ABSTRACT
Regioselective enzymatic acylation of proanthocyanidin is proposed and investigated as a method by which to improve the solubility of proanthocyanidins in the oil phase and maintain its oxidation resistance. Experimental results indicate that butanol functions as the best solvent in the studied reaction, in which Lipase Novozym435 is used as biological catalyst enzyme and the molar ratio of lauric acid to proanthocyanidins is 4:1. To increase the esterification conversion, we propose the addition of molecular sieve at 5 h. The product was separated by TLC, and results indicate an optimal solvent ratio of ethyl acetate: petroleum ether: acetic acid = 2:3:0.5. This condition can effectively separate the ester and proanthocyanidins, achieving an esterification yield of 60.9%.
KEYWORDS: esterification, lipase-catalyzed, Novozym435, proanthocyanidins, peroxide value
Introduction
Flavonoids are widely present in natural plant drugs, which act as free radical scavengers, and provide oxidation resistance, among other biological functions. Procyanidins are significant active substances with flavonoids. Procyanidin can prevent free radical oxidation with anti-aging, anti-inflammatory, anti-tumor, and immunomodulatory characteristics.1 Procyanidins are flavonoids, which naturally appear in the form of glucosides. Glucosides are polar molecules, most of which exhibit low solubility and stability in non-polar media, such as oil. The esterification of the hydroxyl functional groups by fatty acids can improve the hydrophobic nature of procyanidins, fat-soluble flavonoids which primarily enrich human adipose tissue and cell membranes. The primary physiological role of flavonoids is to promote fat and lipid metabolism; additionally, the esterified product is easy to incorporate into the cell membrane, which allows the elimination of fat-soluble “junk” attached to the cell surface.2 There are also many phenolic hydroxyl groups in proanthocyanidins (Fig. 1), though their redox potential is lower and the antioxidant time is inadequate. When aliphatic chains are connected to proanthocyanidins, their stability increases and their antioxidant timeliness become extended.3
Figure 1.
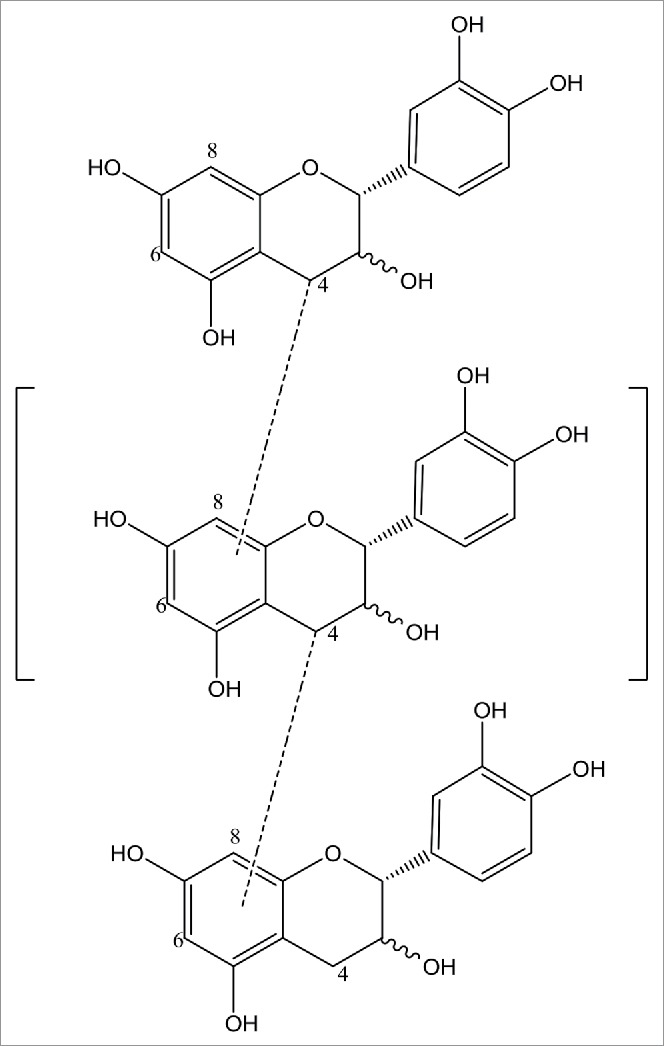
The structure of proanthocyanidins.
Esterification reactions using chemical methods typically proceed through 3 steps (protect group—esterification—deprotect group). However, an enzymatic approach may be more suitable to such reactions due to the regioselectivity of enzymes, which allows the process to be conducted under mild temperature and pressure conditions and provide good selectivity and stereospecificity. Previous studies have indicated that the position of the lipase esterification reaction is closely related to the reaction solvents used, acyl donor chain length, the molecular sieve and enzymes (Table 1).
Table 1.
Selective esterification of flavonoids as related to lipases, fatty acids and solvents.4-12
| Lipase | Reaction solvent | Fatty acid (acyl donor) |
|---|---|---|
| Novozym 435 (Candida antarctica lipaseB) | 2-methyl-2-butanol | palmitic acid palmitic acid methyl ester |
| C. antarctica | n-butanol | lauric acid |
| C. cylindrica | THF | myristic acid |
| P. cepacia | t-BME | stearic acid |
| Rizophus jaÍanicus | CH2Cl2 | adipic acid |
| M. miehei | acetone | azelaic acid |
| Porcine pancreas lipase | tert-butanol | dodecandioic acid |
| subtilisin | tert-amyl alcohol | hexade-candioic acid |
| Streptomyces rochei or Aspergillus niger obtained | n-hexane | 11-mercaptoundecanoic acid |
The present study describes the single-step acylation of proanthocyanidins catalyzed by the immobilized lipase Novozym 435, in order to increase the solubility of proanthocyanidins in apolar media. Through esterification, the introduction of the long carbon chains can increase the hydrophilic and hydrophobic balance of proanthocyanidins.
Experiment
Materials and methods
Proanthocyanidin content (UV) ≥95% (JS90148–20 mg, Shanghai Golden Harvest Biotech Co., Ltd.). The palmitic, stearic acid, and lauric acid used in the experiment are CP grade (Institute of Tianjin, Guangfu Fine Chemicals); Lipase Novozym 435 Novzymes (China, Biology Technology Limited Company); molecular sieve (China-America Shanghai Global Molecular sieve Limited Company); methanol (HPLC grade) (Honeywell Trading (Shanghai) Co., Ltd.); tert-amyl alcohol, butanol, isopentanol, tertiary butyl alcohol and n-hexane are CP grade (Beijing Chemistry Reagent Company); chromatography silica gel, 300–400 order (Qingdao Ocean Chemistry Factory); deionized water.
Analytical method
HPLC analysis is performed using a Shimadzu liquid chromatographic (LC-10ATVP) system. A Diamonsil C18 column (250 mm × 4.6 mm i.d.,10 μm) is employed to separate samples. The detector is set to 280 nm and injection volume is 10µl, methanol: 0.4% phosphoric acid = 1: 1 is adopted as mobile phase, with the flow rate equal to 1.0ml/min. All HPLC analyses are performed at (30 ± 1)°C. The retention time of proanthocyanidins is equal to 3.28 min, and the working calibration curve based on proanthocyanidins standard solutions demonstrated strong linearity over the range of 2.0–10.0 mg. The regression line was represented by y = 71386x + 14416 (R2 = 0.9998, n = 5), where y represents the peak value of proanthocyanidin content and x represents the proanthocyanidin concentration in mg.
During the reaction, 0.2 ml of reaction solution were periodically removed, diluted with ethanol in 10 mL volumetric flasks, and then filtered through 0.45 µm membranes (Chromatography Science and Technology Co., Tianjin, China). The contents of proanthocyanidin samples were calculated prior to HPLC analysis.
Clear super oxide anion radical
Oxygen free radicals can be released by pyrogallol under alkaline conditions. A super oxide anion radical and NBT form purple compounds, which can be measured at a wavelength of 530 nm. The absorption value (A) reflects O2- content. A sample of 2.5 ml of 0.1 mol/L Tris-HCl (pH 8.2) buffer solution 2.5 is collected in a tube; samples are then preheated in a warm water bath for 20 min at 25°C. Then, 2.0 ml of various sample concentrations are added to 0.6 ml of 0.98 mmol/L NBT and 0.3 ml of 10 mmol/L pyrogallol. After four minutes of reaction in the water bath at 25°C 0.1 ml of 8 mol/L HCl is immediately added to terminate the reaction. The absorption values A were then determined by measuring absorbance at a wavelength of 530 nm. Use distilled water instead of a blank sample. Results were determined at a clearance rate E% = (A blank-A sample) / A blank × 100%.
Clear hydroxyl radical
An·OH group is produced by EDTA -Na- Fe (II) - H2O2 (Fenton) system, which can result in the fading of crocus sativus red T; the degree of fade can be determined by measurement according to the calorimetric method, which is related to the OH content. In a reaction system, there are the solution of the phosphoric acid buffer pH = 7.4, Crocus sativus red T(520 µg/ml) 0.2 ml, 2 mmol EDTA-2Na-Fe(II) 0.7 ml, different concentrations of selected liquid 1.0 ml, 6% of H2O2 0.4 ml, mix 30 min in 37°C water. Then absorbance is measured by spectrophotometry at a wavelength of 520nm. Experimental results are determined according to clearance E % = (A sample - A blank) / A sample ×100 %.
Determination of peroxide value (POV)
Peroxide value (POV) is the measure of degree of oxidation or rancidity that occurs within a reaction, which determines the concentration of peroxides in an oil or fat. In order to determine the peroxide values of cotton seed oils, a sample of approximately 5 g of cotton seed oil was weighed accurately and dissolved in 30 ml of an acetic acid and chloroform mixture (3:2 V/V), prior to the addition of 0.5 ml of a saturated KI solution. The mixture was allowed to stand for one minute before the addition of 30 ml of distilled water. The entire mixture was then titrated with 0.1 N Na2S2O3 solution, using 1 ml of 0.5% starch as an indicator. The POV was then calculated from the following relationship: 6
where:V = volume of Na2S2O3 usedN = normality of Na2S2O3W = sample weight
Experimental methods
The water content in organic medium greatly influences catalytic esterification reactions. High water content typically results in low conversion yield; therefore, water treatment was conducted prior to the reaction. Proanthocyanidins, palmitic, stearic acid and lauric acid were dried in a dryer for a period of one week. A 4A molecular sieve prior to the activation of 4A (150°C, 24 h) is placed in a solution of tert-amyl alcohol, butanol, isopentanol, tertiary butyl alcohol and n-hexane for 5 d.
A fixed ratio of proanthocyanidin and acyl donor (such as palmitic, stearic acid and lauric acid) are placed in a solvent (such as tert-amyl alcohol, butanol, isopentanol, tertiary butyl alcohol and n-hexane). When the reaction temperature reaches 50–60°C, Novozym435 lipase (4 mg/ml) is added. The bottle is shaken for several hours, after which 100 g 4A molecular sieves are added in order remove the water produced by esterification. The reaction is completed after 96 h, at which point the enzyme and molecular sieve are filtered before the products are isolated by TLC.
Results and discussion
The effect of solvent on the reaction
In a 250 ml bottle, 0.5 g proanthocyanidin, 2.0 g lauric acid, 1.5 g lipase Novozym 435, and 150 ml solvent (tert-amyl alcohol, butanol, isopentanol, tertiary butyl alcohol and n-hexane) are combined. A total of 5 such flasks are placed in a temperature-controlled shaking bed at 55°C for 5 h. Then, the molecular sieve are added to the 5 flasks, according to 1 L reaction liquid adding 100 g molecular sieves 4A, continue the reaction, after 96 h, stop reaction. Experimental data are presented in Fig. 2 and Table 2.
Figure 2.
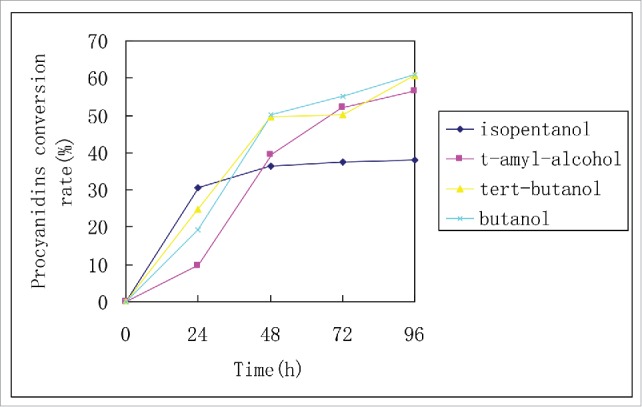
Effect of solvent on reaction.
Table 2.
Comparison of 3 solvents.
| Solvent | Conversion rate (%) | Notes |
|---|---|---|
| Tert-butanol | 60.5 | Solid at room temperature, analytical difficulties |
| Butanol | 60.9 | Easy to operate, high conversion rate |
| Hexane | - | Procyanidin has poor solubility in this solvent |
| Tert -amyl alcohol | 56.6 | Easy to operate, high conversion rate, but Procyanidin has poor solubility in this solvent |
| Isopentanol | 38.0 | Easy to operate, low conversion rate. |
As shown in Table 2, experimental results indicate that butanol is the optimal tested reaction solvent, demonstrating easy operation at the highest conversion rate of up to 60.9%.
The effect of acyl donors
In a 250 ml bottle 0.5 g proanthocyanidins, 2.0 g fatty acid (palmitic, stearic acid, lauric acid) 1.5 g lipase Novozym 435, and 150 ml butanol are combined. A total of 3 flasks are placed in a temperature-controlled shaking bed at 55°C for 5 h. Then, add the molecular sieve in every flask, according to 1 L reaction liquid adding 100 g molecular sieves 4A, continue the reaction, after 96 h, stop reaction. The molecular sieve and product are separated by filtration, and the liquid mixtures are then separated by TLC. Experimental results indicate that the optimal tested ratio of developing solvent was ethyl acetate: petroleum ether: acetic acid = 2:3:0.5. This condition can effectively separate ester from proanthocyanidin, exhibiting an Rf value of proanthocyanidin equal to 0.37 and an Rf value of ester equal to 0.82. Analysis of the proanthocyanidin and esterification products was conducted by IR and NMR. Experimental data are shown in Table 3 and Figs. 3 and 4.
Table 3.
Synthesis conversion rate achieved by various fatty acids.
| Fatty acid | Conversion rate (%) |
|---|---|
| stearic acid | 48.5 |
| palmitic | 58.3 |
| lauric acid | 60.9 |
Figure 3.
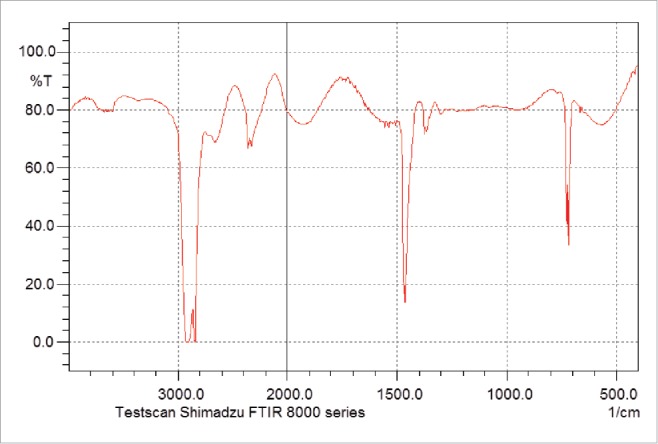
IR of proanthocyanidin.
Figure 4.
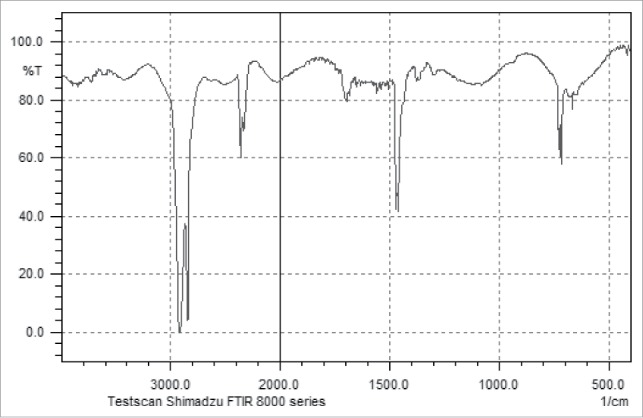
IR of proanthocyanidin lauric acid ester.
The stretching vibration of carbonyl compounds is generally observed at 1750–1670 cm−1 generally. As compared to Figs. 3 and 4, results demonstrate, carbonyl peaks at 1700 cm−1, indicating the generation of the laurate procyanidin ester.
Investigation of the structure of 3-O-laurate procyanidin ester by 1H-NMR reveals the following results: 1H-NMR (300 MHz,DMSO-d6): δ(ppm) 8.29 (s,1H),8.08 (s,1H),7.98 (s,1H),7.81(s,2H),7.76 (m, 1H),7.35 (bs,1H), 6.05 (d, J = 2.3 Hz, 1H),6.03 (d, J = 2.3 Hz, 1H ), 5.61 (m,1H), 5.11 (s.1H), 3.03 (m,2H), 2.18 (t, J = 6.3 Hz, 2H) 1.29–1.06 (m,18H), 0.90 (t, J = 7.2 Hz, 3H). As compared to the procyanidins 1H-NMR, the displacement which represents the proton mother nucleus of 3-O-laurate procyanidin ester does not change; 1.29–1.06 ppm represents a series of aliphatic chain protons, verifying the generation of laurate procyanidins ester. Additionally a new triple peak appears at 2.18 ppm, indicating that the protons of the hydroxyl group attached to induce the esterification reaction. The presence of no other new peaks in the vicinity prove that the product obtained is the 3-O-laurate procyanidins ester (Fig. 5).
Figure 5.
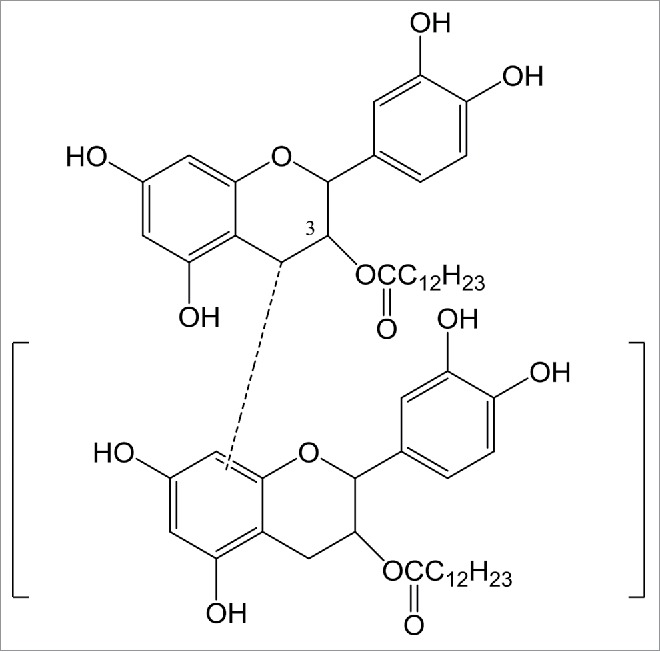
The structure of 3-O-laurate Procyanidins ester.
The influence of molecular sieve
In a 250 ml bottle, 0.5 g proanthocyanidin, 2.0 g lauric acid, 1.5 g lipase Novozym 435, and 150 ml butanol are combined. A total of 4 flasks are placed in a temperature-controlled shaking bed at 55°C. After 5, 12, and 24 h, different molecular sieves are added to different flasks, according to 1 L reaction liquid adding 100 g molecular sieves 4A, continue the reaction, after 96 h, stop reaction. Experimental data are shown in Fig. 6.
Figure 6.
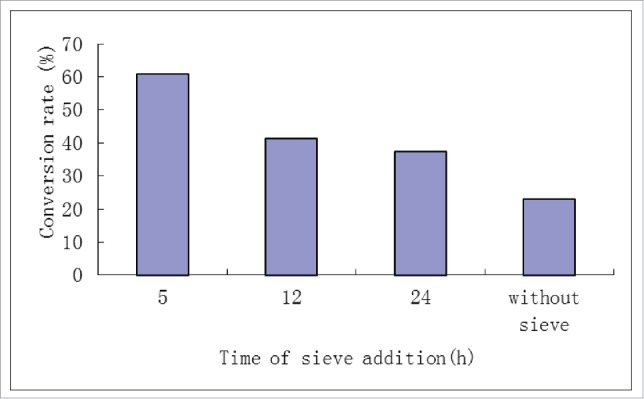
The conversion rate of procyanidins in different time adding the molecular sieve.
As shown in Fig. 6, the earlier the addition of the sieve to the reaction, the higher the resulting esterification rate of procyanidins. The esterification reaction is a reversible reaction, while the formation of the esterification produced also generates water; the 4A molecular sieve is used as a water absorbing agent to improve the procyanidin ester product yield.
Clear hydroxyl and super oxide anion radicals
Comparison of antioxidant activity of procyanidins procyanidins stearate ester vitamins E and BHT, vitamins E and BHT are antioxidants which are always used in oil.
As shown in Table 4, the clear hydroxyl radical and the super oxide anion radical of procyanidin appear with the greatest frequency; the frequency of the procyanidin stearate ester is lower than that of procyanidin, but higher than that of BHT and VE. These results indicate that the procyanidin stearate ester demonstrate the best antioxidant activity. Procyanidin esters can provide reductive protons such as procyanidins, to capture the process of peroxide-generated reactive intermediate radicals. Procyanidins are a mixture of several monomers; monomers of the antioxidant have synergistic effects, which strengthen the antioxidant properties of procyanidins. The antioxidant mechanism is similar to that of vitamin E BET. The structure of procyanidin esters reduces the hydroxyl proton, which removes free radicals, thus suspending the free radical chain reaction and ceasing fat oxidation. Flavonoid glycosides modified by esterification introduce molecules with long hydrocarbon chains, thus increasing the fat-soluble properties and balancing affinities, simultaneously increasing fat compatibility and improving oxidation resistance.
Table 4.
Clearance rate of the clear hydroxyl radical ·OH and the super oxide anion radical O2−.
| ·OH | O2-· | |
|---|---|---|
| Sample | E% | E% |
| Procyanidins esters | 58.34 | 60.21 |
| Procyanidins | 67.72 | 69.18 |
| BHT | 64.40 | 50.21 |
| VE | 65.85 | 52.18 |
Peroxide value (POV) determination of procyanidin esters
According to the peroxide value experimental method, the peroxide value of natural flavonoid procyanidins, procyanidin stearate esters and the antioxidative activity of the standard antioxidant BHT were evaluated. The experimental results are presented in Fig. 7.
Figure 7.
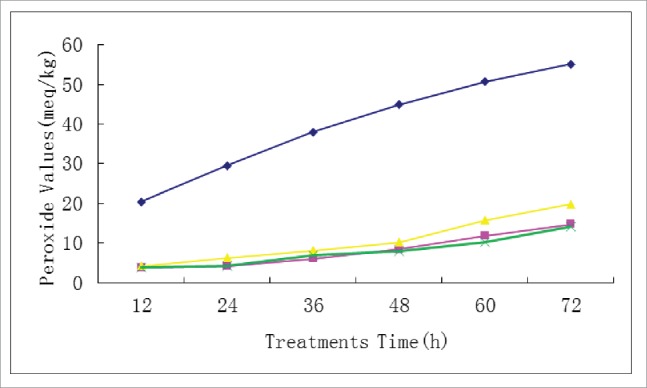
Antioxidative Activity of procyanidin esters.♦Blank ▴BET ▪ Procyanidins esters × Procyanidins.
As shown in Fig. 7, the peroxide value of procyanidin and the esterification product are lower than that of the commonly-used antioxidant BET. The peroxide value of the product of procyanidin esterification is less prior to 48 h, while the peroxide value of procyanidins decrease after 48 h.
Conclusions
The purpose of esterification is to increase the lipophilic properties of procyanidin, and to enhance its antioxidant activity in oil. This reaction is catalyzed by Lipase Novozym435 in order to improve the conversion yield. Water produced during esterification is continuously removed by the addition of 4A molecular sieves 5 h after initiation of the reaction. Experimental results demonstrate that butanol is the optimal reaction solvent. The optimal proportion of procyanidin to fatty acid is 1:4, the optimal reaction temperature is 55–60°C, the highest conversion yield obtained was 60.9%, and product identification is verified by NMR to be the 3-OH of procyanidin(see Fig. 1 and Fig. 5) and fatty acid occur to ester. The adopted technology and obtained theoretical approach in this study offer important significance and instructive value to the molecular modification of bioflavonoids and other natural products.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was financially supported by the 2014 Beijing Natural Science Foundation - Beijing Institute of Science and Technology jointly funded projects L140006, National Natural Science Foundation of China (Grant No. 11475020, 11475198, 11275216 and 11435002), project (No. PXM2016_014209_000023) for building virtual teaching and researching team to enhance teaching quality and project (No. PXM2016_014209_000049) for building emerging major by joint training to enhance the quality of graduate.
References
- [1].Prieur C, Rigaud J, Cheynier V, Moutounet M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994; 36(3):781-4; http://dx.doi.org/ 10.1016/S0031-9422(00)89817-9 [DOI] [Google Scholar]
- [2].Gabetta B, Fuzzati N, Griffini A. Characterization of proanthocyanidins from grape seeds. Fitoterapia 2000; 71:162-75; PMID:10727813; http://dx.doi.org/ 10.1016/S0367-326X(99)00161-6 [DOI] [PubMed] [Google Scholar]
- [3].Saint N, Provost C, Vivas N. Comparative study of polyphenol scavenging activities assessed by different methods. Agric J Food Chem. 1999; 47(2):425-31; PMID:10563911; http://dx.doi.org/ 10.1021/jf980700b [DOI] [PubMed] [Google Scholar]
- [4].Bruno D, Monica L, Giannantonio S, Giacomo C, Sergio R. Regioselective acylation of polyhydroxylated natural compounds catalyzed by Candida Antarctica lipase B (Novozym 435) in organic solvents. J Mol Catalysis B: Enzymatic 1997; 3:193-201; http://dx.doi.org/ 10.1016/S1381-1177(96)00055-0 [DOI] [Google Scholar]
- [5].Sergio Riva. 2002. Enzymatic modification of the sugar moieties of natural glycosides. Journal of Molecular Catalysis B:Enzymatic. 19-20# 43-54. [Google Scholar]
- [6].Chunli G, Patrick M, David A, MacManus, Evgeny NV. Novel enzymatic approach to the synthesis of flavonoid glycosides and their esters. Biotechnol Bioengineer 2000; 71:235-43; PMID:11291033; http://dx.doi.org/ 10.1002/1097-0290(2000)71:3%3c235::AID-BIT1013%3e3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- [7].Gayot S, Santarelli X, Coulon D. Modification of flvonoid using lipase in non-conventional media: effect of the water content. J Biotechnol 2003; 101:29-36; PMID:12523967; http://dx.doi.org/ 10.1016/S0168-1656(02)00286-9 [DOI] [PubMed] [Google Scholar]
- [8].Danieli B, De Bellis P. Enzyme-mediated regioselective acylations of flavonoid disaccharidemonogly cerides. Helv Chim Acta 1990; 73:1837-44; PMID:11674146; http://dx.doi.org/ 10.1002/hlca.1990073070511674146 [DOI] [Google Scholar]
- [9].Sakai M, Suzuki M, Nanjo F. 3-O-acylated catechins and method of producing same. European Patent 0618203A1. 1994-10-05 [Google Scholar]
- [10].Patti A, Piattelli M, Nicolosi G. Use of Mucor miehei lipase in the preparation of long chain 3-O-acylcatechins. J Mol Catal B: Enzym 2000; 10:577-82; http://dx.doi.org/ 10.1016/S1381-1177(00)00140-5 [DOI] [Google Scholar]
- [11].Ardhaoui M, Falcimaigne AJ, Ognier S. Effect of acyl donor chain length and substitutions pattern on the enzymatic acieration of favonoids. Biotechnol 2004; 110(3):265-71. [DOI] [PubMed] [Google Scholar]
- [12].Kontogianni A, Skouridou V, Sereti V, Stamatis H, Kolisis FN. Lipase-catalyzed esterification of rutin and naringin with fatty acids of medium carbon chain. J Mol Catalysis B: Enzymatic 2003; 21:59-62; http://dx.doi.org/ 10.1016/S1381-1177(02)00139-X [DOI] [Google Scholar]


