Abstract
Rationale: Syndecan-1 is a cell surface heparan sulfate proteoglycan primarily expressed in the lung epithelium. Because the influenza virus is tropic to the airway epithelium, we investigated the role of syndecan-1 in influenza infection.
Objectives: To determine the mechanism by which syndecan-1 regulates the lung mucosal response to influenza infection.
Methods: Wild-type (WT) and Sdc1−/− mice were infected with a H1N1 virus (PR8) as an experimental model of influenza infection. Human and murine airway epithelial cell cultures were also infected with PR8 to study the mechanism by which syndecan-1 regulates the inflammatory response.
Measurement and Main Results: We found worsened outcomes and lung injury in Sdc1−/− mice compared with WT mice after influenza infection. Our data demonstrated that syndecan-1 suppresses bronchial epithelial apoptosis during influenza infection to limit widespread lung inflammation. Furthermore, we determined that syndecan-1 attenuated apoptosis by crosstalking with c-Met to potentiate its cytoprotective signals in airway epithelial cells during influenza infection.
Conclusions: Our work shows that cell-associated syndecan-1 has an important role in regulating lung injury. Our findings demonstrate a novel mechanism in which cell membrane–associated syndecan-1 regulates the innate immune response to influenza infection by facilitating cytoprotective signals through c-Met signaling to limit bronchial epithelial apoptosis, thereby attenuating lung injury and inflammation.
Keywords: influenza, lung injury, syndecan-1, proteoglycan, c-Met
At a Glance Commentary
Scientific Knowledge on the Subject
The influenza virus initially infects the airway epithelium, which induces the robust innate immune response necessary to contain the pathogen. However, exuberant inflammation can be harmful and result in excessive lung injury that significantly contributes to mortality. The airway epithelium has an important role in regulating the inflammatory response after influenza infection.
What This Study Adds to the Field
This study further defines mechanisms by which the airway epithelium controls lung injury after influenza infection. We show that syndecan-1 regulates the lung epithelial innate immune response to influenza by limiting cellular apoptosis through crosstalk with the c-Met receptor. Our data demonstrate a novel mechanism of action by which the transmembrane syndecan-1, and not the shed ectodomain, regulates the lung inflammatory response. Furthermore, we provide evidence that limiting programmed cell death of the lung epithelium has dramatic effects on attenuating lung inflammation after influenza infection. Thus, our work defines a unique mechanism by which membrane-bound syndecan-1 regulates cytoprotective signals within the lung epithelium and can potentially be therapeutically targeted to limit lung injury.
Influenza is a devastating respiratory disease that causes up to 500,000 deaths annually (1). The virus is tropic for the airway epithelium, where it replicates, causing epithelial death and tracheobronchitis (2). Lethality from influenza typically occurs with the development of acute respiratory distress syndrome (ARDS) secondary to viral bronchopneumonia, and may commonly occur with a concomitant bacterial superinfection (1, 3). The high mutability of the viral genome (i.e., antigenic drift) prevents long-term immunity and necessitates yearly vaccinations (4). More concerning is the appearance of novel influenza strains from viral genetic rearrangement (i.e., antigenic shift), which leads to pandemic strains (5).
Syndecan-1 is a proteoglycan that is primarily expressed by epithelia (6). All syndecans (1 through 4) are type I transmembrane proteins that contain similar structural domains. The syndecan-1 ectodomain contains attachment sites for glycosaminoglycan side chains, which can bind other proteins (e.g., chemokines or growth factors) primarily through its heparan sulfate chains. The transmembrane domain appears to regulate dimerization, whereas the cytoplasmic domain interacts with scaffolding and signaling molecules.
The biological functions of syndecan-1 in the lungs are elicited during various injuries. Our previous work demonstrated that cell-surface syndecan-1 regulates re-epithelialization in the lungs (7–9). In this situation, shedding of syndecan-1 is a mechanism of turning off the cell surface receptor, and excessive shedding may have adverse effects because of loss of receptor function (10, 11). Syndecan-1 has also been found to regulate lung inflammation, but the mechanisms have all involved the function of the shed ectodomain (12–16). An unexplored area of how syndecan-1 regulates lung inflammation is the ability of the transmembrane protein to modulate cellular function while it is intact on the cell surface. Syndecan-1 can associate with a variety of co-receptors on the cell membrane to modulate cellular signaling and function (17).
Because the lung epithelium is the primary location of influenza infection and is where syndecan-1 is expressed to regulate lung immunity, we postulated that syndecan-1 affects the host response to viral infection through regulation of co-receptors while associated on the cell membrane. We found that syndecan-1 has a cytoprotective role that limits viral-induced apoptosis, which in turn attenuates lung injury after influenza infection. Moreover, syndecan-1 confers an anti-apoptotic signal by acting on the cell surface to potentiate signaling through c-Met, the hepatocyte growth factor (HGF) receptor.
Some of the results of these studies have been previously reported in the form of abstracts (18, 19).
Methods
Influenza Model
A/PR/8/34 (PR8) stocks, a mouse-adapted H1N1 influenza, were expanded by incubating them in embryonated chicken eggs as previously described (20). Wild-type (WT) and Sdc1−/− mice (C57BL/6) were intranasally instilled with an H1N1 virus (A/PR/8/34; PR8; 250–500 plaque-forming units in 50 μl of sterile phosphate-buffered saline).
Cell Culture
The creation of BEAS-2b cells stably expressing human syndecan-1 shRNA (Sdc1 KD) and Sdc1 KD cells that express WT and mutant syndecan-1 were previously validated (7, 9). Primary cultures of airway epithelial cells grown at an air–liquid interface (ALI) were created from WT and Sdc1−/− mice as previously described (7).
Statistics
Statistical significance was determined using the Student’s t test or the two-way analysis of variance. P < 0.05 was considered statistically significant. Survival analysis was calculated by using the log-rank test. All data points are means ± SEM unless stated otherwise.
Results
Syndecan-1 Regulates the Host Response by Attenuating Disease after Influenza Infection
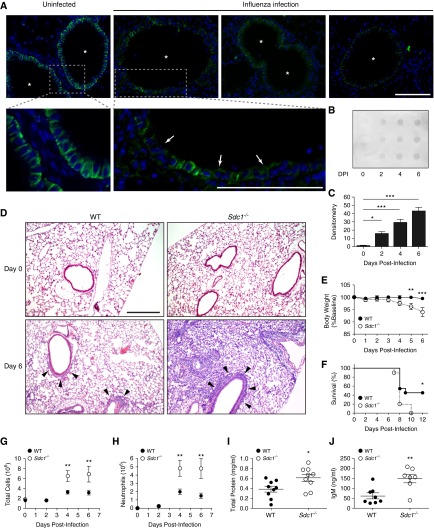
Syndecan-1 is primarily expressed by the lung epithelium with airway epithelial cells having the highest expression (Figure 1A). After PR8 infection, syndecan-1 was shed from the lung epithelium (Figures 1A–1C). WT mice had inflammatory cell infiltration largely confined to the peri-bronchiolar region as expected with influenza (Figure 1D). However, Sdc1−/− mice had extensive alveolitis in addition to the peri-bronchiolar inflammation. Furthermore, body weight loss, a good indicator of disease severity, was greater in Sdc1−/− mice than WT mice (Figure 1E). More importantly, syndecan-1 conferred protection from lethality because WT mice had better survival after influenza compared with Sdc1−/− mice (Day 12 postinfection: 46% vs. 0%, respectively; P < 0.05; Figure 1F).
Figure 1.
Syndecan-1 limits lung inflammation and injury during influenza infection. Wild-type (WT) and Sdc1−/− mice were infected intranasally with 250 plaque-forming units of PR8 virus. (A) Syndecan-1 expression (green) lines the basolateral surface of the airway epithelium at baseline (*airway lumen). After influenza infection (Day 6), syndecan-1 expression is diminished throughout the lung epithelium (arrows). Scale bar, 100 μm. (B) Syndecan-1 dot blot of bronchoalveolar lavage (BAL) from influenza-infected mice shows shedding into the airspaces after infection. (C) Densitometry of dot blot showing a significant increase in syndecan-1 shedding after influenza infection. Day 1 (0.901 ± 0.76), Day 2 (15.56 ± 2.61), Day 3 (29.28 ± 3.99), Day 4 (42.76 ± 4.80); n = 3 per group. (D) Representative hematoxylin and eosin images of control (Day 0) and virus-infected lungs from WT and Sdc1−/− mice. Peribronchiolar infiltrates are evident in both genotypes (arrowhead). However, Sdc1−/− mice have more widespread lung injury during influenza infection. Scale bar, 200 μm. (E) Body weight loss (WT: n = 24 for Days 0–4; n = 10 for Days 5–6; WT: n = 22 for Days 0–4; n = 9 for Days 5–6) and (F) survival in WT and Sdc1−/− mice after influenza infection (n = 11 and 10, respectively). (G–J) Analysis of BAL fluid from mice during influenza infection for (G) total cell count (n = 3–15), (H) neutrophils (n = 3 – 14), (I) total protein (n = 9), and (J) IgM levels (n = 7–8). Data shown as mean ± SEM. DPI = days postinfection. *P < 0.05; **P < 0.01; ***P < 0.001.
Influenza-infected Sdc1−/− mice also had more inflammatory cells (Figure 1G), neutrophils (Figure 1H), total protein (Figure 1I), and IgM (Figure 1J) in the bronchoalveolar lavage (BAL) than WT mice. Furthermore, there were significantly higher expression of the pro-inflammatory chemokines and viral response genes in Sdc1−/− compared with WT animals (see Figures E1A–1E in the online supplement). However, influenza viral load (see Figures E1F–E1H) and secretion of IL-1β and IL-18 (see Figures E1I and E1J) were similar between genotypes. Together, these findings indicate that syndecan-1 dampens the inflammatory response to influenza infection, thereby limiting widespread lung injury and protecting the host from death.
Lung Epithelial Syndecan-1 Regulates the Host Response to Influenza Infection
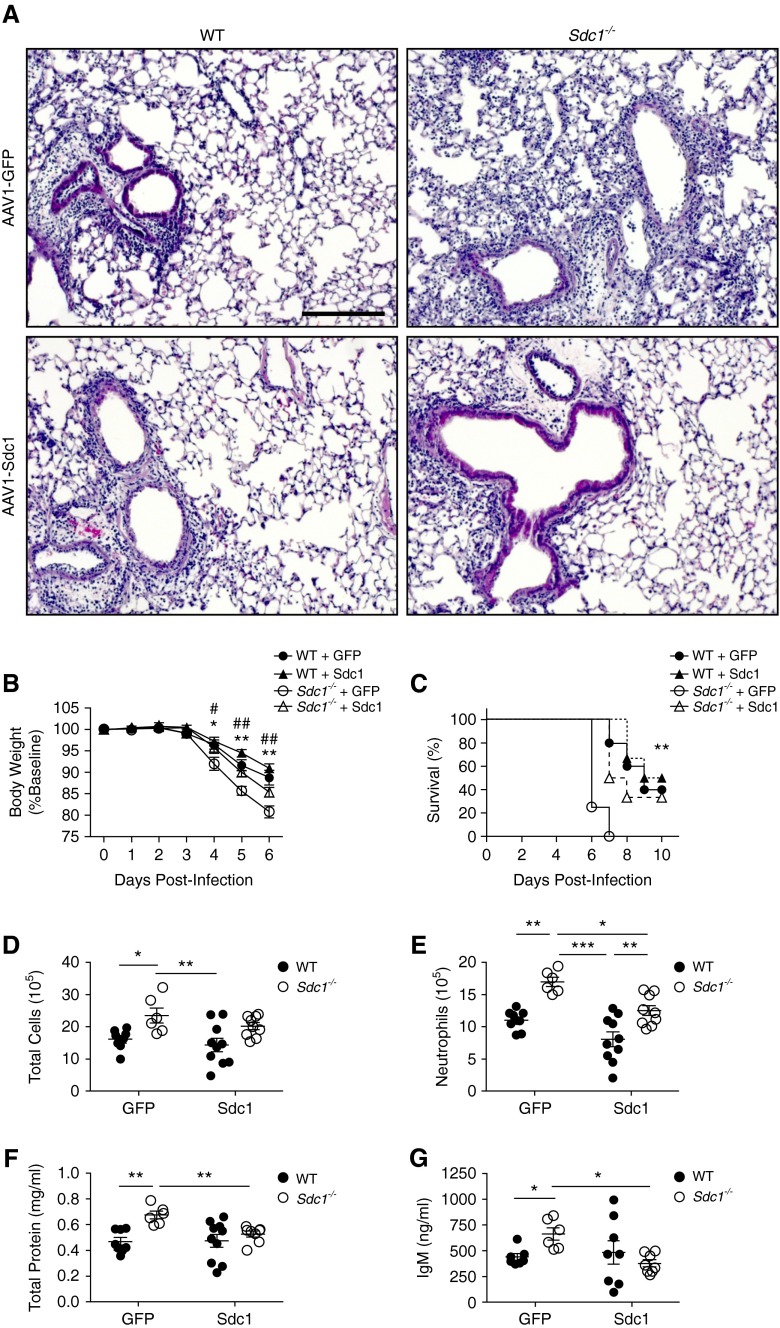
Syndecan-1 expression is primarily confined to the lung epithelium (Figure 1A). To confirm that influenza-mediated inflammation was controlled by lung epithelial expression of syndecan-1, we used an adeno-associated virus (AAV) vector to transduce syndecan-1 within the lung epithelium (see Figure E2 in the online supplement) (21). Restoration of lung epithelial expression of syndecan-1 in Sdc1−/− mice improved lung injury, weight loss, and survival compared with control conditions that expressed green fluorescent protein in Sdc1−/− mice (Figures 2A–2C). Lung permeability and inflammation were also reduced in Sdc1−/− mice with epithelial expression of syndecan-1 compared with controls (Figures 2D–2G). These data establish that syndecan-1 expression in the lung epithelial compartment regulates lung inflammation during influenza infection.
Figure 2.
Lung epithelial expression of syndecan-1 reduces lung inflammation and injury during influenza infection. Adeno-associated virus serotype 1 (AAV1) vectors were intranasally instilled into wild-type (WT) and Sdc1−/− mice to transduce either green fluorescent protein (GFP) or syndecan-1 in the lung epithelium, and mice were subsequently infected intranasally with 500 plaque-forming units of PR8 virus. (A) Representative hematoxylin and eosin images of virus-infected lungs from WT and Sdc1−/− mice transduced with either GFP or syndecan-1 show diminished alveolitis in Sdc1−/− mice transduced with AAV-Sdc1 compared with AAV-GFP. Scale bar, 200 μm. (B) Body weight loss (n = 6–10; *P < 0.01; **P < 0.001 WT + GFP vs. Sdc1−/− + GFP; #P < 0.05; ##P < 0.01 Sdc1−/− + GFP vs. Sdc1−/− + Sdc1) and (C) survival (n = 4–6; *P < 0.001) after influenza infection. (D–G) Analysis of bronchoalveolar lavage fluid from mice (n = 6–10) during influenza infection for (D) total cell count, (E) neutrophils, (F) total protein, and (G) IgM levels. *P < 0.05; **P < 0.01; ***P < 0.001. All data are shown as mean ± SEM.
Syndecan-1 Regulates Bronchial Epithelial Apoptosis during Influenza Infection
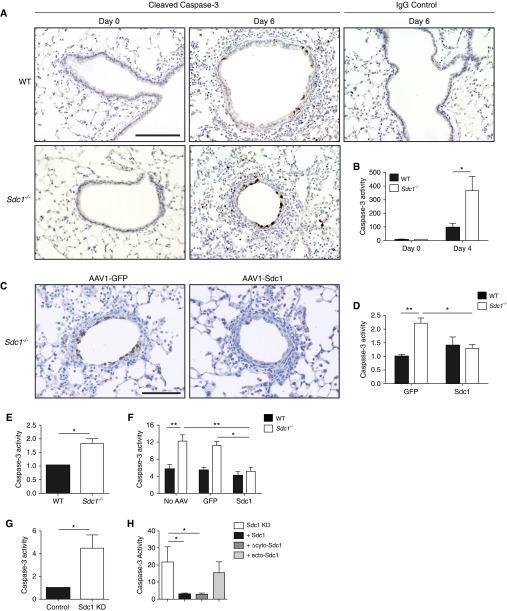
Influenza infection mediates much of its early damage by inducing apoptosis within the lung epithelium (22–26). We found that airway epithelial cells were apoptotic after influenza infection (Figure 3A) and more abundant in Sdc1−/− mice than WT mice (see Figure E3 in the online supplement). Caspase-3 activity was also higher in Sdc1−/− (365.7 ± 106.4) compared with WT conditions (96.04 ± 30.42) after infection (Figure 3B). When syndecan-1 expression was replenished within the lung epithelium of Sdc1−/− mice, caspase-3 activity was reduced in comparison to control Sdc1−/− mice (Figures 3C and 3D).
Figure 3.
Syndecan-1 expression by the lung epithelium attenuates apoptosis during influenza infection. (A) Cleaved caspase-3 immunohistochemistry (brown; scale bar, 200 μm) and (B) lung homogenate caspase-3 activity (normalized to Day 0; n = 5, *P < 0.05) in influenza-infected lungs from wild-type (WT) and Sdc1−/− mice (250 plaque-forming units). (C) Cleaved caspase-3 immunohistochemistry (brown; scale bar, 200 μm) and (D) lung homogenate caspase-3 activity (normalized to the WT GFP condition) in influenza-infected lungs (500 plaque-forming units) from WT and Sdc1−/− mice transduced with either AAV1-GFP (1.0 ± 0.07 vs. 2.21 ± 0.19, respectively) or AAV1-Sdc1 (1.39 ± 0.31 vs. 1.29 ± 0.14, respectively; n = 6–10, *P < 0.05; **P < 0.01). (E–H) Caspase-3 activity was measured in lysates of (E) WT and Sdc1−/− air–liquid interface (ALI) cultures infected with PR8 (n = 4, *P < 0.05); (F) WT and Sdc1−/− ALI cultures transduced with either AAV carrying GFP (control) or murine syndecan-1 followed by PR8 infection. No AAV: 5.66 ± 1.0 versus 12.23 ± 1.46; GFP: 5.39 ± 0.72 versus 11.2 ± 0.93; Sdc1: 4.16 ± 0.89 versus 5.21 ± 0.91 (n = 3, *P < 0.005; **P < 0.001); (G) Infected BEAS-2b cells that had syndecan-1 knocked down by small hairpin RNA expression (Sdc1 KD) versus its control (expressing a scrambled shRNA; n = 8, *P < 0.05). (H) Infected Sdc1 KD cells stably express various mutants of mouse syndecan-1. (n = 3, *P < 0.05). Sdc1 KD (21.78 ± 8.77); +Sdc1 (2.86 ± 0.61, full-length syndecan-1); +Δctyo-Sdc1 (2.83 ± 0.63, truncated syndecan-1 lacking the cytoplasmic domain but still tethered to the cell membrane); +ecto-Sdc1 (15.50 ± 6.35, syndecan-1 ectodomain that is secreted from cell simulating shed syndecan-1). All data are shown as mean ± SEM. AAV = adeno-associated virus; GFP = green fluorescent protein; KD = knockdown.
The airway epithelium is the target of influenza, where apoptosis is localized (Figures 3A–3D) (27, 28). We specifically evaluated airway epithelial apoptosis using murine ALI cultures, and we found Sdc1−/− ALI cultures had 1.82-fold more apoptotic activity compared with WT airway epithelium after influenza infection (Figure 3E). The augmented apoptosis in Sdc1−/− conditions could be attenuated by replenishing syndecan-1 expression in Sdc1−/− ALI cultures (Figure 3F). In addition, using the BEAS-2b human bronchial epithelial cell line, cells with suppressed syndecan-1 expression had 4.5-fold augmented apoptosis after influenza infection compared with control cells replete with syndecan-1 (Figure 3G). Moreover, syndecan-1 must be cell-associated with a “shed” ectodomain that has no effect in attenuating apoptosis after influenza infection (Figure 3H). These results demonstrate syndecan-1 restrains apoptosis in both human and murine airway epithelium after influenza infection. Furthermore, our data indicate that syndecan-1 must be membrane bound to modulate cellular apoptosis during influenza infection.
Syndecan-1 Controls Airway Epithelial Apoptosis to Moderate Lung Inflammation after Influenza Infection
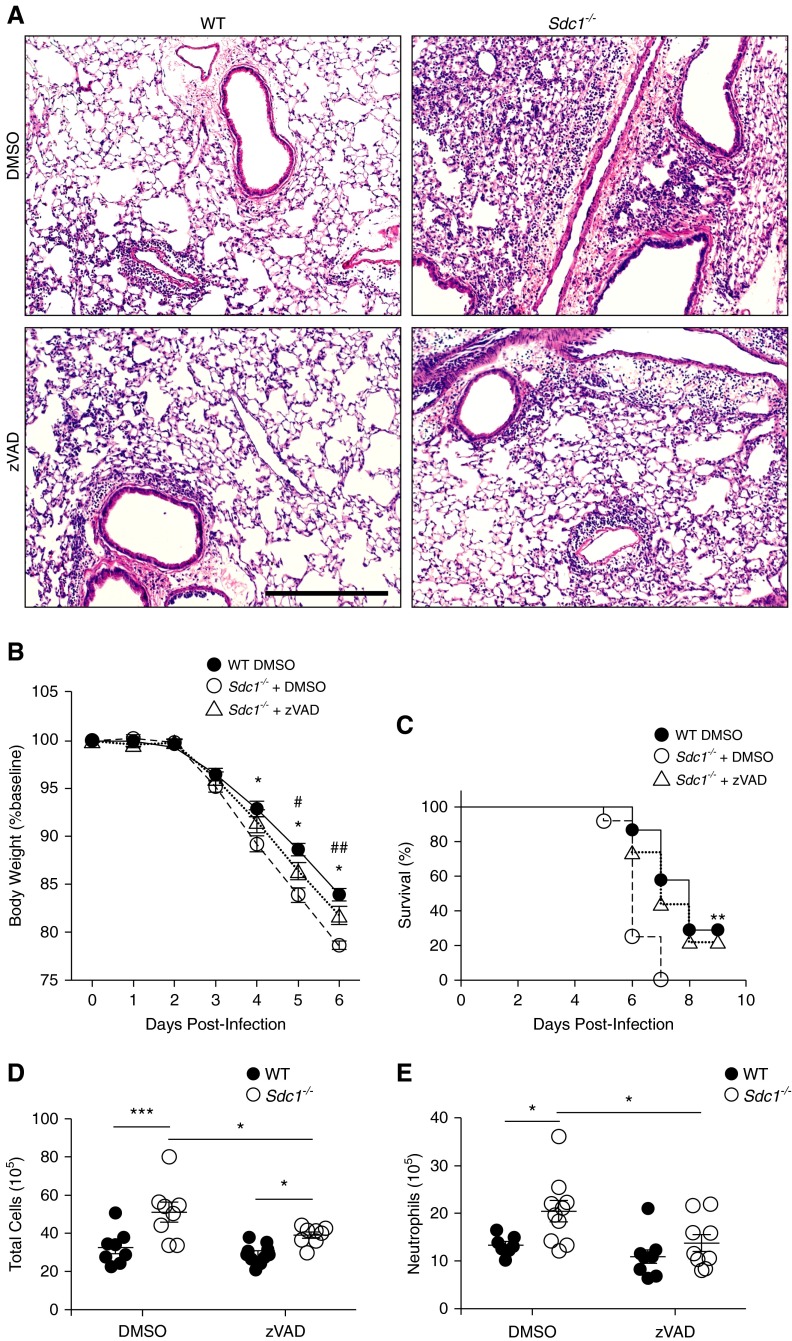
To determine if augmented apoptotic activity in the lungs of infected Sdc1−/− mice caused more lung injury, we treated mice with zVAD, a pan-caspase inhibitor. This treatment attenuated the exuberant apoptosis and significantly improved lung injury, weight loss, and survival in infected Sdc1−/− mice, bringing the levels closer to those seen in infected WT mice (see Figure E4 in the online supplement; Figures 4A– 4C). Moreover, zVAD reduced lung inflammation in Sdc1−/− mice with relatively little impact on WT mice (Figures 4D and 4E; see Figure E5 in the online supplement). Altogether, our data demonstrate reduction of the excessive lung epithelial apoptosis in Sdc1−/− mice after influenza infection attenuates lung injury and inflammation.
Figure 4.
Suppressing apoptosis reduces lung injury and increases survival in Sdc1−/− mice during influenza infection. Wild-type (WT) and Sdc1−/− mice treated with zVAD during influenza infection (500 plaque-forming units). (A) Representative hematoxylin and eosin images of influenza infected lung tissue 6 days postinfection. Scale bar, 200 μm. (B) Weight loss in WT and Sdc1−/− mice after influenza infection (n = 8–10). *P < 0.005 for WT + dimethyl sulfoxide (DMSO) versus Sdc1−/− + DMSO; #P < 0.05; ##P < 0.01 for Sdc1−/− + DMSO versus Sdc1−/− + zVAD. (C) Survival in WT and Sdc1−/− mice after influenza infection (n = 15). **P < 0.01. WT + zVAD conditions had no significant difference compared with WT + DMSO conditions and was removed in B and C for improved visualization of the graphs. (D) Total cell count and (E) neutrophils in the bronchoalveolar lavage 6 days postinfection (n = 8–10). *P < 0.05; ***P < 0.001. All data are shown as mean ± SEM.
Syndecan-1 Potentiates c-Met Signaling in the Bronchial Epithelium during Influenza Infection
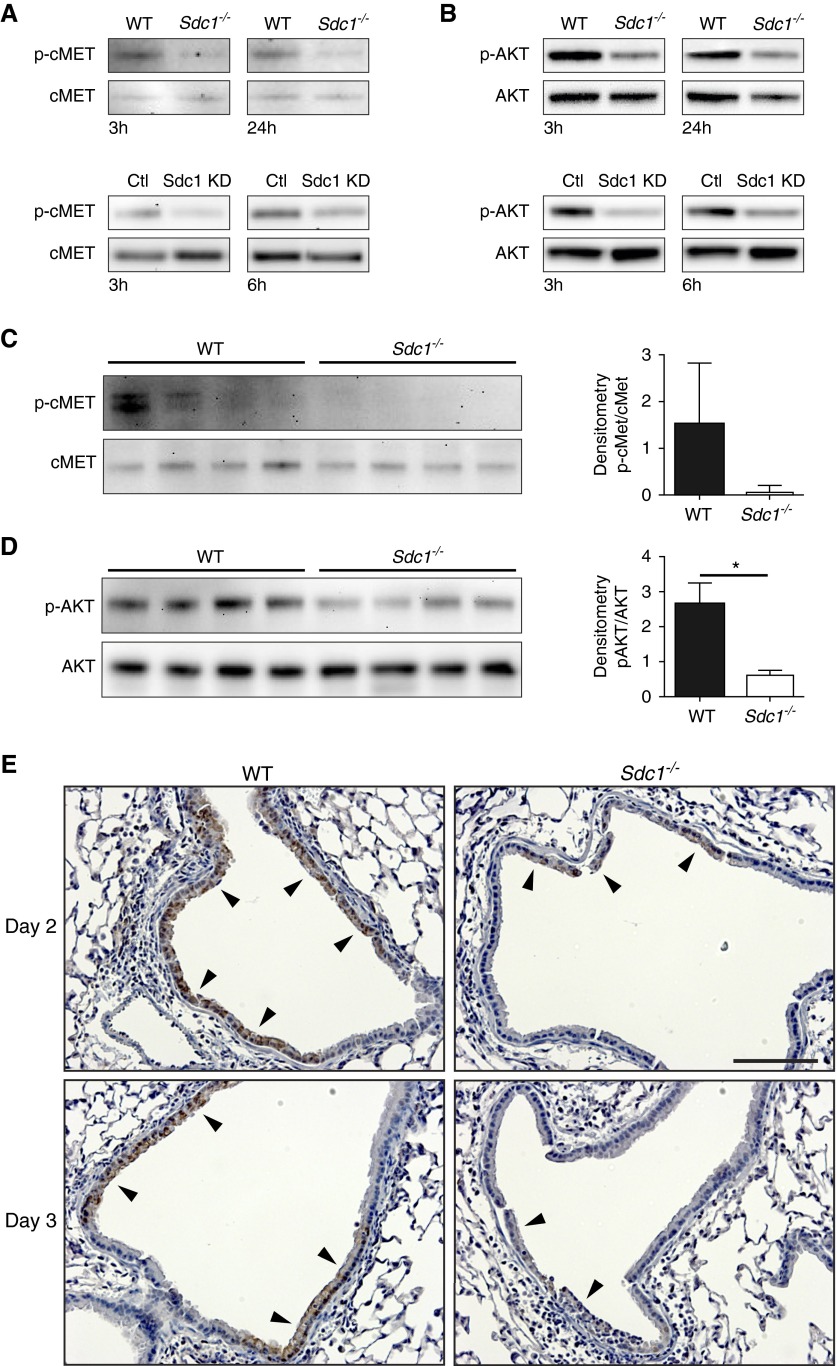
c-Met has been found to attenuate lung injury and apoptosis in bronchial epithelial cells (29, 30), so we evaluated if syndecan-1 facilitated c-Met signaling during influenza infection. Influenza-infected cells had more c-Met activation in murine and human lung bronchial epithelial cells that expressed syndecan-1 (Figure 5A). c-Met mediates its pro-survival and anti-apoptotic effects via AKT (31), and AKT activation was also dampened in influenza-infected murine and human bronchial epithelial cultures lacking syndecan-1 expression (Figure 5B). Immunoblotting lung homogenates from influenza-infected mice also revealed differential activation of c-Met (Figure 5C) and AKT (Figure 5D) with more activation in the lungs of WT mice compared with Sdc1−/− mice after influenza infection. Furthermore, immunohistochemistry for activated AKT revealed more intense staining within the airway epithelium of WT mice compared with Sdc1−/− mice (Figure 5E). These data demonstrate that syndecan-1 facilitates activation of c-Met and its downstream effector, AKT, in the lung epithelium after infection.
Figure 5.
Syndecan-1 facilitates activation of c-Met and its downstream effector, AKT, after influenza infection. Western blot analysis for total and activated (A) c-Met and (B) AKT in PR8 infected wild-type (WT) and Sdc1−/− air–liquid interface cultures and BEAS-2b cells that had syndecan-1 knocked down (Sdc1 KD) and its control. Representative results of three independent experiments are shown. Immunoblot for total and activated (C) c-Met and (D) AKT in whole lung homogenates of influenza-infected WT and Sdc1−/− mice (500 plaque-forming units; Day 3 post-infection; n = 4). Densitometry results: c-Met (1.52 ± 1.31 vs. 0.06 ± 0.15); AKT (2.64 ± 0.62 vs. 0.60 ± 0.15). *P < 0.05. (E) Immunohistochemistry for pS473AKT (brown; arrowheads) in influenza-infected mice reveal less intense expression in the airway epithelium of Sdc1−/− mice. Scale bar, 100 μm. Ctl = control.
To determine if manipulation of this signaling pathway could affect outcomes after influenza infection, we delivered an activated (myristolated) AKT via AAV vectors to the lung epithelium (32). We found that the augmented apoptosis in Sdc1−/− cultures during influenza infection could be suppressed to comparable levels with WT conditions by the expression of activated AKT (see Figure E6A in the online supplement). Moreover, activated AKT expressed in the lung epithelium in vivo improved outcomes after influenza infection (see Figures E6B and E6C). These data provide direct evidence that suppression of apoptosis specifically within the epithelial compartment attenuates the severity of disease in Sdc1−/− mice during influenza infection and highlight the importance of syndecan-1 in regulating lung bronchial epithelial apoptosis via the c-Met/AKT axis during the onset of influenza infection.
HGF-stimulated Signaling Is Dampened In Syndecan-1–Deficient Bronchial Epithelial Cells
HGF (scatter factor) is the only known ligand for c-Met (29). Therefore, HGF stimulation of cells specifically activates c-Met, which allowed us to interrogate syndecan-1 regulation of this pathway. Murine and human bronchial epithelial cells treated with HGF revealed greater activation of c-Met in syndecan-1–expressing cells compared with cells deficient in syndecan-1 expression (Figure 6A; see Figure E7 in the online supplement). Furthermore, differential AKT activation was also demonstrated (Figure 6B; see Figure E7) and mirrored the c-Met results. Together, these data further demonstrate that c-Met signaling is enhanced by syndecan-1 when stimulated by its cognate ligand, HGF.
Figure 6.
Syndecan-1 promotes c-Met signaling and downstream functions when stimulated with hepatocyte growth factor (HGF). Western blot analysis for total and activated (A) c-Met and (B) AKT in HGF stimulated wild-type (WT) and Sdc1−/− air–liquid interface cultures and BEAS-2b cells that had syndecan-1 knocked down (Sdc1 KD) and its control. Representative results of three independent experiments are shown. (C) Proliferation of control and Sdc1 KD cells were measured after HGF stimulation using CyQuant (Life Technologies, Carlsbad, CA). Various cell lines are respectively labeled. The proliferation index is the number of cells with HGF stimulation compared with cells without HGF stimulation per cell line; n = 3. *P < 0.05; **P < 0.005; ***P < 0.001. (D) Sdc1 KD cells that stably express various mutants of mouse syndecan-1 were stimulated with HGF, and the proliferation index was determined. n = 3, *P < 0.05. (E) Caspase-3 activity was measured in control and Sdc1 KD BEAS-2b cells infected with PR8 virus with or without treatment with HGF or the c-Met inhibitor, SGX-523 (n = 3). Control (PR8 only: 1.55 ± 0.071, HGF: 1.16 ± 0.03, c-Met inhibitor: 1.82 ± 0.05). Sdc1 KD (PR8 only: 2.69 ± 0.16, HGF: 2.53 ± 0.21, c-Met inhibitor: 2.86 ± 0.12). All data are shown as mean ± SEM. Ctl = control; ecto-Sdc1 = syndecan-1 ectodomain that is secreted from cell simulating shed syndecan-1; MTEC = primary mouse tracheal epithelial cells; Sdc1 = full-length syndecan-1; Δctyo-Sdc1 = truncated syndecan-1 lacking the cytoplasmic domain but still tethered to the cell membrane.
We predicted other cellular functions of c-Met activation would be diminished in conditions that lack syndecan-1 expression. The mitogenic effects of c-Met signaling were blunted in the absence of syndecan-1 (Figures 6C and 6D). Furthermore, the syndecan-1 ectodomain must be tethered to the cell membrane to augment HGF-mediated cell proliferation (Figure 6D). In contrast, a shed ectodomain had no effect on c-Met function. These findings are congruous with our previous data that demonstrated that syndecan-1 regulates c-Met function only when associated with the cell membrane.
Our findings suggest beneficial effects from c-Met-AKT activation during influenza infection. When BEAS-2b cells (that express syndecan-1) were infected with PR8, apoptosis was attenuated with HGF and enhanced with c-Met inhibition (Figure 6E). In contrast, apoptosis was minimally affected by HGF or c-Met inhibitor in BEAS-2b cells that lacked syndecan-1 expression. A similar trend was found when WT and Sdc1−/− ALI cultures were treated with HGF and a c-MET inhibitor (see Figure E8 in the online supplement). These data indicate that c-Met requires the presence of syndecan-1 to be fully functional in mediating its pro-survival effects.
Cytoprotective Effects from c-Met Signaling Is Blunted in Sdc1−/− Conditions In Vivo during Influenza Infection
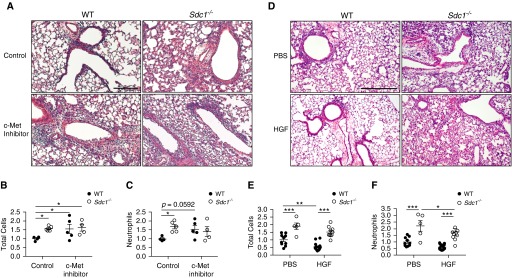
Next, we modulated c-Met activity in vivo to test the ability of syndecan-1 to regulate its protective effects during influenza infection. c-Met inhibition in mice during influenza infection augmented lung injury and inflammation in WT conditions, but this had minimal effect on Sdc1−/− conditions (Figures 7A–7C). Conversely, when HGF was given to influenza-infected mice, WT mice treated with HGF had improvement in lung inflammation, whereas Sdc1−/− mice had no effect on the total BAL cell count and only a modest decline in neutrophils (Figures 7D–7F). Moreover, HGF improved survival and attenuated weight loss in WT mice after influenza infection (see Figure E9 in the online supplement). Furthermore, we found c-Met inhibition augmented and HGF treatment attenuated lung apoptosis during influenza infection in vivo (see Figure E10 in the online supplement). Because c-Met is primarily expressed by the epithelium, these results provide additional proof that modulating lung epithelial apoptosis can regulate the inflammatory response during influenza infection. These data demonstrate that syndecan-1 modulates c-Met signaling to provide cytoprotective signals to the lung epithelium and limits inflammation after influenza infection.
Figure 7.
c-Met stimulation is protective during influenza infection in vivo and requires syndecan-1 for optimal signaling. (A–C) Wild-type (WT) and Sdc1−/− mice were infected with PR8 and concurrently treated with c-Met inhibitor (SGX-523) or control. (A) Representative hematoxylin and eosin–stained lung sections from Day 6 postinfection demonstrate worsened lung injury in WT conditions treated with c-Met inhibitor compared with phosphate-buffered saline (PBS). c-Met inhibition had minimal effect in Sdc1−/− mice. Scale bar, 200 μm. (B) Total cellular and (C) neutrophil infiltrates into the airspaces 6 days postinfection (n = 4–5; normalized to WT control conditions). *P < 0.05. All data are shown as mean ± SEM. (D–F) WT and Sdc1−/− mice were infected with PR8 and concurrently treated with hepatocyte growth factor (HGF) or PBS control. (D) Representative hematoxylin and eosin–stained lung sections from Day 6 postinfection demonstrate improvement in lung injury in WT conditions treated with HGF compared with PBS. HGF had minimal effect on Sdc1−/− mice. Scale bar, 200 μm. (E) Total cellular and (F) neutrophil infiltrates into the airspaces 6 days postinfection (n = 5–16; normalized to WT PBS conditions). All data are shown as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Although the mechanisms that lead to lung injury and inflammation after influenza infection are not fully understood, much of the mortality that occurs during the yearly flu epidemic, especially among otherwise healthy people, is because of an excessive host response. Our study indicates that syndecan-1 is an important host survival factor that moderates inflammation during influenza infection. Previous studies have found that lung inflammation is regulated by the shed ectodomain (12–16). Our results show that syndecan-1, as a transmembrane receptor on the cell surface, also has an important role in regulating lung injury and inflammation. Moreover, our work unravels a novel mechanism whereby syndecan-1 crosstalks with c-Met to potentiate its cytoprotective effects and moderates inflammation during influenza infection by controlling epithelial apoptosis.
Inflammation is clearly necessary to fight influenza infection, but an exaggerated response characterized by exuberant and dysregulated cytokines results in widespread lung injury that can cause the host to succumb. Our data indicate syndecan-1 functions as an important cytoprotective factor in the lung epithelium during influenza infection to limit inflammation and minimize lung injury. Because our studies evaluated early time points, these findings implicate syndecan-1 in governing innate immunity during influenza infection, which is consistent with the fact that clinical outcomes are determined by mucosal innate immune responses (33). Previous studies have found that alveolar cell apoptosis is an important process for the adaptive immune response to this viral pathogen (26, 34). Our data suggest that syndecan-1 also controls the alveolar apoptotic response during influenza infection, but that the role of syndecan-1 in regulating the adaptive immune response has yet to be delineated.
The lung epithelium is not solely responsible for regulating the inflammatory response, but because of its location, it functions as a first responder to quickly sense pathogenic insults and coordinate inflammatory cell influx into the airspaces (14). An interesting aspect of these data is that controlling the initial response in the airway epithelial compartment can limit the overall lung injury. Influenza is primarily tropic to the airway epithelium, and an ensuing alveolitis can occur even in the absence of viral dissemination (28, 35). ARDS can happen with extra-pulmonary diseases (36), so the fact that airway pathology causes alveolitis is not overly surprising. Airway specific inflammation has been experimentally shown to cause broader lung injury (37, 38). The proximity of the terminal bronchioles to the alveolar spaces could allow for paracrine signals between these compartments that mediate the lung injury response after influenza. Recent evidence has also implicated the endothelium in propagating the cytokine storm during influenza (39), and the bronchial epithelium could potentially crosstalk with the endothelial compartment to mediate the recruitment of inflammatory cells.
IL-1β and IL-18 levels were identical between WT and Sdc1−/− mice, indicating that syndecan-1 regulates lung injury independent of inflammasome activation. Congruous to this finding, our data demonstrated that apoptosis of the bronchial epithelial cells regulates lung inflammation, whereas the inflammasome response is moderated by hematopoietic cells during influenza infection (40). Epithelial apoptosis is predominantly a proviral event that augments lung injury (22–26), and programmed cell death has a prominent role in regulating lung inflammation and injury (41–43). Influenza mediates many of its cytotoxic effects through induction of apoptosis, and our data and studies by others demonstrate that blocking apoptosis during influenza infection significantly reduces lung injury and mortality (26, 44, 45). Simplistically, apoptotic death of the epithelium breaks down the nature barriers that prevent lung edema. However, apoptotic cells can also act as a stimulus to promote lung injury (41–43). Furthermore, impaired clearance of apoptotic cells can lead to autoimmune disease, augment septic shock and lung injury, and result in secondary necrosis, which is an additional pro-inflammatory signal (46–51). Factors released from the apoptotic cell can also modulate phagocytic cell migration and tissue repair (52–58). Thus, intrinsic mechanisms that function to mitigate bronchial epithelial apoptosis in response to infection would predictably reduce tissue injury and inflammation, thereby promoting recovery and survival.
Apoptosis is also necessary for viral proliferation (24, 25, 59). However, the augmented apoptosis in Sdc1−/− animals had no effect on viral titer compared with WT conditions. One possible explanation is that the caspase activity in WT conditions exceeds a maximal threshold necessary for viral proliferation; therefore, augmented apoptosis in Sdc1−/− conditions had no further effect. In addition, because of the complexities of the entire animal, other factors not modeled in culture may affect viral proliferation in vivo. Our results are not unique because others have also shown that augmented apoptosis has no effect on viral titers in vivo in the acute inflammatory phase of infection (26).
Unlike previous studies that found a significant role for the shed syndecan-1 ectodomain in mediating lung inflammation (12–16), our data delineated a new mechanism by which syndecan-1 functions on the cell surface as a cytoprotective signaling modulator to control lung inflammation after injury. We found that syndecan-1 regulates the apoptotic response in cells by potentiating c-Met signaling. As the receptor for HGF, c-Met activates an important pathway that limits lung injury (29). The bronchial epithelium is the predominant location of c-Met expression in the lungs and also primarily produces HGF in the early phase after bacterial injury (30, 60, 61). Furthermore, the HGF/c-Met signaling axis is a pro-survival signal in the bronchial epithelium that promotes proliferation and suppresses apoptosis after injury (30, 62).
Mice treated with a c-Met inhibitor had worsening lung injury after influenza infection. c-Met inhibitors are currently being studied in clinical trials as treatments for cancer (63), and our data would suggest that patients might become susceptible to more severe lung injury after influenza infection. HGF treatment significantly improved total cell counts in WT mice, but neutrophils, although trending to improvement, were not statistically significant. Several possibilities exist that limit the efficacy of intranasally delivered HGF. The dose of HGF given may not sufficiently saturate c-Met receptors. Moreover, receptor–ligand interactions require proper compartmentalization that may not be effectively replicated with exogenously administered HGF (31). HGF could also non-specifically bind cell debris, and expose the extracellular matrix or inflammatory cells, thus reducing the effective dose. As we continue to further our understanding of how syndecan-1 regulates c-Met activation, this research may lead to alternative methods (e.g., pharmacologic) that can circumvent these therapeutic limitations while manipulating this pathway to dampen lung injury after influenza infection.
Sdc1−/− mice are fertile and develop without any abnormalities in contrast to Met−/− mice (64, 65). This divergence in phenotypes most likely is because syndecan-1 has the capability to tune c-Met activity and is not absolutely necessary for its function. Moreover, the functional role of syndecan-1 is only elicited in perturbed states, which may suggest syndecan-1 modulation of c-Met is only necessary in non-homeostatic, non-developmental situations. Analogously, syndecan-1 interacts with and modulates the insulin growth factor-1 receptor, which when deficient, causes severe pulmonary developmental issues and neonatal lethality (66–68). In contrast, hypomorphic expression of insulin growth factor-1 receptor had no developmental abnormalities (66). In a similar fashion, lower levels of c-Met activation in the absences of syndecan-1 may be sufficient for normal development. What is the evolutionary advantage for syndecan-1 potentiation of c-Met signaling? One possibility is that the lung epithelium gains a migratory phenotype as part of the reparative programming when syndecan-1 is shed from the cell surface (7–9). After the epithelial surface is reestablished, cells must re-differentiate and regain the expression of syndecan-1. Those that do not will be more susceptible to death, which has a teleological advantage to prevent pathological outcomes that may benefit from less differentiated, pro-migratory cells (e.g., cancer).
We have identified syndecan-1 as an important pro-survival factor in the lung epithelium that limits acute lung injury after influenza infection. We provide evidence for a novel mechanism in which transmembrane syndecan-1 potentiates c-Met activity, thereby providing anti-apoptotic signals that limit epithelial cell death after influenza infection. Because apoptosis is no longer considered a quiescent process, syndecan-1 may be a factor that controls lung inflammation by regulating the apoptotic response in the lung epithelium. Identifying methods to control this pathway or limit the shedding of syndecan-1 after infection may lead to new therapeutics to limit lung injury.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Ying Wang for her technical support of the animal work in this manuscript.
Footnotes
Supported by the National Institutes of Health (NIH) grants HL120947 (P.C.), HL103868 (P.C.), and HL089455 (W.C.P.); the American Heart Association Grant-in-Aid (P.C.); the Cystic Fibrosis Foundation Research Development Program; and the Cystic Fibrosis Research Translation Center (NIH P30 DK089507).
Author Contributions: Performed experiments: R.B., L.G., S.Y.S., T.P.B., Y.H., T.P., V.L., and B.L.M. Designed the studies: R.B. and P.C. Analyzed the data: R.B., J.K.M., W.C.P., and P.C. Wrote the manuscript: R.B., W.C.P., and P.C.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201509-1878OC on March 9, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Short KR, Kroeze EJBV, Fouchier RAM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis. 2014;14:57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- 2.Kuiken T, Riteau B, Fouchier RA, Rimmelzwaan GF. Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr Opin Virol. 2012;2:276–286. doi: 10.1016/j.coviro.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 5.Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 2003;4:926–937. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Abacherli LE, Nadler ST, Wang Y, Li Q, Parks WC. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting α2β1 integrin activation. PLoS One. 2009;4:e6565. doi: 10.1371/journal.pone.0006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altemeier WA, Schlesinger SY, Buell CA, Parks WC, Chen P. Syndecan-1 controls cell migration by activating Rap1 to regulate focal adhesion disassembly. J Cell Sci. 2012;125:5188–5195. doi: 10.1242/jcs.109884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altemeier WA, Schlesinger SY, Buell CA, Brauer R, Rapraeger AC, Parks WC, Chen P. Transmembrane and extracellular domains of syndecan-1 have distinct functions in regulating lung epithelial migration and adhesion. J Biol Chem. 2012;287:34927–34935. doi: 10.1074/jbc.M112.376814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teng YH-F, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012;31:3–16. doi: 10.1016/j.matbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bass MD, Morgan MR, Humphries MJ. Syndecans shed their reputation as inert molecules. Sci Signal. 2009;2:pe18. doi: 10.1126/scisignal.264pe18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Park PW, Kheradmand F, Corry DB. Endogenous attenuation of allergic lung inflammation by syndecan-1. J Immunol. 2005;174:5758–5765. doi: 10.4049/jimmunol.174.9.5758. [DOI] [PubMed] [Google Scholar]
- 13.Park PW, Pier GB, Hinkes MT, Bernfield M. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 2001;411:98–102. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 15.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114:3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashida A, Bartlett AH, Foster TJ, Park PW. Staphylococcus aureus β-toxin induces lung injury through syndecan-1. Am J Pathol. 2009;174:509–518. doi: 10.2353/ajpath.2009.080394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brauer R, Schlesinger SY, Hastings BL, McGuire JK, Parks WC, Chen P. Syndecan-1 attenuates lung injury during influenza infection by activating survival signals via c-Met [abstract] Am J Respir Crit Care Med. 2014;189:A3967. doi: 10.1164/rccm.201509-1878OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brauer R, Schlesinger SY, Birkland TP, Ge L, Huang Y, McGuire JK, Parks WC, Chen P. LSC Abstract – Syndecan-1 attenuates lung injury during influenza infection by activating survival signals via c-Met (winner of the LSC 2015 Young Investigator William MacNee Award) [abstract] Eur Respir J. 2015;46:PA3022. [Google Scholar]
- 20.Brauer R, Chen P. Influenza virus propagation in embryonated chicken eggs. J Vis Exp. 2015 doi: 10.3791/52421. (97): 10.3791/52421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limberis MP, Vandenberghe LH, Zhang L, Pickles RJ, Wilson JM. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther. 2009;17:294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinshaw VS, Olsen CW, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. In vivo induction of apoptosis by influenza virus. J Gen Virol. 1995;76:2869–2873. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- 24.Wurzer WJ, Planz O, Ehrhardt C, Giner M, Silberzahn T, Pleschka S, Ludwig S. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 2003;22:2717–2728. doi: 10.1093/emboj/cdg279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wurzer WJ, Ehrhardt C, Pleschka S, Berberich-Siebelt F, Wolff T, Walczak H, Planz O, Ludwig S. NF-κB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. J Biol Chem. 2004;279:30931–30937. doi: 10.1074/jbc.M403258200. [DOI] [PubMed] [Google Scholar]
- 26.Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, Mack M, Kuziel WA, Corazza N, Brunner T, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blazejewska P, Koscinski L, Viegas N, Anhlan D, Ludwig S, Schughart K. Pathogenicity of different PR8 influenza A virus variants in mice is determined by both viral and host factors. Virology. 2011;412:36–45. doi: 10.1016/j.virol.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 28.Heaton NS, Langlois RA, Sachs D, Lim JK, Palese P, tenOever BR. Long-term survival of influenza virus infected club cells drives immunopathology. J Exp Med. 2014;211:1707–1714. doi: 10.1084/jem.20140488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L924–L940. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 30.Okada M, Sugita K, Inukai T, Goi K, Kagami K, Kawasaki K, Nakazawa S. Hepatocyte growth factor protects small airway epithelial cells from apoptosis induced by tumor necrosis factor-α or oxidative stress. Pediatr Res. 2004;56:336–344. doi: 10.1203/01.PDR.0000134255.58638.59. [DOI] [PubMed] [Google Scholar]
- 31.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 32.Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oshansky CM, Gartland AJ, Wong S-S, Jeevan T, Wang D, Roddam PL, Caniza MA, Hertz T, Devincenzo JP, Webby RJ, et al. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am J Respir Crit Care Med. 2014;189:449–462. doi: 10.1164/rccm.201309-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unkel B, Hoegner K, Clausen BE, Lewe-Schlosser P, Bodner J, Gattenloehner S, Janßen H, Seeger W, Lohmeyer J, Herold S. Alveolar epithelial cells orchestrate DC function in murine viral pneumonia. J Clin Invest. 2012;122:3652–3664. doi: 10.1172/JCI62139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guarner J, Shieh WJ, Dawson J, Subbarao K, Shaw M, Ferebee T, Morken T, Nolte KB, Freifeld A, Cox N, et al. Immunohistochemical and in situ hybridization studies of influenza A virus infection in human lungs. Am J Clin Pathol. 2000;114:227–233. doi: 10.1309/HV74-N24T-2K2C-3E8Q. [DOI] [PubMed] [Google Scholar]
- 36.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 37.Cheng D-S, Han W, Chen SM, Sherrill TP, Chont M, Park G-Y, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-κB pathway. J Immunol. 2007;178:6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Adler KB, Erjefalt J, Bai C. Airway epithelial dysfunction in the development of acute lung injury and acute respiratory distress syndrome. Expert Rev Respir Med. 2007;1:149–155. doi: 10.1586/17476348.1.1.149. [DOI] [PubMed] [Google Scholar]
- 39.Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MBA, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 42.Hagimoto N, Kuwano K, Kawasaki M, Yoshimi M, Kaneko Y, Kunitake R, Maeyama T, Tanaka T, Hara N. Induction of interleukin-8 secretion and apoptosis in bronchiolar epithelial cells by Fas ligation. Am J Respir Cell Mol Biol. 1999;21:436–445. doi: 10.1165/ajrcmb.21.3.3397. [DOI] [PubMed] [Google Scholar]
- 43.Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol. 2001;158:153–161. doi: 10.1016/S0002-9440(10)63953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 45.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, Ravichandran KS. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493:547–551. doi: 10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi VD, Kalvakolanu DV, Cross AS. Simultaneous activation of apoptosis and inflammation in pathogenesis of septic shock: a hypothesis. FEBS Lett. 2003;555:180–184. doi: 10.1016/s0014-5793(03)01271-7. [DOI] [PubMed] [Google Scholar]
- 48.Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 49.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184:547–560. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peter C, Wesselborg S, Herrmann M, Lauber K. Dangerous attraction: phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis. 2010;15:1007–1028. doi: 10.1007/s10495-010-0472-1. [DOI] [PubMed] [Google Scholar]
- 52.Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, Li C-Y. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3:ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lauber K, Bohn E, Kröber SM, Xiao Y-J, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 54.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peter C, Waibel M, Radu CG, Yang LV, Witte ON, Schulze-Osthoff K, Wesselborg S, Lauber K. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- 57.Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, Griffiths R, Barbour SE, Milstien S, Spiegel S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bournazou I, Pound JD, Duffin R, Bournazos S, Melville LA, Brown SB, Rossi AG, Gregory CD. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest. 2009;119:20–32. doi: 10.1172/JCI36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mühlbauer D, Dzieciolowski J, Hardt M, Hocke A, Schierhorn KL, Mostafa A, Müller C, Wisskirchen C, Herold S, Wolff T, et al. Influenza virus-induced caspase-dependent enlargement of nuclear pores promotes nuclear export of viral ribonucleoprotein complexes. J Virol. 2015;89:6009–6021. doi: 10.1128/JVI.03531-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, Yamamoto H, Ii T, Oishi K, Nagatake T. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol. 2001;24:608–615. doi: 10.1165/ajrcmb.24.5.4292. [DOI] [PubMed] [Google Scholar]
- 61.Singh-Kaw P, Zarnegar R, Siegfried JM. Stimulatory effects of hepatocyte growth factor on normal and neoplastic human bronchial epithelial cells. Am J Physiol. 1995;268:L1012–L1020. doi: 10.1152/ajplung.1995.268.6.L1012. [DOI] [PubMed] [Google Scholar]
- 62.Skibinski G, Elborn JS, Ennis M. Bronchial epithelial cell growth regulation in fibroblast cocultures: the role of hepatocyte growth factor. Am J Physiol Lung Cell Mol Physiol. 2007;293:L69–L76. doi: 10.1152/ajplung.00299.2006. [DOI] [PubMed] [Google Scholar]
- 63.Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol. 2012;9:314–326. doi: 10.1038/nrclinonc.2012.71. [DOI] [PubMed] [Google Scholar]
- 64.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 65.Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Genet. 2000;25:329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 66.Epaud R, Aubey F, Xu J, Chaker Z, Clemessy M, Dautin A, Ahamed K, Bonora M, Hoyeau N, Fléjou J-F, et al. Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis. PLoS ONE. 2012;7:e48071. doi: 10.1371/journal.pone.0048071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beauvais DM, Rapraeger AC. Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation. J Cell Sci. 2010;123:3796–3807. doi: 10.1242/jcs.067645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rapraeger AC, Ell BJ, Roy M, Li X, Morrison OR, Thomas GM, Beauvais DM. Vascular endothelial-cadherin stimulates syndecan-1-coupled insulin-like growth factor-1 receptor and cross-talk between αVβ3 integrin and vascular endothelial growth factor receptor 2 at the onset of endothelial cell dissemination during angiogenesis. FEBS J. 2013;280:2194–2206. doi: 10.1111/febs.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.