Abstract
Chondrogenesis and endochondral ossification are precisely controlled by cellular interactions with surrounding matrix proteins and growth factors that mediate cellular signaling pathways. Here, we report that extracellular matrix protein 1 (ECM1) is a previously unrecognized regulator of chondrogenesis. ECM1 is induced in the course of chondrogenesis and its expression in chondrocytes strictly depends on parathyroid hormone–related peptide (PTHrP) signaling pathway. Overexpression of ECM1 suppresses, whereas suppression of ECM1 enhances, chondrocyte differentiation and hypertrophy in vitro and ex vivo. In addition, target transgene of ECM1 in chondrocytes or osteoblasts in mice leads to striking defects in cartilage development and endochondral bone formation. Of importance, ECM1 seems to be critical for PTHrP action in chondrogenesis, as blockage of ECM1 nearly abolishes PTHrP regulation of chondrocyte hypertrophy, and overexpression of ECM1 rescues disorganized growth plates of PTHrP-null mice. Furthermore, ECM1 and progranulin chondrogenic growth factor constitute an interaction network and act in concert in the regulation of chondrogenesis.—Kong, L., Zhao, Y.-P., Tian, Q.-Y., Feng, J.-Q., Kobayashi, T., Merregaert, J., Liu, C.-J. Extracellular matrix protein 1, a direct targeting molecule of parathyroid hormone–related peptide, negatively regulates chondrogenesis and endochondral ossification via associating with progranulin growth factor.
Keywords: ECM1, PTHrP, PGRN
In the growth plate, endochondral ossification in long bone growth requires tight regulation of chondrogenesis, which corresponds to well-organized resting, proliferative, prehypertrophic, and hypertrophic zones. Alterations in this regulation disrupts the normal chondrocyte differentiation program, which results in a disorganized growth plate architecture and, ultimately, in skeletal dysplasia with pronounced limb shortening (1). To date, however, much is left to be elucidated concerning regulation of chondrogenesis during development.
Extracellular matrix protein 1 (ECM1) is a multifunctional protein involved in the regulation of ureteric bud patterning and branching (2), lung regeneration in postpneumonectomy mice (3), and various disease processes, including lipoid proteinosis (4–6), lichen sclerosus (7–10), and ulcerative colitis (11, 12). ECM1 plays a critical role in the acquisition of immune tolerance and allergic responses via particular T-cell subsets, such as CD4+CD25+ regulatory T cells and Th2 cells (13). It has been reported that ECM1 is a late response gene of parathyroid hormone–related peptide (PTHrP) in a microarray assay (14). Recombinant ECM1 inhibits alkaline phosphatase activity and mineralization of mouse embryonic metatarsals in vitro (15). Moreover, binding between ECM1 and other ECMs, including cartilage oligomeric matrix protein, perlecan, collagen type IV, fibronectin, laminin 332, phospholipid scramblase 1, matrix metallopeptidase 9, and fibulin-1C/1D and 3, has been reported by our laboratory and by other researchers (16–18). ECM1–carbohydrate interactions were established through in vitro binding experiments with hyaluronic acid, chondroitin sulfate A, and heparin (19); however, whether ECM1 regulates chondrogenesis in vitro and cartilage development in vivo remains largely unknown. In this study, we present comprehensive in vitro, ex vivo, and in vivo evidence that demonstrates that ECM1 acts as an important downstream mediator of PTHrP signaling in chondrogenesis. Progranulin (PGRN) is a chondrogenic growth factor (20) that interacts with a disintegrin and metalloproteinase with thrombospondin type 1 motif-7, another downstream molecule of PTHrP signaling (21), and plays a protective role in endochondral ossification and homeostasis of cartilage matrix (20, 22, 23). Here, we demonstrate that ECM1 regulates chondrogenesis, at least in part, via interaction with PGRN.
MATERIALS AND METHODS
Generation of ECM1 transgenic mice
All animal studies were performed in accordance with institutional guidelines and were approved by the Institutional Animal Care and Use Committee of New York University (approval 09112-02). To create ECM1 transgene, a 5.1-kb DNA fragment covering the entire encoding region of human ECM1 cDNA (AF023694) was prepared by high-fidelity PCR. ECM1 cDNA was subcloned into the SwaI sites of an expression vector that contained chondrocyte-specific Col IIa promoter and enhancer sequence to create Col 2a1–ECM1 plasmid. Transgenic mice were produced by microinjecting the plasmid into the pronuclei of fertilized eggs from F1 mice (C57/BL6). In a similar way, we cloned the same DNA fragment mentioned above under the 2.3-kb fragment of the collagen type I promoter as described previously (24). Genomic DNA was amplified by transgene-specific PCR using primers that were derived from human ECM1 cDNA.
Immunohistochemistry
For formalin-fixed paraffin, 5-μm-thick sections of indicated embryonic murine limbs were immunostained for the target cytokines mentioned in the experiments. Sections were pretreated with 0.1% trypsin for 30 min at 37°C, followed by 3% bovine serum albumin and 20% goat serum blocking solution for 1 h at room temperature to reduce nonspecific staining. Polyclonal anti-ECM1, anti-Col2, anti-Col10, anti–ADAMTS-7, and anti-PGRN antibodies were used in the current study as previously reported (21, 25). Antibodies mentioned above were diluted at 1:100 and incubated overnight at 4°C. Binding of primary antibodies was detected by using indicated biotinylated secondary antibodies (Vectastain ABC Elite kit; Vector Laboratories, Burlingame, CA, USA) diluted at 1:200 and incubated for 1 h at 37°C, followed by ABC peroxidase (Vector Laboratories) at 37°C for 1 h, and developed with DAB (Sigma-Aldrich, St. Louis, MO, USA) for 2 min at room temperature. Sections were counterstained with methyl green (Dako, Carpinteria, CA, USA).
Expression and purification of recombinant proteins
For expression of GST fusion proteins, appropriate plasmids for indicated fragments of ECM1a were subcloned into the pGEX-3X and transformed into Escherichia coli DH5 (Thermo Fisher Scientific Life Sciences, Waltham, MA, USA). Fusion proteins were affinity purified on glutathione-agarose beads. To remove GST moiety from GST-fused catalytic domain, 50 μg purified GST fusion protein was incubated with 1 μg of Xa factor (New England Biolabs, Beverly, MA, USA) in 20 μl of 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, and 2 mM CaCl2 at 23°C for 8 h. Reaction was terminated by the addition of 2 μM dansyl-Glu-Gly-Arg-chloromethyl ketone (New England Biolabs) and incubated at room temperature for 1 min. Completion of the cleavage was verified by SDS-PAGE, and the resultant GST moiety was removed by using GSH-Sepharose 4B beads (Amersham, Piscataway, NJ, USA).
His-δF/COOH and His-δP/COOH were purified by affinity chromatography using a HiTrap chelating column (Amersham). In brief, bacteria lysates that were supplemented with 20 mM HEPES (pH 7.5) and 0.5 M NaCl were applied to the HiTrap chelating column. The column was washed with HSB buffer (40 mM HEPES, pH 7.5, 1 M NaCl, and 0.05% Brij 35) that contained 10 mM imidazole, and the His-δF/COOH and His-δP/COOH were eluted with HSB buffer that contained 300 mM imidazole.
Recombinant intact PGRN and ECM1 were expressed and purified from corresponding stable lines that were derived from human embryonic kidney 293 (HEK293) cells as described previously (26).
Micro–computed tomography assay
Before histologic processing, paraformaldehyde-fixed femurs of 3-wk-old wild-type (WT) and Col 1a–ECM1 transgenic mice were evaluated with micro–computed tomography (micro-CT) using a Scanco vivaCT40 cone-beam scanner (Scanco Medical, Brüttisellen, Switzerland) with 55 kVp source and 145 µAmp current. We scanned the samples at a resolution of 10.5 µm. Scanned images from each group were evaluated at the same thresholds to allow 3-dimensional structural reconstruction. For cancellous bone analysis, bone tissue of 0.8-mm thickness at a distance of 0.16 mm from the proximal end of the distal femur growth plate was selected from the image as the region of interest. Trabecular thickness and bone volume/total volume (BV/TV) were calculated. For cortical bone analysis, cortical thickness and diameter of femur were presented at midshaft of diaphysis.
RNA preparation and real-time PCR
Total RNA was extracted from indicated cells by using RNeasy kit (Qiagen, Valencia, CA, USA). Of total RNA, 1 μg/sample was reverse transcribed by using the ImProm-II reverse transcription system (Promega, Madison, WI, USA). The following sequence specific primers were synthesized: 5′-TGGTGGAGCAGCAAGAGCAA-3′ and 5′-CAGTGGACAGTAGACGGAGGAAA-3′ for mouse Col II; 5′-CTGCTGC TAATGTTCTTGAC-3′ and 5′-ACTGGAATCCCT TTACTCTTT-3′ for mouse Col X; 5′-CCT GAA GCA GAG GAA GAT GGA-3′ and 5′-GGG ATC AGC TGA ACA GGG TAA-3′ for Sox5; 5′-CGCTCGCAATACGACTACGC-3′ and 5′-TAGAGCCCTGAGCCCTGTCC-3′ for mouse Sox9; 5′-CAGTGGAGTGTCCTGGTATT-3′ and 5′-GATCTCCGCGATCAGATGGT-3′ for mouse PTHrP; 5′-GCTCGTGCCTCTTGCCTACA-3′ and 5′-CGTGTTCTCCTCGTCCTTGA-3′ for mouse Indian hedgehog (IHH); 5′-GTGAGCCATGATTCGCCTCGG-3′ and 5′-CACCAGGTTCACCAGGATTGCC-3′ for human Col II; and 5′-CCCTTTTTGCTGCTAGTATCC-3′ and 5′-CTGTTGTCCAGGTTTTCCTGGCAC-3′ for human Col X. The following pair of oligonucleotides was used as internal controls: 5′-AGGTCGGTGTGAACGGATTTG-3′ and 5′-TGTAGACCATGTAGTTGAGGTCA-3′ for mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH); and 5′-ATGACATCAAGAAGGTGGTG-3′ and 5′-CATACCAGGAAATGAGCTTG-3′ for human GAPDH. Reactions were performed in a 50 μl Sybr green PCR volume in a 96-well optical reaction plate formatted in the 7300 sequence detection system (Applied Biosystems, Foster City, CA, USA) under the following PCR conditions: 40 cycles at 95°C for 15 s and at 60°C for 1 min. The transcript of GAPDH mRNA was employed as an internal control for RNA quality. For each gene, 3 independent PCRs from the same reverse-transcription sample were performed. In addition, quantitative RT-PCR experiments were carried out in 3 biologic replicates. The presence of a single specific PCR product was verified by using melting curve analysis, confirmed on an agarose gel, and further sequenced by Applied Biosystems sequencing system.
Construction of small interfering RNA of ECM1
Mouse ECM1 was targeted for small interfering RNA (siRNA) by using mammalian expression pSuper vector (OligoEngine) according to the manufacturer instructions. To generate each siRNA, equimolar amounts of complementary sense and antisense strands were mixed and annealed slowly by cooling to 10°C in a 50-μl reaction buffer (100 mM NaCl and 50 mM HEPES, pH 7.4). Anealed oligonucleotides were inserted into the BglII/HindIII sites of pSuper vector. Resulting plasmids and control vector pSuper were transfected into C3H10T1/2 cells by using Lipofectamine 2000 reagent (Thermo Fisher Life Sciences), and the level of ECM1 was monitored by using real-time PCR and immunofluorescence cell staining. Data demonstrated that the siRNA 5′-CGAGGAGAAGGACTTAAAG-3′ was able to efficiently reduce the expression of mouse ECM1.
Chondrogenesis of human mesenchymal stem cells
Chondrogenic differentiation was induced by placing 2.5 × 105 human mesenchymal stem cells [hMSCs; provided by Drs. Glyn D. Palmer and Steven B. Abramson (New York University School of Medicine, New York, NY, USA)] into the defined chondrogenic medium and subjecting cells to gentle centrifugation (800 g for 5 min) in a 15-ml conical polypropylene tube, after which the cap was loosened and the tube was placed in the incubator, where the cells adhered to one another and consolidated into a cell pellet within 24 h. Three to 4 d later, hMSCs had formed a 1-mm ball in the bottom of the tube. Chondrogenic medium was made with fresh bone morphogenetic protein 2 (BMP-2; 100 ng/ml) changed every 3–4 d in the presence or absence of ECM1 conditioned medium. Medium was changed by careful aspiration because the cell pellets were free floating. If cell pellets were found attached to the tube wall, they were gently dislodged with a pipette tip. Pellets were collected at various time points, as indicated, and the expressions of chondrogenic marker genes were determined with real-time PCR.
Culture of fetal mouse bone explants
Fetal mouse metatarsals were dissected from fetal C57/BL6 mice (15-d-old embryos) and cultured in DMEM (Thermo Fisher Scientific Life Sciences) that contained 1% heat-inactivated fetal calf serum (Thermo Fisher Scientific Life Sciences) and 100 U penicillin–streptomycin per milliliter in the absence or presence of various stimuli as indicated for 5 d in sextuple. PTHrP (10−7 M) was used as a positive control as previously reported because it was known to strongly inhibit mineralization in this system. For staining of bone and cartilage via alizarin red and alcian blue staining, respectively, the explants were placed in 4% paraformaldehyde in PBS for overnight fixation. Subsequently, explants were placed in staining solution (0.05% alizarin red, 0.015% alcian blue, 5% acetic acid in 70% ethanol) for 45–60 min. Digital images of stained bones were analyzed. For safranin O–fast green staining, explants were fixed in 96% alcohol and processed for paraffin embedding. Sections were stained with 0.1% safranin O (orange stain) to evaluate cartilage matrices and with 0.03% fast green to evaluate morphologic features as previously described.
Solid-phase binding assay
Microtiter plates (96-well EIA/RIA plates; Costar, Badhoevedorp, The Netherlands) were coated with various amounts (0.001–5.000 µg) of purified ECM1 in 100 µl of Tris-buffered saline (TBS) buffer (50 mM Tris-HCl, 150 mM NaCl, pH 7.4) overnight at 4°C. Wells were blocked with 1% bovine serum albumin in TBS buffer for 3 h at 37°C. After washing with TBS and 0.05% Tween, 100 µl of 50 µg/ml of PGRN was added to each well, followed by addition of 10 mM CaCl2. Samples were allowed to bind overnight at 4°C. Bound protein from the liquid phase was detected by mouse polyclonal antibody against PGRN, followed by a secondary anti-mouse antibody conjugated with horseradish peroxidase (Antigenix America, Huntington Station, NY, USA) and 5-amino-2-hydroxybenzoic acid as a substrate, with absorbance measured at 490 nm in an ELISA reader.
Transfection of cells
HEK293-EBNA, C3H10T1/2, ATDC5, and rat chondrosarcoma cells were seeded at 5 × 106 cells/well in a 6-well plate in medium that contained 10% fetal calf serum (Thermo Fisher Scientific Life Sciences). Cells were grown overnight and transfected the following day with 6 μg of DNA using Lipofectamine 2000 (Thermo Fisher Scientific Life Sciences) according to the manufacturer protocol. Stable lines were selected with 1 mg/ml of G418 (Sigma-Aldrich) and maintained with 200 μg/ml of G418. To obtain the conditioned medium from various stable lines, cells were washed once with serum-free DMEM to remove any remaining serum, and 1 ml of serum-free DMEM with or without 100 μg/ml heparin was added to each culture and incubated for an additional 48 h.
Pull down assay
Pull-down assay was performed for identification of binding between various fragments of ECM1 and PGRN, according to a previously reported protocol (22). Yeast expression vectors pDBleu and pPC86 (both from Thermo Fisher Scientific Life Sciences) are fusion vectors for the linkage of proteins to Gal4 DNA-binding domain and to VP16 transactivation domain, respectively. Fragments encoding mouse ECM1 were amplified by PCR and cloned in frame into the SalI/NotI sites of pDBleu to serve as bait in the screening assay. Bacterial expression pBAD TOPO vector (Thermo Fisher Scientific Life Sciences) was used to produce His-tagged proteins in E. coli. Constructs that encoded ECM1 fused to GST were described previously. Fragments of intact δF/COOH (360–540 aa) and partial C δP/COOH (360–480 aa) terminal of ECM1 were subcloned into the pBAD TOPO vector according to manufacturer instructions to generate the indicated plasmids. All constructs were verified by nucleic acid sequencing, and subsequent analysis was performed by using BLAST software (www.ncbi.nlm.nih.gov/blast/). Purified proteins were conjugated to glutathione-sepharose beads and incubated with recombinant PGRN. After washing, bound proteins were immunoblotted with anti-PGRN antibody. The experiments were repeated ≥3 times.
Assay of protein–protein interactions by using the yeast-2 hybrid system
A yeast-2 hybrid assay was performed, following a previously reported protocol (27), for identification of binding between various fragments of PGRN and ECM1. Independent yeast colonies were analyzed for interaction of 2 proteins, one of which was fused to the Gal4 DNA binding domain and the other, to the VP16 transactivation domain. Our published procedures were followed for growing and transforming the yeast strain MAV203 with the selected plasmids, and determining β-galactosidase activity and growth phenotypes on selective media lacking tryptophan, leucine, histidine, and uracil. To quantify interactions, yeast colonies were harvested, lysed, and activity determined by a liquid assay for β-galactosidase. β-Galactosidase activity was monitored at 420 nm, and 1 U β-galactosidase activity was defined as the amount capable of hydrolyzing 1 μmol o-nitrophenyl-β-d-galactopyranoside to o-nitrophenol and d-galactose per minute per cell.
Statistical test
Results were expressed as means ± sem. Statistical analysis was conducted by using a Student’s t test in SPSS software (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
ECM1 expression is induced during the course of chondrogenesis
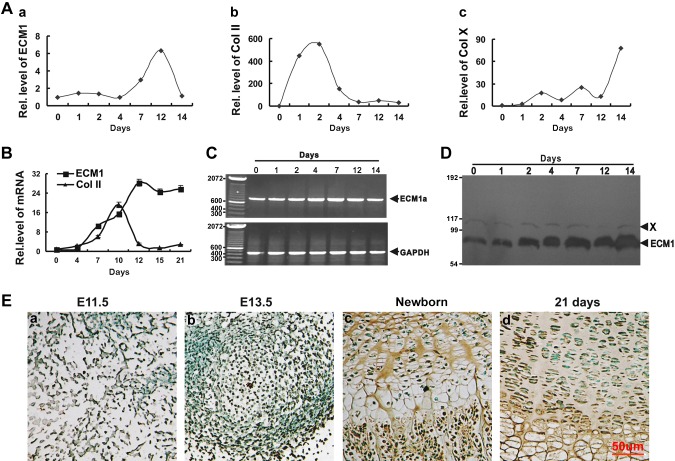
To investigate the expression pattern of ECM1 during chondrocyte differentiation, C3H10T1/2 progenitor cells were incubated in the presence of 300 ng/ml recombinant BMP-2. Cells were harvested at indicated time points, and ECM1 and markers for proliferative and hypertrophic chondrocytes, including Col II and Col X, were assessed via real-time PCR. As shown in Fig. 1A, the level of ECM1 was relatively low until d 4, and at d 7, ECM1 level was elevated and continued to rise until peaking at d 12. After d 12, ECM1 level dramatically declined at the stage of terminal differentiation, which was marked by the increase in Col X expression. Conversely, ECM1 level of hMSCs that were cultured in pellets was elevated from d 7, reached peak level near d 12, and remained at high levels even during terminal differentiation stage (Fig. 1B). Traditional RT-PCR was performed on C3H10T1/2 cells to determine which of the 3 isoforms of ECM1, ECM1a, ECM1b, and/or ECM1c (28), is distributed in chondrocytes. Results showed that only ECM1a was detectable, which suggested that ECM1a is the singular isoform expressed in chondrocytes (Fig. 1C). We next examined the protein level of ECM1. C3H10T1/2 cells were cultured in micromass and harvested at various time points, followed by Western blotting (Fig. 1D). ECM1 protein was markedly elevated at d 2 and remained at high levels at subsequent time points. A nonspecific protein, designated X, served as an internal control (Fig. 1D). To characterize temporal and spatial expression patterns of ECM1 protein during skeletal development of WT mice, we performed immunohistochemistry assays at multiple time points, including at previously reported critical developmental stages, embryonic d 11.5 (E11.5) and E13.5, and at postnatal developmental stages, newborn and 21-d-old mice. As revealed in Fig. 1E, ECM1 expression is detectable in staining of the proliferating mesenchymal cells at E11.5 (Fig. 1Ea) and was clearly evident in the center of the condensation and around it at E13.5 (Fig. 1Eb). ECM1 demonstrated prominent expression in the ECM of proliferating and prehypertrophic zones in growth plates of newborn mice (Fig. 1Ec). In the case of the 21-d-old mice, ECM1 was expressed extensively in the matrix of growth plate (Fig. 1Ed). This set of experiments implies that the expression profile of ECM1 is closely linked to chondrogenesis throughout the entire process of cartilage formation.
Figure 1.
Expression of ECM1 in the course of chondrogenesis in vitro and in the growth plate chondrocytes in vivo. A) Expression of ECM1 (a), Col II (b), and Col X (c) in the course of chondrogenesis of a micromass culture of C3H10T1/2 cells assayed by real-time PCR. Experiments were carried out in biologic replicates (n = 3). B) Differential expression of ECM1 during chondrogenesis of a pellet culture of hMSCs assayed by real-time PCR. Experiments were carried out in biologic replicates (n = 3). C) Specific expression of ECM1a isoform, but not 1b or 1c, in the course of chondrogenesis, evaluated by PCR assay. C3H10T1/2 cells were cultured as described for panel A, and ECM1a was detected via PCR and confirmed by sequence analysis. D) Levels of ECM1 and internal control (nonspecific protein, labeled X) during chondrogenesis were detected by using Western blot. The process was the same as for panel A. E) Temporal and spatial expression of ECM1 during chondrogenesis in vivo assayed by immunohistochemistry. Sections of long bone from various embryonic and postnatal developmental stages of mice (a, E11.5; b, E13.5; c, newborn; d, 21-d-old mice) were stained with anti-ECM1 antiserum (brown) and counterstained with methyl green (green). Immunostaining revealed positive cytoplasmic staining in and around chondrocytes. Each experiment was repeated ≥3 times. Units are arbitrary and normalized values were calibrated against controls, here given the value of 1. Rel. level, relative level.
ECM1 negatively regulates chondrocyte differentiation in vitro
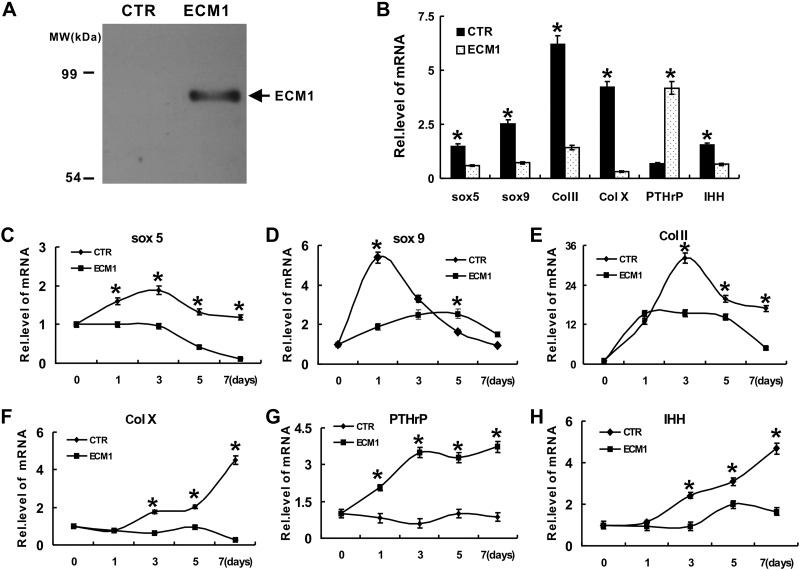
We next sought to determine the role of ECM1 in chondrogenesis. We generated a control and an ECM1 stable line on the basis of HEK293-EBNA cells, Western blotting was performed, and ectopic expression of ECM1 in its stable line is shown in Fig. 2A. Pellet culture of hMSCs in ECM1-conditioned medium (collected from HEK293-EBNA cells that were stably transfected with an ECM1 expression plasmid) was used as experimental group and was assessed by real-time PCR using primers for Col II, Col X, Sox9, Sox5, PTHrP, and IHH. Cells incubated with conditioned medium from control HEK293-EBNA cells (not expressing ectopic ECM1) were used as control group. Cytotoxicity was ruled out via a commercial kit as described in Materials and Methods. As shown in Fig. 2B, ECM1 was a potent inhibitor of chondrocyte differentiation, as it suppressed expression of marker genes for chondrogenesis, including Col II, Col X, Sox9, Sox5, and IHH, and enhanced expression of PTHrP. Furthermore, this potent inhibition of chondrocyte differentiation was verified by using micromass cultures of both pcDNA3.1 vector control and ECM1 stable cell lines. Both groups were stimulated with 300 ng/ml BMP-2 protein for different time points. Real-time PCR was performed, and levels of chondrogenic markers, including Col II, Col X, Sox9, Sox5, and IHH, were dramatically repressed, whereas PTHrP was increased in the ECM1 overexpression group (Fig. 2C–H).
Figure 2.
Recombinant ECM1 protein negatively affects chondrocyte differentiation. A) Characterization of recombinant ECM1a protein. Purified recombinant human ECM1a (10 ng/μl in 0.2% bovine serum albumin) was detected by Western blotting with a polyclonal anti-ECM1 antibody. B) Effect of ECM1 conditioned medium on expression of molecules that control chondrocyte maturation assayed by real-time PCR. Pellet culture of hMSCs in either control or ECM1-conditioned medium for 7 d, and real-time PCR was performed. Experiments were carried out in biologic replicates (n = 3). C–H) Recombinant ECM1a protein regulated the expression of genes critical for chondrogenesis, including Sox5 (C), Sox9 (D), Col II (E), Col X (F), PTHrP (G), and IHH (H), assayed by real-time PCR. Micromass cultures of C3H10T1/2 cells were stimulated with 300 ng/ml BMP-2 protein for various time points as indicated in presence or absence of ECM1, and levels of mRNAs were determined by using real-time PCR. Experiments were carried out in biologic replicates (n = 3). Units are arbitrary and normalized values were calibrated against controls, here given the value of 1. Rel. level, relative level. Values are means ± sd. *P < 0.05 vs. PBS treatment control group.
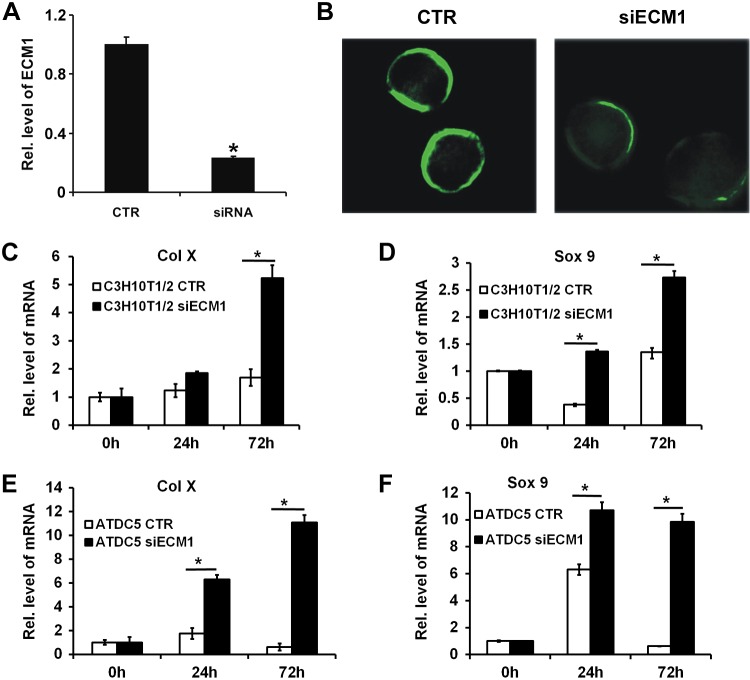
To investigate whether suppression of ECM1 promoted chondrogenesis, ECM1 siRNA was constructed. Real-time PCR (Fig. 3A) and cellular immunostaining (Fig. 3B) were performed in C3H10T1/2 cells to confirm successful transfection. Thereafter, C3H10T1/2 and ATDC5 cells that were transfected with control vector or ECM1 siRNA were cultured in micromass. Both groups were stimulated with 300 ng/ml BMP-2 protein for different time points, followed by real-time PCR for levels of chondrogenic marker genes, including Sox9 and Col X. As shown in Fig. 3C–F, ECM1 siRNA enhanced chondrogenesis in both cell lines, as confirmed by remarkably elevated gene levels of Sox9 and Col X at different time points.
Figure 3.
Knockdown of ECM1 enhances chondrocyte differentiation. A) pSuper-ECM1 (siECM1), an ECM1 siRNA construct, reduced >70% of endogenous ECM1 mRNA as measured by real-time PCR. B) siECM1 largely reduces ECM1 protein level detected by cell immunostaining. C–F) Blocking ECM1 expression stimulates chondrocyte marker genes, including Col X (C, E) and Sox9 (D, F), in both C310T1/2 (C, D) and ATDC5 (E, F) cells, assayed by real-time PCR. Experiments were carried out in biologic replicates (n = 3). Units are arbitrary and normalized values were calibrated against controls, here given the value of 1. Rel. level, relative level. Values are means ± sd. *P < 0.05 vs. control group.
Tissue specific overexpression of ECM1 leads to disorganized chondrogenesis and ossification in growth plate
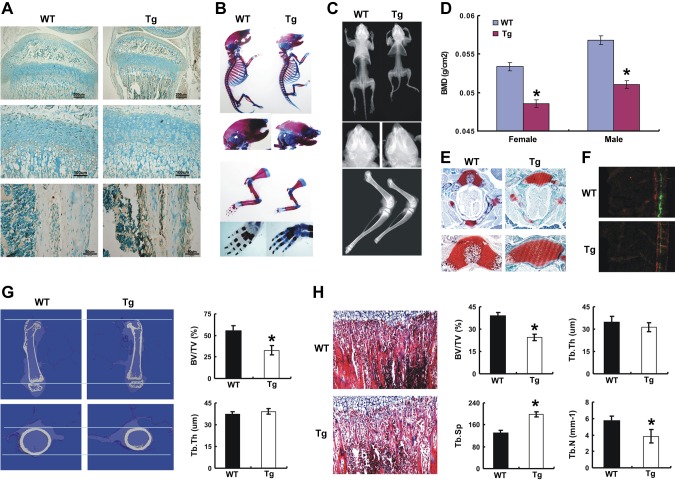
Deckers and colleagues (15) have reported that ECM1 suppressed ossification in vitro, and our findings reported herein reveal that ECM1 negatively regulates chondrogenesis in vitro. These results prompted us to investigate the role of ECM1 in vivo. We thus generated Col Ia–ECM1 and Col IIa1–ECM1 transgenic mice with a protocol described in Materials and Methods. For Col Ia–ECM1 transgenic mice, immunohistochemistry confirmed that ECM1 was overexpressed in osteoblasts of the intramedulary cavity but not growth plate (Fig. 4A). Alcian blue/alizarin red whole-mount staining of 3-wk-old mice revealed that osteoblast overexpression of ECM1 delayed mineralization in transgenic mice, which was illustrated by comparatively reduced red staining in the calvaria, forelimb, and hindlimb (Fig. 4B). Representative X-ray images of whole animals and hindlimbs at 3 wk showed remarkable reductions of skeletal length and bone volume in transgenic mice (Fig. 4C). Dual-energy X-ray absorptiometry scan indicated significantly lower bone mineral density in transgenic mice at 3 wk compared with WT mice (Fig. 4D). Figure 4E shows that transgenic mice displayed abnormal ossification center formation during development, which was determined through distribution of safranin O stain in vertebral ossification center. Moreover, the bone formation rate indicated by the distance between the 2 consecutive labels was significantly decreased in transgenic mice (Fig. 4F). Micro-CT indicated that ECM1 transgenic mice displayed a shorter length and a smaller diameter of the femur, and the parameters of bone quality, including BV/TV, was significantly diminished in transgenic mice (Fig. 4G). Moreover, bone quality on the basis of histology of long bone revealed that ECM1 overexpression in osteoblasts dramatically diminished several morphometric parameters, including BV/TV, trabecular thickness, trabecular separation, and trabecular number (Fig. 4H).
Figure 4.
Targeted overexpression of ECM1 in osteoblast leads to short-limbed dwarfism and a delay in endochondral ossification in young mice. A) Increased ECM1 expression in newborn transgenic mouse osteoblasts detected by immunohistochemistry. B) Skeletal preparation with alcian blue/alizarin red staining of newborn WT and TG mice. Representative pictures shown as whole-mount view and high magnification of forelimbs and hind limbs. C) Representative pictures of whole body from WT and ECM1 transgenic mice at 3 wk old assayed by X-ray. D) Dual-energy X-ray absorptiometry scan analysis for the total bone mineral density (BMD) in WT and transgenic mice at 3 wk; n = 6 in each group. E) Delayed formation of ossification center in transgenic mice as detected by safranin O staining of the vertebra of 3-wk-old mice. F) Calcein double labeling in 4-wk-old mice. Bone formation rate (BFR) represented by the distance between 2 green labels, and BFR was compared between WT and transgenic mice; n = 5 for each group. G) Coronal section of middle femurs were collected and bone quality was compared in 3-wk-old WT and transgenic mice assayed by micro-CT. H) Morphologic assessment of bone quality was performed in the trabecular bone of tibia from WT and ECM1 transgenic mice, and parameters were statistically analyzed. Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness. Data show means ± sem. *P < 0.05 between WT and transgenic mice; n = 6. Original magnification, ×40.
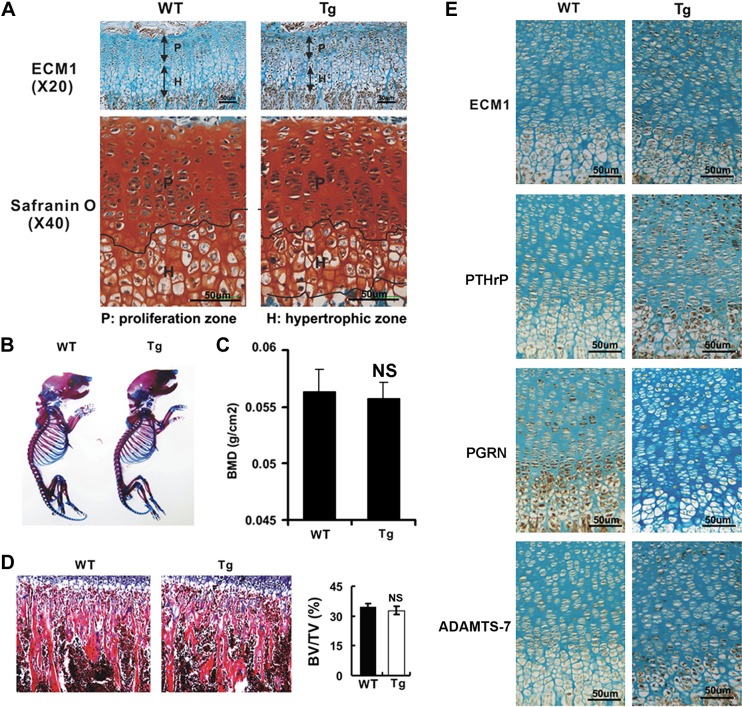
For Col IIa1–ECM1 transgenic mice, immunohistochemistry revealed high expression of ECM1, specifically in proliferating and prehypertrophic zones of proximal tibia growth plate in these transgenic mice (Fig. 5A). Safranin O staining demonstrated that thickness of hypertrophic zone was dramatically reduced (Fig. 5A), although there was no remarkable difference in body status (Fig. 5B). DXA data reveal that transgenic mice displayed no significant difference in bone density compared with WT littermates (Fig. 5C). Furthermore, bone morphologic analysis did not show significant difference in BV/TV of trabecular bone between the 2 genotypes (Fig. 5D). Immunohistochemistry was performed to determine the expression pattern of known ECM1-associated chondrogenesis-related molecules in the growth plate of Col IIa1–ECM1 transgenic mice. As shown in Fig. 5E, PTHrP and ADAMTS-7 levels were elevated in the growth plate of ECM1 transgenic mice, whereas PGRN became almost undetectable. This implies that ECM1 overexpression might also play a critical role in the chondrogenic process in the growth plate in vivo.
Figure 5.
Targeted overexpression of ECM1 in chondrocytes leads to alteration of growth plate in vivo. A) Expression pattern of ECM1 (upper panels) and alteration of hypertrophic zone (lower panels) in growth plate of ECM1 transgenic mice (Col IIa1–ECM1 Tg) compared with WT littermates assayed by histology. Slides from growth plate of WT and transgenic mice were taken, followed by safranin O staining and immunohistochemistry against ECM1; n = 6, respectively. B) Skeletal preparation with alcian blue/alizarin red staining of newborn WT and transgenic mice. Representative pictures were shown as whole-mount view. C) Dual-energy X-ray absorptiometry scan analysis for total bone mineral density (BMD) in WT and transgenic mice at 3 wk. Bone density is significantly lower in male transgenic mice but not in female mice; n = 6 in each group. D) No significant difference in BV/TV is observed between ECM1 transgenic and WT mice assayed by bone morphology analysis of tibia sections. E) Alteration of signals for chondrogenesis-related markers, including ECM1, PTHrP, PGRN, and ADAMTS-7 (TS-7), in growth plate of transgenic mice detected by immunohistochemistry. NS, not significant. Tg:Col IIa1–ECM1 transgenic mice. Values are means ± sd. *P < 0.05 vs. WT control group.
PTHrP induced expression of ECM1 in chondrocyte and maintained expression of ECM1 in growth plate
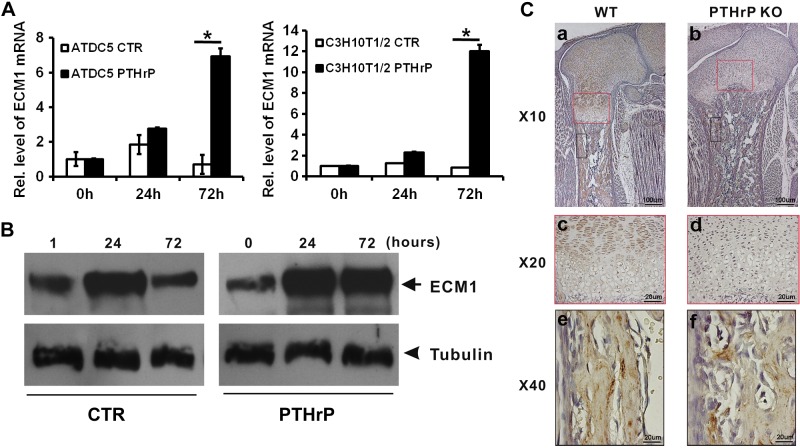
ECM1 is a known late-responsive gene of PTHrP in chondrocytes (14). In the study, we used C3H10T1/2 and chondroprogenitor ATDC5 cells to examine whether ECM1 was the downstream molecule of PTHrP in chondrogenesis. Micromass cultures of both C3H10T1/2 and ATDC5 cells were pretreated with 300 ng/ml BMP-2 for 1 wk, followed by culturing with or without 10−7 M of PTHrP for various time points. Level of ECM1 mRNA was measured by real-time PCR (Fig. 6A). ECM1 mRNA was slightly increased at d 1 but dramatically reduced by d 3 in PTHrP-untreated control C3H10T1/2 cells, whereas PTHrP treatment markedly enhanced the level of ECM1 mRNA at d 1, and its level was increased to more than 6-fold at d 3 (Fig. 6A, left). In the case of ATDC5 cells, ECM1 mRNA did not show an obvious increase from d 1 to 3 in absence of PTHrP; however, in the presence of PTHrP, ECM1 mRNA level increased by d 1 and had increased to >10-fold at d 3 (Fig. 6A, right). Moreover, dramatic induction of ECM1 by PTHrP was also visualized at the protein level via Western blotting in ATDC5 cells (Fig. 6B). Taken together, these findings demonstrate that ECM1 is a PTHrP-inducible gene, and both transcription and translation levels of ECM1 were elevated in presence of PTHrP, especially in the late stage of chondrogenesis. To investigate PTHrP-induced ECM1 expression pattern in vivo, immunohistochemistry for ECM1 was performed on sections of long bone from 18.5-d-old WT control (PTHrP+/+) and PTHrP-deficient (PTHrP−/−) mouse embryos. ECM1 demonstrated prominent expression in the long bone and in proliferating and prehypertrophic zones of the embryonic growth plates of control mice (Fig. 6Ca, c). Surprisingly, in PTHrP−/− mice, ECM1 expression was dramatically impaired in the growth plate (Fig. 6Cb, d) but not in other locations, such as long bone, of the same samples (Fig. 6Ce, f), which indicated that expression of ECM1 in growth plate requires PTHrP signaling.
Figure 6.
ECM1 is a PTHrP-inducible protein in chondrogenesis. A) PTHrP elevated RNA level of ECM1 in a time-dependent manner as measured by real-time PCR. ATDC5 and C310T1/2 cells were cultured in absence or presence of PTHrP for indicated time points, and real-time PCR was performed. Experiments were carried out in biologic replicates (n = 3). B) PTHrP elevated protein level of ECM1 in a time-dependent manner assayed by Western blotting. ATDC5 cells were cultured as described in panel A, then total protein was collected and Western blotting was performed against ECM1. β-Tubulin was used as an internal control. C) Deficiency of PTHrP resulted in specific loss of ECM1 expression in growth plate cartilage (red boxes) but not bone tissue (black boxes). Tibias were collected from both WT and PTHrP KO mice, and immunohistochemistry for ECM1 was performed. Brown color indicated the expression of ECM1. Values are means ± sd. *P < 0.05 vs. PBS treatment group.
ECM1 was a key downstream target of PTHrP and mediated PTHrP action in growth plate
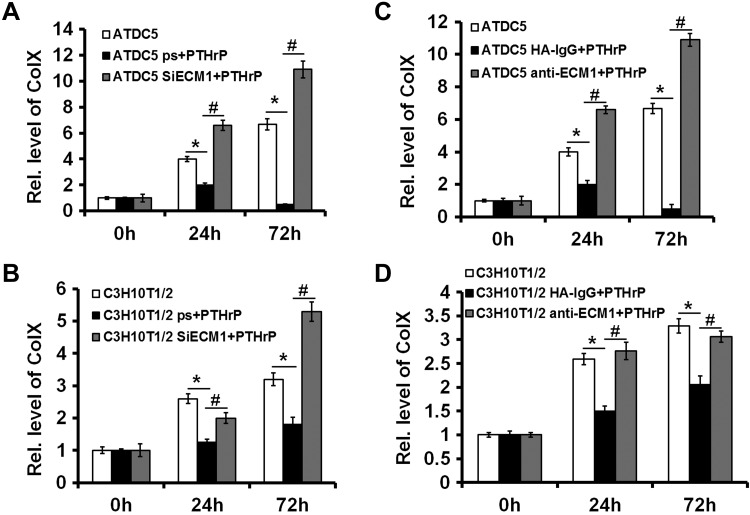
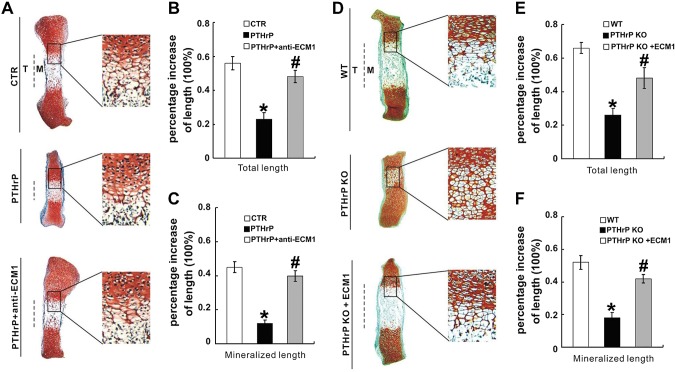
PTHrP signaling is known to repress chondrocyte hypertrophy (29). In the current study, we investigated whether ECM1 is required for PTHrP-mediated inhibition of chondrocyte hypertrophy. ATDC5 and C3H10T1/2 cells that were stably transfected with pSuper vector (control) or pSuper-ECM1 were cultured in micromass with stimulation of 300 ng/ml BMP-2 for 5 d, followed by treatment with 10−7 M PTHrP for another 3 d. Level of hypertrophic biomarker Col X was determined by real-time PCR. As shown in Fig. 7A, B, PTHrP strongly inhibited Col X expression in cells transfected with control vectors; however, PTHrP-mediated inhibition of Col X was impaired or even reversed when ECM1 was knocked down by pSuper-ECM1 in both ATDC5 and C3H10T1/2 cells. The requirement of ECM1 for PTHrP-mediated inhibition on Col X expression was further verified in both ATDC5 and C3H10T1/2 cells via an antibody-blocking assay, and the blockage of ECM1 also rescued the expression of Col X in presence of PTHrP (Fig. 7C, D). Moreover, fetal mouse metatarsals were cultured, and, in line with previous reports (15, 21), PTHrP potently inhibited chondrocyte hypertrophy, mineralization, and bone length. However, additional use of anti-ECM1 antibody largely abolished the PTHrP-mediated inhibition of these activities (Fig. 8A–C). These findings suggest that PTHrP-mediated chondrocyte differentiation and endochondral bone growth, at least in part, depends on ECM1. Accordingly, we sought to determine whether ECM1 compensation was able to rescue defects seen in growth plates of PTHrP−/− embryos. As shown in Fig. 8D–F, disorganized PTHrP−/− growth plates, including accelerated chondrocyte hypertrophy and shortening of bone length, were largely corrected in the presence of recombinant ECM1.
Figure 7.
ECM1 is required for the normal function of PTHrP in vitro and ex vivo. A, B) Knockdown of ECM1 via siRNA almost abolished PTHrP-mediated inhibition of Col X expression in ATDC5 (A) and C3H10T1/2 (B) cells assayed by real-time PCR. C, D) Knockdown of ECM1 via ECM1 antibody greatly impaired PTHrP-mediated inhibition of Col X expression in ATDC5 (C) and C3H10T1/2 (D) cells detected by real-time PCR. Experiments were carried out in biologic replicates (n = 3). Rel. level, relative level; siECM1, pSuper-ECM1. Values are means ± sd. *P < 0.05 vs. PBS treatment control group; #P < 0.05 between PTHrP treatment and additional administration of pSuper-ECM1.
Figure 8.
ECM1 is required for the normal function of PTHrP ex vivo. A) ECM1 blocking antibody completely neutralized PTHrP-mediated chondrocyte hypertrophy, mineralization, and bone length detected by safranin O–fast green staining of metatarsals. Metatarsals were explanted from 15-d-old mouse embryos and cultured in the absence [control (ctr)] or presence of PTHrP (10−7 M), with or without anti-ECM1 (1 µg/ml) antibodies. Explants were cultured for 5 d and safranin O–fast green staining was observed by using low-power and high-power microphotography. B, C) Percent increase in total (B) and mineralization (C) length of metatarsal bones. Metatarsals were cultured as described above, total or mineralization length was determined, and percent increase was calculated [percent increase = (length at d 5 − length at d 0)/length at d 0]. D) ECM1 corrects defects in chondrocyte hypertrophy in metatarsal bones of PTHrP-null embryos. Metatarsals were explanted from 14.5-d-old WT and PTHrP−/− mouse embryos and cultured in the absence [control (ctr)] or presence of ECM1-conditioned medium. Explants were cultured for 5 d, and safranin O staining was observed. E, F) Percent increase in total (E) and mineralization (F) length of metatarsal bones. Rel. level, relative level; siECM1, pSuper-ECM1. Values are means ± sd. *P < 0.05 vs. PBS treatment control group. #P < 0.05 between PTHrP alone treatment group and additional use of ECM1 antibody (B, C). #P < 0.05 between PTHrP knockout group and additional use of ECM1(E, F).
ECM1 directly associated with PGRN growth factor and inhibited the chondroinductive activity of PGRN
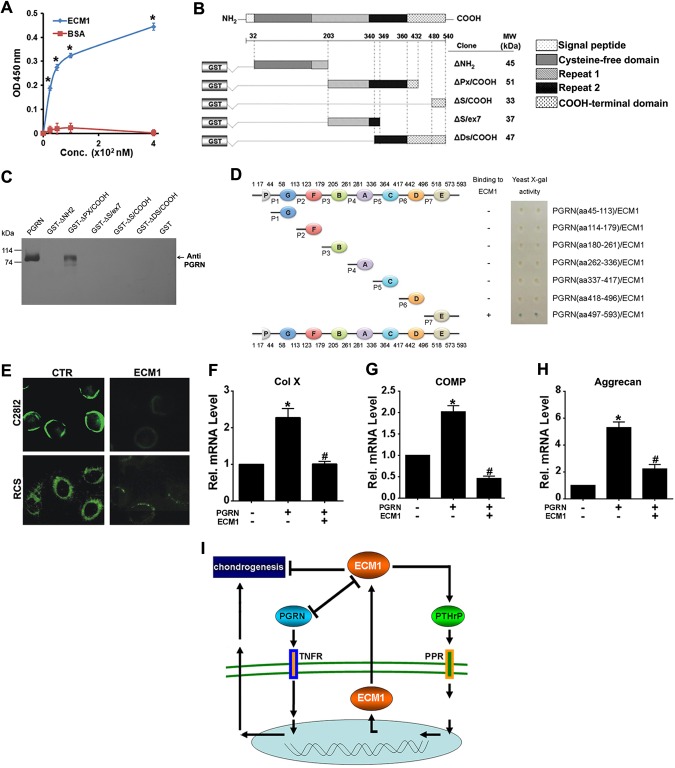
The attested critical role of PGRN in developmental chondrogenesis (20, 23), together with our observation of dramatically reduced PGRN expression in growth plate of Col IIa1–ECM1 transgenic mice (Fig. 4E), prompted us to determine whether ECM1 interplays with PGRN in chondrogenesis. We performed a solid-phase binding assay to test for direct binding between ECM1a and PGRN. As shown in Fig. 9A, ECM1a bound to PGRN in a dose-dependent manner. To demonstrate the specific domain of ECM1 required for binding to PGRN, various functional domains of ECM1 were cultured (Fig. 9B), followed by pull-down experiments against PGRN. The result revealed that ΔPx/COOH domain was responsible for PGRN–ECM1 binding (Fig. 9C). In addition, various motifs of PGRN were constructed, and a yeast-2 hybrid assay was performed to determine the PGRN binding sites for ECM1. As demonstrated in Fig. 9D, granulin E was identified as the PGRN fragment responsible for interaction with ECM1. Of importance, addition of recombinant ECM1 in culture medium markedly reduced the cell-surface localization of PGRN (Fig. 9E). Moreover, ECM1 repressed PGRN-mediated stimulation of chondrogenesis, as indicated by expression levels of Col X, cartilage oligomeric matrix protein, and aggrecan (Fig. 9F–H). Collectively, it seems that ECM1 and PGRN form an interplay network and act in concert to regulate chondrogenesis.
Figure 9.
ECM1 directly associated with PGRN growth factor and inhibited the chondroinductive activity of PGRN. A) ECM1 associated with PGRN directly (solid-phase binding). Microtiter plate coated with PGRN was incubated with various amounts of ECM1 or bovine serum albumin (BSA), and ECM1 bound to PGRN was detected by corresponding antibodies. B) Schematic representation of various ECM1a deletion mutants fused to GST. Amino acid residue numbers are indicated. The predicted molecular weights (kDa) of each recombinant fusion fragment are shown on the right. C) ΔPx/COOH domain of ECM1 is directly associated with PGRN assayed by pull-down assay. Purified ECM1 mutants fused to GST were separated on SDS-PAGE and visualized by Coomassie blue staining. D) PGRN unit E and its linker regions were responsible for binding with ECM1 protein, assayed by yeast-2 hybrid. All motifs of PGRN were isolated and cultured, and yeast-2 hybrid was performed. Schematic diagram of PGRN constructs used to map those of its fragments that bind to ECM1 (left). Galactosidase assays (right). E) ECM1 reduced PGRN localization on surface of C28i2 and rat chondrosarcoma cells chondrocyte progenitor cell lines. F–H) ECM1a inhibited PGRN-stimulated chondrogenesis. Total RNA was prepared from micromass cultures of C3H10T1/2 cells treated with 250 ng/ml recombinant PGRN in the absence or presence of 250 ng/ml recombinant ECM1 for 7 d, and mRNA expression of Col X (F), cartilage oligomeric matrix protein (COMP) (G), aggrecan (H), and GAPDH (serving as an internal control) were examined by RT-PCR. I) A proposed model for the role and regulation of ECM1 in chondrogenesis. ECM1, a downstream mediator of PTHrP, forms a positive regulatory loop with PTHrP and negatively regulates chondrogenesis. In addition, ECM1 also associates with PGRN, a chondrogenic growth factor that binds to TNF receptor (TNFR). Reciprocal inhibition between ECM1 and PGRN may contribute, at least in part, to ECM1-mediated suppression of chondrogenesis. PPR, PTH/PTHrP receptor. Values are means ± sd. *P < 0.05 vs. PBS treatment control group. #P < 0.05 between PGRN alone treatment group and additional use of ECM1.
DISCUSSION
Chondrogenesis in the growth plate is crucial for endochondral ossification during growth and development. Among all molecules involved, PTHrP is a well-established factor in maintenance of the growth plate (30–33). Microarray has proven that ECM1 is a late-response, downstream molecule of PTHrP signaling, which suggests that ECM1 may be a critical mediator for PTHrP regulation of chondrogenesis (14). Here, we found that ECM1 was expressed during chondrogenesis in vitro and during growth plate development in vivo. Of interest, this expression pattern was altered dramatically in presence or absence of PTHrP, which implies that ECM1 expression depends on PTHrP. Moreover, blockage of ECM1 remarkably abolished, whereas administration of exogenous ECM1 greatly enhanced, the effect of PTHrP. It is well established that growth plate is disordered in PTHrP knockout mice (34, 35). In the current study, we found that ECM1 supplementation rescued growth plate defects caused by absence of PTHrP. Collectively, ECM1 seems to be a key downstream molecule of PTHrP and forms a positive feedback loop with PTHrP.
To study the role of ECM1 in skeletogenesis in vivo, we generated 2 tissue-specific ECM1 transgenic mice, including Col I–ECM1 transgenic mice and Col IIa1–ECM1 transgenic mice. In Col I–ECM1 transgenic mice, ECM1 was specifically overexpressed in osteoblasts, which led to dwarf-like changes in body status, delayed bone growth, and declining bone quality. This result is consistent with previous in vitro results concerning the role of ECM1 in osteoblastogenesis (15). In Col IIa1–ECM1 transgenic mice, although there was no obvious phenotype, immunohistochemistry revealed altered levels of chondrogenic marker genes in the growth plate, among which PTHrP and ADAMTS-7 were elevated. Altered expression of chondrogenic markers, together with the finding that ECM1 was induced in response to PTHrP treatment, may suggest that ECM1 and PTHrP constitute a positive feedback loop in the regulation of chondrogenesis. We previously reported that ADAMTS-7 was a key downstream molecule of PTHrP that negatively regulated chondrogenesis and played a detrimental role in pathogenesis of osteoarthritis (21, 22, 25, 36, 37); therefore, it is possible that ECM1 enhanced PTHrP level, which subsequently promoted expression of ADAMTS-7.
ECM1 largely corrects accelerated chondrocyte hypertrophy of fetal PTHrP-null metatarsals, and skeletal phenotypes of mice that overexpress ECM1 resemble the PTHrP transgenic phenotype, which suggests a genetic interaction between PTHrP and ECM1 in vivo. Mice that are homozygous for the PTHrP-null mutation die postnatally and exhibit widespread abnormalities of endochondral bone development (38); however, whether overexpression of ECM1 may normalize PTHrP-null skeletal phenotype remains to be demonstrated. ECM1 transgenic and PTHrP-null double mutants may have reversal of the early exit of proliferation that occurs in PTHrP-null mutants as well as the restoration of normal chondrocyte proliferation rates, which are decreased in PTHrP-null mutants. Furthermore, overexpression of ECM1 may prevent or reverse premature hypertrophic differentiation that occurs in PTHrP-null mutants (38, 39). It has been reported that chondrocyte-specific overexpression of PTHrP causes a profound delay in the developmental program of chondrocyte differentiation and endochondral ossification (40), which is quite similar to the observed phenotype of our Col IIa1–ECM1 transgenic mice. It is expected that both ECM1 overexpression, investigated in this study, and deficiency of ECM1 may lead to abnormal development of growth plate cartilage, which are similar to developmental abnormalities observed in PTHrP genetically modified mice (38–40).
In Col IIa1–ECM1 transgenic mice, immunohistochemistry revealed markedly diminished level of PGRN in the growth plate. PGRN is a growth factor with multiple functions (41, 42), and is expressed in various cells. PGRN plays a critical role in diverse physiologic and disease processes, such as wound healing (43), tumorigenesis (41), inflammation (42, 44–48), and degeneration of the nervous system (49–51). It has been found that PGRN activates ERK1/2 signaling and enhances chondrogenesis during developmental and regenerative processes (20, 52). PGRN is expressed in human articular cartilage, and its level is significantly elevated in cartilage of patients with osteoarthritis and rheumatoid arthritis (22). Moreover, PGRN is expressed in the growth plate and plays a crucial role in chondrocyte proliferation and differentiation, and in endochondral ossification of the growth plate during development (20, 21). We previously reported binding of PGRN to TNF receptors, which protected against cartilage destruction in inflammatory arthritis (42, 44, 53, 54). In the current study, ECM1 transgenic mice exhibited dramatically reduced signal of PGRN in growth plate, and ECM1 inhibited cell-surface localization of PGRN. This suggests that ECM1 might sequester PGRN, which inhibits PGRN from binding to its receptor, and, thus, blocks PGRN function. Furthermore, various protein–protein interaction assays revealed that PGRN directly bound to ECM1 and antagonized the chondrogenesis mediated by PGRN. On the basis of previously published data and data obtained in this study, we present a proposed model (Fig. 9I) wherein ECM1 forms a positive feedback loop with PTHrP and negatively regulates chondrogenesis via its interaction with PGRN chondrogenic growth factor. This study links together 2 important signaling pathways during chondrogenesis and cartilage development, that is, PTHrP and PGRN, through ECM1. Future studies are warranted to clarify the crosstalk and interplay between PTH/PTHrP and PGRN pathways, which will not only better our understanding of the signaling pathways in control of cartilage and skeletal development, but may lead to innovative therapies for cartilage regeneration and other musculoskeletal diseases by selectively targeting these pathways.
Acknowledgments
The authors thank Dr. Brendan Lee (Baylor College of Medicine, Houston, TX, USA) for providing both Col II– and Col I–specific transgenic constructs used for generating ECM1 transgenic mice in this study, and Aubryanna Hettinghouse (New York University School of Medicine) for assistance in preparing the manuscript. This work was supported in part by U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grants R01-AR062207 and R01-AR061484), and a Disease Targeted Research Grant from the Rheumatology Research Foundation (to C.-J.L.).
Glossary
- BMP-2
bone morphogenetic protein 2
- BV/TV
bone volume/total volume
- ECM1
extracellular matrix protein 1
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HEK293
human embryonic kidney 293
- hMSC
human mesenchymal stem cell
- IHH
Indian hedgehog
- micro-CT
micro–computed tomography
- PGRN
progranulin
- PTHrP
parathyroid hormone–related peptide
- siRNA
small interfering RNA
- TBS
Tris-buffered saline
- WT
wild-type
REFERENCES
- 1.Kronenberg H. M. (2003) Developmental regulation of the growth plate. Nature 423, 332–336 [DOI] [PubMed] [Google Scholar]
- 2.Paroly S. S., Wang F., Spraggon L., Merregaert J., Batourina E., Tycko B., Schmidt-Ott K. M., Grimmond S., Little M., Mendelsohn C. (2013) Stromal protein Ecm1 regulates ureteric bud patterning and branching. PLoS One 8, e84155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamoto K., Gibney B. C., Lee G. S., Ackermann M., Konerding M. A., Tsuda A., Mentzer S. J. (2013) Migration of CD11b+ accessory cells during murine lung regeneration. Stem Cell Res. (Amst.) 10, 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamada T., McLean W. H., Ramsay M., Ashton G. H., Nanda A., Jenkins T., Edelstein I., South A. P., Bleck O., Wessagowit V., Mallipeddi R., Orchard G. E., Wan H., Dopping-Hepenstal P. J., Mellerio J. E., Whittock N. V., Munro C. S., van Steensel M. A., Steijlen P. M., Ni J., Zhang L., Hashimoto T., Eady R. A., McGrath J. A. (2002) Lipoid proteinosis maps to 1q21 and is caused by mutations in the extracellular matrix protein 1 gene (ECM1). Hum. Mol. Genet. 11, 833–840 [DOI] [PubMed] [Google Scholar]
- 5.Hamada T., Wessagowit V., South A. P., Ashton G. H., Chan I., Oyama N., Siriwattana A., Jewhasuchin P., Charuwichitratana S., Thappa D. M., Jeevankumar B., Lenane P., Krafchik B., Kulthanan K., Shimizu H., Kaya T. I., Erdal M. E., Paradisi M., Paller A. S., Seishima M., Hashimoto T., McGrath J. A. (2003) Extracellular matrix protein 1 gene (ECM1) mutations in lipoid proteinosis and genotype-phenotype correlation. J. Invest. Dermatol. 120, 345–350 [DOI] [PubMed] [Google Scholar]
- 6.Quirici M. B., da Rocha A. J. (2013) Teaching NeuroImages: lipoid proteinosis (Urbach-Wiethe disease): typical findings in this rare genodermatosis. Neurology 80, e93 [DOI] [PubMed] [Google Scholar]
- 7.Chan I., Oyama N., Neill S. M., Wojnarowska F., Black M. M., McGrath J. A. (2004) Characterization of IgG autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Clin. Exp. Dermatol. 29, 499–504 [DOI] [PubMed] [Google Scholar]
- 8.Oyama N., Chan I., Neill S. M., South A. P., Wojnarowska F., Kawakami Y., D’Cruz D., Mepani K., Hughes G. J., Bhogal B. S., Kaneko F., Black M. M., McGrath J. A. (2004) Development of antigen-specific ELISA for circulating autoantibodies to extracellular matrix protein 1 in lichen sclerosus. J. Clin. Invest. 113, 1550–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyama N., Chan I., Neill S. M., Hamada T., South A. P., Wessagowit V., Wojnarowska F., D’Cruz D., Hughes G. J., Black M. M., McGrath J. A. (2003) Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet 362, 118–123 [DOI] [PubMed] [Google Scholar]
- 10.Edmonds E., Barton G., Buisson S., Francis N., Gotch F., Game L., Haddad M., Dinneen M., Bunker C. (2011) Gene expression profiling in male genital lichen sclerosus. Int. J. Exp. Pathol. 92, 320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung C., Hugot J. P. (2009) Inflammatory bowel diseases: the genetic revolution. Gastroenterol. Clin. Biol. 33(Suppl 3), S123–S130 [DOI] [PubMed] [Google Scholar]
- 12.Fisher S. A., Tremelling M., Anderson C. A., Gwilliam R., Bumpstead S., Prescott N. J., Nimmo E. R., Massey D., Berzuini C., Johnson C., Barrett J. C., Cummings F. R., Drummond H., Lees C. W., Onnie C. M., Hanson C. E., Blaszczyk K., Inouye M., Ewels P., Ravindrarajah R., Keniry A., Hunt S., Carter M., Watkins N., Ouwehand W., Lewis C. M., Cardon L., Lobo A., Forbes A., Sanderson J., Jewell D. P., Mansfield J. C., Deloukas P., Mathew C. G., Parkes M., Satsangi J.; Wellcome Trust Case Control Consortium (2008) Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn’s disease. Nat. Genet. 40, 710–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Zhang Y., Liu Z., Wu X., Zheng Y., Tao Z., Mao K., Wang J., Lin G., Tian L., Ji Y., Qin M., Sun S., Zhu X., Sun B. (2011) ECM1 controls TH2 cell egress from lymph nodes through re-expression of S1P1. Nat. Immunol. 12, 178–185 [DOI] [PubMed] [Google Scholar]
- 14.Hoogendam J., Farih-Sips H., van Beek E., Löwik C. W., Wit J. M., Karperien M. (2007) Novel late response genes of PTHrP in chondrocytes. Horm. Res. 67, 159–170 [DOI] [PubMed] [Google Scholar]
- 15.Deckers M. M., Smits P., Karperien M., Ni J., Tylzanowski P., Feng P., Parmelee D., Zhang J., Bouffard E., Gentz R., Löwik C. W., Merregaert J. (2001) Recombinant human extracellular matrix protein 1 inhibits alkaline phosphatase activity and mineralization of mouse embryonic metatarsals in vitro. Bone 28, 14–20 [DOI] [PubMed] [Google Scholar]
- 16.Sercu S., Zhang L., Merregaert J. (2008) The extracellular matrix protein 1: its molecular interaction and implication in tumor progression. Cancer Invest. 26, 375–384 [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto N., Terlizzi J., Aho S., Brittingham R., Fertala A., Oyama N., McGrath J. A., Uitto J. (2006) Extracellular matrix protein 1 inhibits the activity of matrix metalloproteinase 9 through high-affinity protein/protein interactions. Exp. Dermatol. 15, 300–307 [DOI] [PubMed] [Google Scholar]
- 18.Kong L., Tian Q., Guo F., Mucignat M. T., Perris R., Sercu S., Merregaert J., Di Cesare P. E., Liu C. J. (2010) Interaction between cartilage oligomeric matrix protein and extracellular matrix protein 1 mediates endochondral bone growth. Matrix Biol. 29, 276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sercu S., Zhang M., Oyama N., Hansen U., Ghalbzouri A. E., Jun G., Geentjens K., Zhang L., Merregaert J. H. (2008) Interaction of extracellular matrix protein 1 with extracellular matrix components: ECM1 is a basement membrane protein of the skin. J. Invest. Dermatol. 128, 1397–1408 [DOI] [PubMed] [Google Scholar]
- 20.Feng J. Q., Guo F. J., Jiang B. C., Zhang Y., Frenkel S., Wang D. W., Tang W., Xie Y., Liu C. J. (2010) Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. FASEB J. 24, 1879–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai X. H., Wang D. W., Kong L., Zhang Y., Luan Y., Kobayashi T., Kronenberg H. M., Yu X. P., Liu C. J. (2009) ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol. Cell. Biol. 29, 4201–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo F., Lai Y., Tian Q., Lin E. A., Kong L., Liu C. (2010) Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 62, 2023–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu K., Zhang Y., Ilalov K., Carlson C. S., Feng J. Q., Di Cesare P. E., Liu C. J. (2007) Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J. Biol. Chem. 282, 11347–11355 [DOI] [PubMed] [Google Scholar]
- 24.Napierala D., Sun Y., Maciejewska I., Bertin T. K., Dawson B., D’Souza R., Qin C., Lee B. (2012) Transcriptional repression of the Dspp gene leads to dentinogenesis imperfecta phenotype in Col1a1-Trps1 transgenic mice. J. Bone Miner. Res. 27, 1735–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai Y., Bai X., Zhao Y., Tian Q., Liu B., Lin E. A., Chen Y., Lee B., Appleton C. T., Beier F., Yu X. P., Liu C. J. (2014) ADAMTS-7 forms a positive feedback loop with TNF-α in the pathogenesis of osteoarthritis. Ann. Rheum. Dis. 73, 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luan Y., Kong L., Howell D. R., Ilalov K., Fajardo M., Bai X. H., Di Cesare P. E., Goldring M. B., Abramson S. B., Liu C. J. (2008) Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis Cartilage 16, 1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian Q. Y., Zhao Y. P., Liu C. J. (2012) Modified yeast-two-hybrid system to identify proteins interacting with the growth factor progranulin. J. Vis. Exp. 59, 3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mongiat M., Fu J., Oldershaw R., Greenhalgh R., Gown A. M., Iozzo R. V. (2003) Perlecan protein core interacts with extracellular matrix protein 1 (ECM1), a glycoprotein involved in bone formation and angiogenesis. J. Biol. Chem. 278, 17491–17499 [DOI] [PubMed] [Google Scholar]
- 29.Correa D., Hesse E., Seriwatanachai D., Kiviranta R., Saito H., Yamana K., Neff L., Atfi A., Coillard L., Sitara D., Maeda Y., Warming S., Jenkins N. A., Copeland N. G., Horne W. C., Lanske B., Baron R. (2010) Zfp521 is a target gene and key effector of parathyroid hormone-related peptide signaling in growth plate chondrocytes. Dev. Cell 19, 533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanske B., Karaplis A. C., Lee K., Luz A., Vortkamp A., Pirro A., Karperien M., Defize L. H., Ho C., Mulligan R. C., Abou-Samra A. B., Jüppner H., Segre G. V., Kronenberg H. M. (1996) PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273, 663–666 [DOI] [PubMed] [Google Scholar]
- 31.Vortkamp A., Lee K., Lanske B., Segre G. V., Kronenberg H. M., Tabin C. J. (1996) Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273, 613–622 [DOI] [PubMed] [Google Scholar]
- 32.Hirai T., Chagin A. S., Kobayashi T., Mackem S., Kronenberg H. M. (2011) Parathyroid hormone/parathyroid hormone-related protein receptor signaling is required for maintenance of the growth plate in postnatal life. Proc. Natl. Acad. Sci. USA 108, 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J., Liu M., Yang D., Bouxsein M. L., Thomas C. C., Schipani E., Bringhurst F. R., Kronenberg H. M. (2010) Phospholipase C signaling via the parathyroid hormone (PTH)/PTH-related peptide receptor is essential for normal bone responses to PTH. Endocrinology 151, 3502–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi T., Chung U. I., Schipani E., Starbuck M., Karsenty G., Katagiri T., Goad D. L., Lanske B., Kronenberg H. M. (2002) PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development 129, 2977–2986 [DOI] [PubMed] [Google Scholar]
- 35.Guo J., Chung U. I., Kondo H., Bringhurst F. R., Kronenberg H. M. (2002) The PTH/PTHrP receptor can delay chondrocyte hypertrophy in vivo without activating phospholipase C. Dev. Cell 3, 183–194 [DOI] [PubMed] [Google Scholar]
- 36.Lai Y., Bai X., Zhao Y., Tian Q., Liu B., Lin E. A., Chen Y., Lee B., Appleton C. T., Beier F., Yu X. P., Liu C. J. (2014) ADAMTS-7 forms a positive feedback loop with TNF-α in the pathogenesis of osteoarthritis. Ann. Rheum. Dis. 73, 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckland J. (2013) Osteoarthritis: positive feedback between ADAMTS-7 and TNF in OA. Nat. Rev. Rheumatol. 9, 566 [DOI] [PubMed] [Google Scholar]
- 38.Karaplis A. C., Luz A., Glowacki J., Bronson R. T., Tybulewicz V. L., Kronenberg H. M., Mulligan R. C. (1994) Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 8, 277–289 [DOI] [PubMed] [Google Scholar]
- 39.Amizuka N., Warshawsky H., Henderson J. E., Goltzman D., Karaplis A. C. (1994) Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J. Cell Biol. 126, 1611–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karaplis A. C., Kronenberg H. M. (1996) Physiological roles for parathyroid hormone-related protein: lessons from gene knockout mice. Vitam. Horm. 52, 177–193 [DOI] [PubMed] [Google Scholar]
- 41.Bateman A., Bennett H. P. (2009) The granulin gene family: from cancer to dementia. BioEssays 31, 1245–1254 [DOI] [PubMed] [Google Scholar]
- 42.Liu C. J. (2011) Progranulin: a promising therapeutic target for rheumatoid arthritis. FEBS Lett. 585, 3675–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He Z., Ong C. H., Halper J., Bateman A. (2003) Progranulin is a mediator of the wound response. Nat. Med. 9, 225–229 [DOI] [PubMed] [Google Scholar]
- 44.Tang W., Lu Y., Tian Q. Y., Zhang Y., Guo F. J., Liu G. Y., Syed N. M., Lai Y., Lin E. A., Kong L., Su J., Yin F., Ding A. H., Zanin-Zhorov A., Dustin M. L., Tao J., Craft J., Yin Z., Feng J. Q., Abramson S. B., Yu X. P., Liu C. J. (2011) The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332, 478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kessenbrock K., Fröhlich L., Sixt M., Lämmermann T., Pfister H., Bateman A., Belaaouaj A., Ring J., Ollert M., Fässler R., Jenne D. E. (2008) Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J. Clin. Invest. 118, 2438–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin F., Banerjee R., Thomas B., Zhou P., Qian L., Jia T., Ma X., Ma Y., Iadecola C., Beal M. F., Nathan C., Ding A. (2010) Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J. Exp. Med. 207, 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J., Nathan C., Jin W., Sim D., Ashcroft G. S., Wahl S. M., Lacomis L., Erdjument-Bromage H., Tempst P., Wright C. D., Ding A. (2002) Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 111, 867–878 [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y. P., Tian Q. Y., Liu C. J. (2013) Progranulin deficiency exaggerates, whereas progranulin-derived Atsttrin attenuates, severity of dermatitis in mice. FEBS Lett. 587, 1805–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker M., Mackenzie I. R., Pickering-Brown S. M., Gass J., Rademakers R., Lindholm C., Snowden J., Adamson J., Sadovnick A. D., Rollinson S., Cannon A., Dwosh E., Neary D., Melquist S., Richardson A., Dickson D., Berger Z., Eriksen J., Robinson T., Zehr C., Dickey C. A., Crook R., McGowan E., Mann D., Boeve B., Feldman H., Hutton M. (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919 [DOI] [PubMed] [Google Scholar]
- 50.Cruts M., Gijselinck I., van der Zee J., Engelborghs S., Wils H., Pirici D., Rademakers R., Vandenberghe R., Dermaut B., Martin J. J., van Duijn C., Peeters K., Sciot R., Santens P., De Pooter T., Mattheijssens M., Van den Broeck M., Cuijt I., Vennekens K., De Deyn P. P., Kumar-Singh S., Van Broeckhoven C. (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442, 920–924 [DOI] [PubMed] [Google Scholar]
- 51.Wils H., Kleinberger G., Pereson S., Janssens J., Capell A., Van Dam D., Cuijt I., Joris G., De Deyn P. P., Haass C., Van Broeckhoven C., Kumar-Singh S. (2012) Cellular ageing, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J. Pathol. 228, 67–76 [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y. P., Tian Q. Y., Frenkel S., Liu C. J. (2013) The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials 34, 6412–6421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C. J., Bosch X. (2012) Progranulin: a growth factor, a novel TNFR ligand and a drug target. Pharmacol. Ther. 133, 124–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu H., Siegel R. M. (2011) Medicine. Progranulin resolves inflammation. Science 332, 427–428 [DOI] [PMC free article] [PubMed] [Google Scholar]