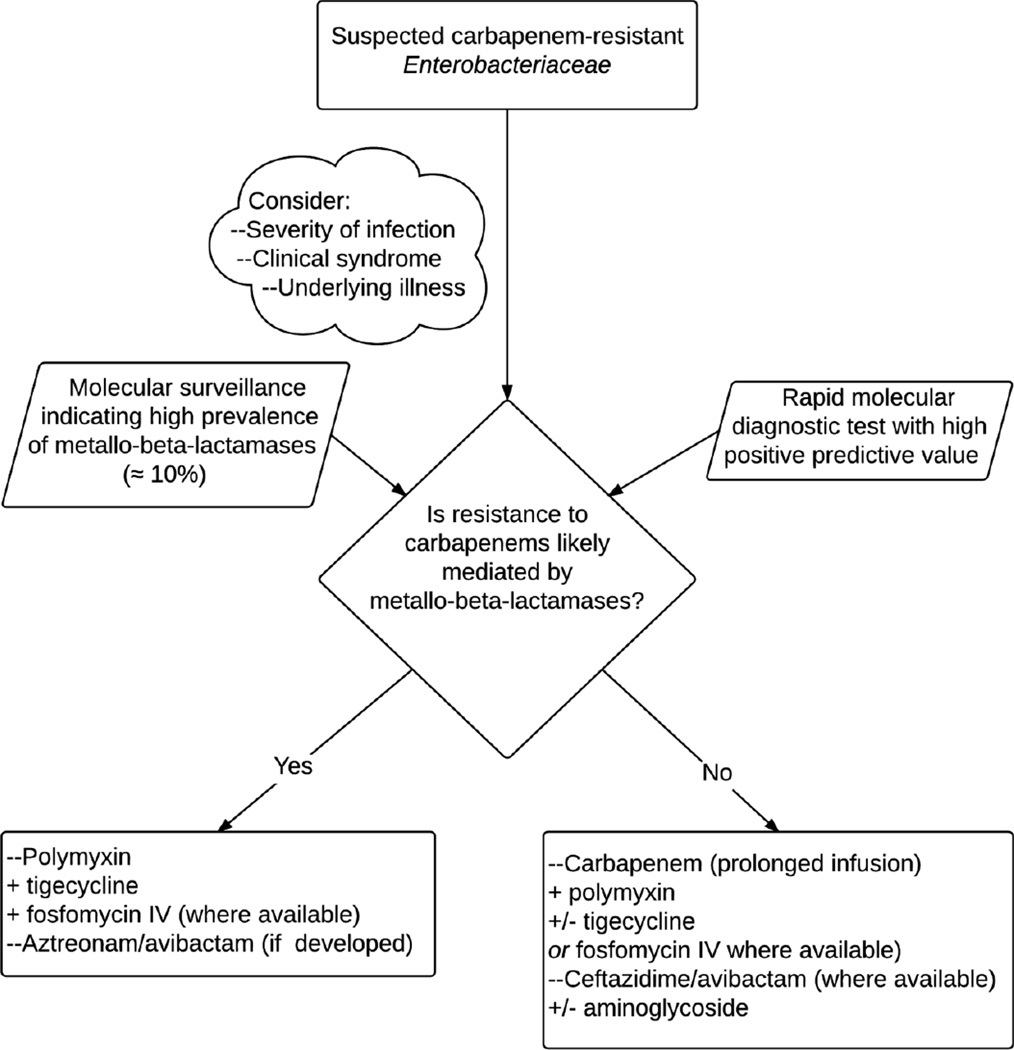
The CRE epidemic has catalyzed progress in our understanding of the mechanisms of resistance to carbapenems and the genetic underpinnings linked to their successful dissemination. Application of these insights to surveillance studies that define the molecular epidemiology of CRE, and the relative predominance of KPC-producing K. pneumoniae ST258, OXA-48 or MBLs in a given locale, is essential to inform prevention and control strategies, and to guide empiric therapy where CRE is suspected (Figure 7). Also, the rapid detection of these ‘high risk’ genotypes as a cause of infection is feasible with various molecular diagnostic platforms already in existence, yet to be widely adopted by microbiology laboratories and clinicians. Detection of genes coding for carbapenemases with rapid molecular testing would permit the judicious and expeditious use of antibiotics active against CRE; conversely, a negative result from a rapid molecular test would encourage the use of carbapenems (or antibiotics of narrower spectrum). The contribution of changes in outer membrane proteins, efflux pumps, and variations in beta-lactamase presence and expression to carbapenem resistance, remain an important caveat when trying to “rule in” or “rule out” the carbapenem-resistant phenotype from genotype. Furthermore, the clinical impact of the various new beta-lactam/carbapenemase inhibitor combinations will depend on the discrimination of the mechanism of resistance by rapid molecular tests. The detection of KPC would support treatment with the novel ceftazidime-avibactam (or imipenem-relebactam) if available. Alternatively, treatment with a combination regimen that includes a polymyxin, tigecycline and carbapenem administered in prolonged infusion is warranted. In cases where OXA-48 or SME are responsible for the CRE phenotype, ceftazidime-avibactam might be the preferred drug, given the resistance of ceftazidime to hydrolysis by these carbapenemases. On the other hand, in cases where a MBL is identified or is the most prevalent carbapenemase, carbapenem MICs are likely to be very high (> 8 µg/ml) and the addition of a carbapenem to a combination regimen may not be of benefit. Similarly, ceftazidime-avibactam would not be indicated, while aztreonam-avibactam, if developed further, would be favored [151]. While this option becomes available, infections caused by CRE that harbor MBLs could be treated with aztreonem in combination with ceftazidime-avibactam; this strategy, as an example of “dual beta-lactam therapy”, may offer additional benefits [165].
Figure 7.
Guide for the empiric treatment of infections where carbapenem-resistant Enterobacteriaceae is suspected, based on the local prevalence of metallo-beta-lactamases and results of rapid molecular diagnostics.
We expect that the introduction of novel agents will improve the prospects of patients with infections caused by CRE. Perhaps it will also usher a transformation in the infectious diseases clinicians practice, because the full potential of those new therapies may only be realized with the adoption of rapid molecular diagnostics. Innovative approaches that assimilate our present and future knowledge about the complexity of resistance mechanisms and genetic heterogeneity in host and bacteria, PK/PD considerations, and systematic clinical experience are necessary to advance in the treatment of infections caused by CRE. In this way, CRE pharmacotherapy could serve as a paradigm of ‘precision medicine’ that informs the future agenda of infectious diseases clinical research.
Article Highlights.
CRE are increasingly common in the United States and globally with mortality approaching 50% in patients with bloodstream infections, comparable to mortality from Ebola virus disease
The main mechanisms responsible for the CRE phenotype include OXA-48 and KPC carbapenemases, as well as the emerging metallo-beta-lacamase NDM-1.
Currently available antibiotics reliable (> 85% activitiy) against CRE include polymyxin B and colistin, tigecycline, fosfomycin and aminoglycosides.
Randomized controlled trials comparing monotherapy with these agents against combination therapy for the treatment of CRE are ongoing. The available observational data suggests a benefit for combination therapy, especially carbapenem-containing regimens against isolates where the carbapenem MIC is < 16 µg/ml and where carbapenems are administered in high doses and prolonged infusion.
Novel therapeutic agents such as the combination of ceftazidime or imipenem with the non-beta-lactam beta-lactamase inhibitor avibactam and relebactam offer activivity against CRE in vitro, except when carbapenem resistance is mediated by MBLs. Although “real life” efficacy of the newly released ceftazidime-avibactam against CRE infections still needs to be demonstrated, it stands as the best treatment option for KPC and OXA-48 producing Enterobacteraiceae.
Molecular surveillance studies that define the epidemiology of CRE in a given locale are essential to inform empiric therapy. An approach whereby molecular diagnostic platforms guide clinicians to choose the best therapy against CRE infections could represent a new paradigm of “precision medicine”.
Acknowledgments
The authors are supported by the Department of Veterans Affairs VISN 10 Geriatrics Research, Education and Clinical Center (F Perez and RA Bonomo), by the Department of Veterans Affairs Office of Research and Development under Award Number I01BX001974, by the National Institute Of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI104681, R01AI072219, R01AI063517 and R01AI100560 (RA Bonomo), and by the Cleveland Translational Science Collaborative funded by the National Center for Research Resources under Award Number U1TR000439 (F Perez). Additionally, RA Bonomo has received grants from AstraZeneca, Merck and Melinta. F Perez has received grants from Pfizer and Steris Corporation. The content is solely the responsibility of the authors and does not represent the official views of the Department of Veterans Affairs or the National Institutes of Health.
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
*=of importance, **= of considerable importance
- 1.Kahan JS, Kahan FM, Goegelman R, et al. Thienamycin, a new beta-lactam antibiotic. I. Discovery, taxonomy, isolation and physical properties. J Antibiot (Tokyo) 1979;32(1):1–12. doi: 10.7164/antibiotics.32.1. [DOI] [PubMed] [Google Scholar]
- 2.Kahan JSK, F M, Goegelman R, Currie SA, Jackson M, Stapley EO, Miller TW, Miller AK, Hendlin D, Mochales S, Hernandez S, Woodruff HS. Abstract XVI. Interscience Conference on Antimicrobial Agents and Chemotherapy; American Society for Microbiology; Chicago. 1976. [Google Scholar]
- 3.Romagnoli MF, Fu KP, Neu HC. The antibacterial activity of thienamycin against multiresistant bacteria-comparison with beta-lactamase stable compounds. J Antimicrob Chemother. 1980;6(5):601–606. doi: 10.1093/jac/6.5.601. [DOI] [PubMed] [Google Scholar]
- 4.Spratt BG, Jobanputra V, Zimmermann W. Binding of thienamycin and clavulanic acid to the penicillin-binding proteins of Escherichia coli K-12. Antimicrob Agents Chemother. 1977;12(3):406–409. doi: 10.1128/aac.12.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011;55(11):4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woodward RB. Penems and related substances. Philos Trans R Soc Lond B Biol Sci. 1980;289(1036):239–250. doi: 10.1098/rstb.1980.0042. ** Robert B. Woodward, perhaps the most creative medicinal chemist who ever lived, turns his attention towards the structure-function relationship of beta-lactams and predicts the superior spectrum of activity of carbapenems.
- 7.Salzmann TN, Ratcliffe RW, Bouffard FA, Christensen BG. A stereocontrolled, enantiomerically specific total synthesis of thienamycin. Philos Trans R Soc Lond B Biol Sci. 1980;289(1036):191–195. doi: 10.1098/rstb.1980.0037. [DOI] [PubMed] [Google Scholar]
- 8.Kropp H, Gerckens L, Sundelof JG, Kahan FM. Antibacterial activity of imipenem: the first thienamycin antibiotic. Rev Infect Dis. 1985;7(Suppl 3):S389–S410. doi: 10.1093/clinids/7.supplement_3.s389. [DOI] [PubMed] [Google Scholar]
- 9. Paterson DL, Ko WC, Von Gottberg A, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39(1):31–37. doi: 10.1086/420816. * A landmark study that established carbapenems as the drug of choice for the treatment of infections caused by beta-lactamase producing organisms.
- 10.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62(9):165–170. ** Crucial report from the U.S. Centers for Disease Control and Prevention establishing carbapenem resistant Enterobacteriacea as an important clinical challenge and a threat to public health.
- 12. Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29(12):1099–1106. doi: 10.1086/592412. ** This study determined that the mortality of infections caused by carbapenem-resistant K. pneumoniae is as high as 40%.
- 13.Perez F, Van Duin D. Carbapenem-resistant Enterobacteriaceae: a menace to our most vulnerable patients. Cleve Clin J Med. 2013;80(4):225–233. doi: 10.3949/ccjm.80a.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother. 2010;65(8):1807–1818. doi: 10.1093/jac/dkq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4(148):148ra116. doi: 10.1126/scitranslmed.3004129. ** Landmark outbreak of carbapenem-resistant K. pneumoniae at the National Institutes of Health Clinical Center which was elucidated with the application of whole-genome sequencing.
- 16.Satlin MJ, Calfee DP, Chen L, et al. Emergence of carbapenem-resistant Enterobacteriaceae as causes of bloodstream infections in patients with hematologic malignancies. Leuk Lymphoma. 2013;54(4):799–806. doi: 10.3109/10428194.2012.723210. [DOI] [PubMed] [Google Scholar]
- 17.Viale P, Giannella M, Lewis R, Trecarichi EM, Petrosillo N, Tumbarello M. Predictors of mortality in multidrug-resistant Klebsiella pneumoniae bloodstream infections. Expert Rev Anti Infect Ther. 2013;11(10):1053–1063. doi: 10.1586/14787210.2013.836057. [DOI] [PubMed] [Google Scholar]
- 18.Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011;17(12):1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 19.Bialek-Davenet S, Criscuolo A, Ailloud F, et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis. 2014;20(11):1812–1820. doi: 10.3201/eid2011.140206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaureguy F, Landraud L, Passet V, et al. Phylogenetic and genomic diversity of human bacteremic Escherichia coli strains. BMC Genomics. 2008;9:560. doi: 10.1186/1471-2164-9-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiedrowski LM, Guerrero DM, Perez F, et al. Carbapenem-resistant Enterobacter cloacae isolates producing KPC-3, North Dakota, USA. Emerg infect Dis. 2014;20(9):1583–1585. doi: 10.3201/eid2009.140344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwab F, Geffers C, Piening B, Haller S, Eckmanns T, Gastmeier P. How many outbreaks of nosocomial infections occur in German neonatal intensive care units annually? Infection. 2014;42(1):73–78. doi: 10.1007/s15010-013-0516-x. [DOI] [PubMed] [Google Scholar]
- 23.Maragakis LL, Winkler A, Tucker MG, et al. Outbreak of multidrug-resistant Serratia marcescens infection in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2008;29(5):418–423. doi: 10.1086/587969. [DOI] [PubMed] [Google Scholar]
- 24.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papp-Wallace KM, Bethel CR, Distler AM, Kasuboski C, Taracila M, Bonomo RA. Inhibitor resistance in the KPC-2 beta-lactamase, a preeminent property of this class A beta-lactamase. Antimicrob Agents Chemother. 2010;54(2):890–897. doi: 10.1128/AAC.00693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Queenan AM, Torres-Viera C, Gold HS, et al. SME-type carbapenem-hydrolyzing class A beta-lactamases from geographically diverse Serratia marcescens strains. Antimicrob Agents Chemother. 2000;44(11):3035–3039. doi: 10.1128/aac.44.11.3035-3039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh TR. Emerging carbapenemases: a global perspective. Int J Antimicrob Agents. 2010;36(Suppl 3):S8–S14. doi: 10.1016/S0924-8579(10)70004-2. [DOI] [PubMed] [Google Scholar]
- 28. Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 2012;67(7):1597–1606. doi: 10.1093/jac/dks121. * Important review focusing on OXA-48, one of the most prevalent mechanisms of resistance to carbapenems in Enterobacteriaceae worldwide.
- 29.Findlay J, Hamouda A, Dancer SJ, Amyes SG. Rapid acquisition of decreased carbapenem susceptibility in a strain of Klebsiella pneumoniae arising during meropenem therapy. Clin Microbiol Infect. 2012;18(2):140–146. doi: 10.1111/j.1469-0691.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 30.Shin SY, Bae IK, Kim J, et al. Resistance to carbapenems in sequence type 11 Klebsiella pneumoniae is related to DHA-1 and loss of OmpK35 and/or OmpK36. J Med Microbiol. 2012;61(Pt 2):239–245. doi: 10.1099/jmm.0.037036-0. [DOI] [PubMed] [Google Scholar]
- 31.Mammeri H, Guillon H, Eb F, Nordmann P. Phenotypic and biochemical comparison of the carbapenem-hydrolyzing activities of five plasmid-borne AmpC beta-lactamases. Antimicrob Agents Chemother. 2010;54(11):4556–4560. doi: 10.1128/AAC.01762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chudackova E, Bergerova T, Fajfrlik K, et al. Carbapenem-nonsusceptible strains of Klebsiella pneumoniae producing SHV-5 and/or DHA-1 beta-lactamases in a Czech hospital. FEMS Microbiol Lett. 2010;309(1):62–70. doi: 10.1111/j.1574-6968.2010.02016.x. [DOI] [PubMed] [Google Scholar]
- 33.van Duin D, Perez F, Rudin SD, et al. Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother. 2014;58(7):4035–4041. doi: 10.1128/AAC.02636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright MS, Perez F, Brinkac L, et al. Population structure of KPC-producing Klebsiella pneumoniae isolates from midwestern U.S. hospitals. Antimicrob Agents Chemother. 2014;58(8):4961–4965. doi: 10.1128/AAC.00125-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deleo FR, Chen L, Porcella SF, et al. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 2014;111(13):4988–4993. doi: 10.1073/pnas.1321364111. ** This important phylogenetic analysis of the core genome of various strains of ST258 K. pneumoniae revelaed two distinct clades that are prevalent in the United States.
- 36.Ramirez MS, Xie G, Johnson S, et al. Genome Sequences of Two Carbapenemase-Resistant Klebsiella pneumoniae ST258 Isolates. Genome Announc. 2014;2(3) doi: 10.1128/genomeA.00558-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limbago BM, Rasheed JK, Anderson KF, et al. IMP-producing carbapenem-resistant Klebsiella pneumoniae in the United States. J Clin Microbiol. 2011;49(12):4239–4245. doi: 10.1128/JCM.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuzon G, Naas T, Correa A, Quinn JP, Villegas MV, Nordmann P. Dissemination of the KPC-2 carbapenemase in non-Klebsiella pneumoniae enterobacterial isolates from Colombia. Int J Antimicrob Agents. 2013;42(1):59–62. doi: 10.1016/j.ijantimicag.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Rojas LJ, Mojica MF, Blanco VM, et al. Emergence of Klebsiella pneumoniae Coharboring KPC and VIM Carbapenemases in Colombia. Antimicrob Agents Chemother. 2013;57(2):1101–1102. doi: 10.1128/AAC.01666-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villegas MV, Lolans K, Correa A, et al. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob Agents Chemother. 2006;50(8):2880–2882. doi: 10.1128/AAC.00186-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adler A, Hussein O, Ben-David D, et al. Persistence of Klebsiella pneumoniae ST258 as the predominant clone of carbapenemase-producing Enterobacteriaceae in post-acute-care hospitals in Israel, 2008–13. J Antimicrob Chemother. 2015;70(1):89–92. doi: 10.1093/jac/dku333. [DOI] [PubMed] [Google Scholar]
- 42. Schwaber MJ, Lev B, Israeli A, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis. 2011;52(7):848–855. doi: 10.1093/cid/cir025. ** Description of actions underatken in Israel to successfully curtail the epidemic of carbapenem resitant Enterobacteriaceae.
- 43.Hammoudi D, Moubareck CA, Aires J, et al. Countrywide spread of OXA-48 carbapenemase in Lebanon: surveillance and genetic characterization of carbapenem-non-susceptible Enterobacteriaceae in 10 hospitals over a one-year period. Int J Infect Dis. 2014;29:139–144. doi: 10.1016/j.ijid.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Carrer A, Poirel L, Yilmaz M, et al. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob Agents Chemother. 2010;54(3):1369–1373. doi: 10.1128/AAC.01312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balkan II, Aygun G, Aydin S, et al. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis. 2014;26:51–56. doi: 10.1016/j.ijid.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Lowman W, Schleicher G. Antimicrobial treatment and outcomes of critically ill patients with OXA-48like carbapenemase-producing Enterobacteriaceae infections. Diagn Microbiol Infect Dis. 2015;81(2):138–140. doi: 10.1016/j.diagmicrobio.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 47.Grundmann H, Livermore DM, Giske CG, et al. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2010;15(46) doi: 10.2807/ese.15.46.19711-en. [DOI] [PubMed] [Google Scholar]
- 48.Oteo J, Saez D, Bautista V, et al. Carbapenemase-producing Enterobacteriaceae in Spain in 2012. Antimicrob Agents Chemother. 2013;57(12):6344–6347. doi: 10.1128/AAC.01513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pena I, Picazo JJ, Rodriguez-Avial C, Rodriguez-Avial I. Carbapenemase-producing Enterobacteriaceae in a tertiary hospital in Madrid, Spain: high percentage of colistin resistance among VIM-1-producing Klebsiella pneumoniae ST11 isolates. Int J Antimicrob Agents. 2014;43(5):460–464. doi: 10.1016/j.ijantimicag.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Sidjabat H, Nimmo GR, Walsh TR, et al. Carbapenem resistance in Klebsiella pneumoniae due to the New Delhi Metallo-beta-lactamase. Clin Infect Dis. 2011;52(4):481–484. doi: 10.1093/cid/ciq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-beta-lactamase-producing Enterobacteriaceae, United States. Emerg infect Dis. 2013;19(6):870–878. doi: 10.3201/eid1906.121515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-beta-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014;312(14):1447–1455. doi: 10.1001/jama.2014.12720. ** Description of outbreak of carbapenem-resistant Enterobacteriaceae associated with endoscopic equipment, illustrating the vulnerability of our healthcare system to metallo-beta-lactamase mediated resistance.
- 53.Villa L, Guerra B, Schmoger S, et al. IncA/C Plasmid Carrying blaNDM-1, blaCMY-16, and fosA3 in a Salmonella enterica Serovar Corvallis Strain Isolated from a Migratory Wild Bird in Germany. Antimicrob Agents Chemother. 2015;59(10):6597–6600. doi: 10.1128/AAC.00944-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zowawi HM, Sartor AL, Balkhy HH, et al. Molecular characterization of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in the countries of the Gulf cooperation council: dominance of OXA-48 and NDM producers. Antimicrob Agents Chemother. 2014;58(6):3085–3090. doi: 10.1128/AAC.02050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin S, Fu Y, Zhang Q, et al. High incidence and endemic spread of NDM-1-positive Enterobacteriaceae in Henan Province, China. Antimicrob Agents Chemother. 2014;58(8):4275–4282. doi: 10.1128/AAC.02813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Zhang J, Liu Y, et al. Molecular characteristics of carbapenemase-producing Enterobacteriaceae in China from 2008 to 2011: predominance of KPC-2 enzyme. Diagn Microbiol Infect Dis. 2014;78(1):63–65. doi: 10.1016/j.diagmicrobio.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 57. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602. doi: 10.1016/S1473-3099(10)70143-2. ** Seminal description of widespread dissemination of New-Delhi metallo-beta-lactamase as a cause of carbapepem-resistance throughout the Indian subcontinent and extending to the United Kingdom.
- 58.Regna PP, Solomons IA, Forscher BK, Timreck AE. Chemical Studies on Polymyxin B. J Clin Invest. 1949;28(5 Pt 1):1022–1027. doi: 10.1172/JCI102133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev. 2008;21(3):449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levin AS, Barone AA, Penco J, et al. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28(5):1008–1011. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]
- 61.Karabinis A, Paramythiotou E, Mylona-Petropoulou D, et al. Colistin for Klebsiella pneumoniae-associated sepsis. Clin Infect Dis. 2004;38(1):e7–e9. doi: 10.1086/380461. [DOI] [PubMed] [Google Scholar]
- 62.Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6(9):589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 63.Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40(9):1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 64.Petersdorf RG, Plorde JJ. Colistin--a reappraisal. JAMA. 1963;183:123–125. [PubMed] [Google Scholar]
- 65.Nation RL, Li J, Cars O, et al. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis. 2014 doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 66. Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55(7):3284–3294. doi: 10.1128/AAC.01733-10. ** A very important study that defines the way colistin is dosed, as well as raising fundamental questions about the ability of colistin to be used as monotherapy to treat infections in critically ill patients
- 67.Bergen PJ, Landersdorfer CB, Zhang J, et al. Pharmacokinetics and pharmacodynamics of 'old' polymyxins: what is new? Diagn Microbiol Infect Dis. 2012;74(3):213–223. doi: 10.1016/j.diagmicrobio.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Landersdorfer CB, Nation RL. Colistin: how should it be dosed for the critically ill? Semin Respir Crit Care Med. 2015;36(1):126–135. doi: 10.1055/s-0034-1398390. ** Comprehensive review article that synthesizes the current clinical and pharmacologic insights about colistin.
- 69.Karaiskos I, Friberg LE, Pontikis K, et al. Colistin Population Pharmacokinetics after Application of a Loading Dose of 9 MU Colistin Methanesulfonate (CMS) in Critically Ill Patients. Antimicrob Agents Chemother. 2015 doi: 10.1128/AAC.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pike M, Saltiel E. Colistin- and polymyxin-induced nephrotoxicity: focus on literature utilizing the RIFLE classification scheme of acute kidney injury. Journal of pharmacy practice. 2014;27(6):554–561. doi: 10.1177/0897190014546116. [DOI] [PubMed] [Google Scholar]
- 71.Yousef JM, Chen G, Hill PA, Nation RL, Li J. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother. 2012;67(2):452–459. doi: 10.1093/jac/dkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sirijatuphat R, Limmahakhun S, Sirivatanauksorn V, Nation RL, Li J, Thamlikitkul V. Preliminary clinical study of the effect of ascorbic acid on colistin-associated nephrotoxicity. Antimicrob Agents Chemother. 2015;59(6):3224–3232. doi: 10.1128/AAC.00280-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yousef JM, Chen G, Hill PA, Nation RL, Li J. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob Agents Chemother. 2011;55(9):4044–4049. doi: 10.1128/AAC.00328-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mammina C, Bonura C, Di Bernardo F, et al. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2012;17(33) [PubMed] [Google Scholar]
- 75.Bogdanovich T, Adams-Haduch JM, Tian GB, et al. Colistin-resistant, Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae belonging to the international epidemic clone ST258. Clin Infect Dis. 2011;53(4):373–376. doi: 10.1093/cid/cir401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother. 2011;55(2):593–599. doi: 10.1128/AAC.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kontopoulou K, Protonotariou E, Vasilakos K, et al. Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 beta-lactamase resistant to colistin. J Hosp Infect. 2010;76(1):70–73. doi: 10.1016/j.jhin.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 78.Giacobbe DR, Del Bono V, Trecarichi EM, et al. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect. 2015 doi: 10.1016/j.cmi.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 79.Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol. 2014;5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poirel L, Jayol A, Bontron S, et al. The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70(1):75–80. doi: 10.1093/jac/dku323. [DOI] [PubMed] [Google Scholar]
- 81.Cannatelli A, D'Andrea MM, Giani T, et al. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob Agents Chemother. 2013;57(11):5521–5526. doi: 10.1128/AAC.01480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cannatelli A, Di Pilato V, Giani T, et al. In vivo evolution to colistin resistance by PmrB sensor kinase mutation in KPC-producing Klebsiella pneumoniae is associated with low-dosage colistin treatment. Antimicrob Agents Chemother. 2014;58(8):4399–4403. doi: 10.1128/AAC.02555-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wright MS, Suzuki Y, Jones MB, et al. Genomic and Transcriptomic Analyses of Colistin-Resistant Clinical Isolates of Klebsiella pneumoniae Reveal Multiple Pathways of Resistance. Antimicrob Agents Chemother. 2015;59(1):536–543. doi: 10.1128/AAC.04037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Llobet E, Campos MA, Gimenez P, Moranta D, Bengoechea JA. Analysis of the networks controlling the antimicrobial-peptide-dependent induction of Klebsiella pneumoniae virulence factors. Infect Immun. 2011;79(9):3718–3732. doi: 10.1128/IAI.05226-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hendlin D, Stapley EO, Jackson M, et al. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 86.Stapley EO, Hendlin D, Mata JM, et al. Phosphonomycin. I. Discovery and in vitro biological characterization. Antimicrob Agents Chemother. 1969;9:284–290. [PubMed] [Google Scholar]
- 87.Gobernado M. Fosfomycin. Rev Esp Quimioter. 2003;16(1):15–40. [PubMed] [Google Scholar]
- 88.Kaase M, Szabados F, Anders A, Gatermann SG. Fosfomycin susceptibility in carbapenem-resistant Enterobacteriaceae from Germany. J Clin Microbiol. 2014;52(6):1893–1897. doi: 10.1128/JCM.03484-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother. 2010;54(1):526–529. doi: 10.1128/AAC.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Michalopoulos AS, Livaditis IG, Gougoutas V. The revival of fosfomycin. Int J Infect Dis. 2011;15(11):e732–e739. doi: 10.1016/j.ijid.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56(11):5744–5748. doi: 10.1128/AAC.00402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis. 2008;46(7):1069–1077. doi: 10.1086/527442. [DOI] [PubMed] [Google Scholar]
- 93.Pontikis K, Karaiskos I, Bastani S, et al. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents. 2014;43(1):52–59. doi: 10.1016/j.ijantimicag.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 94.Akova M, Daikos GL, Tzouvelekis L, Carmeli Y. Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria. Clin Microbiol Infect. 2012;18(5):439–448. doi: 10.1111/j.1469-0691.2012.03823.x. [DOI] [PubMed] [Google Scholar]
- 95.Arca P, Rico M, Brana AF, Villar CJ, Hardisson C, Suarez JE. Formation of an adduct between fosfomycin and glutathione: a new mechanism of antibiotic resistance in bacteria. Antimicrob Agents Chemother. 1988;32(10):1552–1556. doi: 10.1128/aac.32.10.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang Y, Shen P, Wei Z, et al. Dissemination of a clone carrying a fosA3-harbouring plasmid mediates high fosfomycin resistance rate of KPC-producing Klebsiella pneumoniae in China. Int J Antimicrob Agents. 2015;45(1):66–70. doi: 10.1016/j.ijantimicag.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 97.Stein GE, Babinchak T. Tigecycline: an update. Diagn Microbiol Infect Dis. 2013;75(4):331–336. doi: 10.1016/j.diagmicrobio.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother. 2005;49(1):220–229. doi: 10.1128/AAC.49.1.220-229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin Infect Dis. 2005;41(Suppl 5):S333–S340. doi: 10.1086/431674. [DOI] [PubMed] [Google Scholar]
- 100.MacGowan AP. Tigecycline pharmacokinetic/pharmacodynamic update. J Antimicrob Chemother. 2008;62(Suppl 1):i11–i16. doi: 10.1093/jac/dkn242. [DOI] [PubMed] [Google Scholar]
- 101.Castanheira M, Sader HS, Deshpande LM, Fritsche TR, Jones RN. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-beta-lactamase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2008;52(2):570–573. doi: 10.1128/AAC.01114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dixit D, Madduri RP, Sharma R. The role of tigecycline in the treatment of infections in light of the new black box warning. Expert Rev Anti Infect Ther. 2014;12(4):397–400. doi: 10.1586/14787210.2014.894882. [DOI] [PubMed] [Google Scholar]
- 103.Bassetti M, Eckmann C, Bodmann KF, et al. Prescription behaviours for tigecycline in real-life clinical practice from five European observational studies. J Antimicrob Chemother. 2013;68(Suppl 2):ii5–ii14. doi: 10.1093/jac/dkt140. [DOI] [PubMed] [Google Scholar]
- 104.Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56(4):2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 106.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Pascale G, Montini L, Pennisi M, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care. 2014;18(3):R90. doi: 10.1186/cc13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brust K, Evans A, Plemmons R. Tigecycline in treatment of multidrug-resistant Gram-negative bacillus urinary tract infections: a systematic review. J Antimicrob Chemother. 2014;69(10):2606–2610. doi: 10.1093/jac/dku189. [DOI] [PubMed] [Google Scholar]
- 109.van Duin D, Cober E, Richter SS, et al. Impact of therapy and strain type on outcomes in urinary tract infections caused by carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2014 doi: 10.1093/jac/dku495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Duin D, Cober ED, Richter SS, et al. Tigecycline therapy for carbapenem-resistant Klebsiella pneumoniae (CRKP) bacteriuria leads to tigecycline resistance. Clin Microbiol Infect. 2014;20(12):O1117–O1120. doi: 10.1111/1469-0691.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruzin A, Visalli MA, Keeney D, Bradford PA. Influence of transcriptional activator RamA on expression of multidrug efflux pump AcrAB and tigecycline susceptibility in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2005;49(3):1017–1022. doi: 10.1128/AAC.49.3.1017-1022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Veleba M, Schneiders T. Tigecycline resistance can occur independently of the ramA gene in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2012;56(8):4466–4467. doi: 10.1128/AAC.06224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Villa L, Feudi C, Fortini D, Garcia-Fernandez A, Carattoli A. Genomics of KPC-producing Klebsiella pneumoniae sequence type 512 clone highlights the role of RamR and ribosomal S10 protein mutations in conferring tigecycline resistance. Antimicrob Agents Chemother. 2014;58(3):1707–1712. doi: 10.1128/AAC.01803-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ababneh M, Harpe S, Oinonen M, Polk RE. Trends in aminoglycoside use and gentamicin-resistant gram-negative clinical isolates in US academic medical centers: implications for antimicrobial stewardship. Infect Control Hosp Epidemiol. 2012;33(6):594–601. doi: 10.1086/665724. [DOI] [PubMed] [Google Scholar]
- 115.Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother. 2011;55(12):5893–5899. doi: 10.1128/AAC.00387-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Galani I, Souli M, Daikos GL, et al. Activity of plazomicin (ACHN-490) against MDR clinical isolates of Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. from Athens, Greece. J Chemother. 2012;24(4):191–194. doi: 10.1179/1973947812Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Livermore DM, Mushtaq S, Warner M, et al. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother. 2011;66(1):48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- 118.Almaghrabi R, Clancy CJ, Doi Y, et al. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother. 2014;58(8):4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhanel GG, Lawson CD, Zelenitsky S, et al. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev Anti Infect Ther. 2012;10(4):459–473. doi: 10.1586/eri.12.25. [DOI] [PubMed] [Google Scholar]
- 120.Garcia-Salguero C, Rodriguez-Avial I, Picazo JJ, Culebras E. Could plazomicin alone or in combination be a therapeutical option against carbapenem-resistant Acinetobacter baumannii? Antimicrob Agents Chemother. 2015 doi: 10.1128/AAC.00873-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2010;65(6):1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 122.Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58(4):2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee GC, Burgess DS. Treatment of Klebsiella pneumoniae carbapenemase (KPC) infections: a review of published case series and case reports. Ann Clin Microbiol Antimicrob. 2012;11:32. doi: 10.1186/1476-0711-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rafailidis PI, Falagas ME. Options for treating carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2014;27(6):479–483. doi: 10.1097/QCO.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 125.Perez F, Adachi J, Bonomo RA. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin Infect Dis. 2014;59(Suppl 5):S335–S339. doi: 10.1093/cid/ciu612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58(2):654–663. doi: 10.1128/AAC.01222-13. ** This is is an attempt at a systematically reviewing the evidence regarding the treatment of carbapenem-resistant Enterobacteriaceae. The authors' conclusion is that such a systematic review and meta-analysis is not possible given the heterogeneity in the available evidence
- 127.Zusman O, Avni T, Leibovici L, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother. 2013;57(10):5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jernigan MG, Press EG, Nguyen MH, Clancy CJ, Shields RK. The combination of doripenem and colistin is bactericidal and synergistic against colistin-resistant, carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2012;56(6):3395–3398. doi: 10.1128/AAC.06364-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elemam A, Rahimian J, Doymaz M. In vitro evaluation of antibiotic synergy for polymyxin B-resistant carbapenemase-producing Klebsiella pneumoniae. J Clin Microbiol. 2010;48(10):3558–3562. doi: 10.1128/JCM.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hong JH, Clancy CJ, Cheng S, et al. Characterization of porin expression in Klebsiella pneumoniae Carbapenemase (KPC)-producing K. pneumoniae identifies isolates most susceptible to the combination of colistin and carbapenems. Antimicrob Agents Chemother. 2013;57(5):2147–2153. doi: 10.1128/AAC.02411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011;55(6):3002–3004. doi: 10.1128/AAC.01420-10. ** Early but very important report describing the use of double-carbapenem therapy for carbapenem-producing Enterobacteriaceae
- 132.Samonis G, Maraki S, Karageorgopoulos DE, Vouloumanou EK, Falagas ME. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa clinical isolates. Eur J Clin Microbiol Infect Dis. 2012;31(5):695–701. doi: 10.1007/s10096-011-1360-5. [DOI] [PubMed] [Google Scholar]
- 133.Souli M, Galani I, Boukovalas S, et al. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrob Agents Chemother. 2011;55(5):2395–2397. doi: 10.1128/AAC.01086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70(7):2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 135.Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis. 2013;56(2):272–282. doi: 10.1093/cid/cis857. [DOI] [PubMed] [Google Scholar]
- 136.Dulhunty JM, Roberts JA, Davis JS, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56(2):236–244. doi: 10.1093/cid/cis856. [DOI] [PubMed] [Google Scholar]
- 137.Huttner A, Harbarth S, Hope WW, Lipman J, Roberts JA. Therapeutic drug monitoring of the beta-lactam antibiotics: what is the evidence and which patients should we be using it for? J Antimicrob Chemother. 2015 doi: 10.1093/jac/dkv201. [DOI] [PubMed] [Google Scholar]
- 138.Tabah A, De Waele J, Lipman J, et al. The ADMIN-ICU survey: a survey on antimicrobial dosing and monitoring in ICUs. J Antimicrob Chemother. 2015;70(9):2671–2677. doi: 10.1093/jac/dkv165. [DOI] [PubMed] [Google Scholar]
- 139.Michalopoulos A, Virtzili S, Rafailidis P, Chalevelakis G, Damala M, Falagas ME. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin Microbiol Infect. 2010;16(2):184–186. doi: 10.1111/j.1469-0691.2009.02921.x. [DOI] [PubMed] [Google Scholar]
- 140.Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev. 2010;23(1):160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Drawz SM, Papp-Wallace KM, Bonomo RA. New beta-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother. 2014;58(4):1835–1846. doi: 10.1128/AAC.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lahiri SD, Mangani S, Jahic H, et al. Molecular Basis of Selective Inhibition and Slow Reversibility of Avibactam against Class D Carbapenemases: A Structure-Guided Study of OXA-24 and OXA-48. ACS Chem Biol. 2014 doi: 10.1021/cb500703p. [DOI] [PubMed] [Google Scholar]
- 143.Livermore DM, Mushtaq S, Warner M, et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-Producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55(1):390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Endimiani A, Choudhary Y, Bonomo RA. In vitro activity of NXL104 in combination with beta-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob Agents Chemother. 2009;53(8):3599–3601. doi: 10.1128/AAC.00641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aktas Z, Kayacan C, Oncul O. In vitro activity of avibactam (NXL104) in combination with beta-lactams against Gram-negative bacteria, including OXA-48 beta-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents. 2012;39(1):86–89. doi: 10.1016/j.ijantimicag.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 146.Castanheira M, Sader HS, Farrell DJ, Mendes RE, Jones RN. Activity of ceftaroline-avibactam tested against Gram-negative organism populations, including strains expressing one or more beta-lactamases and methicillin-resistant Staphylococcus aureus carrying various staphylococcal cassette chromosome mec types. Antimicrob Agents Chemother. 2012;56(9):4779–4785. doi: 10.1128/AAC.00817-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sader HS, Castanheira M, Flamm RK, Farrell DJ, Jones RN. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from U.S. medical centers in 2012. Antimicrob Agents Chemother. 2014;58(3):1684–1692. doi: 10.1128/AAC.02429-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Flamm RK, Sader HS, Farrell DJ, Jones RN. Ceftazidime-avibactam and comparator agents tested against urinary tract isolates from a global surveillance program (2011) Diagn Microbiol Infect Dis. 2014;80(3):233–238. doi: 10.1016/j.diagmicrobio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 149.Wang X, Zhang F, Zhao C, et al. In vitro activities of ceftazidime-avibactam and aztreonam-avibactam against 372 Gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob Agents Chemother. 2014;58(3):1774–1778. doi: 10.1128/AAC.02123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Badal RYK, Motyl M, Hoban D, Hackel M, Biedenbach D, Bouchillon S. Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 2014. Impact of MK-7655 on Imipenem MICs of a Large Collection of Enterobacteriaceae and Pseudomonas aeruginosa Isolates. [Google Scholar]
- 151.Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother. 2013;68(10):2286–2290. doi: 10.1093/jac/dkt178. [DOI] [PubMed] [Google Scholar]
- 152.Endimiani A, Hujer KM, Hujer AM, Pulse ME, Weiss WJ, Bonomo RA. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011;55(1):82–85. doi: 10.1128/AAC.01198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. In vivo efficacy of humanized exposures of Ceftazidime-Avibactam in comparison with Ceftazidime against contemporary Enterobacteriaceae isolates. Antimicrob Agents Chemother. 2014;58(11):6913–6919. doi: 10.1128/AAC.03267-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. Efficacy of humanized carbapenem and ceftazidime regimens against Enterobacteriaceae producing OXA-48 carbapenemase in a murine infection model. Antimicrob Agents Chemother. 2014;58(3):1678–1683. doi: 10.1128/AAC.01947-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Docquier JD, Calderone V, De Luca F, et al. Crystal structure of the OXA-48 beta-lactamase reveals mechanistic diversity among class D carbapenemases. Chem Biol. 2009;16(5):540–547. doi: 10.1016/j.chembiol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 156.Jumes PR ML, Gutierrez M, Li X, Stoch SA, Wagner JA, Butterton JR. 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, CA: American Society for Microbiology; 2012. [Google Scholar]
- 157.Blizzard TA, Chen H, Kim S, et al. Discovery of MK-7655, a beta-lactamase inhibitor for combination with Primaxin(R) Bioorg Med Chem Lett. 2014;24(3):780–785. doi: 10.1016/j.bmcl.2013.12.101. [DOI] [PubMed] [Google Scholar]
- 158.Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, Phase II trial. J Antimicrob Chemother. 2013;68(5):1183–1192. doi: 10.1093/jac/dks523. [DOI] [PubMed] [Google Scholar]
- 159.Vazquez JA, Gonzalez Patzan LD, Stricklin D, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Current medical research and opinion. 2012;28(12):1921–1931. doi: 10.1185/03007995.2012.748653. [DOI] [PubMed] [Google Scholar]
- 160. Paul M, Carmeli Y, Durante-Mangoni E, et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother. 2014;69(9):2305–2309. doi: 10.1093/jac/dku168. ** Comprehensive review exploring combination therapy for carbapenem-resistant bacteria, concluding that there is no evidence-based support for most combination therapies, including colistin/carbapenem combinations
- 161.Michel JB, Yeh PJ, Chait R, Moellering RC, Jr, Kishony R. Drug interactions modulate the potential for evolution of resistance. Proc Natl Acad Sci U S A. 2008;105(39):14918–14923. doi: 10.1073/pnas.0800944105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Munck C, Gumpert HK, Wallin AI, Wang HH, Sommer MO. Prediction of resistance development against drug combinations by collateral responses to component drugs. Sci Transl Med. 2014;6(262):262ra156. doi: 10.1126/scitranslmed.3009940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rodriguez de Evgrafov M, Gumpert H, Munck C, Thomsen TT, Sommer MO. Collateral resistance and sensitivity modulate evolution of high-level resistance to drug combination treatment in Staphylococcus aureus. Mol Biol Evol. 2015 doi: 10.1093/molbev/msv006. [DOI] [PubMed] [Google Scholar]
- 164.Nichol D, Jeavons P, Fletcher AG, et al. Steering Evolution with Sequential Therapy to Prevent the Emergence of Bacterial Antibiotic Resistance. PLoS computational biology. 2015;11(9):e1004493. doi: 10.1371/journal.pcbi.1004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rahme C, Butterfield JM, Nicasio AM, Lodise TP. Dual beta-lactam therapy for serious Gram-negative infections: is it time to revisit? Diagn Microbiol Infect Dis. 2014;80(4):239–259. doi: 10.1016/j.diagmicrobio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 166.Nicolau DP. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis. 2008;47(Suppl 1):S32–S40. doi: 10.1086/590064. [DOI] [PubMed] [Google Scholar]
- 167.Dandekar PK, Maglio D, Sutherland CA, Nightingale CH, Nicolau DP. Pharmacokinetics of meropenem 0.5 and 2 g every 8 hours as a 3-hour infusion. Pharmacotherapy. 2003;23(8):988–991. doi: 10.1592/phco.23.8.988.32878. [DOI] [PubMed] [Google Scholar]
- 168.Krueger WA, Bulitta J, Kinzig-Schippers M, et al. Evaluation by monte carlo simulation of the pharmacokinetics of two doses of meropenem administered intermittently or as a continuous infusion in healthy volunteers. Antimicrob Agents Chemother. 2005;49(5):1881–1889. doi: 10.1128/AAC.49.5.1881-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]