ABSTRACT
Almost all multichannel microelectrodes are only applied to the same nucleus. The multiple brain regions synchronization implanted microelectrodes can be implanted in the several brain regions at the same time, when used in the robo-animal, which can reduce the operation process, shorten animals operation time. Due to electrode position relatively fixed, errors caused by each separately implanted electrode were reduced and the animal control effect was greatly increased compared to the original electrodes. The electrode fixed time was also extended. This microelectrode provided beneficial reference function for the study of the free state of small animals in different brain regions.
KEYWORDS: brain regions, microelectrodes, multichannel, robo-pigeon, stimulating
Introduction
The robo-animal research attracts much attention since Talwar S K et al.1,2 reported the rat can be virtually trained by the remote micro-stimulating key brain regions in 2002, and the turtle with microcamera became a “spy” through the electrical stimulated specific neural site. When the shark brain was implanted with a series of electrode, the researcher could control the move of the shark and decode their senses.3 The researcher could control the beetles wing and related behaviors by simultaneously implanted electrodes in the brain and muscle.4 Stimulating the gecko midbrain nuclei can control the turning movement of animals, so as to complete the control of the gecko route.5 The carp's behavior of turning left, turning right, moving forward and backward were successfully induced by electric stimulation in the corpus cerebelli.6 In our teams, the robo-rat and robo-bird have successfully developed.7-9 In the robo-animals research, 3 or 4 pairs of microelectrodes were implanted in the animal brain, which control animals to turn left, turn right and forward. However, when microelectrodes were implanted at different sites, we tend to make microelectrode fixed in animal's different brain regions one by one. For example, we often fasten on a pair of microelectrodes implanted animal brains, and then the other was implanted, until 3 or 4 microelectrodes were fixed, and then connected the FFC platoon line and fixed. This operation method is very time-consuming, and each implant microelectrodes position would cause deviation by man-made factors, it is difficult to achieve effective stimulus of multiple sites at the same time to complete the animal control.
At present, different microelectrodes have been used in the animal experiment. For example, different types of multichannel microelectrode array10,11 and the multichannel microelectrode which can adjust the depth after implantation,12 etc. But almost all these microelectrodes are only applied to the same nucleus. Based on the domestic pigeon as an example, this paper introduces a multiple brain regions synchronization implanted microelectrode that can be implanted in the several brain regions at the same time. Which provide beneficial reference function for the study of the free state of small animals.
Results
Physical parameter of the microelectrode
This multiple brain regions synchronization implanted microelectrode included 4 parts, electrical stimulation unit, which was composed of microwires, printed circuit board, fixed base board central pole and FFC ribbon cable. (1) Each electrical stimulation unit was made of 4 pairs of microelectrodes, which is the stainless steel wire (OD: 100 µm) insulated by the paraxylene, the tip of microelectrodes is 10 µm. Each pair of microelectrodes corresponds to a brain site, 4 pairs of microelectrodes correspond to 4 different brain regions and the length should be designed in advance according to the position of the brain region. The distance between microelectrodes in the same brain region is less than 1mm. Each pair of electrodes can carry out bipolar stimulation. (2) Fixed base board have printed circuit, each circuit wire corresponds to an electrode and FFC ribbon, the central of fixed base board had a hole which used to insert the connecting rod. (3) The connecting rod which is used to fixed the whole electrode structure to the brain stereotaxic instrument. The connecting rod was put into the hole before surgery, when the microelectrode was implanted in the brain region, it was taken out. (4) The FFC ribbon cable used to connect the electrical stimulation device.
Structural composition of this multiple brain regions synchronization implanted microelectrodes are as Fig. 1. According to different experimental requirements, the number of the microelectrode and unit can be adjusted during the designing circuit. The distance between the microelectrode units can also be adjusted according to the position of the brain region. The microelectrode of this research weight about 3 g, when placed on the animal head, it has no effect on the normal activities.
Figure 1.
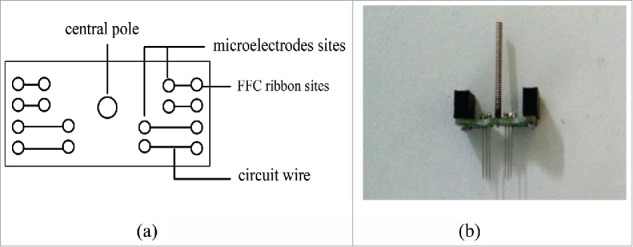
Structural composition of the multiple brain regions synchronization implanted microelectrodes. (A) Plane draft. (B) Physical photo.
Implantation way and the time comparation of original electrode and multiple brain regions synchronization implanted electrode
When the original electrodes implanted in the brain area, each pair of electrodes implanted alone and fixed, until all electrodes were implanted into the specific brain regions, connect FFC ribbon and fixed. The operation time is about 2–2.5 h. Multiple brain regions synchronization implanted microelectrodes included all electrodes and FFC ribbon, can be implanted specific brain regions at the same time, The process takes about 1.5 h. Fig. 2 display the microelectrodes was fixed on the stereotaxic instrument.
Figue2.
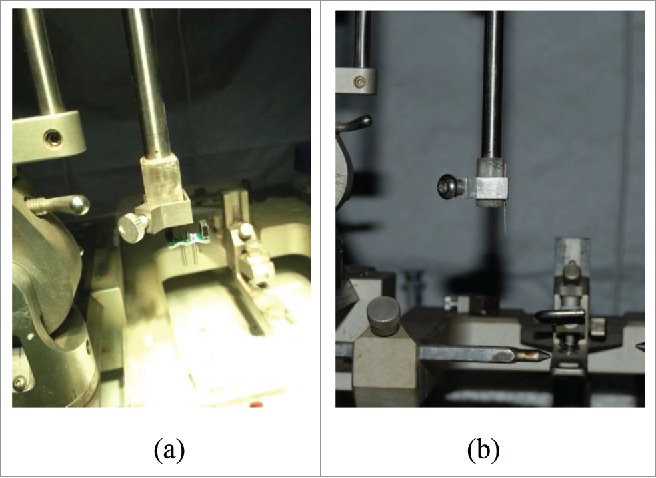
Two kinds of microelectrode are fixed on the stereotaxic instrument. (A) The multiple brain regions synchronization implanted microelectrodes is fixed by the connecting rod. (B) Each pair of original electrodes is implanted alone and fixed.
Electrical stimulation process and comparation of the animal behavioral responses
Electrical stimulation process
Electrical stimulation experiments commenced after recovery of surgery (about 7–10 d). Electrical stimulation device consisted of 2 main components13 (reference the Fig. 3): a micro-embedded computer with a transmitter, and an integrated receiver-microprocessor module mounted on the pigeon's head and connected to the implanted electrodes. Digital commands with stimulation parameters were sent remotely to the receiver-microprocessor module by clicking the buttons on the user interface. The receiver-microprocessor module executed the incoming commands and delivered biphasic TTL pulses to the specified brain region. When stimulating right/left DIVA, domestic pigeons feels very panicky and turn right/left; while the right/left archistriatum was stimulated, the domestic pigeon alarmedly and fleetly run forward.
Figure 3.
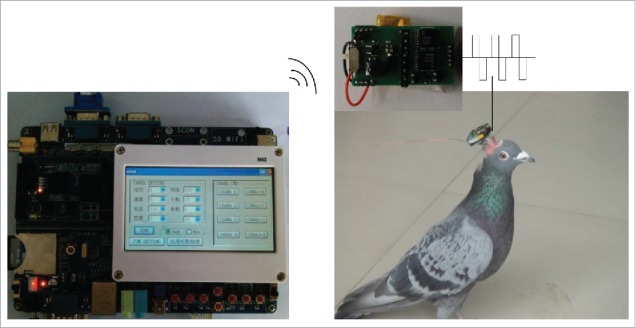
Composition and connection method of the electrical stimulation device.
Comparison of the animal behavioral responses
In this experiment, we respectively implanted original electrodes and multiple brain regions synchronization implanted microelectrodes into the brain regions of 30 domestic pigeons. In the animal that used in the original electrodes, the left and right DIVA of 12 pigeons can be controlled, the left archistriatum of 14 pigeons can be controlled and the right archistriatum of 11 pigeons can be controlled. The numbers of pigeon that all brain regions all can be controlled are 8. In the animal that used in the multiple brain regions synchronization implanted microelectrodes, the left and right DIVA of 13 pigeons can be controlled, the left archistriatum of 12 pigeons can be controlled and the right archistriatum of 14 pigeons can be controlled. The numbers of pigeon that all brain regions all can be controlled are 12. The data of the animal behavior reactions are in Table 1. According to this data, the control effect has no significant difference for single nucleus, but the number of all nuclei can be controlled effectively at the same time are evident increasing. Which shows that the multiple brain regions synchronization implanted microelectrodes designed the relative position of the electrodes in advance, reduced the single electrode deviation. It is very important for animal behavior control. With this microelectrode, we can control the domestic pigeon turn left/right or forward and walk along the specified route (Fig. 4 is drawing according to the video).
Table 1.
Compasation of the animal behavior reaction.
| The effect number |
||||||
|---|---|---|---|---|---|---|
| Totle animal number | left DIVA | right DIVA | left archistriatum | right archistriatum | all brain regions | |
| Original electrodes | 30 | 12 | 12 | 14 | 11 | 8 |
| Current electrodes | 30 | 13 | 13 | 12 | 14 | 12 |
Figure 4.
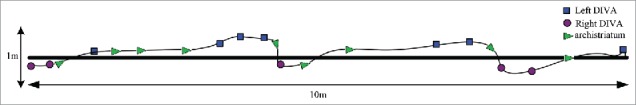
The sketch demonstrates the animal behavioral responses. ▪,•,▸, respectively express stimulating the left DIVA, right DIVA, archistriatum.
Comparison of the electrode fixed time
In the robo-animal study, microelectrode may split away off after which was implanted a period of time. Compared with the original microelectrode, the multiple brain regions synchronization implanted microelectrodes is low, that less animal active collision and extended electrode fixed time. The original microelectrode fixed time about 2 to 3 months, while the multiple brain regions synchronization implanted microelectrodes fixed time about 4 to 6 months.
Discussion
In this paper, the multiple brain regions synchronization implanted microelectrodes reduces the operation process, shorten animals operation time. Due to electrode position relatively fixed, error caused by each separately implanted electrode is reduced and the animal control effect is greatly increased. The electrode fixed time extended.
At present, the various microelectrode processing technology incremental improvements, some microelectrode that is suitable for the different animals experiment have sprung up in recent years. Such as hand-made microelectrode and its propulsion system,12 which can be fixed in animal skull surface, in the process of the experiment, it can adjust electrode depth according to the situation of neurons through the top nut, which is important for the study of animal behavior and neuron specific relationship. Utah microelectrode array14,15 have wide range of application in the brain-machine interface research, but its machining process is relatively complex. Almost all the microelectrode adapted to the same neural site research. In study of the robo-animal, a few sites often need stimulated at the same time. Therefore, the microelectrodes in this study have unmatched advantages, multiple brain regions synchronization implanted microelectrodes design simple, implant process is also simple, and can effect control the animal. It is advantageous to the long-term stimulation of brain function and other chronic electrophysiological experimental study.
There are still many difficult problems in present microelectrode technology, especially the nervous system injury after the microelectrodes implanted the brain region.16-18 This problem influenced the long-term implanted electrodes effectiveness, thus developing better biocompatibility microelectrode, for example, platinum-iridium alloy electrode, or the electrode that can instill nutrient solution at the same time, combining with the existing microelectrode technique based on MEMS technology and microfluidics technology, neurotrophic factors can release to the around implanted microelectrodes through microflow body cavity and pipe, that will promote the injured nerve nutrition and regeneration, can better ease injury problems after microelectrodes implanted. With the continuous development of microelectrode technology research, robo-animals research will rapid promote to a new levels.
Materials and methods
Experiment animal
Adult domestic pigeons of either sex weighing between 400–500 g were used. All pigeons were housed under a normal day/night light cycle with food and water ad libitum, surgery, training and behavior testing protocols were approved by the Committee of animal experimentation and were carefully carried out in accordance with the Guide for the Care and Use of Laboratory Animals (China Ministry of Health).
Animal surgery and electrode implanted process
Animal anesthesia and surgery
Using chloral hydrate 400 mg/kg intramuscular injections with general anesthesia, after the pigeon loses basic reactions, 2% lidocaine hydrochloride injection (including 1/200 000 concentration of epinephrine injection) 0.5 ml subcutaneous injection in the operation area for local anesthesia. After the domestic pigeon loses the pain reflex and corneal reflection, it should be fixed on the brain stereotaxic apparatus. Cut off the feathers above the operation area and medical iodine sterilized, and then cut off the operation area skin and connective tissue; wipe the surface disinfection, exposed location reference point.
Electrode implantation brain region
In this paper, electrode implantation sites are the left and right sides of thalamic nucleus dorsalis intermedius ventralis anterior (DIVA) (AP:5.5 mm, L or R: 2 mm, H:8.2 mm with reference to the bregma) and archistriatum (AP:7.2 mm, L or R: 5.5 mm, H:8.2 mm with reference to the bregma). After stereotactic these brain region, the holes were drilled with a skull drill in the corresponding position.
Electrode placement and fix
The electrode connecting rod was connected to the central hole of the electrical circuit board, and then the connecting rod was fixed on the stereotaxic instrument, in accordance with the stereotactic coordinate, the microelectrodes were slowly implanted to prepared position, filling the gap between the electrode and the brain tissue with EC glue, and then the microelectrodes were fixed with dental cement. The central hole of the electrical circuit was screw off and can be repeated use in the next experiment. The microelectrodes were further fixed with dental cement.
Disinfect the wound at the end of the surgery. It is of great importance to keep the animal warm during the process of its postoperative recovery.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 61305129, 61203370), Natural Science Foundation of Shandong Province (ZR2013CQ017), Qingdao Economic and Technological Development Zone Program (#2013-1-36), Scientific Research Foundation of Shandong University of Science and Technology for Recruited Talents (2015RCJJ071).
References
- [1].Talwar SK, Xu S, Hawley ES, Weiss SA, Moxon KA, Chapin JK. Rat navigation guided by remote control. Nature 2002; 417:37-8; PMID:11986657; http://dx.doi.org/ 10.1038/417037a [DOI] [PubMed] [Google Scholar]
- [2].Xu S, Talwar SK, Hawley ES, Li L, Chapin JK. A multi-channel telemetry system for brain microstimulation in freely roaming animals. J Neurosci Meth 2004; 133:57-63; PMID:14757345; http://dx.doi.org/ 10.1016/j.jneumeth.2003.09.012 [DOI] [PubMed] [Google Scholar]
- [3].Brown S. Stealth sharks to patrol the high seas. New Scientist 2006; 189:30-1. [Google Scholar]
- [4].Hirotaka S, Berry CW, Casey BE, Lavella G, Ying Y, Vanden Brooks JM, Maharbiz MM. A cyborg beetle: Insect flight control through an implantable, tetherless microsystem. MEMS 2008, Tucson, AZ, USA. IEEE 21st International Conference; 2008; 164-7; http://dx.doi.org/10.1109/MEMSYS.2008.4443618. [Google Scholar]
- [5].Wenbo W, Ce G, Jiurong S, Zhendong D Locomotion Elicited by Electrical Stimulation in the Midbrain of the Lizard Gekko gecko. Intelligent Unmanned Systems: Theory and Applications. Berlin, Heidelberg, 2009; 194:145-53; http://dx.doi.org/10.1007/978-3-642-00264-9_9 [Google Scholar]
- [6].Peng Y, Wu Y, Yang Y, Huang R, Wu C, Qi X, Liu Z, Jiang B, Liu Y Study on the control of biological behavior on carp induced by electrophysiological stimulation in the corpus cerebelli. EMEIT 2011, Haerbin, Heilongjiang, China, 2011; 502-5; http:// dx.doi.org/10.1109/EMEIT.2011.6022965 [Google Scholar]
- [7].Yong W, Ruituo H, Min WYB. Study on Intelligent Animals Based on Brain Microstimulation. Chinese J Biomed Eng 2006;25:497-501. [Google Scholar]
- [8].Ruituo H, Xuecheng S, Jun qing Y, Xiaofeng L, Zhihao Y. Robo-animal Navigation Method Guided by Remote Control. J Basic Sci Eng 2010;18:352-8. [Google Scholar]
- [9].Xuecheng S, Ruituo H, Jun qing Y, Hui W. Brain mechanism and methods for robo-animal motor behavior control. Scientia Sinica 2012;42:1130-46. [Google Scholar]
- [10].Chiang B, Fridman GY, Dai C, Rahman MA, Santina CD. Design and performance of a multichannel vestibular prosthesis that restores semicircular canal sensation in rhesus monkey. IEEE Trans Neural Syst Rehabil Eng 2011;19:588-98; PMID:21859631; http://dx.doi.org/ 10.1109/TNSRE.2011.2164937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee HJ, Son Y, Kim J, Lee CJ, Yoon ES, Cho IJ. A multichannel neural probe with embedded microfluidic channels for simultaneous in vivo neural recording and drug delivery. Lab chip 2015;15:1590-7; PMID:25651943; http://dx.doi.org/ 10.1039/C4LC01321B [DOI] [PubMed] [Google Scholar]
- [12].Liu SQ, Wu J, Yang JZ, Tian SH, Chen NH, Lei YL, Peng YP, Wang JH, Ma YY. Methods for single unit recording in behavioring morphine craving rat. Acta Physiologica Sinica 2004;56:735-42; PMID:15614424 [PubMed] [Google Scholar]
- [13].Jun qing Y, Ruituo H, Hui W, Changzhi L, Xuecheng S. A robo-pigeon based on an innovative multi-mode telestimulation system. Bio-Med Mater and Eng 2015;26(Suppl 1):S357-63. [DOI] [PubMed] [Google Scholar]
- [14].Mathews KS, Wark HAC, Normann RA. Assessment of rat sciatic nerve function following acute implantation of high density utah slanted electrode array (25 electrodes/mm 2) based on neural recordings and evoked muscle activity. Muscle nerve 2014;50:417-24; PMID:24638985; http://dx.doi.org/ 10.1002/mus.24171 [DOI] [PubMed] [Google Scholar]
- [15].Xie X, Rieth L, Williams L, Negi S, Bhandari R, Caldwell R, Sharma R, Tathireddy P, Solzbacher F. Long-term reliability of Al2O3 and Parylene C bilayer encapsulated Utah electrode array based neural interfaces for chronic implantation. J Neural Eng 2014;11:026016; PMID:24658358; http://dx.doi.org/ 10.1088/1741-2560/11/2/026016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen KH, Dammann JF, Boback JL, Tenore FV, Otto KJ, Gaunt RA, Bensmaia SJ. The effect of chronic intracortical microstimulation on the electrode-tissue interface. J Neural Eng 2014;11:026004; PMID:24503702; http://dx.doi.org/ 10.1088/1741-2560/11/2/026004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nolta NF, Christensen MB, Crane PD, Skousen JL, Tresco PA. BBB leakage, astrogliosis, and tissue loss correlate with silicon microelectrode array recording performance. Biomaterials 2015;53:753-62; PMID:25890770; http://dx.doi.org/ 10.1016/j.biomaterials.2015.02.081 [DOI] [PubMed] [Google Scholar]
- [18].Parker RA, Davis TS, House PA, Normann RA, Greger B. The functional consequences of chronic, physiologically effective intracortical microstimulation. Prog brain res 2011;194:145-65; PMID:21867801; http://dx.doi.org/ 10.1016/B978-0-444-53815-4.00010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


